Abstract
Background
Transcatheter pulmonary valve placement is an emerging therapy for pulmonary regurgitation and right ventricular outflow tract obstruction in selected patients. The Melody valve was recently approved in the United States for placement in dysfunctional right ventricular outflow tract conduits.
Methods and Results
From January 2007 to August 2009, 136 patients (median age, 19 years) underwent catheterization for intended Melody valve implantation at 5 centers. Implantation was attempted in 124 patients; in the other 12, transcatheter pulmonary valve placement was not attempted because of the risk of coronary artery compression (n=6) or other clinical or protocol contraindications. There was 1 death from intracranial hemorrhage after coronary artery dissection, and 1 valve was explanted after conduit rupture. The median peak right ventricular outflow tract gradient was 37 mm Hg before implantation and 12 mm Hg immediately after implantation. Before implantation, pulmonary regurgitation was moderate or severe in 92 patients (81% with data); no patient had more than mild pulmonary regurgitation early after implantation or during follow-up (≥1 year in 65 patients). Freedom from diagnosis of stent fracture was 77.8±4.3% at 14 months. Freedom from Melody valve dysfunction or reintervention was 93.5±2.4% at 1 year. A higher right ventricular outflow tract gradient at discharge (P=0.003) and younger age (P=0.01) were associated with shorter freedom from dysfunction.
Conclusions
In this updated report from the multicenter US Melody valve trial, we demonstrated an ongoing high rate of procedural success and encouraging short-term valve function. All reinterventions in this series were for right ventricular outflow tract obstruction, highlighting the importance of patient selection, adequate relief of obstruction, and measures to prevent and manage stent fracture.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT00740870.
Keywords: catheterization, heart defects, congenital, tetralogy of Fallot, magnetic resonance imaging
It has been nearly a decade since transcatheter pulmonary valve (TPV) placement was first reported by Bonhoeffer et al1 with a device comprising a valved segment of bovine jugular vein sewn within a balloon-expandable stent. A modified version of the original device, the Melody valve (Medtronic Inc, Minneapolis, Minn), is now approved and commercially available throughout much of the world. In January 2010, the US Food and Drug Administration formally approved the Melody valve for placement in dysfunctional right ventricular (RV) outflow tract (RVOT) conduits under a humanitarian device exemption.
In patients with postoperative RVOT dysfunction, whether pulmonary regurgitation (PR), obstruction, or both, the optimal timing and method of treatment are not always obvious. Although there is clear evidence that PR and RV hypertension are deleterious, the incremental effects of progressive and increasingly chronic RV volume and pressure overload may be difficult to understand.2–6 Despite numerous studies of clinical outcomes in RVOT disease, there are limited data to guide the timing of therapy for RVOT dysfunction.7–10 Surgical conduit or pulmonary valve replacement is the established standard of care, but patients are often managed with PR or RVOT conduit obstruction for many years before referral for surgery. The availability of less invasive options for pulmonary valve placement, such as transcatheter valve implantation, may provide a means of limiting the duration and severity of RV volume and/or pressure overload without increasing the lifetime number of open heart operations, potentially shifting the risk-benefit balance in favor of earlier reintervention in many patients. To determine the ultimate clinical role of TPV, it is imperative that the physiological and adverse effects of this therapy are characterized rigorously.
In a series of reports, Bonhoeffer and colleagues1,10–21 have catalogued their ongoing experience with the Melody valve. In various investigations, they found that Melody implants reduced RVOT obstruction, provided a competent pulmonary valve, improved functional status and peak exercise parameters, and in some patient subsets, improved biventricular function and efficiency. In 2009, we reported early outcomes in the initial 34 patients enrolled in the US Melody TPV trial, the first prospective multicenter study of this valve with a standardized protocol for entry, implantation, and follow-up.22 Implantation was achieved successfully in all but 1 patient, with an acceptable frequency of adverse events and encouraging short-term outcomes. On the basis of these results, we concluded that the Melody platform can be adopted by experienced, properly trained interventional pediatric/congenital cardiologists without a substantial technical learning curve and meets short-term therapeutic objectives in a large majority of rigorously selected patients. Aside from our study and those from Bonhoeffer’s group, there are limited published data on the outcomes of TPV therapy.23–25
The US Melody TPV trial has continued, and the cohort has expanded beyond the initial 30-implant target. After the recent Food and Drug Administration approval of the valve, we evaluated procedural, short-term, and limited midterm results in the cohort enrolled through the middle of August 2009.
Methods
Patients and Study Protocol
The multicenter US Melody TPV trial is an ongoing prospective, nonrandomized study that will follow patients for 5 years after TPV placement. Patients with a spectrum of RVOT conduit dysfunction were enrolled and evaluated in a systematic fashion to assess the safety, procedural success, and short-term effectiveness of the Melody valve. The original study protocol and initial cohort from this trial were reported previously.22 After the initial phase of the trial, several protocol modifications were incorporated through amendments that also extended the cohort from its original target of 30 implants in 4 increments to 35, 70, 120, and finally, 150 implants, and the number of study sites from 3 to 5. For this study, the database was closed for analysis on August 17, 2009, before full enrollment; only patients catheterized by this date were included.
The original inclusion and exclusion criteria22 were modified to allow enrollment of patients with contraindications to magnetic resonance imaging (MRI), patients in whom concomitant transcatheter interventions were indicated, and patients with a bioprosthetic valve not housed in a circumferential conduit as long as the manufacturer-specified inner diameter ranged from 18 to 20 mm. The previously reported catheterization protocol22 was modified to allow concomitant transcatheter interventions at the time of Melody valve implantation at the discretion of the investigator. The follow-up protocol22 was modified to eliminate the 1-month visit and all computed tomography pulmonary angiography evaluations.
Patients with a dysfunctional RVOT conduit or bioprosthetic pulmonary valve were identified by investigators at each of the 5 study sites: Children’s Hospital Boston, Children’s Hospital of New York, Miami Children’s Hospital, Seattle Children’s Hospital, and Nationwide Children’s Hospital. Patients meeting the previously published and aforementioned criteria22 who wished to be considered for inclusion in the trial were asked to provide written informed consent before preimplantation imaging, after which a screening echocardiogram was performed. If the prespecified hemodynamic criteria were met, the evaluation was completed. Patients meeting only the echocardiographic PR criterion (severe, with RV dilation and/or dysfunction for New York Heart Association [NYHA] class I; moderate or greater for NYHA class II and above) were categorized as having a primary implantation indication of PR, those meeting only the RVOT gradient threshold (mean gradient ≥40 mm Hg for NYHA class I; ≥35 mm Hg for NYHA class II and above) were categorized as having a primary implantation indication of RVOT obstruction, and those meeting both criteria for their NYHA class were categorized as having mixed disease.
The study was conducted under an investigational device exemption (No. G050186), and all versions of and amendments to the protocol were approved by the Food and Drug Administration, the Center for Devices and Radiological Health, and the Institutional Review Board at each institution. The trial is registered in ClinicalTrials.gov (identifier: NCT00740870).
Catheterization and Valve Implantation
The catheterization protocol for the US trial, including specifications for predilation, balloon sizing, and evaluation of coronary compression, was summarized in detail in our prior report.22 As noted above, concomitant procedures, which were not permitted in the first 35 implanted patients, were subsequently allowed.
Follow-Up Evaluation
Follow-up evaluations were conducted at prespecified intervals at the implanting center as previously reported.22 For each evaluation, data were recorded by the investigator and entered into a Web-based data collection system, which is maintained by the sponsor of the trial, Medtronic Inc. Raw data from all echocardiograms, MRI studies, and exercise tests were forwarded to core laboratories, which repeated all required measurements and entered them into the same Web-based data collection system. Thus, for each study, both site and core laboratory data were recorded. For data subjected to core review, including echocardiographic, MRI, and exercise data, only patients implanted and measurements entered into the system by the core investigators by the time of database closure (August 17, 2009) were used for this report. Unless otherwise specified, only core readings of echocardiographic, MRI, and exercise data are presented. Data elements not subjected to core review, including catheterization data, vital status, NYHA classification, radiographic data, ECG data, and reports of adverse events and reinterventions, were included through database closure. Because of the inevitable delay between follow-up evaluation and core laboratory reading, clinical data and on-site measurements were usually entered into the data collection system before core readings. Thus, when the database was closed to generate the data set used for this analysis, more clinical follow-up data were available than core data. Data obtained by August 17, 2009, but not yet entered into the database were subsequently added to the analysis at the time of manuscript revision.
Statistical Analysis
Data on adverse events are presented for all patients who underwent catheterization. Procedural results are presented for all patients in whom TPV placement was attempted. Follow-up imaging and data are presented for the 6-, 12-, and 24-month evaluations for all patients with core laboratory data available at the specified time point; exercise data are presented for the preimplantation and 6-month follow-up time points. Analysis of survival free from reintervention was performed for the entire catheterized cohort, including patients who died or underwent early postimplantation RVOT reintervention. Other freedom-from-event analyses, including freedom from Melody valve dysfunction (moderate or greater PR, mean Doppler RVOT gradient ≥40 mm Hg, or reintervention), freedom from a second TPV, and freedom from diagnosis of stent fracture, were performed for all patients in whom a valve was implanted and in place for >24 hours. For analysis of freedom from reintervention or placement of a second TPV, patients who did not meet event criteria were censored at the last date they were known to be alive and in follow-up. For analysis of freedom from valve dysfunction and diagnosis of stent fracture, those without valve dysfunction or stent fracture were censored at the most recent evaluation time point for which core echocardiography data or site radiographic data were available, respectively. Because a temporal window was allowed for each standard study evaluation, estimates of freedom from diagnosis of stent fracture are reported for time points at the end of each evaluation window (eg, 14 months for the 1-year evaluation). Time-to-event analyses were performed with Kaplan–Meier analysis. Factors associated with shorter freedom from TPV dysfunction were assessed by Cox regression, with variables significant at P<0.05 on univariable analysis entered into a forward stepwise multivariable model. The following predictor variables were included in the univariable analysis of freedom from TPV dysfunction: procedure order, age, diagnosis, conduit type and size, primary implantation indication, preimplantation and early postimplantation hemodynamics, and existing or new bare metal stents in the RVOT. Hazard ratios (HRs) are presented with 95% confidence intervals (CIs). Predictor variables not specified in the text were not significant by univariable analysis. Wilcoxon signed-rank test was used to evaluate the change in continuous paired data (from before implantation to 6 months after), with the Hochberg26 procedure used to adjust for multiple comparisons. Adjusted P values are presented, with value of P≤0.05 considered statistically significant. Statistical analyses were performed with SAS (SAS Institute Inc, Cary, NC) version 9.1 software.
Results
Patients
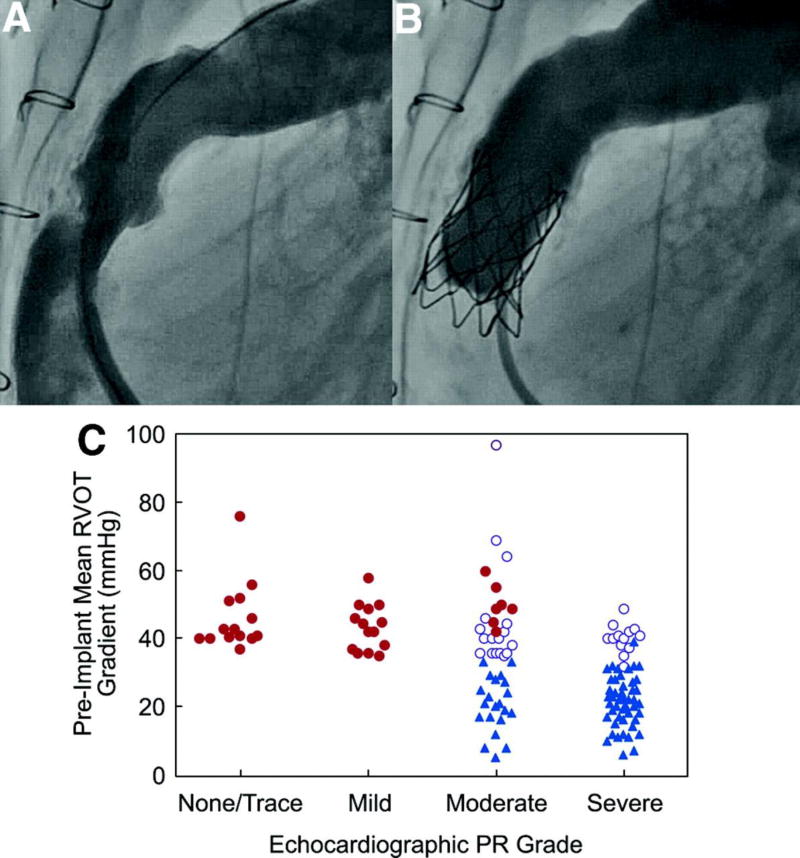
From January 2007 through August 19, 2009, 136 patients (87 male, 64%) were enrolled and underwent cardiac catheterization at a median age of 19 years (7 to 53 years). Diagnostic and conduit-related data are summarized in Table 1. Baseline right-sided hemodynamics are summarized in Table 2. PR was moderate or severe before implantation in 81% of patients with available data. The primary implantation indication among catheterized patients was PR in 70 patients (52%), RVOT obstruction in 36 (26%), and mixed disease in 30 (22%). As noted in Methods, the primary indication was assigned on the basis of screening echocardiographic data, but this categorization did not always reflect the presence of mixed disease (Figure 1). For example, the median directly measured RV pressure and peak RVOT gradient among patients with a PR indication were 58 and 23 mm Hg, whereas in those with a primary indication of obstruction or mixed disease, these measures were 70 and 43.5 mm Hg, respectively. Similarly, many patients with a primary indication of RVOT obstruction had PR and RV dilation, with the MRI-derived PR fraction in this subgroup ranging as high as 55% (median, 4.5%) and indexed RV end-diastolic volume as high as 225 mL/m2 (median, 95 mL/m2).
Table 1.
Diagnostic and Conduit-Related Data in 136 Patients Undergoing Catheterization
| Original cardiac diagnosis, n (%) | |
|---|---|
| Tetralogy of Fallot | 65 (48) |
| Pulmonary atresia | 40 |
| Pulmonary stenosis | 19 |
| Absent pulmonary valve | 5 |
| Atrioventricular canal | 1 |
| Aortic valve disease after Ross operation | 28 (21) |
| Transposition of the great arteries | 15 (11) |
| Truncus arteriosus | 14 (10) |
| Double-outlet right ventricle | 8 (6) |
| Valvar pulmonary stenosis | 3 (2) |
| Other | 2 (1) |
| NYHA functional class, n (%) | |
| I | 22 (16) |
| II | 91 (67) |
| III | 22 (16) |
| IV | 1 (1) |
| Type of conduit or pulmonary valve, n (%) | |
| Homograft | 103 (76) |
| Bioprosthetic valve or conduit* | 26 (19) |
| Synthetic | 7 (5) |
| Previously placed conduit stent, n (%) | |
| No | 100 (74) |
| Single stent | 23 (17) |
| Multiple stents | 12 (9) |
| No report | 1 (1) |
| Median (range) conduit/valve diameter at the time of surgical implantation, mm | 21 (16–28) |
| Number of prior surgical conduits | |
| Median (range), n | 1 (1–5) |
| >1, n (%) | 65 (48) |
Data reflect the number of patients and the percentage of the catheterized cohort (n=136) or the median and range as appropriate.
Includes bioprosthetic valves, conduits with integrated bioprosthetic valves, and nonhomograft biological conduits (eg, Contegra).
Table 2.
Baseline Right-Sided Hemodynamics Among the 124 Patients Who Underwent TPV Implantation
| Variable | Value |
|---|---|
| Echocardiography (n=120) | |
| RV systolic pressure, mm Hg | 73.4±17.8 |
| Median (range) | 74 (33–149) |
| Mean RVOT gradient, mm Hg | 33.9±14.7 |
| Median (range) | 34.7 (8–80) |
| Maximum instantaneous RVOT gradient, mm Hg | 55.6±23.0 |
| Median (range) | 54.8 (14–130) |
| MRI (n=100) | |
| RV end-diastolic volume, mL | 210.3±96.8 |
| Median (range) | 188 (93–715) |
| Indexed RV end-diastolic volume, mL/m2 | 126.7±51.6 |
| Median (range) | 114.7 (61–365) |
| RV ejection fraction, % | 42.7±13.6 |
| Median (range) | 42.8 (9–91) |
| RV mass, g | 67.6±26.7 |
| Median (range) | 62 (24–171) |
| PR fraction, % | 24.7±16.3 |
| Median (range) | 25.6 (0–79) |
| Catheterization (n=124) | |
| RV systolic pressure, mm Hg | 65.3±17.7 |
| Median (range) | 65 (23–108) |
| PA systolic pressure, mm Hg | 32.5±15.8 |
| Median (range) | 28.5 (13–88) |
| Peak RV-to-PA gradient, mm Hg | 35.6±15.8 |
| Median (range) | 37 (1–70) |
| RV/aortic pressure ratio | 0.71±0.18 |
| Median (range) | 0.74 (0.28–1.09) |
Data are presented as mean±SD and median (range). Echocardiographic and MRI data are not available for all patients, as discussed in the text.
Figure 1.
Angiograms demonstrating (A) preimplantation conduit obstruction and PR and (B) relief of obstruction and a competent valve after TPV. This patient with tetralogy of Fallot and pulmonary atresia had a primary indication of PR assigned on the basis of preimplantation echocardiography, which showed severe PR and a mean echocardiographic RVOT gradient of 18 mm Hg, although the directly measured RVOT gradient was 60 mm Hg at the time of catheterization. At the 2-year follow-up, there was no stent fracture, no PR, and a mean Doppler RVOT gradient of 11 mm Hg. C, Preimplantation mean Doppler RVOT gradient and echocardiographic PR grade are depicted in each patient according to the site-determined primary implantation indication: RVOT obstruction (solid red circles), PR (solid blue triangles), or mixed PR and obstruction (open purple circles). Patients of all NYHA classes are depicted; thus, some patients with moderate PR and a gradient >40 mm Hg are in the mixed indication category (NYHA class II or higher), and others are in the RVOT obstruction category (NYHA class I).
Procedural and Short-Term Outcomes
TPV placement was attempted in 124 of the 136 patients who underwent catheterization. Implantation was not attempted in 12 patients because of the risk of coronary compression in 6 (Figure 2), insufficient RVOT obstruction in 3 with stenosis as the primary implant indication, an anatomically unsuitable conduit (>20 mm in diameter by angiography or balloon sizing) in 2, and indication for concomitant pulmonary artery stenting (disallowed in the initial version of the protocol) in 1. Of these 12 patients, 5 subsequently underwent surgical conduit or pulmonary valve replacement, while conduit replacement was planned (as of the date this manuscript was submitted) in 2, pulmonary arterial stenting was performed in 1, and 4 received medical management. Among patients undergoing TPV placement, the valve was delivered from a femoral venous approach in 120 patients, the right internal jugular vein in 3, and the left subclavian vein in 1. Pre-dilation of the conduit was performed before balloon sizing in all but 2 patients. The angiographic conduit diameter prior to intervention ranged from 5–19.7 mm (median 12.9 mm), and the diameter of the sizing balloon waist (measured after pre-dilation, when performed) ranged from 14–20 mm (median 17 mm).
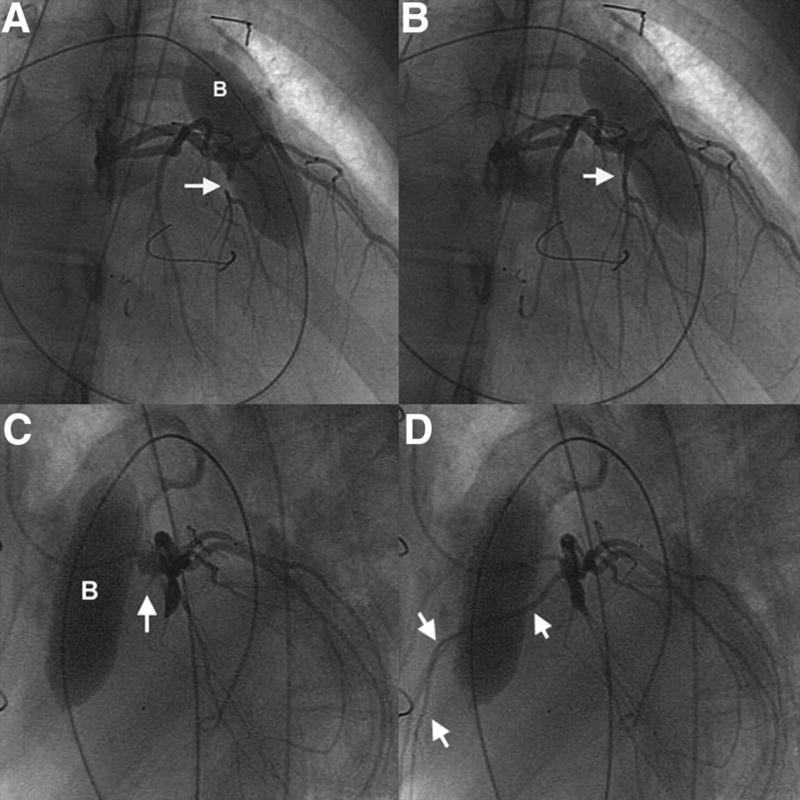
Figure 2.
In this patient with tetralogy of Fallot and pulmonary atresia, the conduit passed over a large anterior RV coronary branch that arose from the proximal left coronary artery. Selective coronary angiograms with simultaneous inflation of an angioplasty balloon (B) in the conduit demonstrate occlusion of this RV branch. Angiography from a right anterior oblique projection shows (A) early occlusion of the anomalous coronary (arrow), with persistent distal contrast, and (B) subsequent resumption of flow as the balloon was deflated (arrow). C and D, Subsequent angiography in a lateral projection demonstrates complete occlusion of the coronary branch with balloon inflation to higher pressure. In C, the arrow indicates the proximal stump of the occluded vessel, which then fills (multiple arrows) as the balloon is deflated in D.
Overall, TPV placement resulted in acute reduction in the RV pressure to a median of 41.5 mm Hg, the peak RVOT pressure gradient (including any subvalvar RVOT obstruction) to a median of 12 mm Hg, and the ratio of RV to aortic pressure to a median of 0.42. Hemodynamic results are presented by primary implantation indication in Table 3. All patients had no or trivial PR, except for 1 who was reported to have moderate angiographic PR but subsequently shown by echocardiography to have none (Figure 3). Additional interventional procedures at the same catheterization were not permitted in the initial 35 implanted patients but were performed in 51 of the subsequent 89 patients who underwent TPV. Concomitant interventions included bare metal stenting of the RVOT in 43 patients (single stent in 25, multiple stents in 18), branch pulmonary artery stenting or angioplasty in 8, coronary artery stenting in 1, inferior vena cava stenting in 1, and atrial septal defect closure in 1. Average procedure and fluoroscopy times were 174±67 and 46±25 minutes.
Table 3.
Pre- and Early Post-TPV Hemodynamics Measured During Catheterization in Patients Undergoing Melody Implantation Stratified by Primary Implantation Indication
| Primary Indication, PR (n=65) | Primary Indication, Obstruction or Mixed (n=59) | |||||
|---|---|---|---|---|---|---|
| Variable | Pre-TPV | Post-TPV | P | Pre-TPV | Post-TPV | P |
| RV systolic pressure, mm Hg | 61.6±20.6 | 47.2±15.0 | 0.001 | 69.4±12.9 | 44.7±10.9 | 0.001 |
| Median (range) | 58 (23–108) | 45 (23–91) | 70 (40–100) | 44.5 (26–92) | ||
| PA systolic pressure, mm Hg | 34.8±14.6 | 34.9±13.1 | 0.94 | 30.1±16.8 | 31.5±11.1 | 0.9 |
| Median (range) | 31 (13–75) | 33 (13–81) | 23.5 (15–88) | 28 (16–80) | ||
| Peak RV-to-PA gradient, mm Hg | 28.1±15.7 | 12.7±7.4 | 0.001 | 43.7±11.4 | 14.4±5.7 | 0.001 |
| Median (range) | 23 (1–69) | 12 (0–37) | 43.5 (20–70) | 14 (2–28) | ||
| Aortic systolic pressure, mm Hg | 93.8±13.5 | 112.5±20.9 | 0.001 | 89.7±12.7 | 104.8±17.2 | 0.001 |
| Median (range) | 91 (75–137) | 109 (77–114) | 88 (62–124) | 105 (50–119) | ||
| RV/aortic pressure ratio | 0.65±0.19 | 0.42±0.12 | 0.001 | 0.78±0.15 | 0.43±0.12 | |
| Median (range) | 0.66 (0.28–1.0) | 0.42 (0.18–0.75) | 0.78 (0.39–1.09) | 0.42 (0.28–0.90) | 0.001 | |
PA indicates pulmonary artery. Data are presented as mean±SD and median (range).
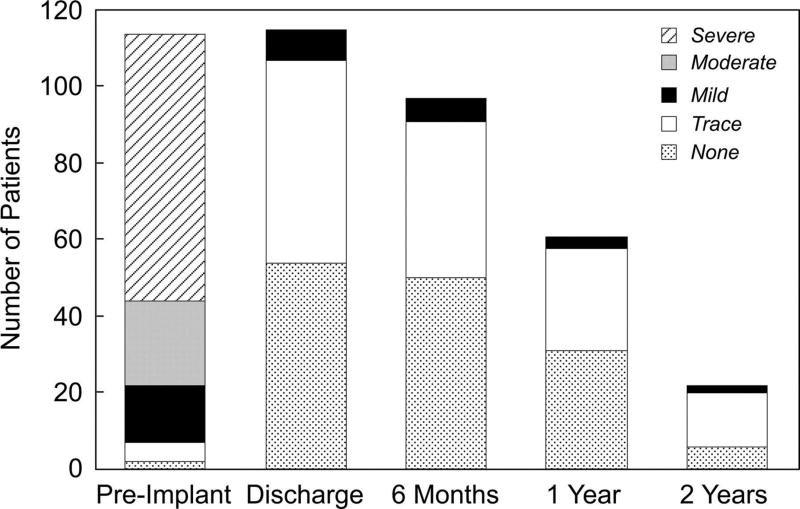
Figure 3.
Bar graph depicting the distribution of echocardiographic PR grades before TPV, at discharge, and at 6-month, 1-year, and 2-year follow-up evaluations among patients who underwent Melody implant. For technical reasons, the degree of PR could not be adequately graded from the preimplantation echocardiogram in 6 patients, the discharge echocardiogram in 3, and the 6-month echocardiogram in 1.
Eight of the 136 patients catheterized (6%) experienced serious procedural adverse events: coronary artery dissection treated with stenting and extracorporeal membrane oxygenation (n=1); conduit rupture treated with emergent surgery (n=1); contained conduit rupture/tear treated with covered stent placement (n=1); wide-complex tachycardia treated with cardioversion (n=1); hypercarbia and elevation of LV filling pressure treated acutely with milrinone and mechanical ventilation (n=1); femoral vein thrombosis treated with anticoagulation, thrombolysis, and balloon angioplasty (n=1); and 2 guidewire-induced perforations of a distal pulmonary artery branch (1 treated with coil occlusion of the injured vessel, 1 self-limited). The patient with a coronary dissection had severe biventricular dysfunction before catheterization and was diagnosed during the procedure, before TPV placement, with previously unrecognized occlusion of the proximal left coronary artery by the surgically placed bioprosthetic valved conduit. After coronary stenting, resuscitation, and TPV implantation, the chronically occluded coronary was recanalized and stented. This patient was able to come off extracorporeal support but subsequently suffered an intracranial hemorrhage and died. The 7 surviving patients with adverse events were discharged within 1 week of implantation; all other patients were discharged from the hospital the day of or the day after the procedure.
Follow-Up
Clinical Status and Functional Capacity
Aside from the patient who died after coronary dissection, all implanted patients were alive at the time of follow-up. Ninety-nine patients had completed the 6-month evaluation, 65 had completed the 1-year evaluation, and 24 had completed the 2-year evaluation (Figure I in the online-only Data Supplement). At the time of database closure, no patients were considered lost to follow-up.
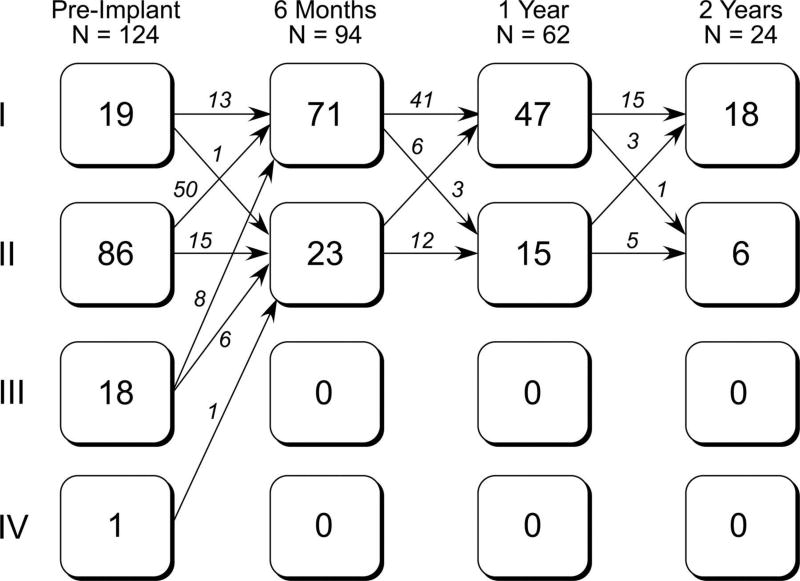
Improvements in NYHA class were observed at the 6-month visit and sustained through the duration of available follow-up in most patients (Figure 4). Across all evaluation intervals, there were 5 instances in which patients declined from NYHA class I to II, 3 of which were associated with stent fracture and recurrent RVOT obstruction. The QRS duration did not change from preimplantation to 6 months (Table 4).
Figure 4.
Flow diagram depicting the number of patients in each NYHA functional class and changes in status from before implantation to the 6-month, 1-year, and 2-year follow-up evaluations.
Table 4.
Pre- and Early Post-TPV Echocardiographic, MRI, and Exercise Test Data on Patients With Paired Preimplantation and 6-Month Postimplantation Testing
| Variable | Pre-TPV | 6-Month Post-TPV | P |
|---|---|---|---|
| Echocardiography (n=98) | |||
| RV pressure, mm Hg | 73.5±17.9 | 55.0±14.6 | 0.001 |
| Median (range) | 74.0 (33–149) | 51.0 (29–106) | |
| Mean RVOT gradient, mm Hg | 33.4±15.0 | 20.0±8.6 | 0.001 |
| Median (range) | 34.3 (8–80) | 17.5 (7–52) | |
| Maximum instantaneous RVOT gradient, mm Hg | 55.0±23.1 | 32.9±13.8 | 0.001 |
| Median (range) | 54.8 (14–130) | 29.2 (13–88) | |
| MRI (n=80) | |||
| RV end-diastolic volume, mL | 205.8±90.2 | 172.7±76.3 | 0.001 |
| Median (range) | 173 (93–574) | 152 (70–451) | |
| Indexed RV end-diastolic volume, mL/m2 | 125.1±49.2 | 103.0±39.5 | 0.001 |
| Median (range) | 111 (62–312) | 98 (48–253) | |
| RV ejection fraction, % | 43.2±14.1 | 42.6±12.3 | 0.9 |
| Median (range) | 43.8 (10–91) | 41.5 (8–68) | |
| RV mass, g | 67.3±27.3 | 57.5±19.3 | 0.001 |
| Median (range) | 62 (24–171) | 53 (25–114) | |
| PR fraction, % | 24.8±14.9 | 2.8±3.1 | 0.001 |
| Median (range) | 26.7 (0–61) | 1.8 (0–13) | |
| Cardiopulmonary exercise testing (n=93) | |||
| Peak relative V̇O2, mL · kg−1 ·/min−1 | 23.9±8.6 | 24.5±7.8 | 0.44 |
| Median (range) | 25.0 (9–59) | 24.9 (9–52) | |
| Percent of predicted peak V̇O2, % | 60.2±18.4 | 62.5±18.7 | 0.40 |
| Median (range) | 61 (20–122) | 63 (21–125) | |
| V̇E/V̇CO2 slope | 30.9±4.6 | 29.3±4.2 | 0.001 |
| Median (range) | 31 (21–48) | 29 (22–44) | |
| Respiratory exchange ratio | 1.11±0.12 | 1.13±0.12 | 0.01 |
| Median (range) | 1.11 (0.87–1.40) | 1.13 (0.88–1.37) | |
| ECG (n=90) | |||
| Heart rate, bpm | 70.4±12.2 | 69.8±13.3 | 0.62 |
| Median (range) | 70 (48–108) | 68 (40–108) | |
| QRS duration, ms | 142.7±32.4 | 142.1±35.2 | 0.73 |
| Median (range) | 146 (80–240) | 148 (61–242) |
Data are presented as mean±SD and median (range). The numbers of patients with follow-up data do not equal the number with clinical follow-up because of the time lag between clinical follow-up evaluation and core laboratory reading/entry of study data, as discussed in Methods, and MRI studies that were not performed because of pacemakers or metallic artifact.
Cardiopulmonary exercise testing was performed at baseline in 113 patients, and paired preimplantation and 6-month data were available in 93 (Table 4). The VE/VCO2 slope was significantly lower (improved) at 6 months; the preimplantation to 6-month change in peak VO2 as a percent of the predicted value was significant before adjustment but not after. These results were no different between patients with a primary implantation indication of PR and those with RVOT obstruction or mixed disease. The respiratory exchange ratio was <1.1 (suggesting submaximal effort) in 40 (43%) of these 93 patients on the preimplantation study and 36 (39%) on the 6-month study.
Hemodynamic Results
Echocardiography
Core echocardiographic measurements were available for the preimplantation evaluation in 120 of the 123 implanted patients with an intact valve at 24 hours, for the early post-TPV study in 118, for the 6-month visit in 98, for the 1-year visit in 61, and for the 2-year visit in 22. Echocardiographic PR, which was moderate or severe before implantation in 81% of patients with available data, was none or trivial at all time points in >90% of patients with follow-up (Figure 3). RV pressure and RVOT gradients were lower at 6 months than before implantation (Table 4). Among patients with paired preimplantation and 12-month postimplantation data, estimated RV pressure was down from a median of 74 mm Hg (33 to 110 mm Hg) to 54 mm Hg (31 to 102 mm Hg), and the mean RVOT gradient was down from 28 mm Hg (8 to 63 mm Hg) to 19 mm Hg (6 to 48 mm Hg; both P=0.001).
MRI Measurements
Preimplantation core laboratory MRI measurements were available in 100 patients, and paired preimplantation and 6-month postimplantation MRI data were available in 80 patients. Eleven patients did not undergo preimplantation MRI because of the presence of a pacemaker or internal defibrillator, and 13 did not undergo MRI for other reasons or because adequate data could not be obtained due to artifact from metallic implants. The PR fraction was down from a median of 26.7% to 1.8%, with a maximum of 11.6%, and only 9 patients had a PR fraction >5%. As summarized in Table 4, RV end-diastolic volume, PR fraction, and RV mass decreased significantly, but RV ejection fraction did not change. These changes were observed regardless of primary implantation indication.
Stent Fracture
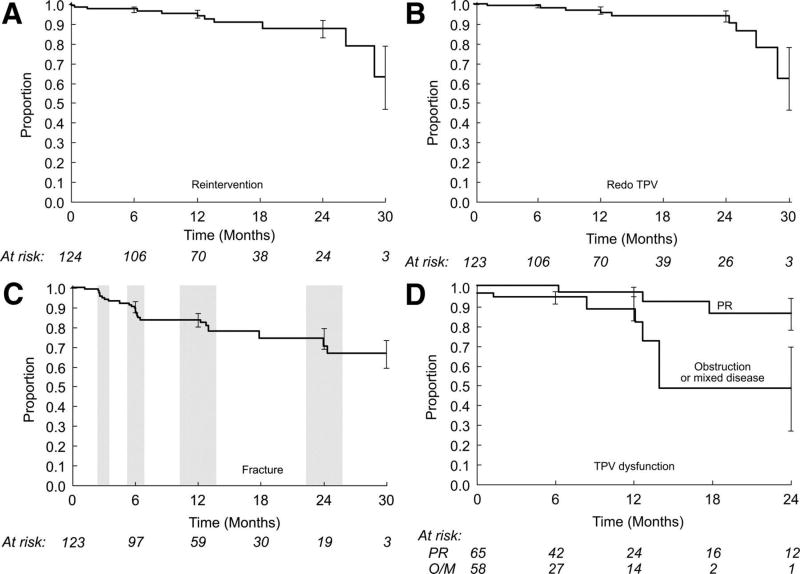
Stent fractures were diagnosed in 25 patients: 8 initially at the 3-month evaluation, 10 at the 6-month evaluation, 4 at the 1-year evaluation, and 3 at the 2-year evaluation. In all but one of these patients, the fracture was initially defined as minor, with ≥1 individual struts fractured but no loss of stent integrity. One stent fracture was major at the time of identification (defined as multiple strut fractures with a loss of stent integrity), and 6 progressed from minor to major during the course of follow-up. As depicted in Figure 5, freedom from diagnosis of stent fracture was 83.7±3.7% at 7.5 months (after all 6-month evaluations were complete) and 77.8±4.3% at 14 months (after all available 1-year evaluations were complete).
Figure 5.
Kaplan–Meier curves depicting (A) survival free from RVOT reintervention, (B) freedom from placement of a second Melody valve, (C) freedom from diagnosis of stent fracture, and (D) freedom from Melody valve dysfunction, with separate curves for patients with a primary implantation indication of PR and those with an indication of RVOT obstruction or mixed disease (O/M). Error bars indicate SE. The shaded regions in the graph showing freedom from diagnosis of stent fracture indicate the follow-up windows for the 3-, 6-, 12-, and 24-month evaluations.
Melody Valve Dysfunction and Reintervention
Including the patient who underwent emergent conduit replacement for conduit rupture during the implantation procedure, 11 patients underwent RVOT reintervention after TPV. Nine of these patients received a second TPV for stent fracture and recurrent RVOT obstruction (1 as a second reintervention after prior redilation of the Melody valve), 1 underwent redilation of the original Melody valve without placement of a second TPV, and 1 had emergent conduit replacement, as discussed above and previously.22 Among the 10 patients who underwent catheter-based reintervention, the median directly measured RVOT gradient was 47.5 mm Hg (16 to 68 mm Hg) before reintervention and fell to 9 mm Hg (6 to 11 mm Hg) after reintervention.
Among the 9 patients who underwent placement of a second TPV, the primary indication for the original Melody valve implantation was PR in 4, RVOT obstruction in 3, and mixed PR and obstruction in 2. Two of the 4 with a primary implantation indication of PR had a peak RVOT gradient <20 mm Hg at the initial catheterization.
Survival free from RVOT reintervention was 95.4±2.1% at 1 year and 87.6±4.5% at 2 years. Freedom from a second TPV was 96.9±2.0% at 1 year and 90.4±4.4% at 2 years (Figure 5). Freedom from TPV dysfunction was 93.5±2.4% at 1 year and 85.6±4.7% at 2 years. By multivariable Cox regression analysis, a higher mean RVOT gradient on discharge echocardiography (univariable HR, 3.3; 95% CI, 1.4 to 7.4 per 10 mm Hg; P=0.003; multivariable HR, 3.2; 95% CI, 1.5 to 6.8 per 10 mm Hg; P=0.003) and younger age (univariable HR, 0.85; 95% CI, 0.74 to 0.98 per year; P=0.008; multivariable HR, 0.86; 95% CI, 0.73 to 0.99 per year; P=0.01) were associated with shorter freedom from TPV dysfunction. A primary implantation indication of RVOT obstruction or mixed disease was the only other independent variable that was significant by univariable but not multivariable analysis (univariable HR, 5.7; 95% CI, 1.4 to 23.5; P=0.01; Figure 5).
Adverse Events
Aside from procedural adverse events, stent fractures, recurrent obstruction, and reinterventions summarized above, the only adverse events reported during follow-up consisted of right heart failure associated with pulmonary hypertension (n=1) and worsening tricuspid regurgitation in the setting of recurrent RVOT obstruction, which was subsequently treated with placement of a second TPV (n=1).
Discussion
In this expanded cohort of 136 patients referred for Melody valve implantation as part of the US investigational device exemption trial, we substantiated the findings of our initial 34-patient cohort22 with 2 additional sites and nearly 100 additional implantations. Serious adverse events were uncommon, and implantation was achieved successfully in all but 1 patient, who experienced a conduit rupture that was treated with surgical conduit replacement. There was 1 death, which resulted from complications of a coronary artery dissection that occurred before TPV implantation in a patient with severe biventricular dysfunction and a coronary artery that was found to be occluded by the conduit before intervention.
Assessment of the Melody valve can be divided into evaluation of device performance and clinical impact, each of which can be thought of in the short term and longer term.
Valve Performance
The hemodynamic effectiveness of the Melody valve in this series was similar to findings reported by Bonhoeffer’s group and in our earlier report.11,15,22 Pulmonary valve competence was maintained in patients for as long as they were followed up, and RV end-diastolic volume was significantly smaller at 6 months than before implantation. Directly measured and Doppler RVOT gradients were significantly lower after TPV implantation but not eliminated completely in most patients with obstruction. Although we did not observe significant changes in RV function in this series, it has been shown in other cohorts that RV pressure and/or volume unloading with valve placement is associated with improved RV and septal strain,25 which we did not measure, and with improved biventricular function.12,14,19
The appropriate outcome measure for assessing longer-term TPV function is debatable. Freedom from RVOT reintervention or reoperation is a clear, temporally defined outcome but is based on various factors other than valve function, including judgment about hemodynamic and clinical criteria for reintervention, and may be inadequate as a measure of valve performance. Hemodynamic criteria are preferable but are limited in their temporal resolution (ie, only ascertained when specific evaluation is performed) and may be inaccurate because of difficulty imaging the RVOT and/or other technical factors. In this study, we analyzed various outcomes, including survival free from RVOT reintervention, freedom from a second TPV, and freedom from TPV dysfunction, a composite outcome defined as moderate or greater PR, a mean RVOT gradient ≥40 mm Hg, or RVOT reintervention. In most of our patients, relief of obstruction was maintained throughout the follow-up, but recurrent obstruction, usually associated with stent fracture, led to RVOT reintervention in 10 patients, 9 of whom received a second Melody valve. Freedom from TPV dysfunction was 93.5±2.4% at 1 year and 85.7±4.7% at 2 years. On multivariable analysis, a higher echocardiographic RVOT gradient at the time of discharge and younger age were associated with shorter freedom from dysfunction. A primary implantation indication of RVOT obstruction or mixed disease was associated with shorter freedom from TPV dysfunction on univariable but not multivariable analysis.
When Lurz et al15 assessed risk factors for RVOT reoperation, which were for obstruction, conduit rupture, or valve malposition in all cases (ie, not PR), they found shorter freedom from reoperation in their first 50 patients than in subsequent patients and in patients with a residual RVOT gradient ≥25 mm Hg after TPV placement. An important determinant of the improved outcomes in their more recent experience was their adoption of a “valve-in-valve” approach to TPV dysfunction, with concentric placement of a second Melody valve within the first, usually with additional bare metal stents implanted to buttress the conduit.16 In contrast to their experience, we found no time-order effect or obvious learning curve in our experience, which is likely due in part to the rigorous, standardized inclusion criteria of the US trial.
Recurrent or new RVOT obstruction after TPV implantation, usually associated with stent fracture, is one of the major issues confronting this technology. Simply by virtue of the dynamic implant environment and the intrinsic characteristics of metals used for balloon-expandable stents, RVOT stents are at ongoing risk for fatigue and fracture.13,27 The response of the device to fracture may vary from patient to patient, depending on a variety of factors, and it is possible that some device fractures will not become hemodynamically or clinically important. In our cohort, device fracture was not necessarily associated with valve dysfunction when the fracture was identified, and it is unknown whether stent fractures will inevitably lead to device failure and consequent RVOT obstruction. Although we estimated freedom from diagnosis of stent fracture in this analysis, which was just under 80% at the end of the 1-year evaluation window, we elected to defer evaluation of risk factors for stent fracture in light of ongoing diagnosis of fractures at the 1- and 2-year evaluations and the potential importance of conduit-related factors not included in the original study database. Additional data are being collected to address these issues.
Clinical Impact
The clinical implications of treating RVOT dysfunction are both immediate and longer term. In the short term, a large majority of patients in this series demonstrated improved functional capacity, as evidenced by improvement in NYHA status. There was also a modest improvement in the VE/VCO2 slope. Coats et al12,14 previously reported that some exercise parameters improved after TPV in patients with predominant RVOT obstruction and not in those with primary PR, but this was not true in our cohort. Unfortunately, a high percentage of patients had a respiratory exchange ratio that suggested submaximal effort on both preimplantation and 6-month exercise studies, which may limit our ability to assess the impact of TPV placement on exercise cardiopulmonary function.
Although there is mounting evidence that chronic PR and RV hypertension are detrimental, the longer-term implications of therapies that reduce RV volume and pressure load have yet to be determined.2–10,28–30 A recent study demonstrated short-term improvement in biventricular efficiency with placement of a bare metal stent to relieve pressure overload followed by implantation of a Melody valve to relieve volume overload, providing support for the intuitive argument that normalization of RVOT hemodynamics is beneficial.19 The extent to which such improvement depends on the underlying health of the RV is unknown, and the longer-term effects of such interventions are not well characterized. Additional work in this area is important to more completely understand the indications for and impact of treating RVOT dysfunction.
Safety
In this series and prior reports, serious adverse events associated with Melody TPV were relatively uncommon. In their cohort of 155 patients, Lurz et al15 reported no procedural mortality and significant complications managed surgically in ≈3% of patients. In our series, procedural adverse events occurred in 6% of patients, with 1 death and 1 complication managed surgically. Conduit rupture and PA injury resulting from wire perforation were the most common procedural adverse events (2 patients each). Awareness of the potential for these potentially serious complications is essential to the safe use of TPV technology, and further investigation is necessary to better understand, prevent, and manage them. As discussed above, stent fracture, recurrent RVOT obstruction, and subsequent reintervention were the only device-related adverse events during follow-up.
The most common reason that patients enrolled and catheterized in this study did not receive a TPV was a perceived high risk of coronary artery compression with stent and/or valve placement. As discussed in our prior article and highlighted by other investigators, it is essential to assess the risk of coronary artery compression when placing an RVOT stent or TPV.22,27,31,32 In patients with cardiac defects treated with RVOT reconstruction such as tetralogy of Fallot and complex malposition or transposition of the great arteries, coronary arterial anatomy may be anomalous.33–35 Moreover, because the aortic root is relatively anterior in these anomalies, the origins of even “normal” coronary arteries are typically displaced relative to normal. In such circumstances, the RVOT conduit may pass directly over a major coronary branch, placing the coronary artery at risk for compression and obstruction if a rigid stent is expanded in the conduit and displaces it toward the heart. Some such patients, including 1 in this series, are found to have coronary compromise resulting from simply compression by the surgical conduit. In addition, in patients with otherwise normal anatomy who undergo a Ross procedure, the coronary arteries may be at risk for compression, particularly if the left coronary artery is implanted into the autograft in a relatively high or anterior location or if the conduit lies low across the outflow region. In any event, it may not be possible to predict the risk of coronary compression with noninvasive imaging; even if the relationship between the conduit and a coronary appears close, stenting may displace the conduit away from the coronary or the portion of the conduit near the coronary may be distant from the target site for the stent. Thus, it is essential that the risk of coronary compression is investigated before TPV implantation with screening aortic or coronary angiography and, in cases with an apparently close anatomic relationship, simultaneous angiography and balloon inflation in the conduit using fluoroscopic projections that optimize resolution of the conduit-coronary relationship.
Conclusions
In this updated report from the first prospective multicenter TPV trial, we demonstrated an ongoing high rate of procedural success and encouraging short-term function of the Melody valve. The addition of 2 sites to the original trial protocol supports the conclusion that this technology can be adopted safely and effectively by properly trained, experienced interventional pediatric/congenital cardiologists. The fact that all reinterventions in this series were for RVOT obstruction highlights the importance of appropriate patient selection, adequate relief of obstruction at the time of Melody valve placement, and measures to prevent or manage stent fracture. Additional data and longer follow-up are necessary to understand the optimal indications for and timing of pulmonary valve placement in patients with postoperative RVOT dysfunction, as well as the ultimate role of TPV therapy in this population.
Supplementary Material
Acknowledgments
Source of Funding
The clinical trial reported in this study was sponsored and funded by Medtronic Inc.
This work was supported by the National Institutes of Health under award number: T32HL007572. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosures
Drs McElhinney, Hellenbrand, Zahn, Jones, Cheatham, and Vincent act in consultant roles for Medtronic Inc, the manufacturer of the Melody valve, and all authors act as investigators and/or proctors. Dr Cheatham also acts as a consultant for NuMed Inc.
References
- 1.Bonhoeffer P, Boudjemline Y, Saliba Z, Merckx J, Aggoun Y, Bonnet D, Acar P, Le Bidois J, Sidi D, Kachaner J. Percutaneous replacement of pulmonary valve in a right-ventricle to pulmonary-artery prosthetic conduit with valve dysfunction. Lancet. 2000;356:1403–1405. doi: 10.1016/S0140-6736(00)02844-0. [DOI] [PubMed] [Google Scholar]
- 2.Shimazaki Y, Blackstone EH, Kirklin JW. The natural history of isolated congenital pulmonary valve incompetence: surgical implications. Thorac Cardiovasc Surg. 1984;32:257–259. doi: 10.1055/s-2007-1023399. [DOI] [PubMed] [Google Scholar]
- 3.Carvalho JS, Shinebourne EA, Busst C, Rigby ML, Redington AN. Exercise capacity after complete repair of tetralogy of Fallot: deleterious effects of residual pulmonary regurgitation. Br Heart J. 1992;67:470–473. doi: 10.1136/hrt.67.6.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eyskens B, Reybrouck T, Bogaert J, Dymarkowsky S, Daenen W, Dumoulin M, Gewillig M. Homograft insertion for pulmonary regurgitation after repair of tetralogy of Fallot improves cardiorespiratory exercise performance. Am J Cardiol. 2000;85:221–225. doi: 10.1016/s0002-9149(99)00640-2. [DOI] [PubMed] [Google Scholar]
- 5.Gatzoulis MA, Balaji S, Webber SA, Siu SC, Hokanson JS, Poile C, Rosenthal M, Nakazawa M, Moller JH, Gillette PC, Webb GD, Redington AN. Risk factors for arrhythmia and sudden cardiac death late after repair of tetralogy of Fallot: a multicentre study. Lancet. 2000;356:975–981. doi: 10.1016/S0140-6736(00)02714-8. [DOI] [PubMed] [Google Scholar]
- 6.Therrien J, Siu S, Harris L, Dore A, Niwa K, Janousek J, Williams WG, Webb G, Gatzoulis MA. Impact of pulmonary valve replacement on arrhythmia propensity late after repair of tetralogy of Fallot. Circulation. 2001;103:2489–2494. doi: 10.1161/01.cir.103.20.2489. [DOI] [PubMed] [Google Scholar]
- 7.Davlouros PA, Karatza AA, Gatzoulis MA, Shore DF. Timing and type of surgery for severe pulmonary regurgitation after repair of tetralogy of Fallot. Int J Cardiol. 2004;97 (suppl 1):91–101. doi: 10.1016/j.ijcard.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 8.Oosterhof T, van Straten A, Vliegen HW, Meijboom FJ, van Dijk AP, Spijkerboer AM, Bouma BJ, Zwinderman AH, Hazekamp MG, de Roos A, Mulder BJ. Preoperative thresholds for pulmonary valve replacement in patients with corrected tetralogy of Fallot using cardiovascular magnetic resonance. Circulation. 2007;116:545–551. doi: 10.1161/CIRCULATIONAHA.106.659664. [DOI] [PubMed] [Google Scholar]
- 9.Mulder BJ, Vliegen HW, van der Wall EE. Diastolic dysfunction: a new additional criterion for optimal timing of pulmonary valve replacement in adult patient with tetralogy of Fallot? Int J Cardiovasc Imaging. 2008;24:867–870. doi: 10.1007/s10554-008-9344-y. [DOI] [PubMed] [Google Scholar]
- 10.Frigiola A, Tsang V, Bull C, Coats L, Khambadkone S, Derrick G, Mist B, Walker F, van Doorn C, Bonhoeffer P, Taylor AM. Biventricular response after pulmonary valve replacement for right ventricular outflow tract dysfunction: is age a predictor of outcome? Circulation. 2008;118 (suppl):S182–S190. doi: 10.1161/CIRCULATIONAHA.107.756825. [DOI] [PubMed] [Google Scholar]
- 11.Khambadkone S, Coats L, Taylor A, Boudjemline Y, Derrick G, Tsang V, Cooper J, Muthurangu V, Hegde SR, Razavi RS, Pellerin D, Deanfield J, Bonhoeffer P. Transcatheter pulmonary valve implantation in humans: initial results in 59 consecutive patients. Circulation. 2005;112:1189–1197. doi: 10.1161/CIRCULATIONAHA.104.523266. [DOI] [PubMed] [Google Scholar]
- 12.Coats L, Khambadkone S, Derrick G, Sridharan S, Schievano S, Mist B, Jones R, Deanfield JE, Pellerin D, Bonhoeffer P, Taylor AM. Physiological and clinical consequences of relief of right ventricular outflow tract obstruction late after repair of congenital heart defects. Circulation. 2006;113:2037–2044. doi: 10.1161/CIRCULATIONAHA.105.591438. [DOI] [PubMed] [Google Scholar]
- 13.Nordmeyer J, Khambadkone S, Coats L, Schievano S, Lurz P, Parenzan G, Taylor AM, Lock JE, Bonhoeffer P. Risk stratification, systematic classification, and anticipatory management strategies for stent fracture after percutaneous pulmonary valve implantation. Circulation. 2007;115:1392–1397. doi: 10.1161/CIRCULATIONAHA.106.674259. [DOI] [PubMed] [Google Scholar]
- 14.Coats L, Khambadkone S, Derrick G, Hughes M, Jones R, Mist B, Pellerin D, Marek J, Deanfield JE, Bonhoeffer P, Taylor AM. Physiological consequences of percutaneous pulmonary valve implantation: the different behaviour of volume- and pressure-overloaded ventricles. Eur Heart J. 2007;28:1886–1893. doi: 10.1093/eurheartj/ehm181. [DOI] [PubMed] [Google Scholar]
- 15.Lurz P, Coats L, Khambadkone S, Nordmeyer J, Boudjemline Y, Schievano S, Muthurangu V, Lee TY, Parenzan G, Derrick G, Cullen S, Walker F, Tsang V, Deanfield J, Taylor AM, Bonhoeffer P. Percutaneous pulmonary valve implantation: impact of evolving technology and learning curve on clinical outcome. Circulation. 2008;117:1964–1972. doi: 10.1161/CIRCULATIONAHA.107.735779. [DOI] [PubMed] [Google Scholar]
- 16.Nordmeyer J, Coats L, Lurz P, Lee TY, Derrick G, Rees P, Cullen S, Taylor AM, Khambadkone S, Bonhoeffer P. Percutaneous pulmonary valve-in-valve implantation: a successful treatment concept for early device failure. Eur Heart J. 2008;29:810–815. doi: 10.1093/eurheartj/ehn073. [DOI] [PubMed] [Google Scholar]
- 17.Kostolny M, Tsang V, Nordmeyer J, Van Doorn C, Frigiola A, Khambadkone S, de Leval MR, Bonhoeffer P. Rescue surgery following percutaneous pulmonary valve implantation. Eur J Cardiothorac Surg. 2008;33:607–612. doi: 10.1016/j.ejcts.2007.12.034. [DOI] [PubMed] [Google Scholar]
- 18.Lurz P, Puranik R, Nordmeyer J, Muthurangu V, Hansen MS, Schievano S, Marek J, Bonhoeffer P, Taylor AM. Improvement in left ventricular filling properties after relief of right ventricle to pulmonary artery conduit obstruction: contribution of septal motion and interventricular mechanical delay. Eur Heart J. 2009;30:2266–2274. doi: 10.1093/eurheartj/ehp258. [DOI] [PubMed] [Google Scholar]
- 19.Lurz P, Nordmeyer J, Muthurangu V, Khambadkone S, Derrick G, Yates R, Sury M, Bonhoeffer P, Taylor AM. Comparison of bare metal stenting and percutaneous pulmonary valve implantation for treatment of right ventricular outflow tract obstruction: use of an x-ray/magnetic resonance hybrid laboratory for acute physiological assessment. Circulation. 2009;119:2995–3001. doi: 10.1161/CIRCULATIONAHA.108.836312. [DOI] [PubMed] [Google Scholar]
- 20.Lurz P, Nordmeyer J, Coats L, Taylor AM, Bonhoeffer P, Schulze-Neick I. Immediate clinical and haemodynamic benefits of restoration of pulmonary valvar competence in patients with pulmonary hypertension. Heart. 2009;95:646–650. doi: 10.1136/hrt.2008.153379. [DOI] [PubMed] [Google Scholar]
- 21.Sridharan S, Coats L, Khambadkone S, Taylor AM, Bonhoeffer P. Images in cardiovascular medicine: transcatheter right ventricular outflow tract intervention: the risk to the coronary circulation. Circulation. 2006;113:e934–e935. doi: 10.1161/CIRCULATIONAHA.105.599514. FREE Full Text. [DOI] [PubMed] [Google Scholar]
- 22.Zahn EM, Hellenbrand WE, Lock JE, McElhinney DB. Implantation of the Melody transcatheter pulmonary valve in patients with dysfunctional right ventricular outflow tract conduits: early results from the U.S. clinical trial. J Am Coll Cardiol. 2009;54:1722–1729. doi: 10.1016/j.jacc.2009.06.034. [DOI] [PubMed] [Google Scholar]
- 23.Romeih S, Kroft LJ, Bokenkamp R, Schalij MJ, Grotenhuis H, Hazekamp MG, Groenink M, de Roos A, Blom NA. Delayed improvement of right ventricular diastolic function and regression of right ventricular mass after percutaneous pulmonary valve implantation in patients with congenital heart disease. Am Heart J. 2009;158:40–46. doi: 10.1016/j.ahj.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 24.Momenah TS, El Oakley R, Al Najashi K, Khoshhal S, Al Qethamy H, Bonhoeffer P. Extended application of percutaneous pulmonary valve implantation. J Am Coll Cardiol. 2009;53:1859–1863. doi: 10.1016/j.jacc.2008.08.061. [DOI] [PubMed] [Google Scholar]
- 25.Moiduddin N, Asoh K, Slorach C, Benson LN, Friedberg MK. Effect of transcatheter pulmonary valve implantation on short-term right ventricular function as determined by two-dimensional speckle tracking strain and strain rate imaging. Am J Cardiol. 2009;104:862–867. doi: 10.1016/j.amjcard.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 26.Hochberg Y. A sharper Bonferroni procedure for multiple tests of significance. Biometrics. 1988;75:800–802. [Google Scholar]
- 27.Peng LF, McElhinney DB, Nugent AW, Powell AJ, Marshall AC, Bacha EA, Lock JE. Endovascular stenting of obstructed right ventricle-to-pulmonary artery conduits: a fifteen-year experience. Circulation. 2006;113:2598–2605. doi: 10.1161/CIRCULATIONAHA.105.607127. [DOI] [PubMed] [Google Scholar]
- 28.Lim C, Lee JY, Kim WH, Kim SC, Song JY, Kim SJ, Choh JH, Kim CW. Early replacement of pulmonary valve after repair of tetralogy: is it really beneficial? Eur J Cardiothorac Surg. 2004;25:728–734. doi: 10.1016/j.ejcts.2004.01.036. [DOI] [PubMed] [Google Scholar]
- 29.Gengsakul A, Harris L, Bradley TJ, Webb GD, Williams WG, Siu SC, Merchant N, McCrindle BW. The impact of pulmonary valve replacement after tetralogy of Fallot repair: a matched comparison. Eur J Cardiothorac Surg. 2007;32:462–468. doi: 10.1016/j.ejcts.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 30.Harrild DM, Berul CI, Cecchin F, Geva T, Gauvreau K, Pigula F, Walsh EP. Pulmonary valve replacement in tetralogy of Fallot: impact on survival and ventricular tachycardia. Circulation. 2009;119:445–451. doi: 10.1161/CIRCULATIONAHA.108.775221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maheshwari S, Bruckheimer E, Nehgme RA, Fahey JT, Kholwadwala D, Hellenbrand WE. Single coronary artery complicating stent implantation for homograft stenosis in tetralogy of Fallot. Cathet Cardiovasc Diagn. 1997;42:405–407. doi: 10.1002/(sici)1097-0304(199712)42:4<405::aid-ccd13>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 32.Perret X, Bonvini RF, Aggoun Y, Verin V. Aberrant right coronary artery occlusion during the percutaneous pulmonary trunk stenting in a patient with tetralogy of Fallot. Heart Vessels. 2008;23:140–143. doi: 10.1007/s00380-007-1023-8. [DOI] [PubMed] [Google Scholar]
- 33.Meng CCL, Eckner FAO, Lev M. Coronary artery distribution in tetralogy of Fallot. Arch Surg. 90:363–365. doi: 10.1001/archsurg.1965.01320090041009. [DOI] [PubMed] [Google Scholar]
- 34.Elliott LP, Amplatz K, Edwards JE. Coronary arterial patterns in transposition complexes. Anatomic and angiocardiographic studies. Am J Cardiol. 1966;17:362–378. doi: 10.1016/0002-9149(66)90219-0. [DOI] [PubMed] [Google Scholar]
- 35.Gordillo L, Faye-Petersen O, de la Cruz MV, Soto B. Coronary arterial patterns in double-outlet right ventricle. Am J Cardiol. 1993;71:1108–1110. doi: 10.1016/0002-9149(93)90583-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.