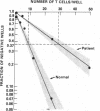
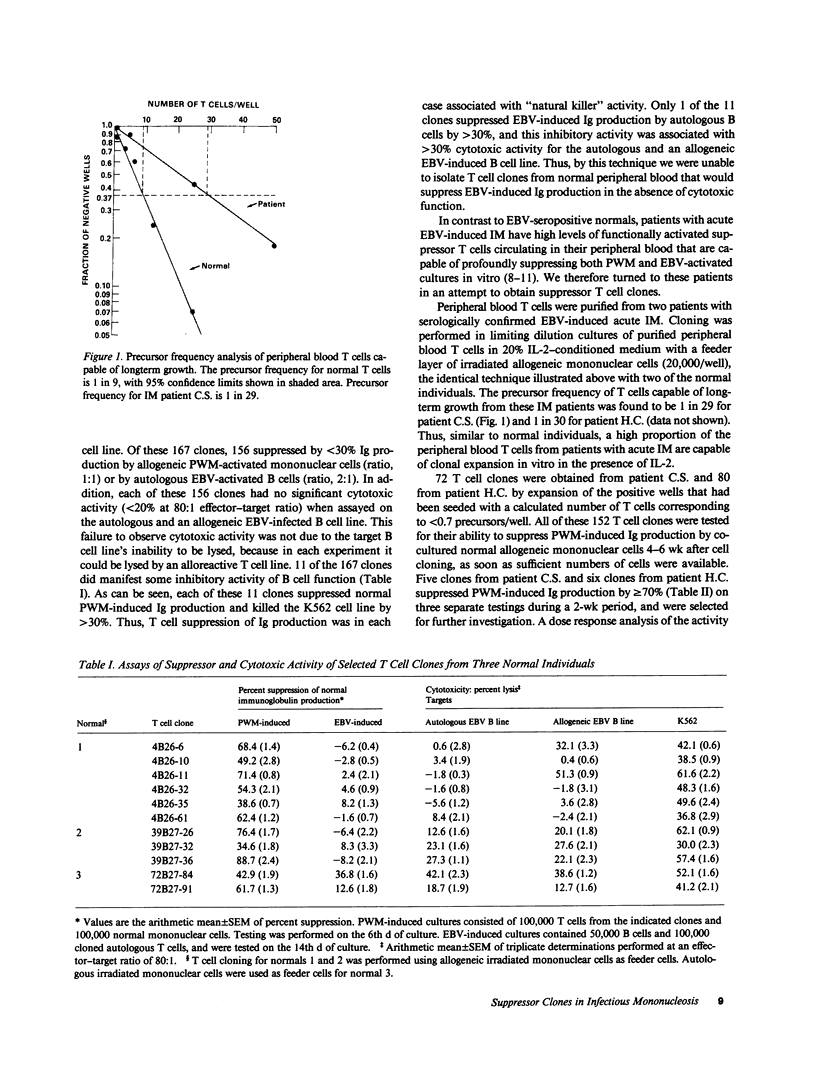
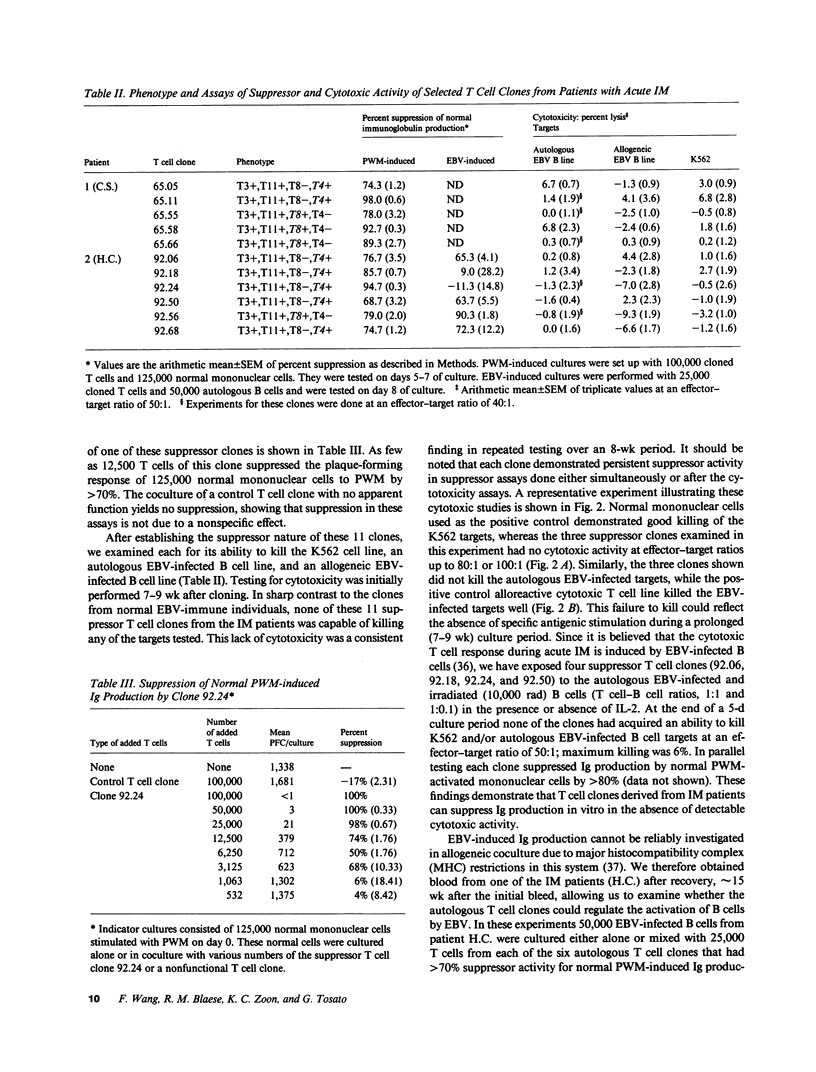
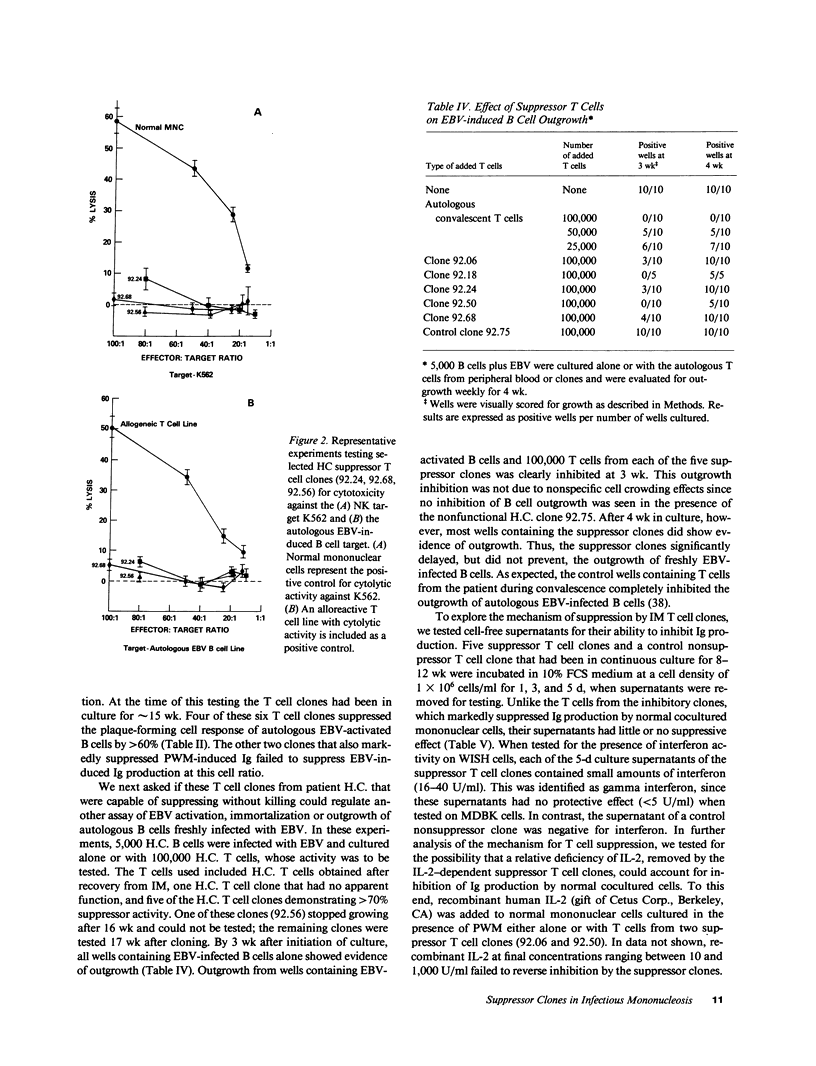
Abstract
Suppression and/or cytotoxicity are believed to play an important role in the defense against Epstein-Barr virus (EBV) infection. To analyze the role of suppressor T cells in relation to EBV, we sought to clone and study these T cells. Analysis of 152 T cell clones derived from the peripheral blood of two patients with acute EBV-induced infectious mononucleosis (IM) yielded 11 highly suppressive clones that had no cytotoxic activity for the natural killer sensitive K562 cell line, an autologous EBV-infected cell line, or an allogeneic EBV-infected B cell line. Four of six suppressor T cell clones also profoundly inhibited EBV-induced immunoglobulin production, and five of five clones delayed the outgrowth of immortalized cells. These results indicate that during acute IM, suppressor T cells capable of inhibiting B cell activation in the absence of cytotoxicity can be identified, and may play a key role in the control of EBV infection.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong J. A. Semi-micro, dye-binding assay for rabbit interferon. Appl Microbiol. 1971 Apr;21(4):723–725. doi: 10.1128/am.21.4.723-725.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin D. C., Berzofsky J. A., East I. J., Gurd F. R., Hannum C., Leach S. J., Margoliash E., Michael J. G., Miller A., Prager E. M. The antigenic structure of proteins: a reappraisal. Annu Rev Immunol. 1984;2:67–101. doi: 10.1146/annurev.iy.02.040184.000435. [DOI] [PubMed] [Google Scholar]
- Crawford D. H., Thomas J. A., Janossy G., Sweny P., Fernando O. N., Moorhead J. F., Thompson J. H. Epstein Barr virus nuclear antigen positive lymphoma after cyclosporin A treatment in patient with renal allograft. Lancet. 1980 Jun 21;1(8182):1355–1356. doi: 10.1016/s0140-6736(80)91800-0. [DOI] [PubMed] [Google Scholar]
- De Waele M., Thielemans C., Van Camp B. K. Characterization of immunoregulatory T cells in EBV-induced infectious mononucleosis by monoclonal antibodies. N Engl J Med. 1981 Feb 19;304(8):460–462. doi: 10.1056/NEJM198102193040804. [DOI] [PubMed] [Google Scholar]
- Diehl V., Henle G., Henle W., Kohn G. Demonstration of a herpes group virus in cultures of peripheral leukocytes from patients with infectious mononucleosis. J Virol. 1968 Jul;2(7):663–669. doi: 10.1128/jvi.2.7.663-669.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber P., Hoyer B. H. Induction of cellular DNA synthesis in human leukocytes by Epstein-Barr virus. Nature. 1971 May 7;231(5297):46–47. doi: 10.1038/231046a0. [DOI] [PubMed] [Google Scholar]
- Gerper P., Whang-Peng J., Monroe J. H. Transformation and chromosome changes induced by Epstein-Barr virus in normal human leukocyte cultures. Proc Natl Acad Sci U S A. 1969 Jul;63(3):740–747. doi: 10.1073/pnas.63.3.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm E. A., Mazumder A., Zhang H. Z., Rosenberg S. A. Lymphokine-activated killer cell phenomenon. Lysis of natural killer-resistant fresh solid tumor cells by interleukin 2-activated autologous human peripheral blood lymphocytes. J Exp Med. 1982 Jun 1;155(6):1823–1841. doi: 10.1084/jem.155.6.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes B. F., Schooley R. T., Payling-Wright C. R., Grouse J. E., Dolin R., Fauci A. S. Emergence of suppressor cells of immunoglobulin synthesis during acute Epstein-Barr virus-induced infectious mononucleosis. J Immunol. 1979 Nov;123(5):2095–2101. [PubMed] [Google Scholar]
- Henle G., Henle W., Diehl V. Relation of Burkitt's tumor-associated herpes-ytpe virus to infectious mononucleosis. Proc Natl Acad Sci U S A. 1968 Jan;59(1):94–101. doi: 10.1073/pnas.59.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henle W., Diehl V., Kohn G., Zur Hausen H., Henle G. Herpes-type virus and chromosome marker in normal leukocytes after growth with irradiated Burkitt cells. Science. 1967 Sep 1;157(3792):1064–1065. doi: 10.1126/science.157.3792.1064. [DOI] [PubMed] [Google Scholar]
- Henle W., Henle G. E., Horwitz C. A. Epstein-Barr virus specific diagnostic tests in infectious mononucleosis. Hum Pathol. 1974 Sep;5(5):551–565. doi: 10.1016/s0046-8177(74)80006-7. [DOI] [PubMed] [Google Scholar]
- Johnsen H. E., Madsen M., Kristensen T. Lymphocyte subpopulations in man: suppression of PWM-induced B-cell proliferation by infectious mononucleosis T cells. Scand J Immunol. 1979;10(3):251–255. doi: 10.1111/j.1365-3083.1979.tb01347.x. [DOI] [PubMed] [Google Scholar]
- Jondal M., Klein G. Surface markers on human B and T lymphocytes. II. Presence of Epstein-Barr virus receptors on B lymphocytes. J Exp Med. 1973 Dec 1;138(6):1365–1378. doi: 10.1084/jem.138.6.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein E., Ernberg I., Masucci M. G., Szigeti R., Wu Y. T., Masucci G., Svedmyr E. T-cell response to B-cells and Epstein-Barr virus antigens in infectious mononucleosis. Cancer Res. 1981 Nov;41(11 Pt 1):4210–4215. [PubMed] [Google Scholar]
- Klein G., Klein E. The changing faces of EBV research. Prog Med Virol. 1984;30:87–106. [PubMed] [Google Scholar]
- Lawrence E. C., Blaese R. M., Martin R. R., Stevens P. M. Immunoglobulin secreting cells in normal human bronchial lavage fluids. J Clin Invest. 1978 Oct;62(4):832–835. doi: 10.1172/JCI109195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipinski M., Fridman W. H., Tursz T., Vincent C., Pious D., Fellous M. Absence of allogeneic restriction in human T-cell-mediated cytotoxicity to Epstein-Barr virus-infected target cells. Demonstration of an HLA-linked control at the effector level. J Exp Med. 1979 Dec 1;150(6):1310–1322. doi: 10.1084/jem.150.6.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotz M., Tsoukas C. D., Fong S., Carson D. A., Vaughan J. H. Regulation of Epstein-Barr virus infection by recombinant interferons. Selected sensitivity to interferon-gamma. Eur J Immunol. 1985 May;15(5):520–525. doi: 10.1002/eji.1830150518. [DOI] [PubMed] [Google Scholar]
- Mangi R. J., Niederman J. C., Kelleher J. E., Jr, Dwyer J. M., Evans A. S., Kantor F. S. Depression of cell-mediated immunity during acute infectious mononucleosis. N Engl J Med. 1974 Nov 28;291(22):1149–1153. doi: 10.1056/NEJM197411282912202. [DOI] [PubMed] [Google Scholar]
- Martin P. J., Shulman H. M., Schubach W. H., Hansen J. A., Fefer A., Miller G., Thomas E. D. Fatal Epstein-Barr-virus-associated proliferation of donor B cells after treatment of acute graft-versus-host disease with a murine anti-T-cell antibody. Ann Intern Med. 1984 Sep;101(3):310–315. doi: 10.7326/0003-4819-101-3-310. [DOI] [PubMed] [Google Scholar]
- Masucci M. G., Bejarano M. T., Masucci G., Klein E. Large granular lymphocytes inhibit the in vitro growth of autologous Epstein-Barr virus-infected B cells. Cell Immunol. 1983 Mar;76(2):311–321. doi: 10.1016/0008-8749(83)90374-x. [DOI] [PubMed] [Google Scholar]
- Moss D. J., Rickinson A. B., Pope J. H. Long-term T-cell-mediated immunity to Epstein-Barr virus in man. I. Complete regression of virus-induced transformation in cultures of seropositive donor leukocytes. Int J Cancer. 1978 Dec;22(6):662–668. doi: 10.1002/ijc.2910220604. [DOI] [PubMed] [Google Scholar]
- Moss D. J., Rickinson A. B., Pope J. H. Long-term T-cell-mediated immunity to Epstein-Barr virus in man. III. Activation of cytotoxic T cells in virus-infected leukocyte cultures. Int J Cancer. 1979 May 15;23(5):618–625. doi: 10.1002/ijc.2910230506. [DOI] [PubMed] [Google Scholar]
- Patel P. C., Dorval G., Menezes J. Cytotoxic effector cells from infectious mononucleosis patients in the acute phase do not specifically kill Epstein-Barr virus genome-carrying lymphoid cell lines. Infect Immun. 1982 Oct;38(1):251–259. doi: 10.1128/iai.38.1.251-259.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purtilo D. T., DeFlorio D., Jr, Hutt L. M., Bhawan J., Yang J. P., Otto R., Edwards W. Variable phenotypic expression of an X-linked recessive lymphoproliferative syndrome. N Engl J Med. 1977 Nov 17;297(20):1077–1080. doi: 10.1056/NEJM197711172972001. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., O'Brien C., Rosenthal P., Schlossman S. F. The cellular basis for viral-induced immunodeficiency: analysis by monoclonal antibodies. J Immunol. 1980 Sep;125(3):1269–1274. [PubMed] [Google Scholar]
- Rickinson A. B., Moss D. J., Pope J. H. Long-term C-cell-mediated immunity to Epstein-Barr virus in man. II. Components necessary for regression in virus-infected leukocyte cultures. Int J Cancer. 1979 May 15;23(5):610–617. doi: 10.1002/ijc.2910230505. [DOI] [PubMed] [Google Scholar]
- Rickinson A. B., Wallace L. E., Epstein M. A. HLA-restricted T-cell recognition of Epstein-Barr virus-infected B cells. Nature. 1980 Feb 28;283(5750):865–867. doi: 10.1038/283865a0. [DOI] [PubMed] [Google Scholar]
- Rocchi G., Felici A., Ragona G., Heinz A. Quantitative evaluation of Epstein-Barr-virus-infected mononuclear peripheral blood leukocytes in infectious mononucleosis. N Engl J Med. 1977 Jan 20;296(3):132–134. doi: 10.1056/NEJM197701202960302. [DOI] [PubMed] [Google Scholar]
- Rosén A., Gergely P., Jondal M., Klein G., Britton S. Polyclonal Ig production after Epstein-Barr virus infection of human lymphocytes in vitro. Nature. 1977 May 5;267(5606):52–54. doi: 10.1038/267052a0. [DOI] [PubMed] [Google Scholar]
- Seeley J., Svedmyr E., Weiland O., Klein G., Moller E., Eriksson E., Andersson K., van der Waal L. Epstein Barr virus selective T cells in infectious mononucleosis are not restricted to HLA-A and B antigens. J Immunol. 1981 Jul;127(1):293–300. [PubMed] [Google Scholar]
- Sullivan J. L., Byron K. S., Brewster F. E., Baker S. M., Ochs H. D. X-linked lymphoproliferative syndrome. Natural history of the immunodeficiency. J Clin Invest. 1983 Jun;71(6):1765–1778. doi: 10.1172/JCI110932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svedmyr E., Jondal M. Cytotoxic effector cells specific for B Cell lines transformed by Epstein-Barr virus are present in patients with infectious mononucleosis. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1622–1626. doi: 10.1073/pnas.72.4.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorley-Lawson D. A., Chess L., Strominger J. L. Suppression of in vitro Epstein-Barr virus infection. A new role for adult human T lymphocytes. J Exp Med. 1977 Aug 1;146(2):495–508. doi: 10.1084/jem.146.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosato G., Blaese R. M. Defective allosuppression in patients with ataxia-telangiectasia. Kroc Found Ser. 1985;19:331–338. [PubMed] [Google Scholar]
- Tosato G., Blaese R. M. Epstein-Barr virus infection and immunoregulation in man. Adv Immunol. 1985;37:99–149. doi: 10.1016/s0065-2776(08)60339-9. [DOI] [PubMed] [Google Scholar]
- Tosato G., Blaese R. M., Yarchoan R. Relationship between immunoglobulin production and immortalization by Epstein Barr virus. J Immunol. 1985 Aug;135(2):959–964. [PubMed] [Google Scholar]
- Tosato G., Magrath I. T., Blaese R. M. T cell-mediated immunoregulation of Epstein Barr virus- (EBV) induced B lymphocyte activation in EBV-seropositive and EBV-seronegative individuals. J Immunol. 1982 Feb;128(2):575–579. [PubMed] [Google Scholar]
- Tosato G., Magrath I., Koski I., Dooley N., Blaese M. Activation of suppressor T cells during Epstein-Barr-virus-induced infectious mononucleosis. N Engl J Med. 1979 Nov 22;301(21):1133–1137. doi: 10.1056/NEJM197911223012101. [DOI] [PubMed] [Google Scholar]
- Tosato G., Straus S., Henle W., Pike S. E., Blaese R. M. Characteristic T cell dysfunction in patients with chronic active Epstein-Barr virus infection (chronic infectious mononucleosis). J Immunol. 1985 May;134(5):3082–3088. [PubMed] [Google Scholar]
- Tursz T., Fridman W. H., Senik A., Tsapis A., Fellous M. Human virus-infected target cells lacking HLA antigens resist specific T-lymphocyte cytolysis. Nature. 1977 Oct 27;269(5631):806–808. doi: 10.1038/269806a0. [DOI] [PubMed] [Google Scholar]
- Twomey J. J. Abnormalities in the mixed leukocyte reaction during infectious mononucleosis. J Immunol. 1974 Jun;112(6):2278–2281. [PubMed] [Google Scholar]
- Yarchoan R., Tosato G., Blaese R. M., Simon R. M., Nelson D. L. Limiting dilution analysis of Epstein-Barr virus-induced immunoglobulin production by human B cells. J Exp Med. 1983 Jan 1;157(1):1–14. doi: 10.1084/jem.157.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler J. L., Drew W. L., Miner R. C., Mintz L., Rosenbaum E., Gershow J., Lennette E. T., Greenspan J., Shillitoe E., Beckstead J. Outbreak of Burkitt's-like lymphoma in homosexual men. Lancet. 1982 Sep 18;2(8299):631–633. doi: 10.1016/s0140-6736(82)92740-4. [DOI] [PubMed] [Google Scholar]
- van Oers M. H., Pinkster J., Zeijlemaker W. P. Quantification of antigen-reactive cells among human T lymphocytes. Eur J Immunol. 1978 Jul;8(7):477–484. doi: 10.1002/eji.1830080706. [DOI] [PubMed] [Google Scholar]