Abstract
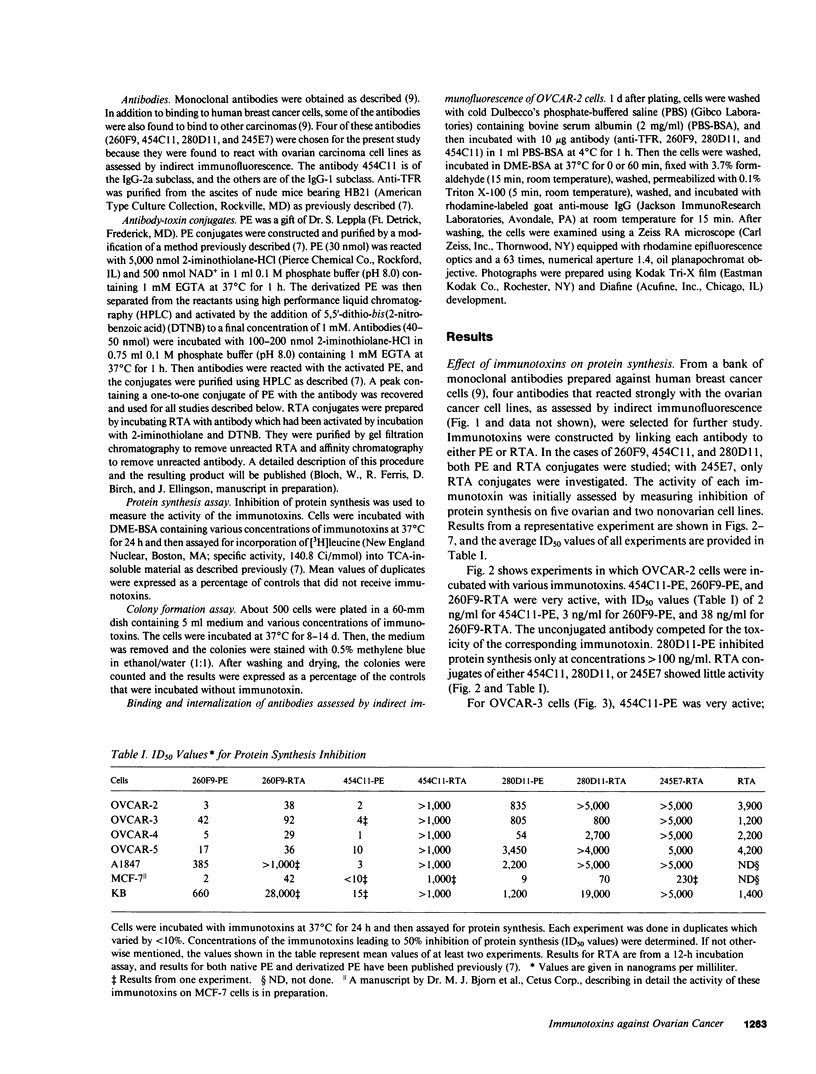
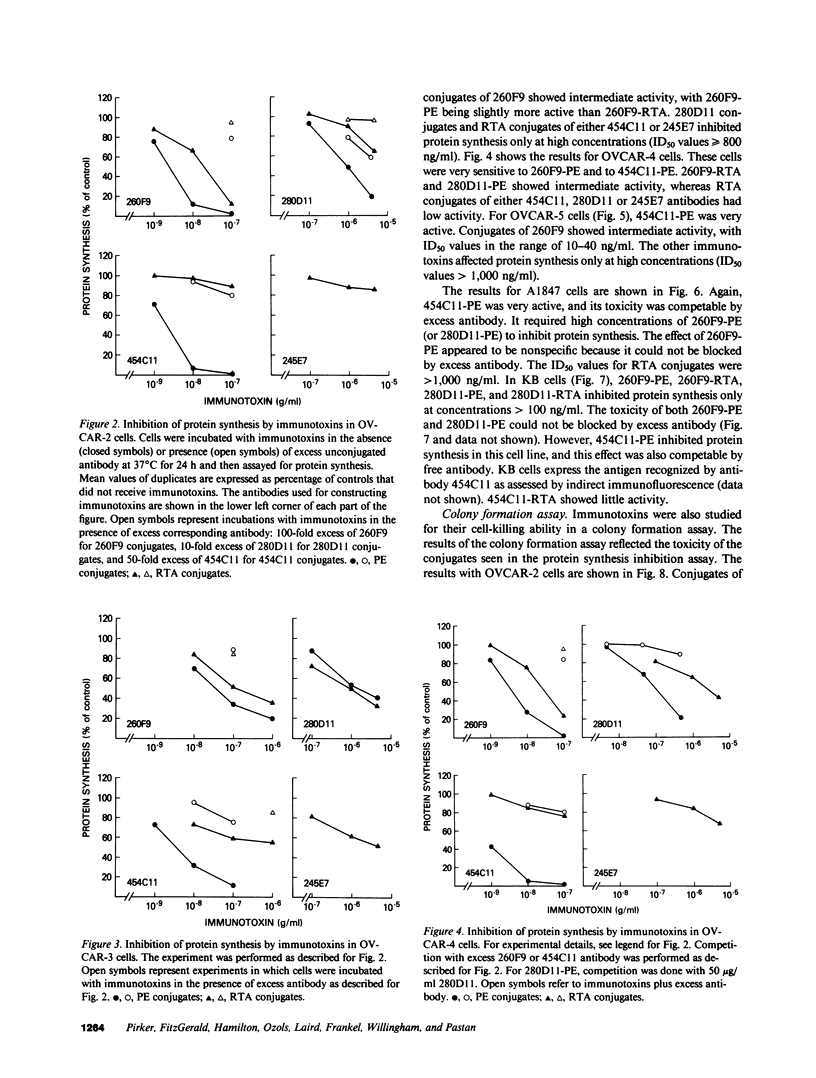
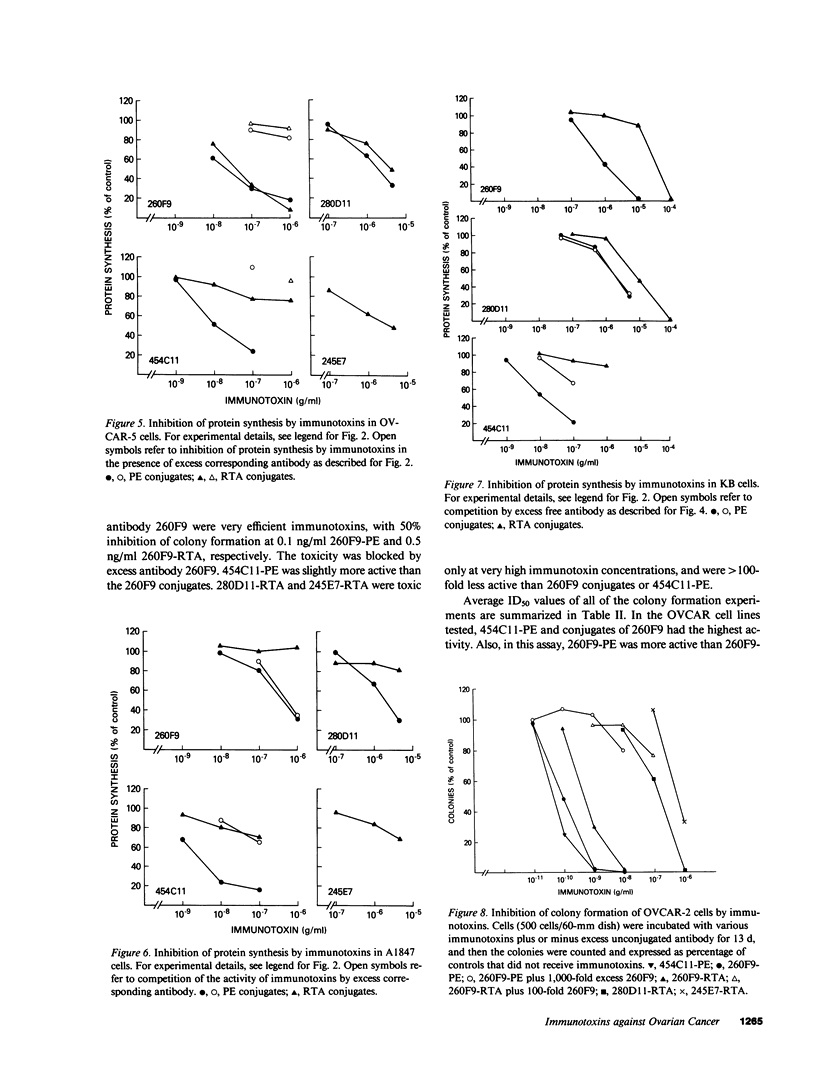
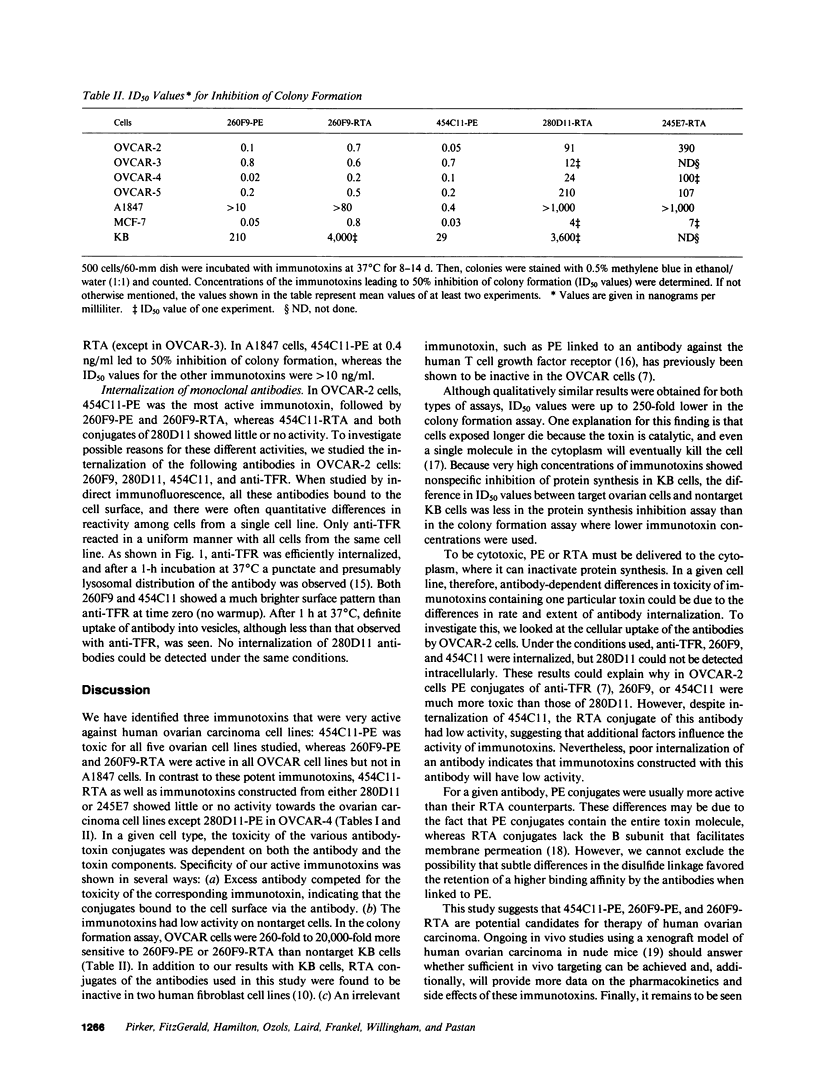
The purpose of the present study was to develop immunotoxins directed against human ovarian carcinoma cells. Four monoclonal antibodies (260F9, 454C11, 280D11, and 245E7) were chosen because they were found to bind to various ovarian carcinoma cell lines. These antibodies were covalently linked to either Pseudomonas exotoxin (PE) or ricin A chain (RTA), and the conjugates were tested against five ovarian cancer cell lines (OVCAR-2, -3, -4, -5; A1847). The ability of the immunotoxins to inhibit both protein synthesis and colony formation was evaluated. Qualitatively similar results were obtained for both types of assays. Usually, PE conjugates were more toxic than their corresponding RTA conjugates. 454C11-PE was very toxic for all ovarian carcinoma lines, whereas 454C11-RTA had low activity. Both 260F9-PE and 260F9-RTA were active in all OVCAR cell lines but not in A1847 cells. 280D11-PE was toxic for OVCAR-4; otherwise, 280D11-PE and RTA conjugates of both 280D11 and 245E7 had little activity. Specificity of immunotoxin action was shown by competition by excess antibody, nontoxicity in nontarget cells, and inactivity of an irrelevant immunotoxin. To investigate the basis of antibody-dependent differences in activity of the various immunotoxins, antibody uptake was studied in OVCAR-2 cells, and the results indicate that antibody internalization is one important factor in the activity of immunotoxins.
Full text
PDF
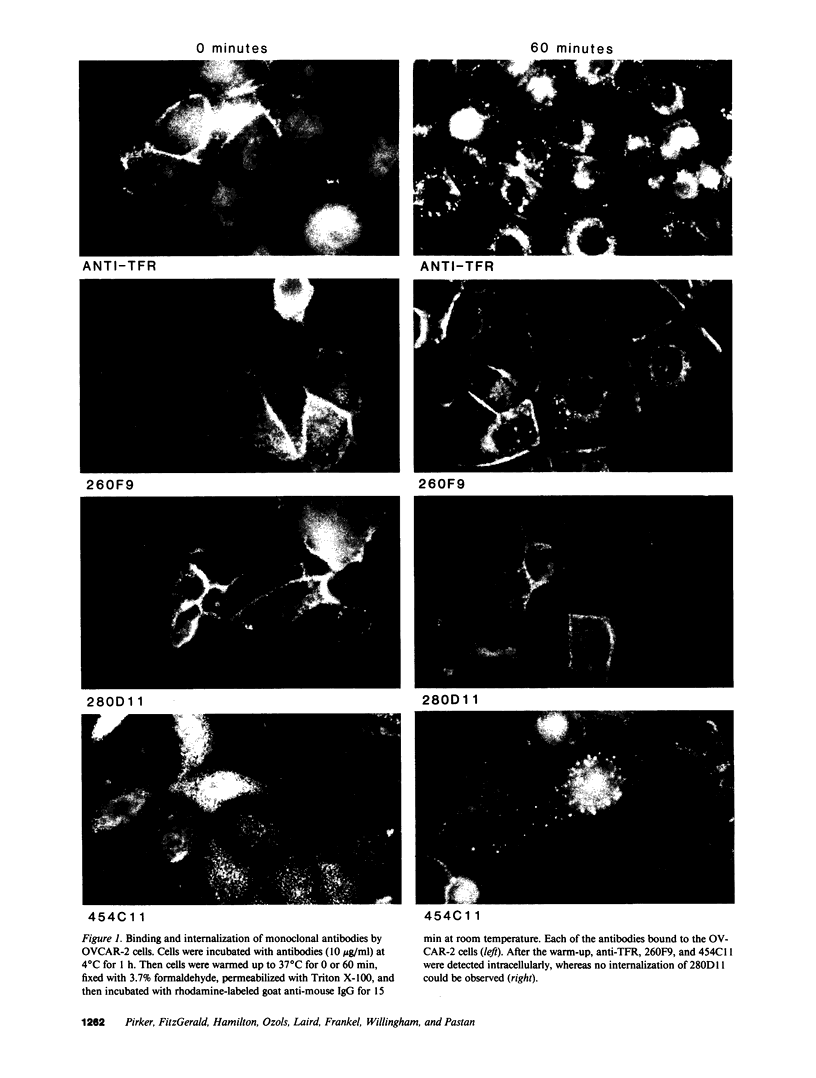

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama S., Seth P., Pirker R., FitzGerald D., Gottesman M. M., Pastan I. Potentiation of cytotoxic activity of immunotoxins on cultured human cells. Cancer Res. 1985 Mar;45(3):1005–1007. [PubMed] [Google Scholar]
- Bjorn M. J., Ring D., Frankel A. Evaluation of monoclonal antibodies for the development of breast cancer immunotoxins. Cancer Res. 1985 Mar;45(3):1214–1221. [PubMed] [Google Scholar]
- Casellas P., Bourrie B. J., Gros P., Jansen F. K. Kinetics of cytotoxicity induced by immunotoxins. Enhancement by lysosomotropic amines and carboxylic ionophores. J Biol Chem. 1984 Aug 10;259(15):9359–9364. [PubMed] [Google Scholar]
- Eiklid K., Olsnes S., Pihl A. Entry of lethal doses of abrin, ricin and modeccin into the cytosol of HeLa cells. Exp Cell Res. 1980 Apr;126(2):321–326. doi: 10.1016/0014-4827(80)90270-0. [DOI] [PubMed] [Google Scholar]
- Feldman G. B., Knapp R. C., Order S. E., Hellman S. The role of lymphatic obstruction in the formation of ascites in a murine ovarian carcinoma. Cancer Res. 1972 Aug;32(8):1663–1666. [PubMed] [Google Scholar]
- FitzGerald D. J., Trowbridge I. S., Pastan I., Willingham M. C. Enhancement of toxicity of antitransferrin receptor antibody-Pseudomonas exotoxin conjugates by adenovirus. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4134–4138. doi: 10.1073/pnas.80.13.4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FitzGerald D. J., Waldmann T. A., Willingham M. C., Pastan I. Pseudomonas exotoxin-anti-TAC. Cell-specific immunotoxin active against cells expressing the human T cell growth factor receptor. J Clin Invest. 1984 Sep;74(3):966–971. doi: 10.1172/JCI111516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel A. E., Ring D. B., Tringale F., Hsieh-Ma S. T. Tissue distribution of breast cancer-associated antigens defined by monoclonal antibodies. J Biol Response Mod. 1985 Jun;4(3):273–286. [PubMed] [Google Scholar]
- Gatter K. C., Brown G., Trowbridge I. S., Woolston R. E., Mason D. Y. Transferrin receptors in human tissues: their distribution and possible clinical relevance. J Clin Pathol. 1983 May;36(5):539–545. doi: 10.1136/jcp.36.5.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliland D. G., Steplewski Z., Collier R. J., Mitchell K. F., Chang T. H., Koprowski H. Antibody-directed cytotoxic agents: use of monoclonal antibody to direct the action of toxin A chains to colorectal carcinoma cells. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4539–4543. doi: 10.1073/pnas.77.8.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton T. C., Young R. C., Louie K. G., Behrens B. C., McKoy W. M., Grotzinger K. R., Ozols R. F. Characterization of a xenograft model of human ovarian carcinoma which produces ascites and intraabdominal carcinomatosis in mice. Cancer Res. 1984 Nov;44(11):5286–5290. [PubMed] [Google Scholar]
- Hamilton T. C., Young R. C., McKoy W. M., Grotzinger K. R., Green J. A., Chu E. W., Whang-Peng J., Rogan A. M., Green W. R., Ozols R. F. Characterization of a human ovarian carcinoma cell line (NIH:OVCAR-3) with androgen and estrogen receptors. Cancer Res. 1983 Nov;43(11):5379–5389. [PubMed] [Google Scholar]
- Hamilton T. C., Young R. C., Ozols R. F. Experimental model systems of ovarian cancer: applications to the design and evaluation of new treatment approaches. Semin Oncol. 1984 Sep;11(3):285–298. [PubMed] [Google Scholar]
- Hopkins C. R., Trowbridge I. S. Internalization and processing of transferrin and the transferrin receptor in human carcinoma A431 cells. J Cell Biol. 1983 Aug;97(2):508–521. doi: 10.1083/jcb.97.2.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglewski B. H., Kabat D. NAD-dependent inhibition of protein synthesis by Pseudomonas aeruginosa toxin,. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2284–2288. doi: 10.1073/pnas.72.6.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Pirker R., FitzGerald D. J., Hamilton T. C., Ozols R. F., Willingham M. C., Pastan I. Anti-transferrin receptor antibody linked to Pseudomonas exotoxin as a model immunotoxin in human ovarian carcinoma cell lines. Cancer Res. 1985 Feb;45(2):751–757. [PubMed] [Google Scholar]
- Ramakrishnan S., Houston L. L. Inhibition of human acute lymphoblastic leukemia cells by immunotoxins: potentiation by chloroquine. Science. 1984 Jan 6;223(4631):58–61. doi: 10.1126/science.6318313. [DOI] [PubMed] [Google Scholar]
- Thorpe P. E., Ross W. C., Brown A. N., Myers C. D., Cumber A. J., Foxwell B. M., Forrester J. T. Blockade of the galactose-binding sites of ricin by its linkage to antibody. Specific cytotoxic effects of the conjugates. Eur J Biochem. 1984 Apr 2;140(1):63–71. doi: 10.1111/j.1432-1033.1984.tb08067.x. [DOI] [PubMed] [Google Scholar]
- Tsukada Y., Hurwitz E., Kashi R., Sela M., Hibi N., Hara A., Hirai H. Chemotherapy by intravenous administration of conjugates of daunomycin with monoclonal and conventional anti-rat alpha-fetoprotein antibodies. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7896–7899. doi: 10.1073/pnas.79.24.7896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitetta E. S., Fulton R. J., Uhr J. W. Cytotoxicity of a cell-reactive immunotoxin containing ricin A chain is potentiated by an anti-immunotoxin containing ricin B chain. J Exp Med. 1984 Jul 1;160(1):341–346. doi: 10.1084/jem.160.1.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitetta E. S., Krolick K. A., Miyama-Inaba M., Cushley W., Uhr J. W. Immunotoxins: a new approach to cancer therapy. Science. 1983 Feb 11;219(4585):644–650. doi: 10.1126/science.6218613. [DOI] [PubMed] [Google Scholar]
- Youle R. J., Neville D. M., Jr Kinetics of protein synthesis inactivation by ricin-anti-Thy 1.1 monoclonal antibody hybrids. Role of the ricin B subunit demonstrated by reconstitution. J Biol Chem. 1982 Feb 25;257(4):1598–1601. [PubMed] [Google Scholar]