Abstract
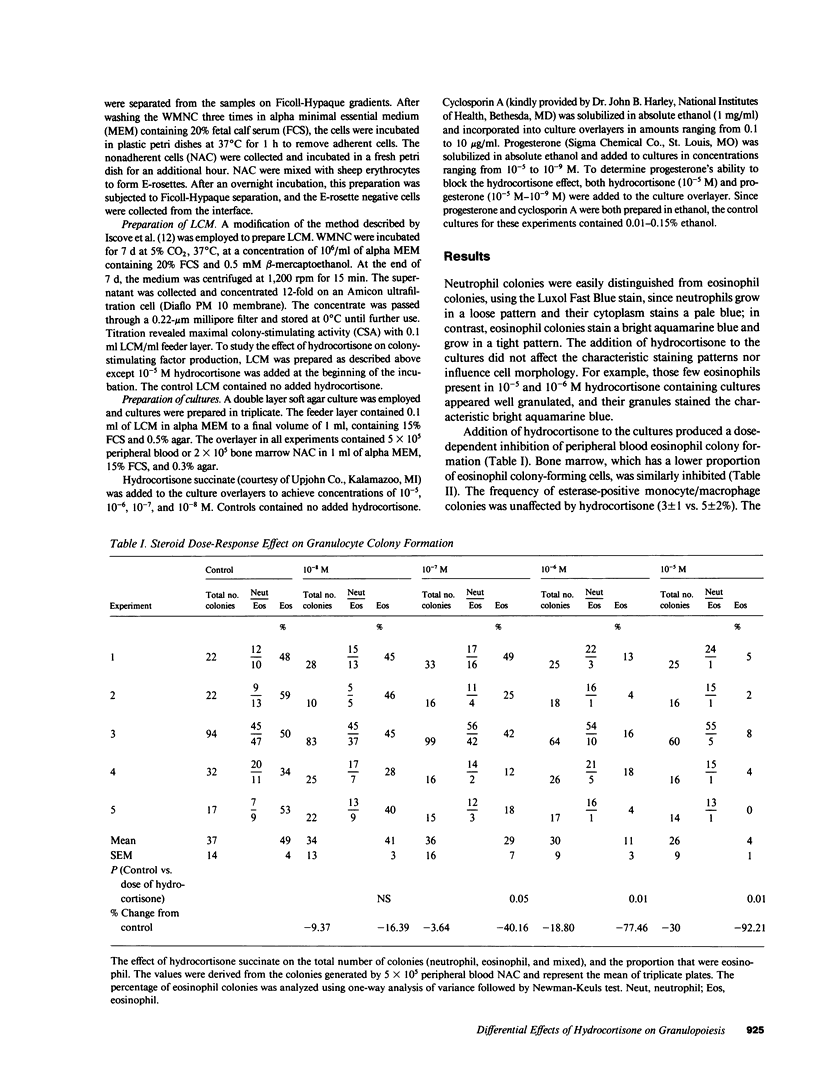
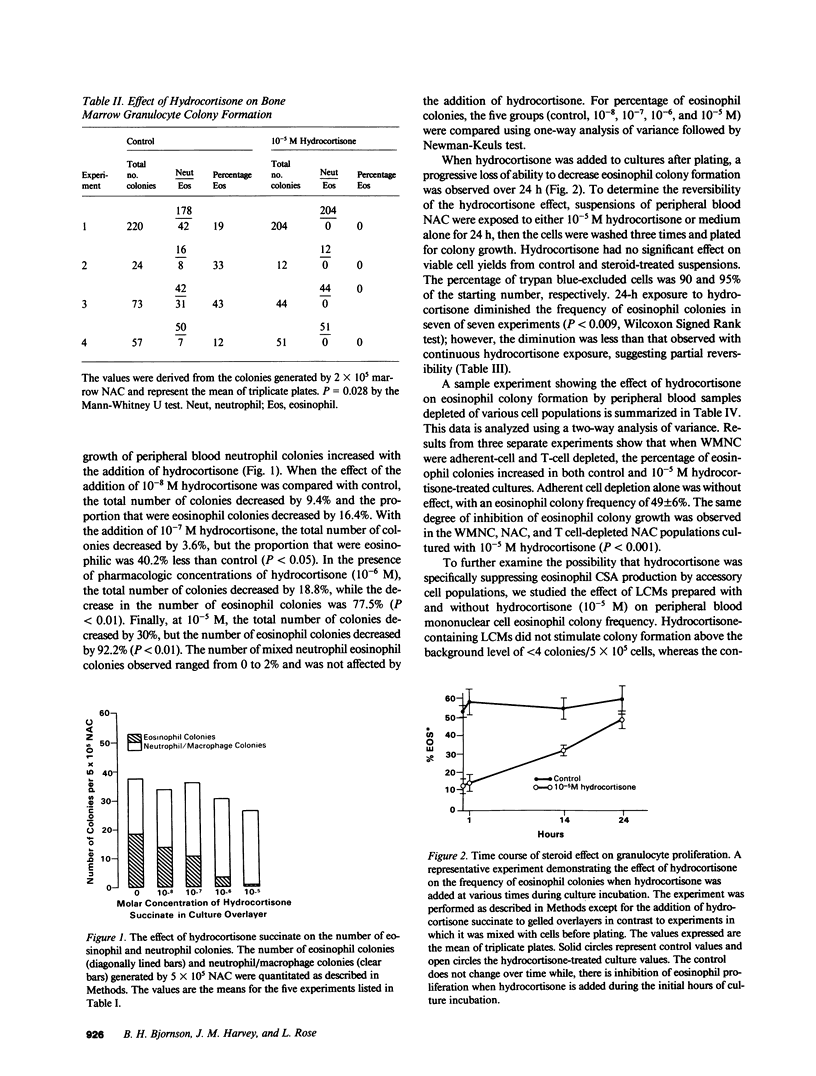
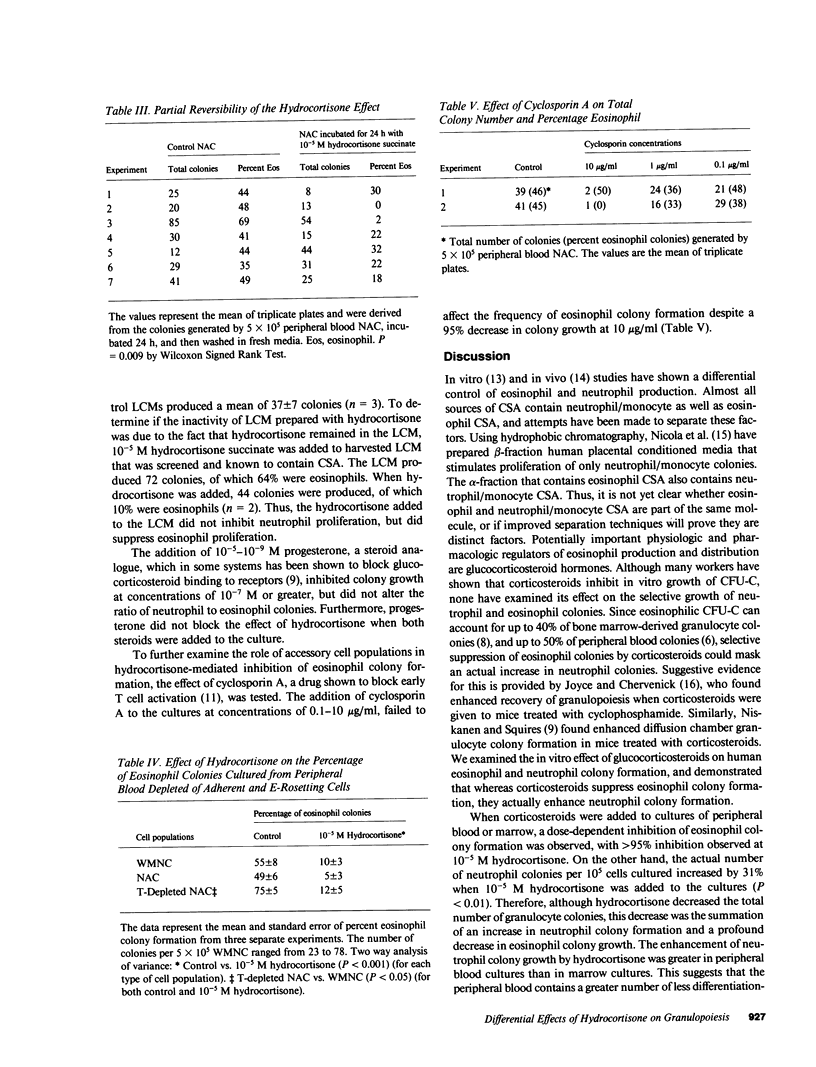
Glucocorticosteroid therapy results in an increase in the number of circulating neutrophils and a decrease in the number of eosinophils. Utilizing the double layer soft agar technique, we examined the effect of physiologic to pharmacologic concentrations of hydrocortisone on the proliferation of human neutrophil progenitors and eosinophil progenitors from peripheral blood and bone marrow. When peripheral blood cultures were studied, eosinophil proliferation was inhibited in a dose-responsive fashion with 10(-8) - 10(-5) M hydrocortisone succinate, and comprised 49 +/- 4% of the colonies in control cultures and only 4 +/- 1% (P less than 0.01) at pharmacologic levels of hydrocortisone (10(-5) M). The number of neutrophil colonies, on the other hand, increased by 31% when 10(-5) M hydrocortisone was added to cultures. In order for corticosteroids to exert this effect, it was necessary to add them within 24 h of the initiation of culture. The effect of hydrocortisone on granulocyte proliferation could not be blocked by progesterone, a structurally analogous steroid. To determine whether hydrocortisone was acting directly on the progenitor cell or via an effector cell, its effect on modulating cell populations and stimulating-factor production was studied. Removal of E-rosetting cells and/or adherent cells did not affect the inhibition of eosinophil colony growth or the enhancement of neutrophil colony growth. Furthermore, addition of the potent inhibitor of T cell function, cyclosporin A, failed to affect eosinophil colony frequency, suggesting that inhibition of T cell function was an unlikely explanation for the observed hydrocortisone effect. Leukocyte conditioned media (LCM), derived from peripheral blood mononuclear cells incubated with hydrocortisone, was devoid of both neutrophil and eosinophil colony-stimulating activity, whereas a control LCM stimulated both neutrophil and eosinophil proliferation. The data suggest that the observed hydrocortisone effect on granulocyte colony formation is unlikely to be mediated by an intermediary, and that hydrocortisone acts directly on progenitor cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker J. B., Barsh G. S., Carney D. H., Cunningham D. D. Dexamethasone modulates binding and action of epidermal growth factor in serum-free cell culture. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1882–1886. doi: 10.1073/pnas.75.4.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartelmez S. H., Dodge W. H., Bass D. A. Differential regulation of spleen cell-mediated eosinophil and neutrophil-macrophage production. Blood. 1980 Mar;55(3):489–493. [PubMed] [Google Scholar]
- Bishop C. R., Athens J. W., Boggs D. R., Warner H. R., Cartwright G. E., Wintrobe M. M. Leukokinetic studies. 13. A non-steady-state kinetic evaluation of the mechanism of cortisone-induced granulocytosis. J Clin Invest. 1968 Feb;47(2):249–260. doi: 10.1172/JCI105721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornson B. H., André-Schwartz J., Desforges J. F. In vitro culture of circulating CFUEOS from normal donors. Exp Hematol. 1982 Mar;10(3):271–276. [PubMed] [Google Scholar]
- Dao C., Metcalf D., Bilski-Pasquier G. Eosinophil and neutrophil colony-forming cells in culture. Blood. 1977 Nov;50(5):833–839. [PubMed] [Google Scholar]
- Dresch C., Johnson G. R., Metcalf D. Eosinophil colony formation in semisolid cultures of human bone marrow cells. Blood. 1977 May;49(5):835–844. [PubMed] [Google Scholar]
- Golde D. W., Bersch N., Quan S. G., Cline M. J. Inhibition of murine granulopoiesis in vitro by dexamethasone. Am J Hematol. 1976;1(4):369–373. doi: 10.1002/ajh.2830010402. [DOI] [PubMed] [Google Scholar]
- Joyce R. A., Chervenick P. A. Corticosteroid effect on granulopoiesis in mice after cyclophosphamide. J Clin Invest. 1977 Aug;60(2):277–283. doi: 10.1172/JCI108775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLGREN J. H., JANUS O. The eosinopenic response to cortisone and ACTH in normal subjects. Br Med J. 1951 Nov 17;2(4741):1183–1187. doi: 10.1136/bmj.2.4741.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf D. Cortisone action on serum colony-stimulating factor and bone marrow in vitro colony-forming cells. Proc Soc Exp Biol Med. 1969 Oct;132(1):391–394. doi: 10.3181/00379727-132-34222. [DOI] [PubMed] [Google Scholar]
- Motulsky H. J., Insel P. A. Adrenergic receptors in man: direct identification, physiologic regulation, and clinical alterations. N Engl J Med. 1982 Jul 1;307(1):18–29. doi: 10.1056/NEJM198207013070104. [DOI] [PubMed] [Google Scholar]
- Nicola N. A., Metcalf D., Johnson G. R., Burgess A. W. Separation of functionally distinct human granulocyte-macrophage colony-stimulating factors. Blood. 1979 Sep;54(3):614–627. [PubMed] [Google Scholar]
- Niskanen E., Squires J. Corticosteroid effect on murine hemopoietic precursor cells in vivo. Blood. 1981 Jun;57(6):1138–1139. [PubMed] [Google Scholar]
- Ralph P., Ito M., Broxmeyer H. E., Nakoinz I. Corticosteroids block newly induced but not constitutive functions of macrophage cell lines: myeloid colony-stimulating activity production, latex phagocytosis, and antibody-dependent lysis of RBC and tumor targets. J Immunol. 1978 Jul;121(1):300–303. [PubMed] [Google Scholar]
- Singer J. W., Samuels A. I., Adamson J. W. Steroids and hematopoiesis. I. The effect of steroids on in vitro erythroid colony growth: structure/activity relationships. J Cell Physiol. 1976 Jun;88(2):127–134. doi: 10.1002/jcp.1040880202. [DOI] [PubMed] [Google Scholar]
- Verma D. S., Spitzer G., Zander A. R., Fisher R., McCredie K. B., Dicke K. A. The myeloid progenitor cell: a parallel study of subpopulations in human marrow and peripheral blood. Exp Hematol. 1980 Jan;8(1):32–43. [PubMed] [Google Scholar]
- Walls R. S. Eosinophil response to alum adjuvants: involvement of T cells in non-antigen-dependent mechanisms. Proc Soc Exp Biol Med. 1977 Dec;156(3):431–435. doi: 10.3181/00379727-156-39951. [DOI] [PubMed] [Google Scholar]
- Wiesinger D., Borel J. F. Studies on the mechanism of action of cyclosporin A. Immunobiology. 1980 Jan;156(4-5):454–463. doi: 10.1016/S0171-2985(80)80078-7. [DOI] [PubMed] [Google Scholar]
- Wong G. L., Lukert B. P., Adams J. S. Glucocorticoids increase osteoblast-like bone cell response to 1,25(OH)2D3. Nature. 1980 May 22;285(5762):254–257. doi: 10.1038/285254a0. [DOI] [PubMed] [Google Scholar]
- Zalman F., Maloney M. A., Patt H. M. Differential response of early erythropoietic and granulopoietic progenitors to dexamethasone and cortisone. J Exp Med. 1979 Jan 1;149(1):67–72. doi: 10.1084/jem.149.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]


