Abstract
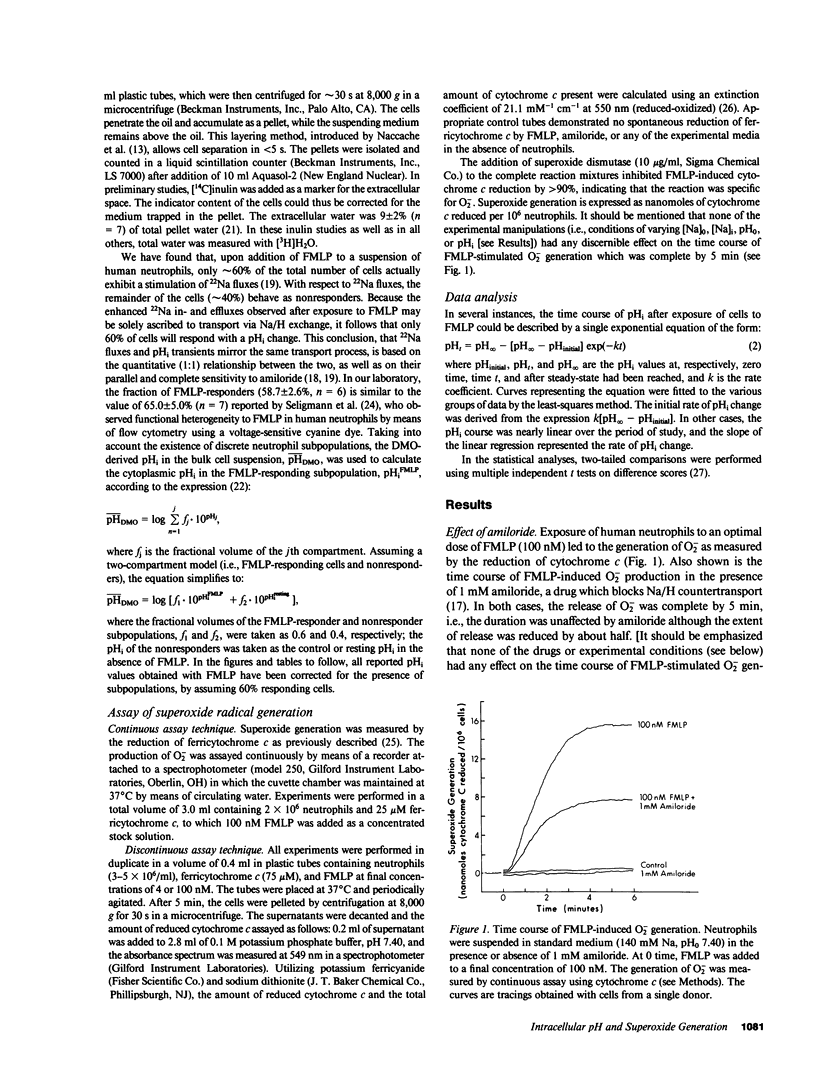
The relationship of intracellular pH (pHi) to superoxide radical (O2-) generation was investigated in chemotactic factor-stimulated human neutrophils. Exposure of cells to 100 nM N-formylmethionyl-leucyl-phenylalanine (FMLP) caused activation of Na/H exchange which, in 140 mM Na medium (pH0 7.40), led to a rise in pHi from 7.22 to 7.80. This pHi change was sensitive to amiloride (apparent Ki 78 microM), an inhibitor of Na/H countertransport. The time course of the alkalinization was similar to that of FMLP-stimulated O2- production, which was complete by 5 min. In the presence of 1 mM amiloride, which nearly blocked the pHi transient elicited by FMLP, or in the absence of external Na, where intracellular acidification was observed in FMLP-stimulated cells, O2- release was still roughly 25-45% of normal. Thus, an alkalinization cannot be an obligatory requirement for O2- generation. By independently varying either pH0, pHi, or the internal or external concentrations of Na, both the direction and magnitude of the FMLP-induced pHi transients could be altered. In each instance, the amount of O2- release correlated directly with pHi and was enhanced by intracellular alkalinization. In the absence of FMLP, a rise in pHi to 7.7-7.8 by exposure of cells to 30 mM NH4Cl, 10 microM monensin (a Na/H exchanging ionophore), or after a prepulse with 18% CO2 did not result in O2- generation. Thus, these results imply that an alkalinization per se is not a sufficient trigger. Neutrophils exposed to 4 nM FMLP exhibited a threefold slower rate of alkalinization (reaching pHi approximately 7.80 by 20-30 min) as compared to that obtained with 100 nM FMLP and did not release significant amounts of O2- under normal incubation conditions. However, these cells could be induced to generate O2- when the degree of alkalinization was enhanced by internal Na depletion or by pretreatment with 18% CO2. Together, these results indicate a modulating effect of pHi on O2- production and suggest that other functional responses of neutrophils may be regulated by their pHi.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M., Curnutte J. T., McMurrich B. J. The particulate superoxide-forming system from human neutrophils. Properties of the system and further evidence supporting its participation in the respiratory burst. J Clin Invest. 1976 Oct;58(4):989–996. doi: 10.1172/JCI108553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bormann B. J., Huang C. K., Mackin W. M., Becker E. L. Receptor-mediated activation of a phospholipase A2 in rabbit neutrophil plasma membrane. Proc Natl Acad Sci U S A. 1984 Feb;81(3):767–770. doi: 10.1073/pnas.81.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boron W. F., Roos A. Comparison of microelectrode, DMO, and methylamine methods for measuring intracellular pH. Am J Physiol. 1976 Sep;231(3):799–809. doi: 10.1152/ajplegacy.1976.231.3.799. [DOI] [PubMed] [Google Scholar]
- Borregaard N., Schwartz J. H., Tauber A. I. Proton secretion by stimulated neutrophils. Significance of hexose monophosphate shunt activity as source of electrons and protons for the respiratory burst. J Clin Invest. 1984 Aug;74(2):455–459. doi: 10.1172/JCI111442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busa W. B., Nuccitelli R. Metabolic regulation via intracellular pH. Am J Physiol. 1984 Apr;246(4 Pt 2):R409–R438. doi: 10.1152/ajpregu.1984.246.4.R409. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- De Togni P., Della Bianca V., Bellavite P., Grzeskowiak M., Rossi F. Studies on stimulus-response coupling in human neutrophils. II. Relationships between the effects of changes of external ionic composition on the properties of N-formylmethionylleucylphenylalanine receptors and on the respiratory and secretory responses. Biochim Biophys Acta. 1983 Feb 22;755(3):506–513. doi: 10.1016/0304-4165(83)90256-8. [DOI] [PubMed] [Google Scholar]
- Della Bianca V., Bellavite P., De Togni P., Fumarulo R., Rossi F. Studies on stimulus-response coupling in human neutrophils. I. Role of monovalent cations in the respiratory and secretory response to N-formylmethionylleucylphenylalanine. Biochim Biophys Acta. 1983 Feb 22;755(3):497–505. doi: 10.1016/0304-4165(83)90255-6. [DOI] [PubMed] [Google Scholar]
- Gerson D. F., Kiefer H., Eufe W. Intracellular pH of mitogen-stimulated lymphocytes. Science. 1982 May 28;216(4549):1009–1010. doi: 10.1126/science.6281887. [DOI] [PubMed] [Google Scholar]
- Green T. R., Wu D. E., Wirtz M. K. The O2- generating oxidoreductase of human neutrophils: evidence of an obligatory requirement for calcium and magnesium for expression of catalytic activity. Biochem Biophys Res Commun. 1983 Feb 10;110(3):973–978. doi: 10.1016/0006-291x(83)91058-6. [DOI] [PubMed] [Google Scholar]
- Johnson J. D., Epel D. Intracellular pH and activation of sea urchin eggs after fertilisation. Nature. 1976 Aug 19;262(5570):661–664. doi: 10.1038/262661a0. [DOI] [PubMed] [Google Scholar]
- Kaczorowski G. J., Barros F., Dethmers J. K., Trumble M. J., Cragoe E. J., Jr Inhibition of Na+/Ca2+ exchange in pituitary plasma membrane vesicles by analogues of amiloride. Biochemistry. 1985 Mar 12;24(6):1394–1403. doi: 10.1021/bi00327a017. [DOI] [PubMed] [Google Scholar]
- Korchak H. M., Weissmann G. Changes in membrane potential of human granulocytes antecede the metabolic responses to surface stimulation. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3818–3822. doi: 10.1073/pnas.75.8.3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korchak H. M., Weissmann G. Stimulus-response coupling in the human neutrophil. Transmembrane potential and the role of extracellular Na+. Biochim Biophys Acta. 1980 Sep 2;601(1):180–194. doi: 10.1016/0005-2736(80)90523-4. [DOI] [PubMed] [Google Scholar]
- L'Allemain G., Paris S., Pouysségur J. Growth factor action and intracellular pH regulation in fibroblasts. Evidence for a major role of the Na+/H+ antiport. J Biol Chem. 1984 May 10;259(9):5809–5815. [PubMed] [Google Scholar]
- McKinney T. D., Burg M. B. Bicarbonate absorption by rabbit cortical collecting tubules in vitro. Am J Physiol. 1978 Feb;234(2):F141–F145. doi: 10.1152/ajprenal.1978.234.2.F141. [DOI] [PubMed] [Google Scholar]
- Mehta J., Spilberg I. Heterologous receptor population for a chemotactic factor F-Met-Leu-Phe on the human neutrophil. Effect of pH and temperature. Inflammation. 1983 Sep;7(3):301–309. doi: 10.1007/BF00917267. [DOI] [PubMed] [Google Scholar]
- Molski T. F., Naccache P. H., Volpi M., Wolpert L. M., Sha'afi R. I. Specific modulation of the intracellular pH of rabbit neutrophils by chemotactic factors. Biochem Biophys Res Commun. 1980 May 30;94(2):508–514. doi: 10.1016/0006-291x(80)91260-7. [DOI] [PubMed] [Google Scholar]
- Naccache P. H., Showell H. J., Becker E. L., Sha'afi R. I. Changes in ionic movements across rabbit polymorphonuclear leukocyte membranes during lysosomal enzyme release. Possible ionic basis for lysosomal enzyme release. J Cell Biol. 1977 Dec;75(3):635–649. doi: 10.1083/jcb.75.3.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naccache P. H., Showell H. J., Becker E. L., Sha'afi R. I. Transport of sodium, potassium, and calcium across rabbit polymorphonuclear leukocyte membranes. Effect of chemotactic factor. J Cell Biol. 1977 May;73(2):428–444. doi: 10.1083/jcb.73.2.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace C. S., Tarvin J. T., Smith J. S. Stimulus-secretion coupling in beta-cells: modulation by pH. Am J Physiol. 1983 Jan;244(1):E3–18. doi: 10.1152/ajpendo.1983.244.1.E3. [DOI] [PubMed] [Google Scholar]
- Pfefferkorn L. C. Transmembrane signaling: an ion-flux-independent model for signal transduction by complexed Fc receptors. J Cell Biol. 1984 Dec;99(6):2231–2240. doi: 10.1083/jcb.99.6.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressman B. Mechanism of action of transport-mediating antibiotics. Ann N Y Acad Sci. 1969 Oct 31;147(19):829–841. doi: 10.1111/j.1749-6632.1969.tb41291.x. [DOI] [PubMed] [Google Scholar]
- Roos A., Boron W. F. Intracellular pH. Physiol Rev. 1981 Apr;61(2):296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- Schiffmann E., Aswanikumar S., Venkatasubramanian K., Corcoran B. A., Pert C. B., Brown J., Gross E., Day A. R., Freer R. J., Showell A. H. Some characteristics of the neutrophil receptor for chemotactic peptides. FEBS Lett. 1980 Aug 11;117(1):1–7. doi: 10.1016/0014-5793(80)80900-8. [DOI] [PubMed] [Google Scholar]
- Segal A. W., Geisow M., Garcia R., Harper A., Miller R. The respiratory burst of phagocytic cells is associated with a rise in vacuolar pH. Nature. 1981 Apr 2;290(5805):406–409. doi: 10.1038/290406a0. [DOI] [PubMed] [Google Scholar]
- Seligmann B. E., Gallin E. K., Martin D. L., Shain W., Gallin J. I. Interaction of chemotactic factors with human polymorphonuclear leukocytes: studies using a membrane potential-sensitive cyanine dye. J Membr Biol. 1980;52(3):257–272. doi: 10.1007/BF01869194. [DOI] [PubMed] [Google Scholar]
- Seligmann B., Chused T. M., Gallin J. I. Human neutrophil heterogeneity identified using flow microfluorometry to monitor membrane potential. J Clin Invest. 1981 Nov;68(5):1125–1131. doi: 10.1172/JCI110356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha'afi R. I., Molski T. F., Naccache P. H. Chemotactic factors activate differentiable permeation pathways for sodium and calcium in rabbit neutrophils. Effect of amiloride. Biochem Biophys Res Commun. 1981 Apr 30;99(4):1271–1276. doi: 10.1016/0006-291x(81)90757-9. [DOI] [PubMed] [Google Scholar]
- Showell H. J., Becker E. L. The effects of external K+ and Na+ on the chemotaxis of rabbit peritoneal neutrophils. J Immunol. 1976 Jan;116(1):99–105. [PubMed] [Google Scholar]
- Showell H. J., Naccache P. H., Sha'afi R. I., Becker E. L. The effects of extracellular K+, Na+ and Ca++ on lysosomal enzyme secretion from polymorphonuclear leukocytes. J Immunol. 1977 Sep;119(3):804–811. [PubMed] [Google Scholar]
- Simchowitz L., Atkinson J. P., Spilberg I. Stimulus-specific deactivation of chemotactic factor-induced cyclic AMP response and superoxide generation by human neutrophils. J Clin Invest. 1980 Oct;66(4):736–747. doi: 10.1172/JCI109911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simchowitz L., Fischbein L. C., Spilberg I., Atkinson J. P. Induction of a transient elevation in intracellular levels of adenosine-3',5'-cyclic monophosphate by chemotactic factors: an early event in human neutrophil activation. J Immunol. 1980 Mar;124(3):1482–1491. [PubMed] [Google Scholar]
- Simchowitz L., Roos A. Regulation of intracellular pH in human neutrophils. J Gen Physiol. 1985 Mar;85(3):443–470. doi: 10.1085/jgp.85.3.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simchowitz L., Spilberg I. Chemotactic factor-induced generation of superoxide radicals by human neutrophils: evidence for the role of sodium. J Immunol. 1979 Nov;123(5):2428–2435. [PubMed] [Google Scholar]
- Simchowitz L., Spilberg I., De Weer P. Sodium and potassium fluxes and membrane potential of human neutrophils: evidence for an electrogenic sodium pump. J Gen Physiol. 1982 Mar;79(3):453–479. doi: 10.1085/jgp.79.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklar L. A., Jesaitis A. J., Painter R. G., Cochrane C. G. The kinetics of neutrophil activation. The response to chemotactic peptides depends upon whether ligand-receptor interaction is rate-limiting. J Biol Chem. 1981 Oct 10;256(19):9909–9914. [PubMed] [Google Scholar]
- Soltoff S. P., Mandel L. J. Amiloride directly inhibits the Na,K-ATPase activity of rabbit kidney proximal tubules. Science. 1983 May 27;220(4600):957–958. doi: 10.1126/science.6302840. [DOI] [PubMed] [Google Scholar]
- Tauber A. I., Goetzl E. J. Structural and catalytic properties of the solubilized superoxide-generating activity of human polymorphonuclear leukocytes. Solubilization, stabilization in solution, and partial characterization. Biochemistry. 1979 Dec 11;18(25):5576–5584. doi: 10.1021/bi00592a009. [DOI] [PubMed] [Google Scholar]
- Winterbourn C. C., Hawkins R. E., Brian M., Carrell R. W. The estimation of red cell superoxide dismutase activity. J Lab Clin Med. 1975 Feb;85(2):337–341. [PubMed] [Google Scholar]
- van GELDER B., SLATER E. C. The extinction coefficient of cytochrome c. Biochim Biophys Acta. 1962 Apr 23;58:593–595. doi: 10.1016/0006-3002(62)90073-2. [DOI] [PubMed] [Google Scholar]
- van Zwieten R., Wever R., Hamers M. N., Weening R. S., Roos D. Extracellular proton release by stimulated neutrophils. J Clin Invest. 1981 Jul;68(1):310–313. doi: 10.1172/JCI110250. [DOI] [PMC free article] [PubMed] [Google Scholar]


