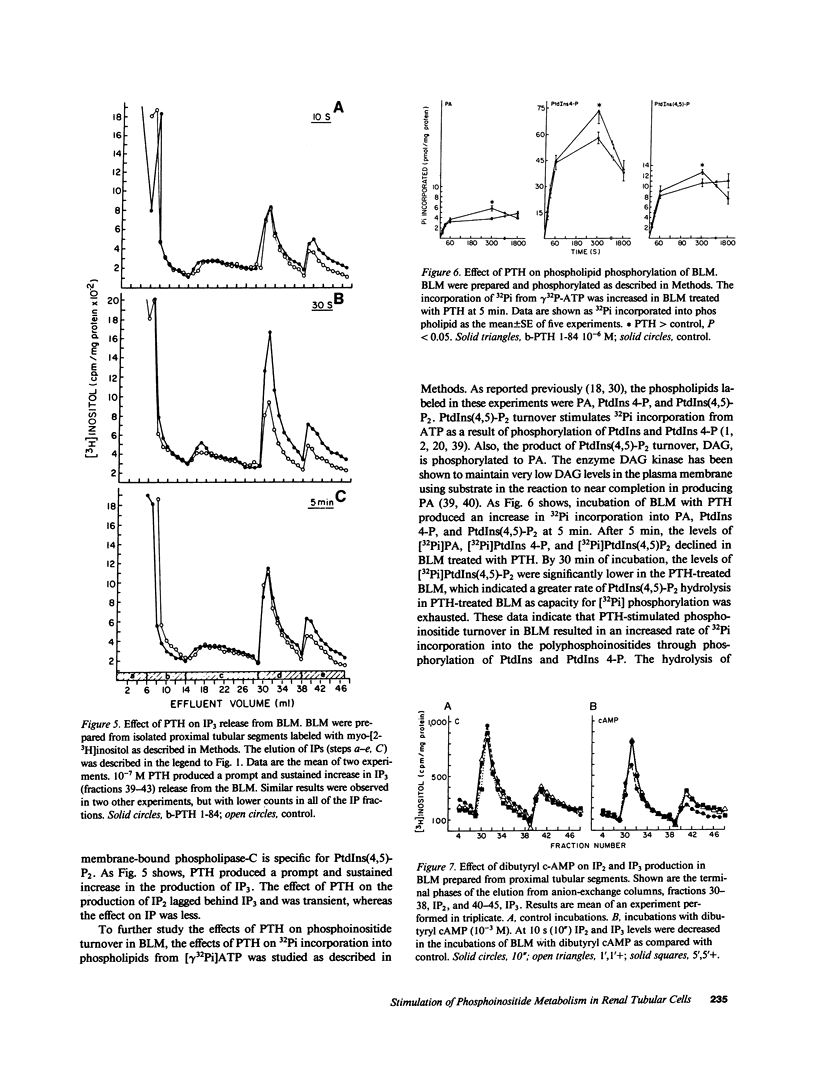
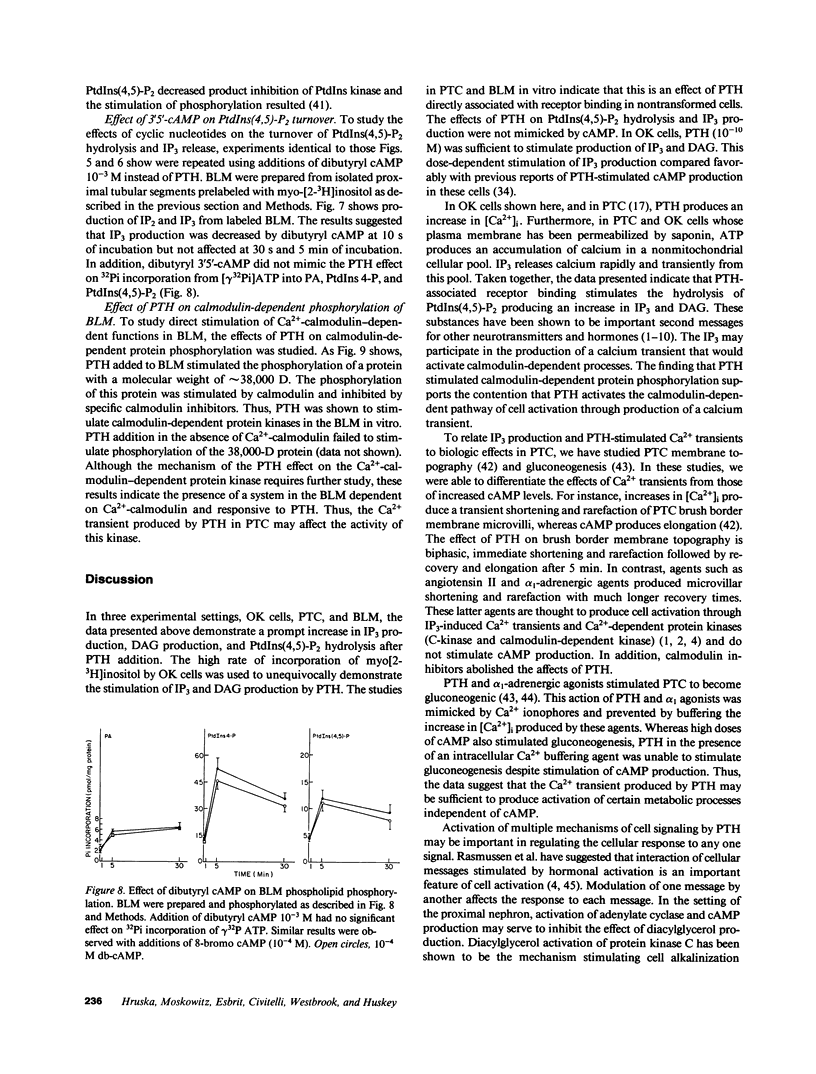
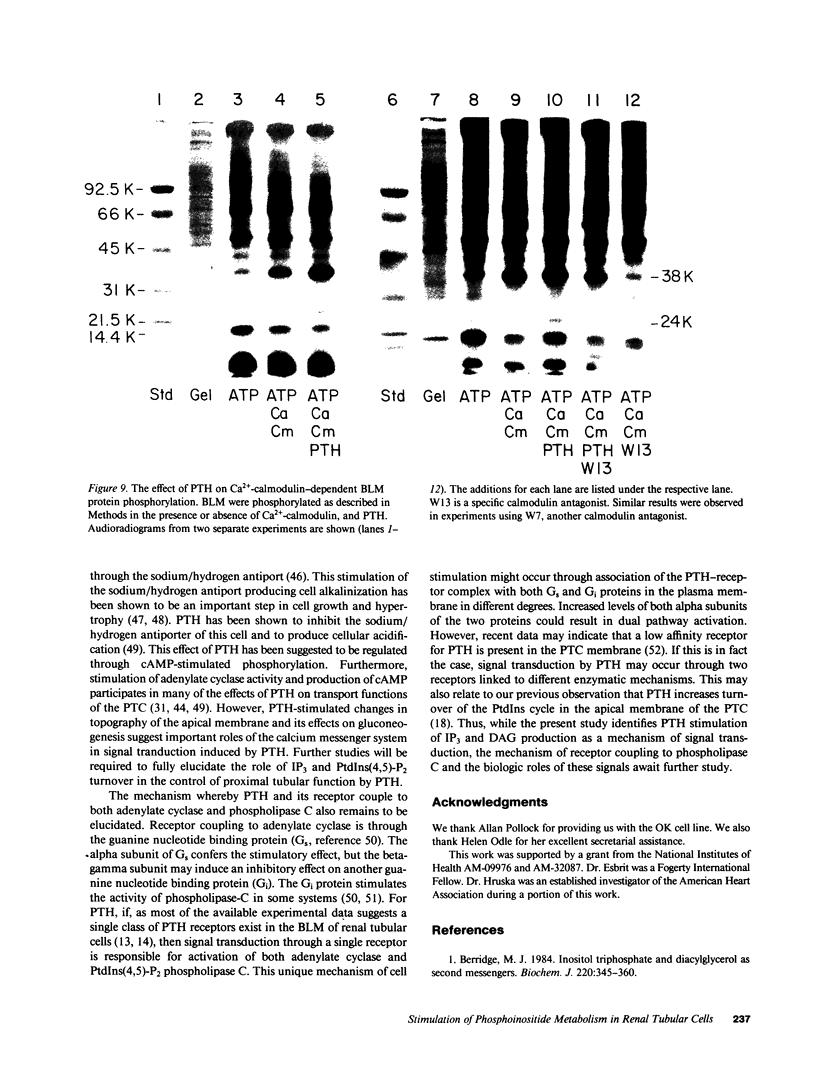
Abstract
Parathyroid hormone (PTH) produced a dose-dependent immediate stimulation of inositol triphosphate and diacylglycerol production in the opossum kidney cell line, primary culture proximal tubular cells, and basolateral membranes from canine proximal tubular segments. The increase in inositol triphosphate production was accompanied by a minor increase in inositol phosphate and no significant increase in inositol bisphosphate production. Associated with the changes in inositol triphosphate and diacylglycerol, there was an immediate hydrolysis of phosphatidylinositol 4'5-bisphosphate. The effect on phospholipid hydrolysis was followed by stimulation of phosphorylation of phosphatidylinositol 4' monophosphate and phosphatidylinositol. PTH produced a sudden increase in cytoplasmic Ca2+ in opossum kidney cells that persisted for approximately 1 min. Inositol triphosphate transiently increased cytoplasmic Ca2+ in saponin-treated opossum kidney and primary culture proximal tubule cells. The effects of PTH were not mimicked by cyclic nucleotides. In fact, cyclic AMP appeared to diminish inositol triphosphate production. These results demonstrate that PTH may activate renal tubular epithelial cells by the production of inositol triphosphate and diacylglycerol.
Full text
PDF


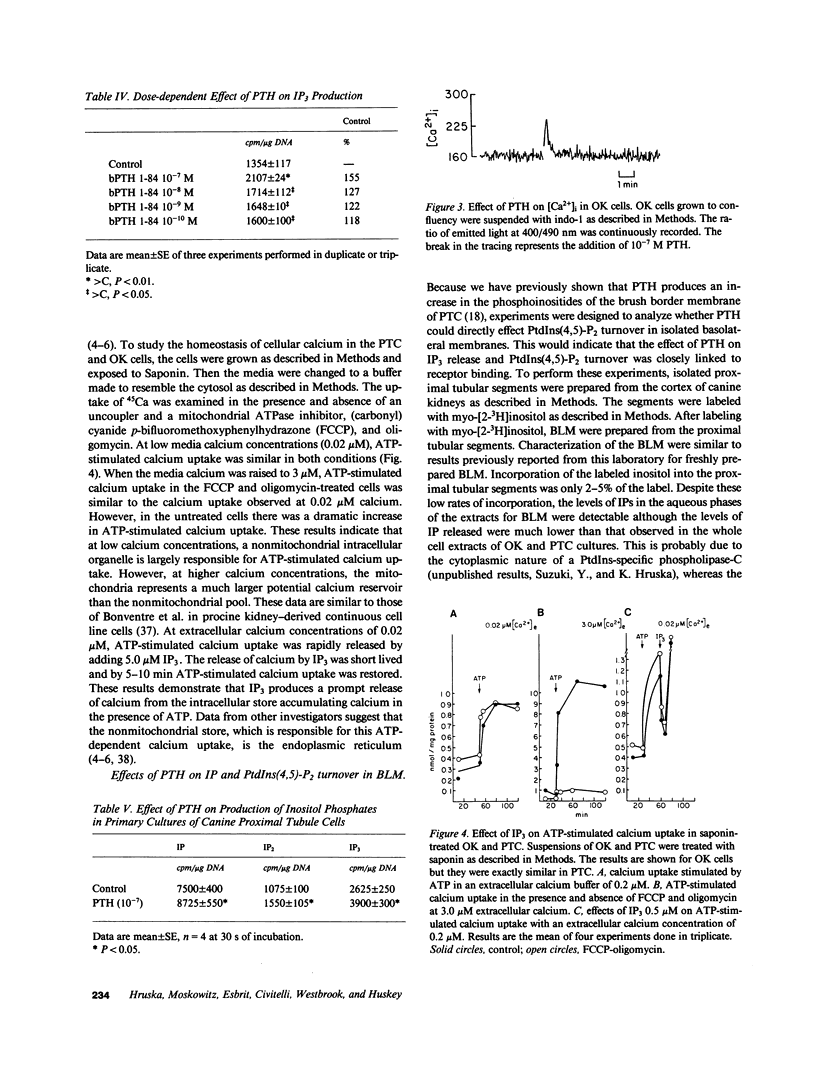


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert P. R., Tashjian A. H., Jr Thyrotropin-releasing hormone-induced spike and plateau in cytosolic free Ca2+ concentrations in pituitary cells. Relation to prolactin release. J Biol Chem. 1984 May 10;259(9):5827–5832. [PubMed] [Google Scholar]
- Bates M. D., Conn P. M. Calcium mobilization in the pituitary gonadotrope: relative roles of intra- and extracellular sources. Endocrinology. 1984 Oct;115(4):1380–1385. doi: 10.1210/endo-115-4-1380. [DOI] [PubMed] [Google Scholar]
- Bellorin-Font E., Martin K. J. Regulation of the PTH-receptor-cyclase system of canine kidney: effects of calcium, magnesium, and guanine nucleotides. Am J Physiol. 1981 Oct;241(4):F364–F373. doi: 10.1152/ajprenal.1981.241.4.F364. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Downes C. P., Hanley M. R. Lithium amplifies agonist-dependent phosphatidylinositol responses in brain and salivary glands. Biochem J. 1982 Sep 15;206(3):587–595. doi: 10.1042/bj2060587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Rapid accumulation of inositol trisphosphate reveals that agonists hydrolyse polyphosphoinositides instead of phosphatidylinositol. Biochem J. 1983 Jun 15;212(3):849–858. doi: 10.1042/bj2120849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bers D. M. A simple method for the accurate determination of free [Ca] in Ca-EGTA solutions. Am J Physiol. 1982 May;242(5):C404–C408. doi: 10.1152/ajpcell.1982.242.5.C404. [DOI] [PubMed] [Google Scholar]
- Bidot-López P., Farese R. V., Sabir M. A. Parathyroid hormone and adenosine-3',5'-monophosphate acutely increase phospholipids of the phosphatidate-polyphosphoinositide pathway in rabbit kidney cortex tubules in vitro by a cycloheximide-sensitive process. Endocrinology. 1981 Jun;108(6):2078–2081. doi: 10.1210/endo-108-6-2078. [DOI] [PubMed] [Google Scholar]
- Blackmore P. F., Bocckino S. B., Waynick L. E., Exton J. H. Role of a guanine nucleotide-binding regulatory protein in the hydrolysis of hepatocyte phosphatidylinositol 4,5-bisphosphate by calcium-mobilizing hormones and the control of cell calcium. Studies utilizing aluminum fluoride. J Biol Chem. 1985 Nov 25;260(27):14477–14483. [PubMed] [Google Scholar]
- Bonventre J. V., Cheung J. Y. Cytosolic free calcium concentration in cultured renal epithelial cells. Am J Physiol. 1986 Feb;250(2 Pt 2):F329–F338. doi: 10.1152/ajprenal.1986.250.2.F329. [DOI] [PubMed] [Google Scholar]
- Chase L. R., Aurbach G. D. Parathyroid function and the renal excretion of 3'5'-adenylic acid. Proc Natl Acad Sci U S A. 1967 Aug;58(2):518–525. doi: 10.1073/pnas.58.2.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke N. G., Dawson R. M. Alkaline O leads to N-transacylation. A new method for the quantitative deacylation of phospholipids. Biochem J. 1981 Apr 1;195(1):301–306. doi: 10.1042/bj1950301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goligorsky M. S., Menton D. N., Hruska K. A. Parathyroid hormone-induced changes of the brush border topography and cytoskeleton in cultured renal proximal tubular cells. J Membr Biol. 1986;92(2):151–162. doi: 10.1007/BF01870704. [DOI] [PubMed] [Google Scholar]
- Grove R. I., Fitzpatrick D., Schimmel S. D. Effect of Ca++ on triphosphoinositide extraction in fusing myoblasts. Lipids. 1981 Sep;16(9):691–693. doi: 10.1007/BF02535065. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
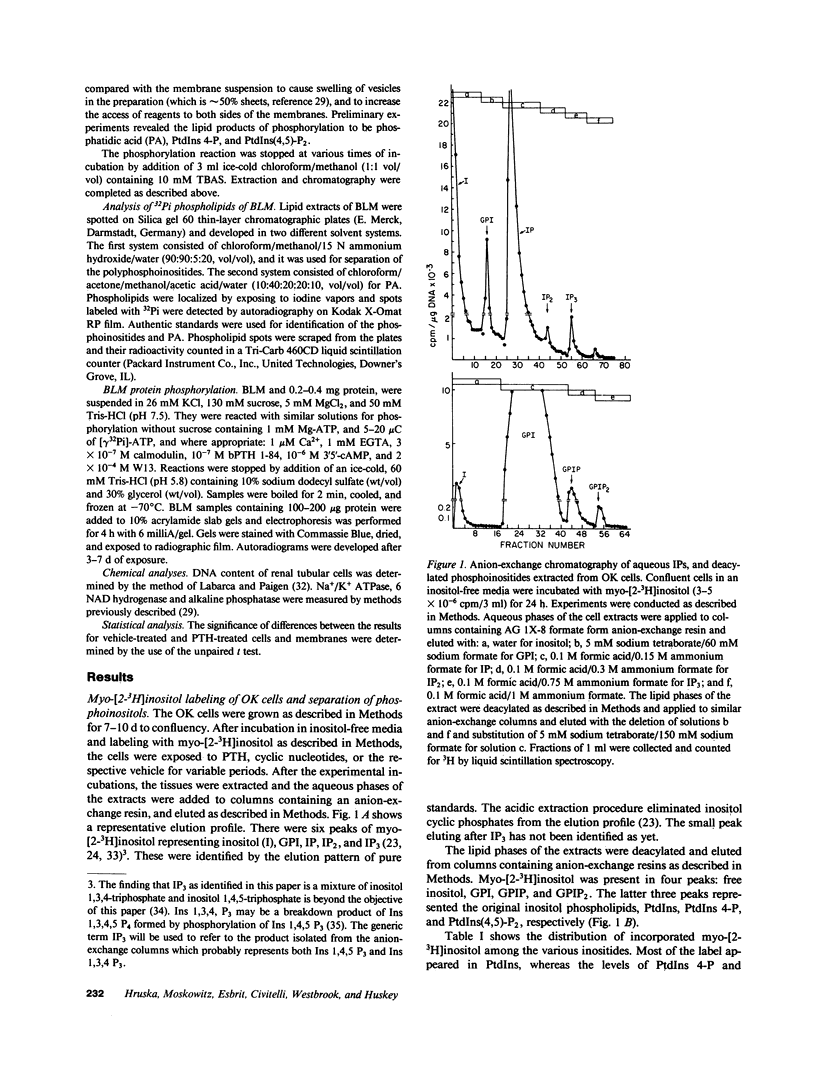
- Hammerman M. R., Hruska K. A. Cyclic AMP-dependent protein phosphorylation in canine renal brush-border membrane vesicles is associated with decreased phosphate transport. J Biol Chem. 1982 Jan 25;257(2):992–999. [PubMed] [Google Scholar]
- Hruska K. A., Goligorsky M., Scoble J., Tsutsumi M., Westbrook S., Moskowitz D. Effects of parathyroid hormone on cytosolic calcium in renal proximal tubular primary cultures. Am J Physiol. 1986 Aug;251(2 Pt 2):F188–F198. doi: 10.1152/ajprenal.1986.251.2.F188. [DOI] [PubMed] [Google Scholar]
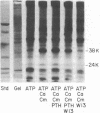
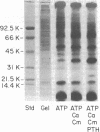
- Hruska K. A., Mills S. C., Khalifa S., Hammerman M. R. Phosphorylation of renal brush-border membrane vesicles. Effect on calcium uptake and membrane content of polyphosphoinositides. J Biol Chem. 1983 Feb 25;258(4):2501–2507. [PubMed] [Google Scholar]
- Irvine R. F., Letcher A. J., Heslop J. P., Berridge M. J. The inositol tris/tetrakisphosphate pathway--demonstration of Ins(1,4,5)P3 3-kinase activity in animal tissues. Nature. 1986 Apr 17;320(6063):631–634. doi: 10.1038/320631a0. [DOI] [PubMed] [Google Scholar]
- Irvine R. F., Letcher A. J., Lander D. J., Downes C. P. Inositol trisphosphates in carbachol-stimulated rat parotid glands. Biochem J. 1984 Oct 1;223(1):237–243. doi: 10.1042/bj2230237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph S. K., Thomas A. P., Williams R. J., Irvine R. F., Williamson J. R. myo-Inositol 1,4,5-trisphosphate. A second messenger for the hormonal mobilization of intracellular Ca2+ in liver. J Biol Chem. 1984 Mar 10;259(5):3077–3081. [PubMed] [Google Scholar]
- Khalifa S., Mills S., Hruska K. A. Stimulation of calcium uptake by parathyroid hormone in renal brush-border membrane vesicles. Relationship to membrane phosphorylation. J Biol Chem. 1983 Dec 10;258(23):14400–14406. [PubMed] [Google Scholar]
- Kojima I., Kojima K., Rasmussen H. Characteristics of angiotensin II-, K+- and ACTH-induced calcium influx in adrenal glomerulosa cells. Evidence that angiotensin II, K+, and ACTH may open a common calcium channel. J Biol Chem. 1985 Aug 5;260(16):9171–9176. [PubMed] [Google Scholar]
- L'Allemain G., Franchi A., Cragoe E., Jr, Pouysségur J. Blockade of the Na+/H+ antiport abolishes growth factor-induced DNA synthesis in fibroblasts. Structure-activity relationships in the amiloride series. J Biol Chem. 1984 Apr 10;259(7):4313–4319. [PubMed] [Google Scholar]
- L'Allemain G., Paris S., Pouysségur J. Growth factor action and intracellular pH regulation in fibroblasts. Evidence for a major role of the Na+/H+ antiport. J Biol Chem. 1984 May 10;259(9):5809–5815. [PubMed] [Google Scholar]
- Labarca C., Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980 Mar 1;102(2):344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Litosch I., Fain J. N. Regulation of phosphoinositide breakdown by guanine nucleotides. Life Sci. 1986 Jul 21;39(3):187–194. doi: 10.1016/0024-3205(86)90529-1. [DOI] [PubMed] [Google Scholar]
- Lo H., Lehotay D. C., Katz D., Levey G. S. Parathyroid hormone-mediated incorporation of 32P-orthophosphate into phosphatidic acid and phosphatidylinositol in renal cortical slices. Endocr Res Commun. 1976;3(6):377–385. doi: 10.3109/07435807609073911. [DOI] [PubMed] [Google Scholar]
- May W. S., Lapetina E. G., Cuatrecasas P. Intracellular activation of protein kinase C and regulation of the surface transferrin receptor by diacylglycerol is a spontaneously reversible process that is associated with rapid formation of phosphatidic acid. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1281–1284. doi: 10.1073/pnas.83.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer V., Weinreb S., Bellorin-Font E., Hruska K. A. Parathyroid hormone stimulation of renal phosphoinositide metabolism is a cyclic nucleotide-independent effect. Biochim Biophys Acta. 1982 Aug 18;712(2):258–267. doi: 10.1016/0005-2760(82)90342-3. [DOI] [PubMed] [Google Scholar]
- Michell R. H. Inositol phospholipids and cell surface receptor function. Biochim Biophys Acta. 1975 Mar 25;415(1):81–47. doi: 10.1016/0304-4157(75)90017-9. [DOI] [PubMed] [Google Scholar]
- Muallem S., Schoeffield M., Pandol S., Sachs G. Inositol trisphosphate modification of ion transport in rough endoplasmic reticulum. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4433–4437. doi: 10.1073/pnas.82.13.4433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y., Takai Y., Kishimoto A., Kikkawa U., Kaibuchi K. Phospholipid turnover in hormone action. Recent Prog Horm Res. 1984;40:301–345. doi: 10.1016/b978-0-12-571140-1.50012-8. [DOI] [PubMed] [Google Scholar]
- Nissenson R. A., Arnaud C. D. Properties of the parathyroid hormone receptor-adenylate cyclase system in chicken renal plasma membranes. J Biol Chem. 1979 Mar 10;254(5):1469–1475. [PubMed] [Google Scholar]
- Pollock A. S., Warnock D. G., Strewler G. J. Parathyroid hormone inhibition of Na+-H+ antiporter activity in a cultured renal cell line. Am J Physiol. 1986 Feb;250(2 Pt 2):F217–F225. doi: 10.1152/ajprenal.1986.250.2.F217. [DOI] [PubMed] [Google Scholar]
- Prentki M., Biden T. J., Janjic D., Irvine R. F., Berridge M. J., Wollheim C. B. Rapid mobilization of Ca2+ from rat insulinoma microsomes by inositol-1,4,5-trisphosphate. Nature. 1984 Jun 7;309(5968):562–564. doi: 10.1038/309562a0. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr A model for receptor-regulated calcium entry. Cell Calcium. 1986 Feb;7(1):1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- Rasmussen H., Goodman D. B. Relationships between calcium and cyclic nucleotides in cell activation. Physiol Rev. 1977 Jul;57(3):421–509. doi: 10.1152/physrev.1977.57.3.421. [DOI] [PubMed] [Google Scholar]
- Rasmussen H. The calcium messenger system (2). N Engl J Med. 1986 May 1;314(18):1164–1170. doi: 10.1056/NEJM198605013141807. [DOI] [PubMed] [Google Scholar]
- Scoble J. E., Mills S., Hruska K. A. Calcium transport in canine renal basolateral membrane vesicles. Effects of parathyroid hormone. J Clin Invest. 1985 Apr;75(4):1096–1105. doi: 10.1172/JCI111803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segre G. V., Rosenblatt M., Reiner B. L., Mahaffey J. E., Potts J. T., Jr Characterization of parathyroid hormone receptors in canine renal cortical plasma membranes using a radioiodinated sulfur-free hormone analogue. Correlation of binding with adenylate cyclase activity. J Biol Chem. 1979 Aug 10;254(15):6980–6986. [PubMed] [Google Scholar]
- Somlyo A. P. Cell physiology: cellular site of calcium regulation. Nature. 1984 Jun 7;309(5968):516–517. doi: 10.1038/309516b0. [DOI] [PubMed] [Google Scholar]
- Teitelbaum A. P., Strewler G. J. Parathyroid hormone receptors coupled to cyclic adenosine monophosphate formation in an established renal cell line. Endocrinology. 1984 Mar;114(3):980–985. doi: 10.1210/endo-114-3-980. [DOI] [PubMed] [Google Scholar]
- Wilson D. B., Bross T. E., Sherman W. R., Berger R. A., Majerus P. W. Inositol cyclic phosphates are produced by cleavage of phosphatidylphosphoinositols (polyphosphoinositides) with purified sheep seminal vesicle phospholipase C enzymes. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4013–4017. doi: 10.1073/pnas.82.12.4013. [DOI] [PMC free article] [PubMed] [Google Scholar]