See Goswami (doi:10.1093/brain/awu296) for a scientific commentary on this article.
Clark et al. present longitudinal MRI data from children at high risk of dyslexia, from before reading instruction began until after dyslexia was diagnosed. Prior to learning to read, children with dyslexia have thinner cortex in visual and auditory processing areas than controls, whereas the “reading network” itself is unaffected.
Keywords: cortical thickness, reading, neuroimaging, paediatric, development
Abstract
Developmental dyslexia is a common reading disorder that negatively impacts an individual’s ability to achieve literacy. Although the brain network involved in reading and its dysfunction in dyslexia has been well studied, it is unknown whether dyslexia is caused by structural abnormalities in the reading network itself or in the lower-level networks that provide input to the reading network. In this study, we acquired structural magnetic resonance imaging scans longitudinally from 27 Norwegian children from before formal literacy training began until after dyslexia was diagnosed. Thus, we were able to determine that the primary neuroanatomical abnormalities that precede dyslexia are not in the reading network itself, but rather in lower-level areas responsible for auditory and visual processing and core executive functions. Abnormalities in the reading network itself were only observed at age 11, after children had learned how to read. The findings suggest that abnormalities in the reading network are the consequence of having different reading experiences, rather than dyslexia per se, whereas the neuroanatomical precursors are predominantly in primary sensory cortices.
Introduction
Reading is a complex cognitive process that develops over many years and is associated with the development and integration of several discrete brain regions to form a reading network (Turkeltaub et al., 2003). Dyslexia is characterized as an unexpected difficulty with learning to read, meaning that despite having otherwise normal intelligence and exposure to training, individuals with dyslexia persistently struggle with reading. Historically it has been difficult to elucidate the underlying causes of dyslexia because, as with all developmental disorders, several different causes may lead to the same end-point (Goswami, 2003). For example, a multitude of studies have demonstrated that dyslexic individuals have impaired phonological awareness, and that measures of phonological skill have predictive value for reading ability (Eden and Zeffiro, 1998; Eckert et al., 2003). However, there are many low-level abnormalities that could lead to impairments in phonological awareness, such as basic auditory processing, magnocellular, cerebellar, attention-shifting, or general sensorimotor deficits (Goswami, 2003). Thus, adopting a neuroconstructivist approach (Goswami, 2003), in which children with a disorder are studied as early as possible, is necessary to draw meaningful inferences about causality in dyslexia and other neurodevelopmental disorders. Understanding the underlying causes of dyslexia may allow for earlier and more focused remediation leading to improved outcomes in predisposed individuals, which would have a large impact on public health.
In the present study we first identified a cohort of subjects in preschool who were at high (n = 26) and low (n = 26) risk for dyslexia and then followed those subjects longitudinally with annual neurocognitive assessments until the sixth grade, when dyslexia could be diagnosed. A subset of the original subject pool (n = 39) chose to participate in three neuroimaging sessions: in the first, third and sixth grades, when the children were ages 6, 8, and 11 years old, respectively. Twelve were excluded for a variety of reasons (see Supplementary material for details), such that 27 subjects were included in the final analysis, 11 of whom developed dyslexia. As Norwegian children at the time of this study did not learn to read until the second grade when they were 7 years old (Helland et al., 2011a), we were able to obtain pre-reading estimates of cortical thickness in the first grade, at age 6. Because we acquired data longitudinally, we were able to quantify how cortical thickness evolved over time.
Materials and methods
Subjects and study design
A questionnaire to determine risk for developing dyslexia was given to caregivers of all 120 Norwegian children enrolled in nine preschools selected from all four counties in western Norway (Helland et al., 2011a). Responses were received from 109 caregivers. From that sample, 52 preschool children (26 at the highest risk and 26 at the lowest risk for developing dyslexia) were originally enrolled in the Bergen Longitudinal Dyslexia Study (http://www.uib.no/project/speakup). Neurocognitive assessments were administered in the fall of 5 years: preschool (baseline, ages 5–6), first grade (pre-MRI 1, ages 6–7), second grade (post-MRI 1, ages 7–8), third grade (pre-MRI 2, ages 8–9), and sixth grade (pre-MRI 3, ages 11–12). MRI scans were acquired in the spring of 3 years: first grade (MRI 1, ages 6–7), third grade (MRI 2, ages 8–9), and sixth grade (MRI 3, ages 11–12). Demographics for the subjects included in the current study are summarized in Table 1 (see Supplementary Material for details). Standard exclusion criteria were applied: mental retardation and diagnoses of any other impairment including attention deficit hyperactivity disorder, neurological impairment, and any visual or hearing impairments on the basis of parental report. The study was approved by the Regional Committee for Medical Research Ethics in Western Norway and Norwegian Social Science Data Services. All parents gave informed consent.
Table 1.
Demographics of subjects who participated in MRI sessions
| Dyslexia | Control | Significance (P-value) | ||
|---|---|---|---|---|
| Gender (male:female) | ||||
| MRI 1 | 3:4 | 6:4 | <0.64 | |
| MRI 2 | 4:5 | 10:6 | <0.43 | |
| MRI 3 | 5:6 | 8:5 | <0.68 | |
| Age, years | ||||
| MRI 1 | 6.6 ± 0.2 | 6.8 ± 0.2 | <0.13 | |
| MRI 2 | 8.7 ± 0.3 | 8.7 ± 0.3 | <0.74 | |
| MRI 3 | 11.9 ± 0.3 | 11.7 ± 0.2 | <0.07 | |
| Handedness (right:left) | ||||
| MRI 1 | 7:0 | 8:2 | <0.49 | |
| MRI 2 | 9:0 | 12:4 | <0.26 | |
| MRI 3 | 11:0 | 9:4 | <0.10 | |
| WPPSI (n = 11 dyslexia, 16 control) | ||||
| Full scale IQ | 100 ± 17 | 104 ± 12 | <0.48 | |
| Verbal IQ | 100 ± 15 | 101 ± 10 | <0.70 | |
| Performance IQ | 101 ± 17 | 105 ± 18 | <0.50 | |
Values are shown as ratios or mean ± standard deviation. Significant group differences in proportions or means were tested using Fisher’s exact test or Student’s t-test, respectively.
WPPSI = Wechsler Preschool and Primary Scale of Intelligence.
Definition of dyslexia
Thirteen of the subjects, 11 of whom participated in the MRI experiments, were identified as having dyslexia in the sixth grade, where dyslexia was defined as scoring below the 25th percentile in two or more of the four literacy tests: Standardized Test of Decoding and Spelling (Standardisert Test i Avkoding og Staving, STAS) reading (score ≤157), STAS spelling (score ≤41), STAS non-word (score ≤ 50), and Carlsten (2002) Reading Test-words per minute (CRT-wpm) (score ≤ 91.3) (Supplementary Table 2) (Helland et al., 2011a). The rationale for using this somewhat liberal criterion is 3-fold. First, Norwegian is a relatively shallow orthography, which is associated in the literature with dyslexia manifesting as normal levels of accuracy and low fluency (Ziegler and Goswami, 2005). Hence, part of our criteria included lower accuracy on a timed reading test in the sixth grade, i.e. CRT-wpm. Second, these subjects were receiving remediation during the first 3 years of this study (Helland et al., 2011b), which would lead to expected improvements in reading and spelling. A more liberal criterion of the 25th percentile still allows us to identify children who profited least from the remediation, and might be thought to have a more serious reading disability. Third, both reading and spelling measures were used, because in a transparent orthography such as Norwegian, some children with a history of dyslexia have been found to read at a functional level, but spelling and writing problems persist (Helland et al., 2011b). The resulting identification of 13 subjects as having dyslexia is ∼12% of the representative sample of 109 families who responded to the original risk questionnaire. The value of 12% is in agreement with most studies that estimate prevalence of dyslexia (Shaywitz et al., 1990).
Imaging
All T1-weighted anatomical images were acquired on the same GE Signa® EXCITE™ 3.0 T scanner at the Haukeland University Hospital, Bergen, Norway. Cortical thickness was estimated using the FreeSurfer image analysis suite (http://surfer.nmr.mgh.harvard.edu/) (Dale et al., 1999), with a modification of substituting FSL’s SIENA (Smith et al., 2002) results for the tissue segmentation, similar to Schumann et al. (2010). Individual cortical surfaces were spherically registered to the average subject that is part of FreeSurfer, the cortical thickness values resampled onto the surface, and spatially smoothed with a Gaussian kernel (5 mm full-width at half-maximum). To run post hoc cross-sectional and longitudinal analyses, e.g. such as estimating effect sizes, the mean cortical thickness value for each subject was extracted within each significant cluster at each time point and further analysed using SPSS 19.0. More details regarding the statistical analyses can be found in the Supplementary material.
Results
Neuroanatomical precursors to dyslexia
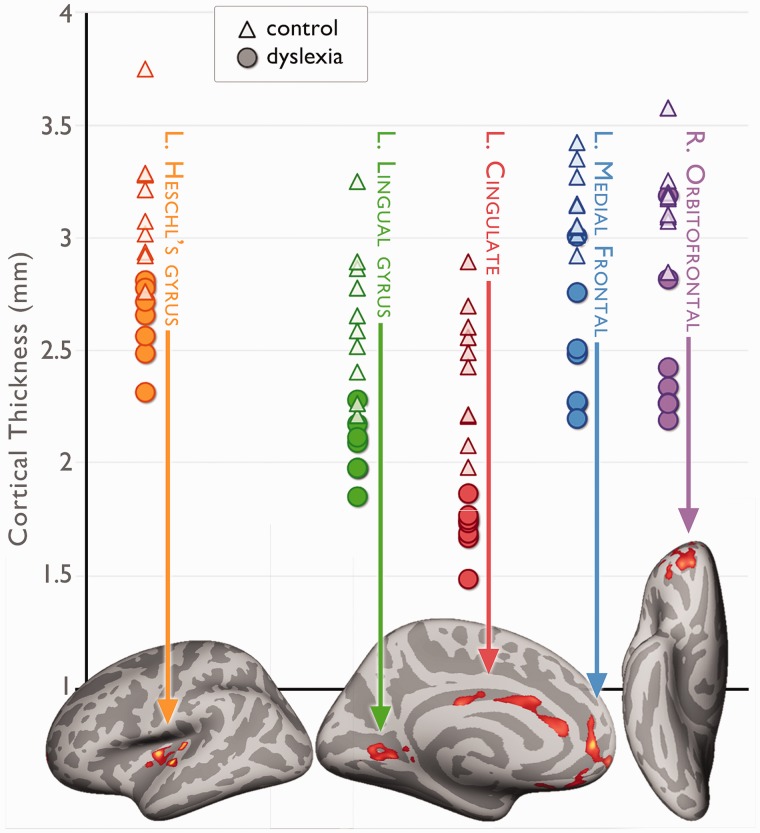
Using only the MRI 1 data, we performed a cross-sectional analysis to identify regions where the cortical thickness was significantly different between those children who later were identified as dyslexic (n = 7) from those who were not (n = 10). At MRI 1, all of the children were ages 6–7 and had not started formal literacy training, and behaviourally were considered pre-reading (Supplementary Table 1). In the main group contrast, the children who later developed dyslexia had thinner cortex in several regions of the left hemisphere: Heschl’s gyrus, lingual gyrus, medial frontal gyrus, middle cingulate gyrus, and an area in the right orbitofrontal cortex (Fig. 1). All of these regions showed strikingly large effects; the largest of which was in the cingulate cortex, where all of the dyslexic children had thinner cortex than all of the control children.
Figure 1.
Early signs of dyslexia. Pre-reading differences in cortical thickness between children who later went on to develop dyslexia (Dys) and those who did not (Ctrl). Images: regions in which Dys<Ctrl before the onset of reading. Raw cortical thickness values are plotted for each of the regions.
Heschl’s gyrus corresponds to primary auditory cortex, and regions that were found to be thinner in our cohort overlap with the left perisylvian regions that Galaburda et al. (1985) identified as having neuronal ectopias in four post-mortem dyslexic males (Galaburda et al., 1985). The portion of the lingual gyrus that we identified corresponds to ventral Brodmann area 18 and is hypothesized to be equivalent to V2 in macaques (Clarke and Miklossy, 1990), which is a low level visual area that has cells sensitive to orientation in addition to other functions (Van Essen and Maunsell, 1983). Cingulate, medial and orbitofrontal regions are typically associated with executive functions, such as cognitive control, motivation, and working memory processes.
Post-literacy anatomical signature of dyslexia
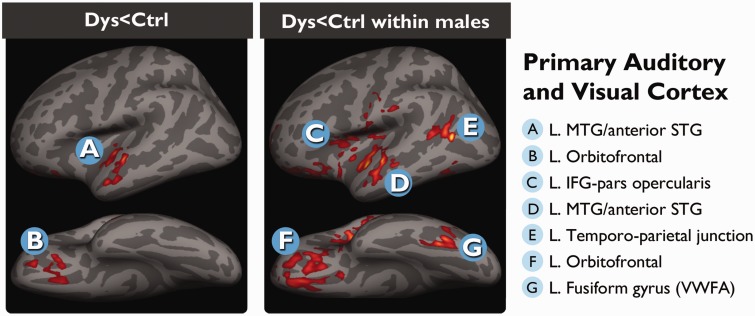
Using only the MRI 3 data, we performed a cross-sectional analysis to identify regions where the cortical thickness was significantly different between children who were identified as having dyslexia (n = 11) from those who were not (n = 13). In the main group contrast, the dyslexic children had thinner cortex in the left orbitofrontal cortex and a region in the anterior segment of the superior temporal cortex that extended inferiorly into the middle temporal gyrus (Fig. 2, left).
Figure 2.
Neuroanatomical signature of dyslexia. Regions of thinner cortex in the left hemisphere observed in children diagnosed with dyslexia (Dys) compared to those who were not (Ctrl). These data are cross-sectional from MRI 3, when the children were in the sixth grade. The left panel shows the whole group differences, whereas the right panel shows the differences when only the males were considered. IFG = inferior frontal gyrus; MTG = middle temporal gyrus; STG = superior temporal gyrus; VWFA = visual word form area.
When only the male subjects were compared, the male dyslexic children (n = 5) exhibited thinner cortex than the male control children (n = 8) in the same two regions identified in the main group contrast. In addition to these, the same pattern (Dyslexic < Control) was observed in three canonical reading areas of the left hemisphere: temporoparietal region, visual word form area of the fusiform gyrus, and inferior frontal gyrus (Fig. 2, right), and two regions in the right hemisphere: the anterior cingulate, and a region that extended from Heschl’s gyrus into the insular cortex (Supplementary Table 3). No differences were found when comparing the female subjects.
Developmental trajectory of neuroanatomical precursors to dyslexia
When studying developmental disorders, such as dyslexia, interpreting differences in cross-sectional data can be complicated due to the dynamic patterns of overproduction and elimination that characterize neurodevelopment (Kantor and Kolodkin, 2003). Interpreting differences in cortical thickness during development is further complicated because as skills develop, some areas of the cortex, e.g. left inferior frontal cortex, have been observed to ‘thicken’, whereas other regions, e.g. primary motor cortex, ‘thin’ (Sowell et al., 2004). Longitudinal data sets such as the one presented here can partially ameliorate these difficulties by observing how cortical thicknesses changes over time within each individual.
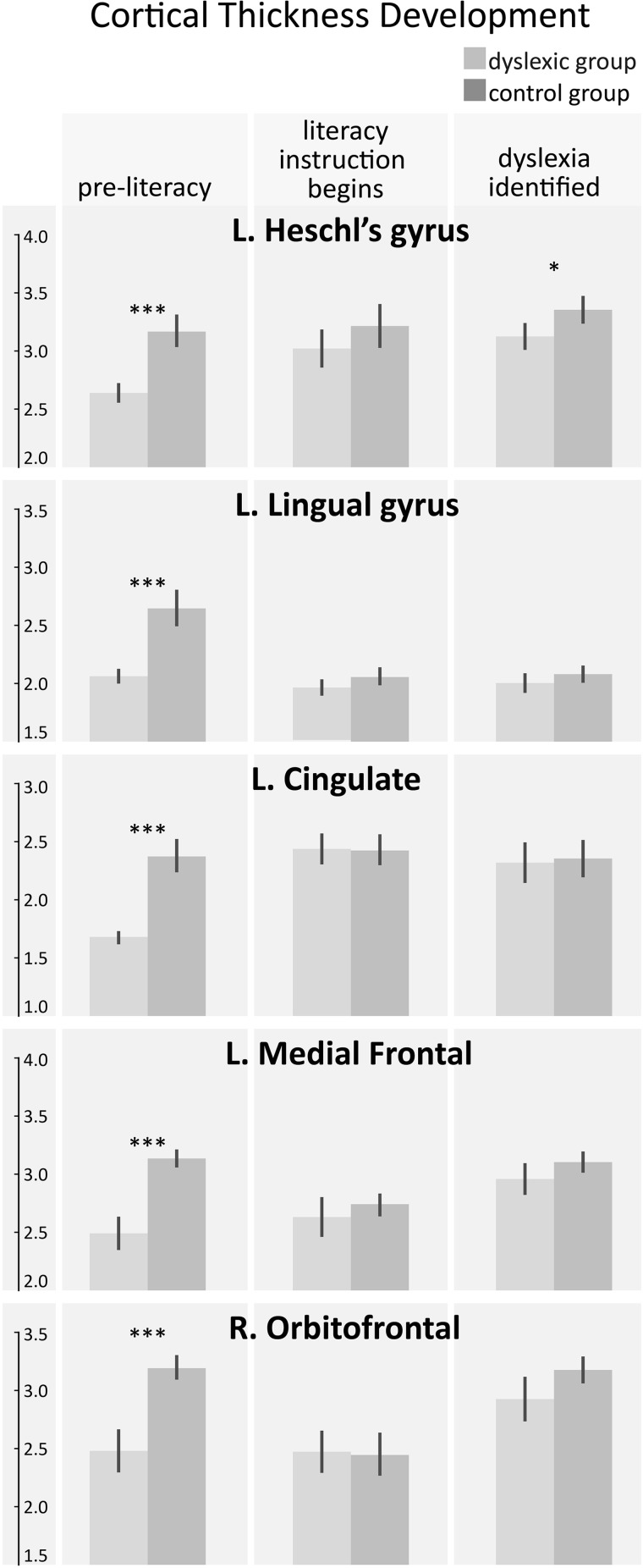
For the five regions of interest identified as being neuroanatomical precursors to dyslexia (Fig. 1), we extracted the mean cortical thickness from each child at each of the three MRI time points for longitudinal analysis in SPSS (Supplementary Table 4). Notably, not all children had data from all three MRI time points, but every child had at least two MRI time points. Almost all of the regions observed to be thinner in the dyslexics before the onset of literacy either thickened over time or stayed the same in the controls. Although these regions also thickened in the dyslexic children, Heschl’s gyrus was still significantly thinner than in control children at the end of the study [F(1,20) = 6.01, P < 0.024; Fig. 3], whereas the frontal and cingulate regions were no longer significantly different between the groups (all P > 0.05, Fig. 3). The sole exception to this pattern was in the lingual gyrus, where significant thinning was observed in the controls, and there were no changes in the cortical thickness of the dyslexics, such that there was no longer a significant group difference at the end of the study (P > 0.05, Fig. 3).
Figure 3.
Developmental trajectories of regions that showed pre-reading differences in cortical thickness. Longitudinal cortical thickness values are shown for the five regions that were different before reading: (A) left Heschl’s gyrus, (B) left lingual, (C) left cingulate, (D) left medial frontal, and (E) right orbitofrontal. The lingual gyrus (B) starts out thicker in the controls and thins over time, while all other regions thicken or stay the same over time. Cross-sectional post hoc t-tests between the two groups are indicated by *P < 0.05, ***P < 0.001.
Discussion
This study is unique because it acquired data from before children began learning to read and followed them longitudinally until after dyslexia was diagnosed. This is important because the neurobiological expression of dyslexia can be separated from the experience-driven changes related to reading development. Recent theories of dyslexia have focused on impaired phonological awareness as being causative (Ramus, 2003); however, impaired phonological awareness itself is a higher-level abstract function that some evidence suggests is an emergent property of reading or at least reciprocally related (Perfetti et al., 1987). Deficits in lower-level auditory processing have been suggested by Goswami (2003) as a potentially viable candidate for a low-level deficit that could lead to the frequently observed phonological awareness deficits. Although, it has been noted elsewhere that lower level perceptual deficits alone are unlikely to explain everything in dyslexia (Ramus, 2003). In our study, we found that children who would later develop dyslexia had thinner cortex in primary auditory and visual areas, in addition to cingulate and frontal regions thought to underlie executive functions, the latter of which have been implicated as being impaired in dyslexia (Helland and Asbjornsen, 2000) and connected to genetic variation (Berninger et al., 2008).
Our findings are consistent with previous studies demonstrating that a significant family history of dyslexia leads to behavioural deficits in auditory processing (Richardson et al., 2003) and structural (Raschle et al., 2011) abnormalities in the lingual gyrus, but extend those findings because our results are specific to dyslexia per se rather than a family history of dyslexia. Our finding of persistently thinner primary auditory cortex, i.e. Heschl’s gyrus, suggest that children who later develop dyslexia have a reduced neuroanatomical capacity to process auditory information before learning to read that may improve but not fully throughout childhood. Notably, a reduced neuroanatomical capacity does not imply a reduced ‘functional’ capacity to process auditory information, as the relationship between cortical thickness and function is not well understood. However, thinner cortex in Heschl’s gyrus is consistent with a functional study demonstrating that babies who are at-risk for developing dyslexia need a larger disparity between two similar speech sounds to identify them as different (Richardson et al., 2003).
Our findings in the lingual gyrus are intriguing for a couple of reasons. The first is that cortical thickness differences were only observed before learning to read, suggesting that, in addition to a reduced neuroanatomical capacity for auditory processing, dyslexic children may also have an early reduction in the neuroanatomical capacity for processing low-level visual information. The longitudinal data, however, shows that the cortical thickness of the dyslexic children stays relatively constant over age, whereas the cortical thickness of the control children starts out thicker and then thins over time. This suggests that children with dyslexia may have a reduced capacity for learning-induced plasticity in visual cortex because there is less grey matter at the beginning to prune in response to experiences.
Previous functional neuroimaging studies (Pugh et al., 2000) have identified a network of left hemispheric regions as being crucial for reading in English and dysfunctional in dyslexia, including: temporoparietal junction, the visual word form area of the fusiform gyrus, and the inferior frontal gyrus. We observed all of the canonical regions to be thinner in the dyslexic males after dyslexia was diagnosed, providing evidence that dyslexia in Norwegian, which has a shallower orthography than English, has a comparable neuroanatomical signature, at least within males. Importantly, while all of these regions were observed to be thinner after significant reading exposure, none of the regions exhibited differences in cortical thickness before the onset of learning to read. Rather, pre-reading differences were identified in lower-level sensory processing areas that project to those areas. The dynamic development of the reading network implies that the success of an intervention should be at least somewhat dependent on the timing of the intervention. For example, interventions targeting basic auditory processing skills (Merzenich et al., 1996) have larger impact if applied early, whereas interventions applied in middle school or adolescence are more effective if they target higher level skills, such as building vocabulary and improving comprehension strategies (Vaughn and Fletcher, 2012).
Funding
This project was supported by the Research Council of Norway through The Leiv Eriksson mobility programme #208916. K.C. was supported by Award Number K99/R00-HD065832 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). This research was supported by grants to K.H. from the Research Council of Norway and West-Norway Health Authority and to T.H. from the Meltzer Foundation, University of Bergen. This study was partially supported by the National Institutes of Health Grants P41-EB015922, R01MH092301 and R01MH094343. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Research Council of Norway, NICHD, NIMH, NIBIB, or NIH.
Supplementary material
Supplementary material is available at Brain online.
Glossary
Abbreviation
- STAS
Standardized Test of Decoding and Spelling (Standardisert Test i Avkoding og Staving)
References
- Berninger VW, Raskind W, Richards T, Abbott R, Stock P. A multidisciplinary approach to understanding developmental dyslexia within working-memory architecture: genotypes, phenotypes, brain, and instruction. Dev Neuropsychol. 2008;33:707–44. doi: 10.1080/87565640802418662. [DOI] [PubMed] [Google Scholar]
- Carlsten CT. Leseprøve 6. klasse bokmål og nynorsk [Reading test 6th grade bokmål and nynorsk] Oslo: Damm & Søn AS; 2002. [Google Scholar]
- Clarke S, Miklossy J. Occipital cortex in man: organization of callosal connections, related myelo- and cytoarchitecture, and putative boundaries of functional visual areas. J Comp Neurol. 1990;298:188–214. doi: 10.1002/cne.902980205. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–94. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Eckert MA, Leonard CM, Richards TL, Aylward EH, Thomson J, Berninger VW. Anatomical correlates of dyslexia: frontal and cerebellar findings. Brain. 2003;126(Pt 2):482–94. doi: 10.1093/brain/awg026. [DOI] [PubMed] [Google Scholar]
- Eden GF, Zeffiro TA. Neural systems affected in developmental dyslexia revealed by functional neuroimaging. Neuron. 1998;21:279–82. doi: 10.1016/s0896-6273(00)80537-1. [DOI] [PubMed] [Google Scholar]
- Galaburda AM, Sherman GF, Rosen GD, Aboitiz F, Geschwind N. Developmental dyslexia: four consecutive patients with cortical anomalies. Ann Neurol. 1985;18:222–33. doi: 10.1002/ana.410180210. [DOI] [PubMed] [Google Scholar]
- Goswami U. Why theories about developmental dyslexia require developmental designs. Trends Cogn Sci. 2003;7:534–40. doi: 10.1016/j.tics.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Helland T, Asbjornsen A. Executive functions in dyslexia. Child Neuropsychol. 2000;6:37–48. doi: 10.1076/0929-7049(200003)6:1;1-B;FT037. [DOI] [PubMed] [Google Scholar]
- Helland T, Plante E, Hugdahl K. Predicting dyslexia at age 11 from a risk index questionnaire at age 5. Dyslexia. 2011a;17:207–26. doi: 10.1002/dys.432. [DOI] [PubMed] [Google Scholar]
- Helland T, Tjus T, Hovden M, Ofte S, Heimann M. Effects of bottom-up and top-down intervention principles in emergent literacy in children at risk of developmental dyslexia: a longitudinal study. J Learn Disabil. 2011b;44:105–22. doi: 10.1177/0022219410391188. [DOI] [PubMed] [Google Scholar]
- Kantor DB, Kolodkin AL. Curbing the excesses of youth: molecular insights into axonal pruning. Neuron. 2003;38:849–52. doi: 10.1016/s0896-6273(03)00364-7. [DOI] [PubMed] [Google Scholar]
- Merzenich MM, Jenkins WM, Johnston P, Schreiner C, Miller SL, Tallal P. Temporal processing deficits of language-learning impaired children ameliorated by training. Science. 1996;271:77–81. doi: 10.1126/science.271.5245.77. [DOI] [PubMed] [Google Scholar]
- Perfetti CA, Beck I, Bell LC, Hughes C. Phonemic knowledge and learning to read are reciprocal: a longitudinal study of first grade children. Merrill-Palmer Q. 1987;33:283–319. [Google Scholar]
- Pugh KR, Mencl WE, Jenner AR, Katz L, Frost SJ, Lee JR, et al. Functional neuroimaging studies of reading and reading disability (developmental dyslexia) Ment Retard Dev Disabil Res Rev. 2000;6:207–13. doi: 10.1002/1098-2779(2000)6:3<207::AID-MRDD8>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Ramus F. Developmental dyslexia: specific phonological deficit or general sensorimotor dysfunction? Curr Opin Neurobiol. 2003;13:212–8. doi: 10.1016/s0959-4388(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Raschle NM, Chang M, Gaab N. Structural brain alterations associated with dyslexia predate reading onset. Neuroimage. 2011;57:742–9. doi: 10.1016/j.neuroimage.2010.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson U, Leppanen PH, Leiwo M, Lyytinen H. Speech perception of infants with high familial risk for dyslexia differ at the age of 6 months. Dev Neuropsychol. 2003;23:385–97. doi: 10.1207/S15326942DN2303_5. [DOI] [PubMed] [Google Scholar]
- Schumann CM, Bloss CS, Barnes CC, Wideman GM, Carper RA, Akshoomoff N, et al. Longitudinal magnetic resonance imaging study of cortical development through early childhood in autism. J Neurosci. 2010;30:4419–27. doi: 10.1523/JNEUROSCI.5714-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaywitz SE, Shaywitz BA, Fletcher JM, Escobar MD. Prevalence of reading disability in boys and girls. Results of the Connecticut Longitudinal Study. JAMA. 1990;264:998–1002. [PubMed] [Google Scholar]
- Smith SM, Zhang Y, Jenkinson M, Chen J, Matthews PM, Federico A, et al. Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. Neuroimage. 2002;17:479–89. doi: 10.1006/nimg.2002.1040. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. J Neurosci. 2004;24:8223–31. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkeltaub PE, Gareau L, Flowers DL, Zeffiro TA, Eden GF. Development of neural mechanisms for reading. Nat Neurosci. 2003;6:767–73. doi: 10.1038/nn1065. [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Maunsell JHR. Hierarchical organization and functional streams in the visual cortex. Trends Neurosci. 1983;6:370–5. [Google Scholar]
- Vaughn S, Fletcher JM. Response to intervention with secondary school students with reading difficulties. J Learn Disabil. 2012;45:244–56. doi: 10.1177/0022219412442157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler JC, Goswami U. Reading acquisition, developmental dyslexia, and skilled reading across languages: a psycholinguistic grain size theory. Psychol Bull. 2005;131:3–29. doi: 10.1037/0033-2909.131.1.3. [DOI] [PubMed] [Google Scholar]