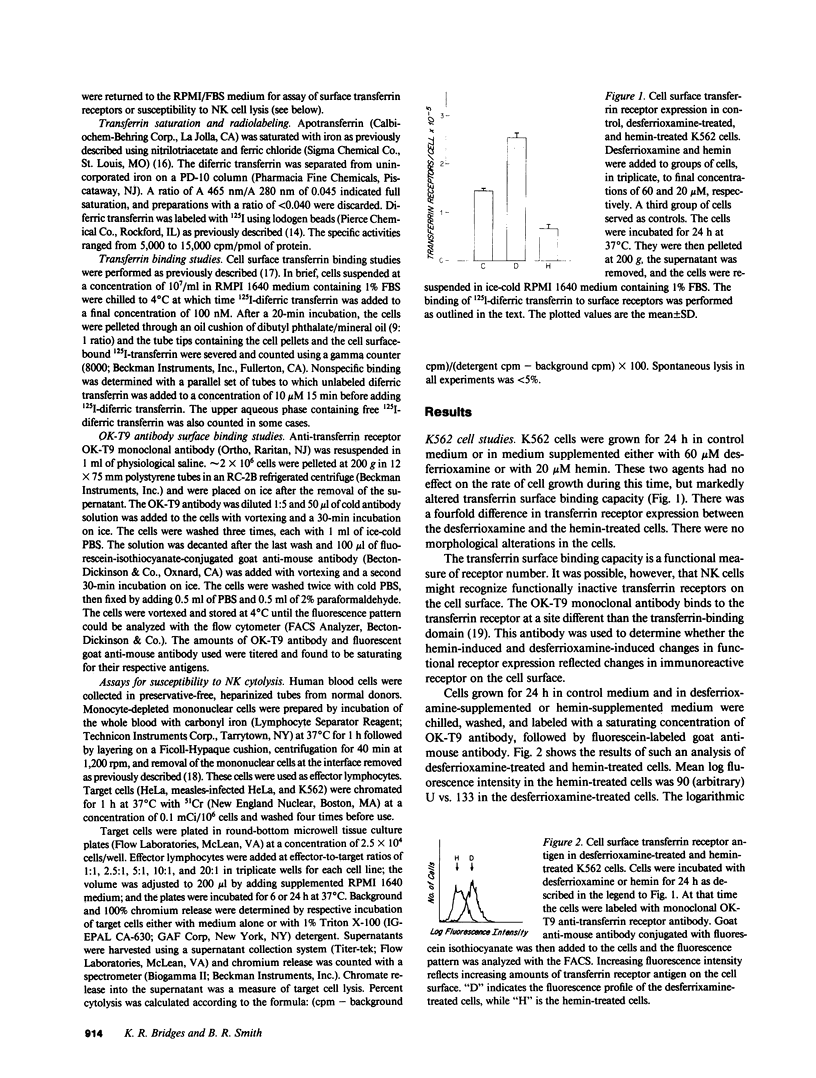
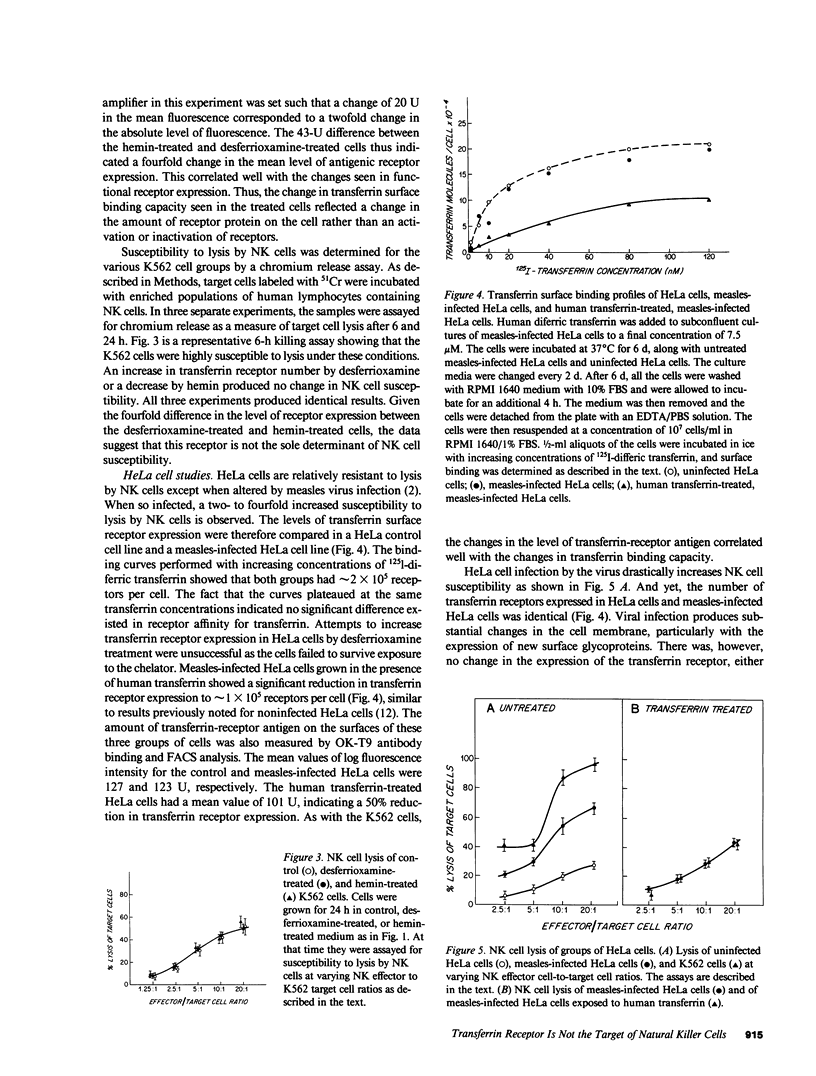
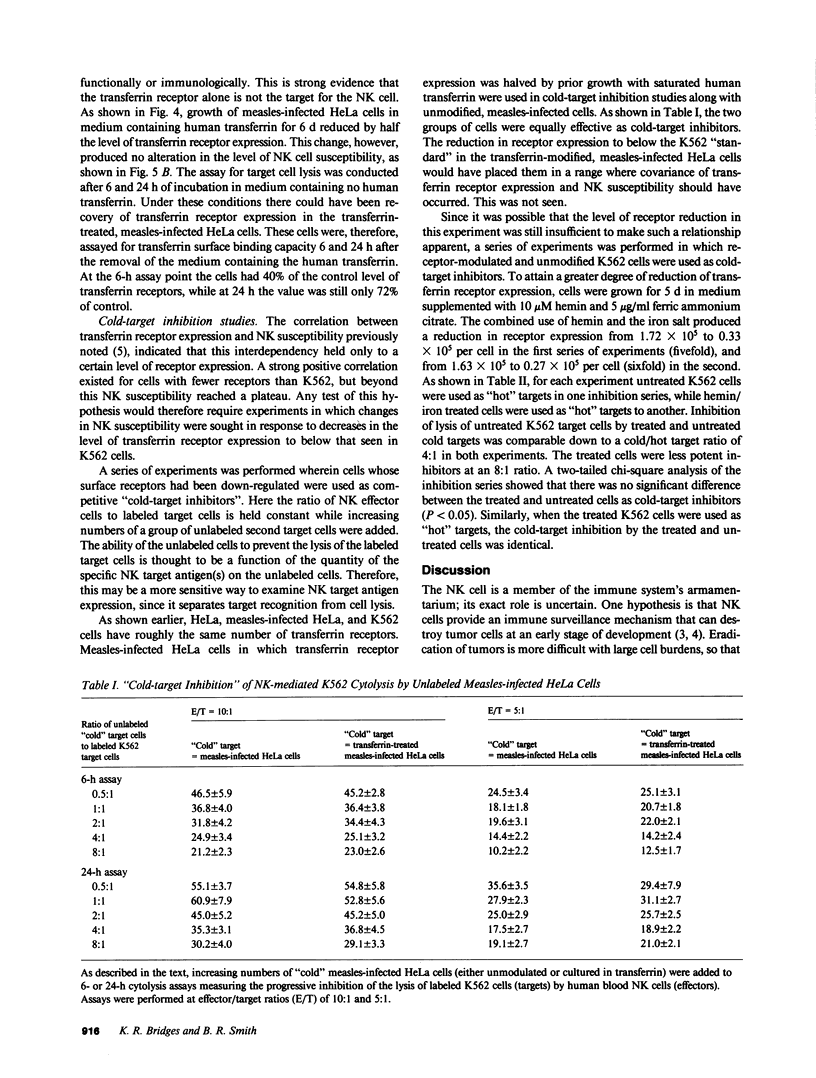
Abstract
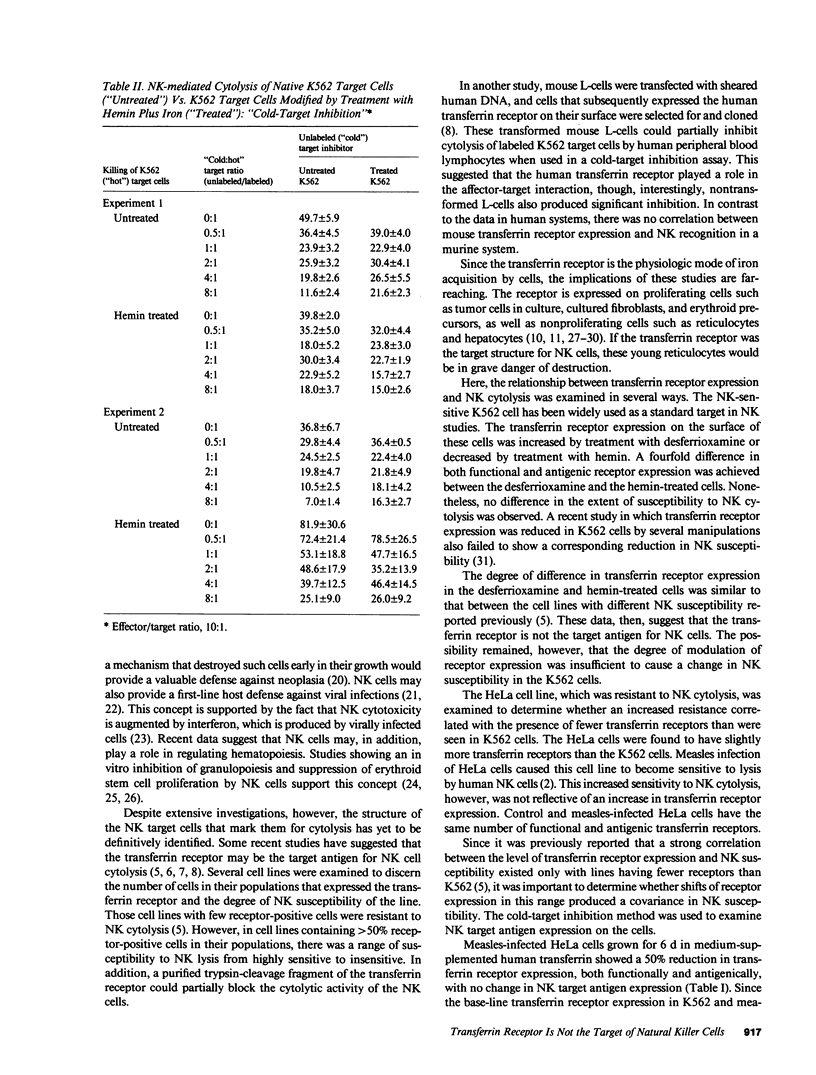
Expression of the transferrin receptor on target cell lines has recently been implicated as a determinant of susceptibility to cytolysis by natural killer (NK) lymphocytes. We have examined this proposed relationship in several ways. First, K562 (a cell line highly vulnerable to NK lysis) cells were grown for 24 h in the iron chelator desferrioxamine. Under these conditions, the cells doubled their surface transferrin receptor expression as determined both by radioligand binding and surface binding of the OK-T9 monoclonal anti-transferrin receptor antibody. In contrast, cells grown for the same period of time in hemin halved their receptor expression. This fourfold change in transferrin receptor expression between the desferrioxamine-treated and hemin-treated cells produced no change in susceptibility to NK cytolysis. Second, HeLa (a cell line which in its native state is very resistant to NK cytolysis) cells were compared with K562 cells with respect to surface transferrin receptor expression. The difference in NK susceptibility of the two cell lines was not reflected in differences in transferrin receptor expression: the K562 cells expressed approximately 1.5 X 10(5) receptors per cell while HeLa cells expressed 2.0 X 10(5) receptors/cell. Third, infection of HeLa cells by measles virus greatly increased their susceptibility to NK lysis but produced no change in surface transferrin receptor expression. Furthermore, when measles-infected HeLa cells were grown for 6 d in medium supplemented with iron-saturated human transferrin they underwent a 50% reduction in receptor expression but no change in NK susceptibility. Finally, possible alterations in the surface expression of NK target antigens on modified cells were further assayed by their ability to serve as cold-target inhibitors of cytolysis of NK-sensitive target cells. We examined two groups of cells in which transferrin receptor expression was reduced. These were the transferrin-treated, measles-infected HeLa cells with the 50% receptor reduction, and K562 cells grown in medium containing hemin and iron salts where the reduction was five- to sixfold relative to control. In neither case was there a change in the apparent expression of NK target antigen(s). We conclude that there is a discordance between transferrin receptor expression and susceptibility to NK cytolysis in the model systems examined. Therefore, it is unlikely that the transferrin receptor per se is the target recognition structure for human NK cells, although a role in concert with other, as yet undefined molecules, cannot be excluded.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aisen P., Listowsky I. Iron transport and storage proteins. Annu Rev Biochem. 1980;49:357–393. doi: 10.1146/annurev.bi.49.070180.002041. [DOI] [PubMed] [Google Scholar]
- Alarcón B., Fresno M. Specific effect of anti-transferrin antibodies on natural killer cells directed against tumor cells. Evidence for the transferrin receptor being one of the target structures recognized by NK cells. J Immunol. 1985 Feb;134(2):1286–1291. [PubMed] [Google Scholar]
- Ault K. A., Weiner H. L. Natural killing of measles-infected cells by human lymphocytes. J Immunol. 1979 Jun;122(6):2611–2616. [PubMed] [Google Scholar]
- Baines M. G., Lafleur F. L., Holbein B. E. Involvement of transferrin and transferrin receptors in human natural killer effector:target interaction. Immunol Lett. 1983;7(1):51–55. doi: 10.1016/0165-2478(83)90055-x. [DOI] [PubMed] [Google Scholar]
- Bancroft G. J., Shellam G. R., Chalmer J. E. Genetic influences on the augmentation of natural killer (NK) cells during murine cytomegalovirus infection: correlation with patterns of resistance. J Immunol. 1981 Mar;126(3):988–994. [PubMed] [Google Scholar]
- Bridges K. R., Cudkowicz A. Effect of iron chelators on the transferrin receptor in K562 cells. J Biol Chem. 1984 Nov 10;259(21):12970–12977. [PubMed] [Google Scholar]
- Dennert G. Cloned lines of natural killer cells. Nature. 1980 Sep 4;287(5777):47–49. doi: 10.1038/287047a0. [DOI] [PubMed] [Google Scholar]
- Dokhélar M. C., Garson D., Testa U., Tursz T. Target structure for natural killer cells: evidence against a unique role for transferrin receptor. Eur J Immunol. 1984 Apr;14(4):340–344. doi: 10.1002/eji.1830140412. [DOI] [PubMed] [Google Scholar]
- Durie B. G., Salmon S. E., Moon T. E. Pretreatment tumor mass, cell kinetics, and prognosis in multiple myeloma. Blood. 1980 Mar;55(3):364–372. [PubMed] [Google Scholar]
- Einhorn S., Blomgren H., Strander H. Interferon and spontaneous cytotoxicity in man. I. Enhancement of the spontaneous cytotoxicity of peripheral lymphocytes by human leukocyte interferon. Int J Cancer. 1978 Oct 15;22(4):405–412. doi: 10.1002/ijc.2910220407. [DOI] [PubMed] [Google Scholar]
- Frazier J. L., Caskey J. H., Yoffe M., Seligman P. A. Studies of the transferrin receptor on both human reticulocytes and nucleated human cells in culture: comparison of factors regulating receptor density. J Clin Invest. 1982 Apr;69(4):853–865. doi: 10.1172/JCI110525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson M., Beran M., Andersson B., Kiessling R. Inhibition of in vitro granulopoiesis by autologous allogeneic human NK cells. J Immunol. 1982 Jul;129(1):126–132. [PubMed] [Google Scholar]
- Herberman R. B., Ortaldo J. R. Natural killer cells: their roles in defenses against disease. Science. 1981 Oct 2;214(4516):24–30. doi: 10.1126/science.7025208. [DOI] [PubMed] [Google Scholar]
- Holmberg L. A., Miller B. A., Ault K. A. The effect of natural killer cells on the development of syngeneic hematopoietic progenitors. J Immunol. 1984 Dec;133(6):2933–2939. [PubMed] [Google Scholar]
- Iacopetta B. J., Morgan E. H. The kinetics of transferrin endocytosis and iron uptake from transferrin in rabbit reticulocytes. J Biol Chem. 1983 Aug 10;258(15):9108–9115. [PubMed] [Google Scholar]
- Iacopetta B. J., Morgan E. H., Yeoh G. C. Transferrin receptors and iron uptake during erythroid cell development. Biochim Biophys Acta. 1982 May 7;687(2):204–210. doi: 10.1016/0005-2736(82)90547-8. [DOI] [PubMed] [Google Scholar]
- Klausner R. D., Van Renswoude J., Ashwell G., Kempf C., Schechter A. N., Dean A., Bridges K. R. Receptor-mediated endocytosis of transferrin in K562 cells. J Biol Chem. 1983 Apr 25;258(8):4715–4724. [PubMed] [Google Scholar]
- Larrick J. W., Cresswell P. Modulation of cell surface iron transferrin receptors by cellular density and state of activation. J Supramol Struct. 1979;11(4):579–586. doi: 10.1002/jss.400110415. [DOI] [PubMed] [Google Scholar]
- Mangan K. F., Hartnett M. E., Matis S. A., Winkelstein A., Abo T. Natural killer cells suppress human erythroid stem cell proliferation in vitro. Blood. 1984 Feb;63(2):260–269. [PubMed] [Google Scholar]
- Mattia E., Rao K., Shapiro D. S., Sussman H. H., Klausner R. D. Biosynthetic regulation of the human transferrin receptor by desferrioxamine in K562 cells. J Biol Chem. 1984 Mar 10;259(5):2689–2692. [PubMed] [Google Scholar]
- Newman R. A., Warner J. F., Dennert G. NK recognition of target structures: is the transferrin receptor the NK target structure? J Immunol. 1984 Oct;133(4):1841–1845. [PubMed] [Google Scholar]
- Nuñez M. T., Glass J. The transferrin cycle and iron uptake in rabbit reticulocytes. Pulse studies using 59Fe, 125I-labeled transferrin. J Biol Chem. 1983 Aug 25;258(16):9676–9680. [PubMed] [Google Scholar]
- Ortaldo J. R., Herberman R. B. Heterogeneity of natural killer cells. Annu Rev Immunol. 1984;2:359–394. doi: 10.1146/annurev.iy.02.040184.002043. [DOI] [PubMed] [Google Scholar]
- Pelicci P. G., Tabilio A., Thomopoulos P., Titeux M., Vainchenker W., Rochant H., Testa U. Hemin regulates the expression of transferrin receptors in human hematopoietic cell lines. FEBS Lett. 1982 Aug 23;145(2):350–354. doi: 10.1016/0014-5793(82)80198-1. [DOI] [PubMed] [Google Scholar]
- Rola-Pleszczynski M., Lieu H. Human natural cytotoxic lymphocytes: definition by a monoclonal antibody of a subset which kills an anchorage-dependent target cell line but not the K-562 cell line. Cell Immunol. 1983 Dec;82(2):326–333. doi: 10.1016/0008-8749(83)90166-1. [DOI] [PubMed] [Google Scholar]
- Sutherland R., Delia D., Schneider C., Newman R., Kemshead J., Greaves M. Ubiquitous cell-surface glycoprotein on tumor cells is proliferation-associated receptor for transferrin. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4515–4519. doi: 10.1073/pnas.78.7.4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timonen T., Reynolds C. W., Ortaldo J. R., Herberman R. B. Isolation of human and rat natural killer cells. J Immunol Methods. 1982;51(3):269–277. doi: 10.1016/0022-1759(82)90393-3. [DOI] [PubMed] [Google Scholar]
- Vodinelich L., Sutherland R., Schneider C., Newman R., Greaves M. Receptor for transferrin may be a "target" structure for natural killer cells. Proc Natl Acad Sci U S A. 1983 Feb;80(3):835–839. doi: 10.1073/pnas.80.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J. H., Kushner J. P., Kaplan J. Regulation of HeLa cell transferrin receptors. J Biol Chem. 1982 Sep 10;257(17):10317–10323. [PubMed] [Google Scholar]
- Young S. P., Aisen P. Transferrin receptors and the uptake and release of iron by isolated hepatocytes. Hepatology. 1981 Mar-Apr;1(2):114–119. doi: 10.1002/hep.1840010205. [DOI] [PubMed] [Google Scholar]
- Zaman Z., Heynen M. J., Verwilghen R. L. Studies on the mechanism of transferrin iron uptake by rat reticulocytes. Biochim Biophys Acta. 1980 Nov 3;632(4):553–561. doi: 10.1016/0304-4165(80)90332-3. [DOI] [PubMed] [Google Scholar]