Abstract
Progression of the infection by host‐specific strains of Fusarium oxysporum and Fusarium arthrosporioides of Orobanche aegyptiaca (Egyptian broomrape) tubercles attached to tomato roots was tracked using light, confocal and electron microscopy. Mycelia transformed with the gene for green fluorescent protein were viewed using a confocal microscope. Fungal penetration was preceded by a rapid loss of starch, with approx. 10 % remaining at 9 h and no measurable starch at 24 h. Penetration into the Orobanche tubercles began by 12 h after inoculation. Hyphae penetrated the outer six cell layers by 24 h, reaching the centre of the tubercles by 48 h and infecting nearly all cells by 72 h. Most of the infected tubercles were dead by 96 h. Breakdown of cell walls and the disintegration of cytoplasm in and around the infected cells occurred between 48 and 96 h. Lignin‐like material increased in tubercle cells of infected tissues over time, but did not appear to be effective in limiting fungal penetration or spread. Callose, suberin, constitutive toxins and phytoalexins were not detected in infected tubercles, suggesting that there are no obvious defence mechanisms to overcome. Both Fusarium spp. pathogenic on Orobanche produced fumonisin‐like ceramide synthase inhibitors, while fusaric acid was produced only by F. oxysporum in liquid culture. The organisms do not have sufficient virulence for field use (based on glasshouse testing), suggesting that virulence should be transgenically enhanced or additional isolates sought.
Key words: Biological control; Fusarium arthrosporioides Sherb.; Fusarium oxysporum Schlechtend.:Fr.; histochemical; lignin; Lycopersicon esculentum; tomato; mycoherbicide; Orobanche aegyptiaca, Egyptian broomrape; starch; transformation
INTRODUCTION
The broomrapes (Orobanche spp.) are obligate parasitic weeds that cause severe damage to hosts (Parker and Riches, 1993). Biocontrol of Orobanche with mycoherbicidal fungi is a promising strategy. Fusarium oxysporum and F. arthrosporioides strains specifically pathogenic to Orobanche have been isolated from diseased shoots and their potential for the biocontrol of O. aegyptiaca has been studied (Amsellem et al., 1999, 2001a, b). These species were transformed to moderate hypervirulence using genes encoding overproduction of auxin (Cohen et al., 2002).
Plant responses to pathogens include the production of elicited plant defence compounds that are toxic to pathogens, and cell wall reinforcements that retard penetration. Low molecular weight phytoalexins are among the elicited plant defence compounds that are often toxic to invading pathogens (Matern and Kneusel, 1988). Callose, lignin and suberin are polymers that can be elicited as plant defences that reinforce cell walls of some species. The callose‐rich papillae that often form, rapidly seal the cells at the points of attempted fungal penetration, or completely encase the penetrated hyphae (Kauss, 1992). Lignin plays a major role in the strengthening of plant cell walls (Mitchell et al., 1999) and can shield cell wall cellulose from fungal cellulase action (Gressel et al., 1983). Suberin can function as a barrier to both water loss and infection by pathogens (Robb et al., 1989).
The virulence of pathogens has been enhanced by adding specific chemical inhibitors that prevent the biosynthesis of elicited plant defences such as callose, lignin and phytoalexin production (Bayles et al., 1990; Sharon et al., 1992; Gressel, 2002). Starch catabolism, a typical host response during fungal attack, supplies energy for metabolic activities such as the production of elicited defences, maintaining osmotic balance, as well as supporting the metabolic activities of the pathogen (Agrios, 1997). Pathogens such as Fusarium spp. often produce phytotoxins such as fumonisins (Abbas and Boyette, 1992) and fusaric acid (Bacon et al., 1996), and protein toxins (Bailey et al., 2000) that assist in overcoming host defences, allowing establishment of the pathogen.
There has been only one previous report on the aetiology of pathogenic fungi on Orobanche. Thomas et al. (1999) reported that conidial suspensions of F. oxysporum f. sp. orthoceras colonized and infected the seeds of Orobanche cumana, a species specific to sunflowers. The fungus was effective in penetrating seed testa. The cell walls of the endosperm were dissolved, cytoplasm degraded, and lipid body membranes were damaged in the infected seeds. The lipid‐ and protein‐rich endosperm was presumably used by the fungus as a nutrient source.
The present study was initiated to determine the biochemical and histochemical responses of Orobanche aegyptiaca to fungal pathogenesis. To the best of our knowledge, this is the first report describing both the fungal infection of Orobanche tubercles, and some of the resulting biochemical changes in the tubercles.
MATERIALS AND METHODS
Fungal strains and culture of plant material
Fusarium oxysporum Schlechtend.:Fr. and F. arthrosporioides Sherb. were isolated from diseased, juvenile, emerging Orobanche aegyptiaca Pers. flower stalks collected from a melon field in northern Israel (Amsellem et al., 2001a). Both fungal strains met Koch’s postulates for primary pathogens. The fungi are deposited at the Collection Nationale de Cultures de Microorganismes (CNCM) at the Institut Pasteur under the accession numbers I‐1622 (F. oxysporum) and I‐1621 (F. arthrosporioides). Transgenic F. oxysporum containing the gfp (green fluorescent protein) gene was used in confocal studies of infection. The cultures of F. oxysporum were prepared using protoplasts that were incubated with plasmid DNA containing the gfp and hygromycin B resistance genes (Maor et al., 1998) and transformed by PEG/CaCl2 mediated transformation, as previously outlined (Cohen, 2001; Cohen et al., 2002). Both fungal strains were maintained on Oxoid potato glucose agar (PDA) or potato glucose broth (PDB).
Seeds of Orobanche aegyptiaca L. (Egyptian broomrape) were kindly provided by the laboratory of Dr Y. Kleifeld of the Newe Yaar Research Center (Ramat Ishay, Israel). Seeds were surface sterilized using ethanol and hypochlorite as outlined by Amsellem et al. (2001a).
Tomato (Lycopersicon esculentum Mill. ‘M82’) seedlings with two or three expanded leaves were kindly provided by Hishtil Inc. (Ashkelon, Israel) in Speedling Insert Trays™. Plants were left to grow for later use as host plants in the Orobanche studies.
Orobanche tubercles growing on tomato roots in polyethylene envelopes were obtained as follows. Approx imately 2000 O. aegyptiaca seeds were pre‐conditioned for 1 week on Whatman GF/A glass fibre paper in each polyethylene‐film envelope (polybag), based on the methods of Parker and Dixon (1983) with modifications for Orobanche (Amsellem et al., 1999). A tomato seedling with three or four expanded leaves and washed roots was fixed inside each polybag containing conditioned Orobanche seeds. Roots were wetted using approx. 40 ml of a modified Hoagland’s solution (Amsellem et al., 2001a). Tomato plants were grown at a constant 25 °C under 70 µmol m–2 s–1 fluorescent lighting with a 14 h light and 10 h dark photoperiod.
After tomato roots had spread (approx. 2 weeks), 2 ml of 5 µg ml–1 GR‐24 (a synthetic strigolactone analogue seed germination stimulant; Jackson and Parker, 1991) was pipetted onto the roots to augment the germination stimulant naturally produced by tomato. Orobanche tubercles 5–10 mm in diameter were ready to be inoculated with the Fusarium spp. 5–7 d after the addition of GR‐24.
Mycelia of F. oxysporum [native or gfp‐transgenic expressing green fluorescent protein prepared as per Cohen et al. (2002)] or F. arthrosporioides were cultivated in PDB and harvested in the late log growth phase, washed to remove medium and spores, resuspended in water containing 0·02 % Tween 80 and fragmented as outlined in Amsellem et al. (2001a). The mycelial suspensions (10 ml of 50 mg ml–1) were sprayed evenly over the exposed tomato roots and tubercles in each bag. Control plants were mock inoculated by spraying with 0·02 % Tween 80. The kinetics of the fungal infection were followed by light, confocal and electron microscopy.
Tubercles were inoculated with 8 h pre‐germinated spores rather than mycelial suspensions (10 ml, containing 107 conidia ml–1) vs. mock‐inoculated for electron microscopy (EM) studies.
Preparation for microscopy
Tissues were fixed, embedded and sectioned following the procedure of Drews et al. (1993) with modifications for Orobanche. Tubercles 5–10 mm long were excised together with several millimetres of attached tomato root and placed in 50 % ethanol, 5 % acetic acid, 3·7 % formaldehyde. Tissues were slowly vacuum‐infiltrated twice in the fixative for 15 min, left for 2 h at room tem perature, and then kept at 4 °C for between 12 h and several days. Fixed tissues were dehydrated in ethanol, cleared with xylenes, and embedded in Paraplast™. The embedded tissues were serial‐sectioned to 7 µm using a Leica RM2055 microtome with a disposable blade. Tissue sections were mounted on Super Frost® Plus glass microscope slides (Menzel‐Glaser Co., Braunscheig, Germany) and were then stained for lignin with safranin O, and counterstained with fast green, according to Jensen (1962).
Infected and control Orobanche tissues were hand‐sectioned and stained for suberin with Sudan IV according to Schneider (1981), and for callose formation with aniline blue (Stone et al., 1984). Callose fluoresced yellow (490 nm excitation and a 520 nm cut‐off filter).
Starch was quantified after infection in hand‐sectioned tubercles stained with I/KI according to Gahan (1984). Tubercles were also collected, weighed and frozen in tubes at each time point. I/KI staining solution was added to each tube and each sample was ground on ice using a motorized pestle at maximum speed for 30 s. Brown–blue‐stained starch grains were counted in aliquots of the suspensions using a 0·1‐mm deep haemocytometer cell.
Growth of F. oxysporum hyphae in the tubercles was examined by confocal microscopy. Optical images of live samples were collected at intervals after inoculation at magnifications of ×200–320 using a Bio‐Rad MRC 1024 laser scanning confocal system. Hand‐sectioned tubercles infected with transgenic F. oxysporum containing the gfp gene were examined for GFP expression at 488 nm excitation (522 nm cut‐off filter) and the Orobanche tissue at 568 nm excitation (585 nm cut‐off). The confocal image sets on optical media were merged and pseudocoloured in the RGB colour format using Bio‐Rad software. The final adjustment for the coloured images was performed using Adobe Photoshop version 5·0. The merged images showed the green fluorescing F. oxysporum hyphae against a red autofluorescent background of the Orobanche tissue.
Infected Orobanche tubercles were fixed by gently shaking with 3 % paraformaldehyde and 2 % glutaraldehyde in cacodylate sucrose buffer (0·1 m sodium cacodylate, 5 mm CaCl2, 0·1 m sucrose, adjusted to pH 7·2 with HCl) for 4 h at 25 °C followed by 48 h at 4 °C. Samples were rinsed in the cacodylate sucrose buffer and post‐fixed at room temperature for 2 h with 1 % osmium tetroxide containing 0·5 % potassium dichromate and 0·5 % potassium hexacyanoferrate in the above cacodylate buffer for 2 h. Samples were rinsed twice in buffer and twice in water. The water was replaced by 1 % aqueous uranyl acetate and samples were incubated for 2 h in darkness. Tissues were rinsed twice in water, dehydrated in a series of 25, 50, 70 and 96 % ethanol solutions for 20 min each, and twice with 100 % ethanol for 30 min. The 100 % ethanol was replaced by a mixture of (1 : 3) Epon (SPI Supplies) : propylenoxide for 12 to 24 h with gentle shaking, which was replaced by mixtures of (1 : 1), (3 : 1) Epon : propylenoxide, and finally pure Epon, each for 12 to 24 h with gentle shaking. Tissues were oriented in blocks with Epon, which hardened overnight at 60 °C. Thin 2‐µm sections were cut using glass knives with a Leica Ultracut OCT ultramicrotome. Sections were stained for light microscopy with Epoxy Tissue Stain (Electron Microscopy Sciences; Fort Washington, PA, USA) containing toluidine blue and basic fuchsin to identify tissues for further EM analysis using 78 nm ultrathin sections cut with a diamond knife. The ultrathin sections were placed on 300 mesh gold grids for viewing using a Philips 410 transmission electron microscope (TEM).
Quantification of disease intensity
Disease intensity was quantified by two methods: (1) thin sectioned tissues stained with Epoxy Tissue Stain were denoted as healthy if the cytoplasm remained intact, diseased if some cytoplasm still remained and dead when cells were devoid of cytoplasm; and (2) the numbers of cells infected were quantified in video images of the tissues fixed for light microscopy that were divided into 150 µm wide concentric sectors. The percentage of infected and healthy cells in each of the sectors was determined. At least four sectioned tubercles were examined at each time point after infection.
The tissues for scanning electron microscopy (SEM) were first fixed as for TEM (above), then post‐fixed for 2 h at 25 °C in 1 % OsO4 without shaking, washed twice in 0·1 m calcium cacodylate buffer pH 7·2, and then washed twice in water. Tissue pieces were dehydrated through a series of ethanol solutions (as above for TEM) and placed in a Pelco critical period dryer flushed with liquid CO2. Tissue pieces were placed on aluminium stubs and held in place with silver mounting paint (SPI Supplies, West Chester, PA, USA). Tissues were coated with Edward’s gold sputter and scanned at 25 KV at magnifications of ×100 and ×1000 with a JEOL JSM‐6400 scanning electron microscope. Sections of infected and mock‐infected tissues were embedded in Paraplast™ (see above for light microscopy).
Assays for anti‐fungal growth inhibitors and phytoalexins
Tubercles 2–3 mm in diameter infected with F. oxysporum and F. arthrosporioides or mock‐infected were extracted 24 and 48 h after infection, fractionated by thin layer chromatography (TLC) and bioassayed according to Sharon and Gressel (1991). TLC plates were bioassayed for constitutive growth inhibitors and phytoalexins by overlaying a suspension of Alternaria alternata spores in agar medium and incubating until the overlaid material became evenly melanized, except where growth inhibitors were present on the plates.
Fusarium oxysporum and F. arthrosporioides culture filtrates were assayed for fusaric acid as per Heitefuss et al. (1960). The culture filtrates were extracted with ethyl acetate, fractionated by TLC and the plates sprayed with bromcresol green, and examined in UV light for the presence of fusaric acid. Acidic compounds react with bromcresol green and appear fluorescent white against a green background.
Ceramide synthase inhibitors were measured in culture filtrates of F. oxysporum and F. arthrosporioides using a direct assay for sphingolipid metabolism according to Hirschberg et al. (1993).
RESULTS AND DISCUSSION
Progression of fungal infection (as viewed under a light microscope)
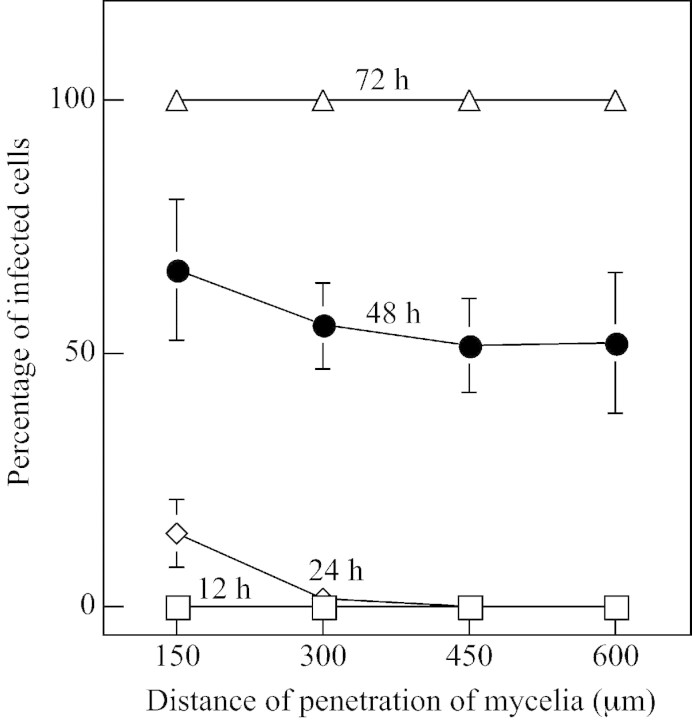
The progression of F. oxysporum infection of Orobanche tubercles was followed after tubercles were infected by fragmented mycelia. Tubercles were collected, sectioned and examined by light microscopy. Orobanche tissue is infected more quickly by mycelia because mycelia immediately begin to grow out in contrast to the lag in growth of germinating spores (Amsellem et al., 1999). Spores generally did not infect uniformly. Tissues inoculated with F. oxysporum mycelia showed no signs of infection for the first 12 h but hyphae were found inside nearly 20 % of the cells in the outer six cell layers of the tissue at 24 h (Figs 1A and 2). Mycelia penetrated directly into the tissue without formation of appressoria. Some of the outer cells collapsed as early as 24 h after infection and the surrounding cells appeared to be devoid of cytoplasm. More than 50 % of the cells in the centre of the tissue were infected by 48 h (Figs 1B and 2) and nearly all cells were infected by 72 h, when some tubercles were already dead (Figs 1C and 2). F. oxysporum grew more or less randomly through and between the infected Orobanche cells. Tubercles were dead by 96 h, when none of the cells had intact cytoplasmic structure, and the cells were full of hyphae (Fig. 1D). Progression of the infection of the tubercles by mycelia is quantified in Fig. 2.
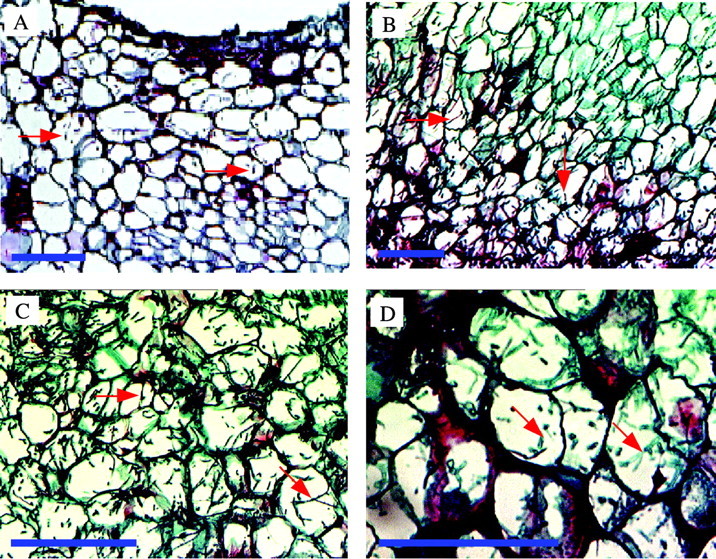
Fig. 1. Progression of infection of Orobanche tubercles by Fusarium arthrosporioides. Sections were stained with safranin O and contrasted with fast‐green. A, Fusarium oxysporum mycelia (green) are in the outer six cell layers at 24 h. B, The mycelia (green) reach the centre of the tubercles by 48 h. C, Higher magnification of infected cells in the centre of the tissue at 72 h. Some of the (green) hyphae are marked (arrows). D, A dead tubercle with cells in the centre of the tissue heavily infused with red‐stained material at 96 h. Representative mycelia denoted by red arrows. Bars = 50 µm.
Fig. 2. Progression of infection of Orobanche by Fusarium oxysporum. Cell viability counts were made in 150 µm concentric sectors superimposed on micrographs of sectioned tissues (bars indicate ± s.e. and are shown where larger than symbols).
Visualization of fungal infection of tubercles (by confocal microscopy)
Intact tubercles infected with transgenic (gfp)F. oxysporum were examined by confocal microscopy. The Orobanche cell walls autofluoresced red and (gfp)F. oxysporum mycelia fluoresced bright green. The transgenic strain of (gfp)F. oxysporum had strong GFP expression whilst retaining pathogenicity. The hyphae are clearly seen on the surface of a tubercle at 24 h (Fig. 3A). Hyphae penetrated into tubercle cells to a minimal depth of 6 µm by 24 h (Fig. 3B–E), 16 µm by 48 h (Fig. 3F–M) and 18 µm by 72 h (Fig. 3N–U). The distance between optical slices was 2 µm, except for K–L in the 48 h series, as well as Q–R and T–U in the 72 h series, where the distance was 4 µm between slices. The autofluorescence of the Orobanche cells was substantially diminished and the hyphae only weakly fluoresced by 96 h. Fungal penetration could only be seen to a depth of 18 µm due to self‐quenching of the tissue.
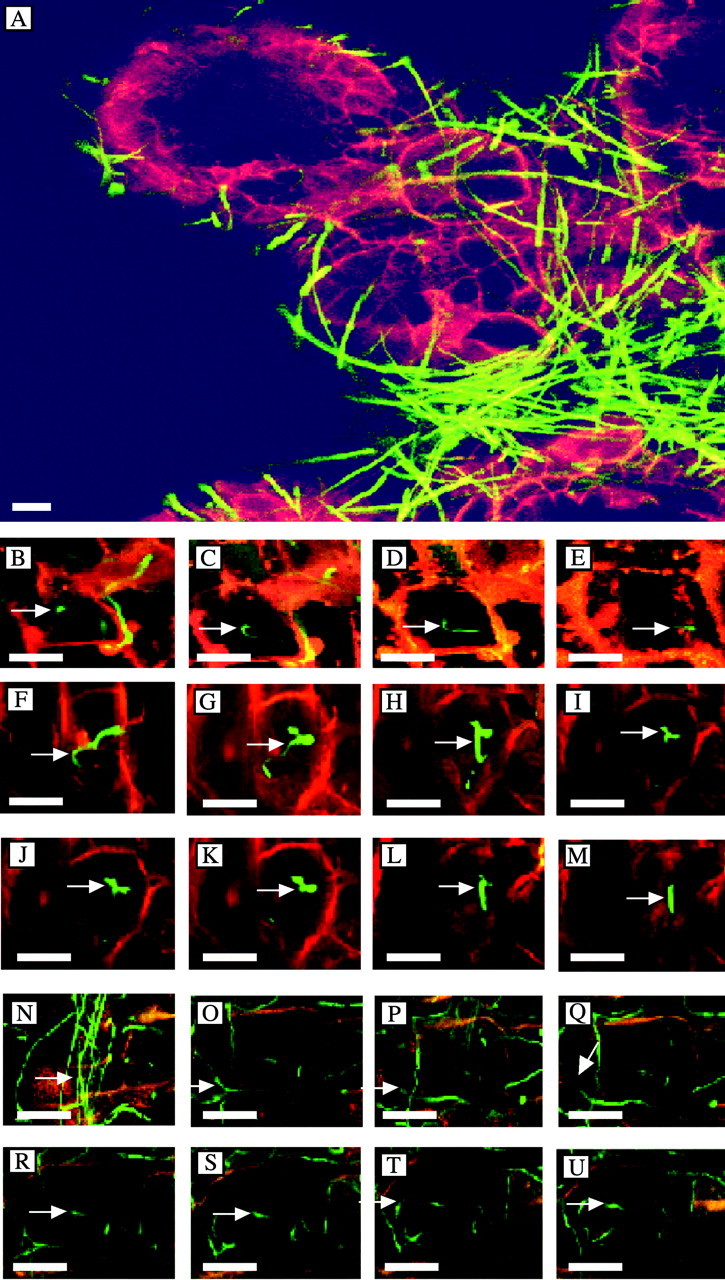
Fig. 3. Infection of Orobanche tubercle tissue by hyphae of transgenic Fusarium oxysporum containing the green fluorescent protein gene (gfp) as visualized by confocal microscopy. A, Overview showing hyphae growing on a rootlet emerging from a tubercle at 24 h. The tubercles were inoculated with fragmented mycelia of (gfp)F. oxysporum. B–U, Penetration of (gfp)F. oxysporum into Orobanche cells. Arrows follow a single hypha into an Orobanche cell beginning at the epidermal cell surface to a depth of 6 µm at 24 h (B–E), 16 µm at 48 h (F–M) and 18 µm at 72 h (N–U). Bars = 25 µm.
Ultrastructural ontogeny of the fungal infection
The progression of spore germination and mycelial growth of F. arthrosporioides and F. oxysporum over the surface of the tubercles was followed by SEM. Spore germination of F. oxysporum and F. arthrosporioides was evident on Orobanche rootlets by SEM at 24 h (Fig. 4A and D); hyphal growth advanced over the rootlets by 48 h (Fig. 4B and E); and the rootlets were covered by the hyphae at 96 h (Fig. 4C and F). The mycelia also grew along the rhizoplane of the tomato roots, without penetration or apparent damage to the tomatoes (Cohen, 2001).
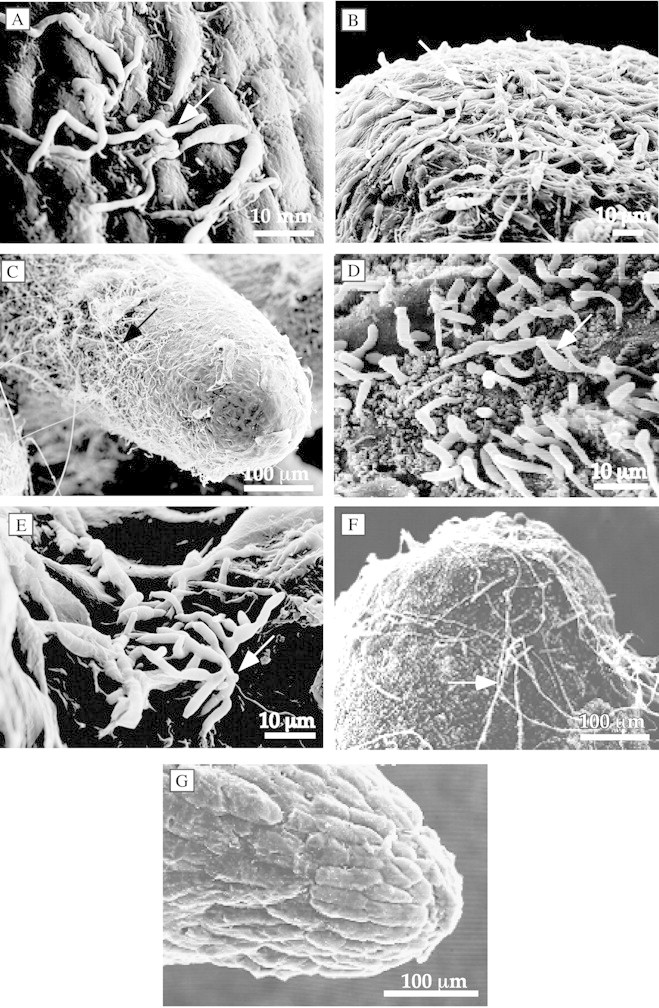
Fig. 4. Progression of the spread of Fusarium oxysporum (A–C) and Fusarium arthrosporioides (D–F) on Orobanche tubercles examined by SEM. Tubercles with mycelia growing on the surface were seen at 24 (A and D), 48 (B and E) and 96 h (C and F) of infection. G, Uninoculated control. Arrows indicate hyphae.
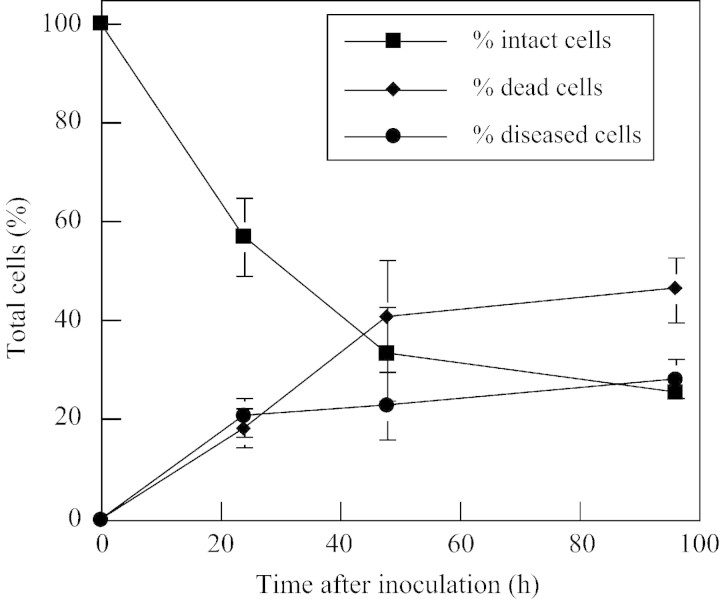
Ultrathin sections were examined in parallel by TEM for structural changes that occur in Orobanche cells after fungal invasion. The mycelia from germinated spores of F. oxysporum (Fig. 5A–D) and F. arthrosporioides (Fig. 5E–H) penetrated the Orobanche epidermal cell walls, and the mycelia were seen by TEM inside the cells at 24 h (Fig. 5A and E). Hyphae had penetrated several cell layers of the tubercles by 48 (Fig. 5B and F) and 72 h (Fig. 5G). The breakdown of cell walls, disintegration of organelles and disappearance of cytoplasm in and around the infected cells occurred by 72 and 96 h, respectively (Fig. 5C and H). Healthy Orobanche tubercle cells with intact organelles and cytoplasm, as well as starch grains are seen in the non‐infected control (Fig. 5D). Starch grains were not visible in any sections of the inoculated tissues in these TEM studies. The numbers of intact, diseased and dead cells in the tubercles inoculated with F. oxysporum were quantified using light microscopy. More than 80 % of the tubercle cells were diseased or dead by 96 h (Fig. 6). A much greater extent of tissue damage was seen by this time when mycelia were used (Fig. 1C), as mycelia infect more rapidly than spores. Nearly all the cells in the tissues were dead by 96 h when mycelia were used for infection, whereas 20 % of the cells were still considered healthy when spores were used.
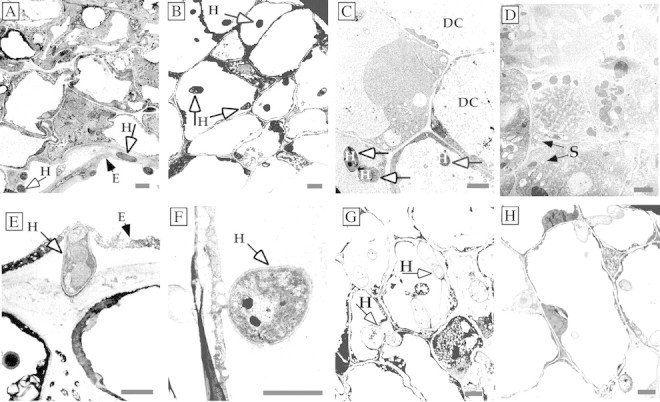
Fig. 5. Tubercles infected with Fusarium oxysporum (A–C) and F. arthrosporioides (D–H) mycelia examined by TEM. Fusarium oxysporum hyphae are inside the epidermal cell wall as well as inside an epidermal cell at 24 h (A and E). Hyphae had penetrated several cell layers of the tubercles by 48 (B and F) and 72 h (G), and the cytoplasm of infected cells and the cytoplasm and cell walls of neighbouring cells were disintegrating by 72 and 96 h, respectively (C and H). Healthy cells with intact organelles in the cytoplasm of the non‐infected control are shown (D). DC, Disintegrated cytoplasm; E, epidermal cell wall; H, hypha; S, starch grains. Bars = 2 µm.
Fig. 6. Quantification of intact, diseased and dead cells in Orobanche tubercles infected with Fusarium oxysporum over the duration of the experiments. At least four sectioned tubercles were examined at each time point and were collected from two to three separate experiments (bars indicate ± s.e. and are shown where larger than symbols).
Infection‐induced appearance of lignin‐like material and cutin
The same tissues that had been used to follow the progression of fungal infection in the light microscopy studies were stained for the detection of lignification and cutinization as putative defences. Epidermal cells of both inoculated (Fig. 7A) and uninfected cells (Fig. 7F) were mildly cutinized. The safranin‐stained material in sub‐epidermal cells is probably lignin‐like material (Siegrist et al., 1994). Sub‐epidermal cells of the outer three to four layers stained red by 24 h, indicating further lignification (Fig. 7B). Up to half of the cells in the outer cell layers stained red by 48 h in the infected tissues (Fig. 7C). More than half of the cells in tissues collected at 72 h appeared to show some lignification (Fig. 7D) and by 96 h nearly all infected cells stained for lignin and were dead (Fig. 7E). The material that stained positively for lignin‐like material was detected in the cytoplasm, but not in the cell walls of infected tubercles where structural enhancement normally takes place (Fig. 7).
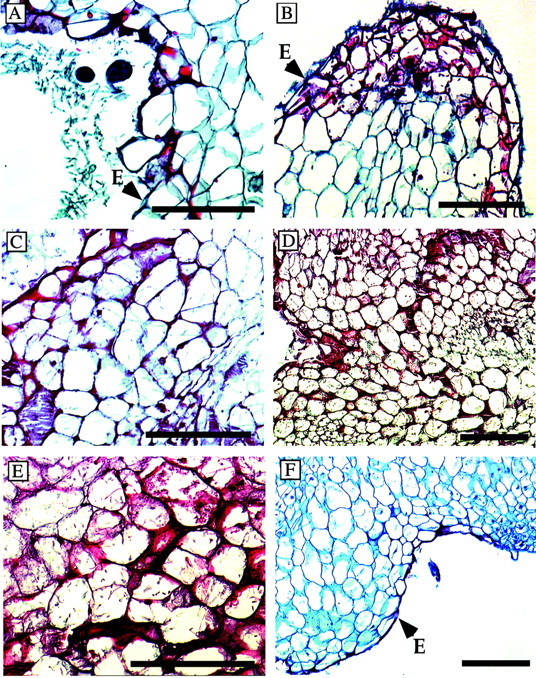
Fig. 7. Increase in lignin‐like material following infection in the tubercle cells. The appearance of lignin‐like material (red) in Orobanche infected by F. oxysporum was detected in the outer and side cell walls of the epidermis at 12 h (A). B, Several cell layers are stained red at 24 h. C, Changes in cell walls progressed as evidenced by the red staining of deeper layers at 48 h; this progression continued at 72 (D) and 96 h (E). F, The epidermal cell layer of control tissues had some safranin‐stained material in the outer surface (possibly cutin), but there was no internal staining. E, Epidermal cell wall. Bars = 100 µm.
Depletion of starch in Orobanche after inoculation with F. oxysporum
There was a dense distribution of starch grains (which appeared white in polarized light) in non‐infected (control) Orobanche tissues (Fig. 8A and B). Starch was still plentiful in the inoculated tissues 1 h after inoculation in kinetic experiments (Fig. 8C), but was greatly depleted by 3 h, well before any mycelium penetrated the tissue (Fig. 8D).
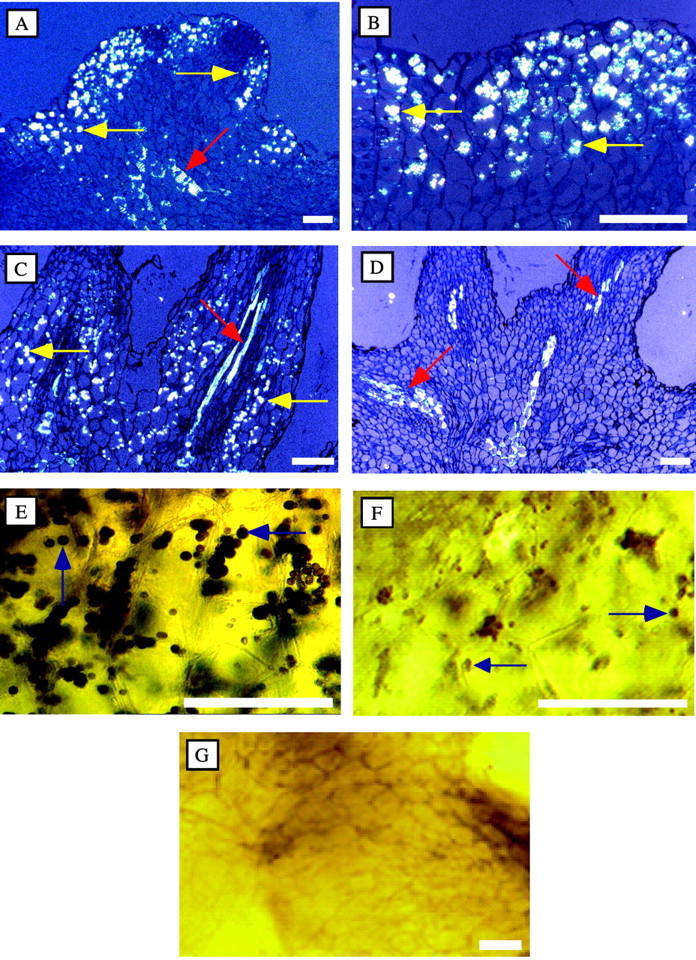
Fig. 8. Resorption of starch in Orobanche infected by Fusarium oxysporum. Starch grains (which appear white in polarized light) are seen in Orobanche tubercle sections in controls (A and B) and at 1 h after F. oxysporum inoculation (C), but were greatly depleted by 3 h (D). Starch stained brown–black with iodine and was plentiful in control tissues (E), much less plentiful in inoculated tubercles at 3 h (F), and absent by 24 h (G). The vascular system (red arrows) observed under polarized light was fluorescent in all samples, although the intensity varied among the tubercles. Yellow and blue arrows indicate starch grains. Bars = 200 µm.
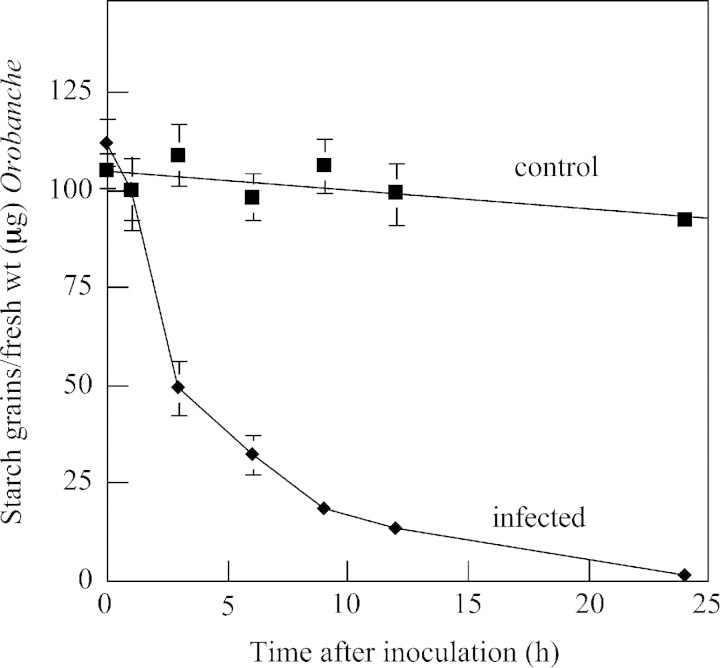
Tubercles were collected at various times after inoculation with F. oxysporum. The distribution of starch was examined and the proportion of starch grains remaining in the Orobanche tissues was quantified. Starch stained brown‐black with I/KI. The stained starch was abundant in the non‐infected control tissues (Fig. 8E); however, the starch content in inoculated tissues was noticeably less by 3 h (Fig. 8F) and absent by 24 h (Fig. 8G). These observations with extracted starch in the control and infected tubercles parallel the histochemical observations of starch content in the tubercles detected in polarized light. The extracted starch content in inoculated tubercles was half that of the non‐inoculated tissue at 3 h, and only 10 % remained by 12 h (Fig. 9). Starch was not detected in tissues inoculated with F. oxysporum in the TEM studies at the earliest time examined (24 h), but was detected in the controls. This complements the kinetic studies.
Fig. 9. Kinetics of disappearance of starch from Fusarium oxysporum‐infected tubercles. Tubercles were inoculated with F. oxysporum mycelia as in Fig. 8. Stained starch grains were counted in aliquots of suspensions of ground tissues. Three replicate samples (two to three tubercles each) were collected at each time point (bars indicate ± s.e. and are shown where larger than symbols).
The monomeric and dimeric sugars derived from starch are possibly used by Orobanche as energy to mobilize a rapid response to the infection, including in the synthesis of lignin‐like materials, or other defence responses. Conversely, fungal pathogens such as F. oxysporum penetrating the cells secrete starch‐degrading enzymes that supply the metabolites needed for growth of the pathogens (Skovgaard and Rosendahl, 1998). Starch disappeared prior to actual fungal penetration of the tubercles. Thus, it seems likely that the fungus activates a signal transduction cascade causing Orobanche to metabolize the starch with endogenous enzymes. Whether Orobanche or Fusarium, or both, utilize these starch catabolites is an open question.
Pathogen‐induced callose, suberin or phytoalexin formation were not observed in Orobanche
Infected Orobanche tissues were stained for callose and observed by fluorescence microscopy. No callose was observed in infected or non‐infected Orobanche tissues, whereas callose was apparent around sites of fungal penetration in a positive control of wheat leaves infected with Erysiphe graminis (Cohen, 2001). No suberin was detected in either infected or non‐infected Orobanche tissues but was observed in the epidermis of a potato tuber used as a positive control (Cohen, 2001).
Plant resistance to specific pathogens is often correlated with increased production of phytoalexins, whereas inhibition or deficiency of phytoalexin production allows greater virulence (Sharon et al., 1992). Infected and non‐infected tubercles were extracted, fractionated on TLC plates and then bioassayed for antifungal agents with an overlay of fungal spores. No clear spots of inhibited fungus were found in any experiment. Unfractionated aqueous extracts of the infected and non‐infected tubercles were also tested for antifungal activity, but none was found. Thus, none of the often used constitutive or pathogen‐induced inhibitors were found (Cohen, 2001).
Phytotoxic metabolites in culture filtrates
Phytotoxic metabolites produced by pathogenic fungi accumulate in the culture filtrates of some fungi (Evidente et al., 2000). Fusarium oxysporum and F. arthrosporioides culture filtrates were each examined for the production of phytotoxins. Semi‐solid cultures of F. oxysporum and F. arthrosporioides were extracted and assayed for the fumonisins FB1 and FB2, but neither was detected. Crude undiluted culture filtrates of F. oxysporum and F. arthro sporioides inhibited ceramide synthase by about 50 % (Cohen, 2001). Other fungal neurotoxins also inhibit this enzyme (Norred et al., 1999). Apparently, these two species secrete non‐fumonisin ceramide synthase inhibitors.
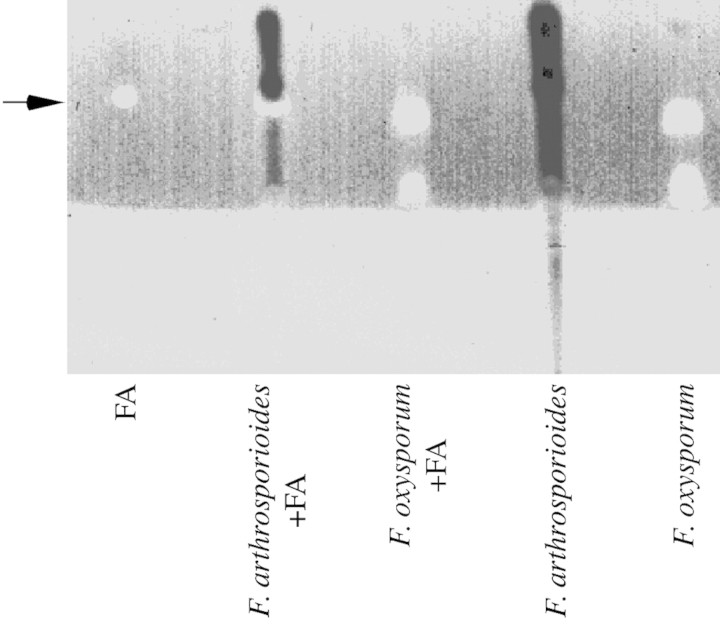
Fusaric acid is commonly produced by many Fusarium species (Bacon et al., 1996). Culture filtrates of F. oxy sporum and F. arthrosporioides were extracted and examined for the detection of fusaric acid. Fusaric acid was only detected in culture filtrates from F. oxysporum (Fig. 10).
Fig. 10. Release of fusaric acid into the culture medium of Fusarium oxysporum. Fusaric acid and other fluorescing materials were found in culture filtrates of F. oxysporum, but not in those of F. arthrosporioides. The figure was scanned in black and white. Authentic fusaric acid (FA) (20 µg) was loaded separately and was used to spike two samples.
CONCLUSIONS
In the study of infection of Orobanche seeds by Fusarium (Thomas et al., 1999), the Fusarium inoculum was augmented with a carbon source to enhance fungal growth. The fungal growth on tubercles in this study was dependent on carbon from the tubercles. Uninfected tubercles scanned by TEM were not rich in lipid bodies (Fig. 5), in contrast to the seeds (Thomas et al., 1999). However, tubercles contained starch that was rapidly degraded and that disappeared from the inoculated tissues before hyphae penetrated into the tissues (Figs 8 and 9). Hyphae penetrated both through and between cells and were found throughout the tubercles by 4 d (Fig. 1A–D), causing organellar and cytoplasmic disintegration. There were no signs of induced cell wall reinforcement in the infected tissues. These mycoherbicidal Fusarium spp. are clearly effective at colonizing Orobanche tubercles with little resistance.
Mycelia of these strains have longer storage viability in a formulation of ‘Stabileze’ (modified starch, sucrose, corn oil and silica) than spores (Amsellem et al., 1999). Additionally, the desiccated mycelia were quicker than fermenter‐derived spores to grow out after rehydration. Rapid growth of the fungal propagules in wet soil is important, allowing the fungi to sporulate before the soil dries. Mycelia were also much quicker than spores in infecting tubercles. Unlike many host–pathogen systems, penetration was directly into the tissue, without need for appressoria. The use of mycelia as mycoherbicides for the control of Orobanche is desirable due to the high infectivity and the long‐term storage and quick germination of the fungi in formulated pellets. The use of mycelial inoculant from asporogenic mutants would preclude off‐target spread of the mycoherbicide (Gressel, 2001).
The virulence on Orobanche could be enhanced by altering hormone balance to disrupt growth patterns. The same strains of mycoherbicidal Fusarium spp. used to infect Orobanche tubercles were transformed with genes for enhanced production of indole‐3‐acetic acid (IAA) (Cohen, 2001; Cohen et al., 2002). The enhanced production of IAA conferred a modicum of hypervirulence to the fungi, which resulted in a reduction in the numbers and size of Orobanche shoots. Having a better understanding of the aetiology of pathogenesis and the nature of the defence responses may lead to the design of still better control organisms and strategies for their use (Gressel, 2002).
ACKNOWLEDGEMENTS
We thank Dr Vera Shinder and Mrs Orna Yeger of the Electron Microscopy Unit, Weizmann Institute, for helpful discussions and technical assistance with light and electron microscopy, and Ms Lena Kapito for general laboratory assistance. This research was supported by a DGF trilateral Israel‐German‐Palestinian project, and a COST 815 travel grant. J.G. holds the Gilbert de Botton Chair of Plant Sciences.
Supplementary Material
Received: 11 February 2002; Returned for revision: 4 June 2002; Accepted: 6 August 2002
References
- AbbasHK, Boyette CD.1992. Phytotoxicity of fumonisin B1 on weed and crop species. Weed Technology 6: 548–552. [Google Scholar]
- AgriosGN.1997. How pathogens attack plants. In: Plant pathology. New York: Academic Press, 63–82. [Google Scholar]
- AmsellemZ, Zidack NK, Quimby PC Jr, Gressel J.1999. Long‐term dry preservation of active mycelia of two mycoherbicidal organisms. Crop Protection 18: 643–649. [Google Scholar]
- AmsellemZ, Kleifeld Y, Kerenyi Z, Hornok L, Goldwasser Y, Gressel J.2001a Isolation, identification, and activity of mycoherbicidal pathogens from juvenile broomrape plants. Biological Control 21: 274–284. [Google Scholar]
- AmsellemZet al.2001b Recent advances in the biocontrol of Orobanche (broomrape) species. BioControl 46: 211–228. [Google Scholar]
- BaconCW, Porter JK, Norred WP, Leslie JF.1996. Production of fusaric acid by Fusarium species. Applied and Environmental Microbiology 62: 4039–4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BaileyBA, Apel‐Birkhold PA, Akingbe OO, Ryan JL, O‘Neill NR, Anderson JD.2000. Nep1 protein from Fusarium oxysporum enhances biological control of opium poppy by Pleospora papaveracea. Phytopathology 90: 812–818. [DOI] [PubMed] [Google Scholar]
- BaylesCJ, Ghemawat MS, Aist JR.1990. Inhibition by 2‐deoxy‐d‐glucose of callose formation, papilla deposition, and resistance to powdery mildew in ml‐o barley mutant. Physiological and Molecular Plant Pathology 36: 63–72. [Google Scholar]
- CohenB.2001. Ontogeny of pathogenesis and the biochemical responses of Orobanche following infection by compatible Fusarium spp. PhD Thesis, Weizmann Institute of Science, Rehovot. [Google Scholar]
- CohenB, Amsellem Z, Maor R, Sharon A, Gressel J.2002. Transgenically‐enhanced expression of indole‐3‐acetic acid (IAA) confers hypervirulence to plant pathogens. Phytopathology 92: 590–596. [DOI] [PubMed] [Google Scholar]
- DrewsGN, Bowman JL, Meyerowitz EM.1993. Negative regulation of the Arabidopsis homeotic gene AGAMOUS by the APETALA2 product. Cell 65: 991–1002. [DOI] [PubMed] [Google Scholar]
- EvidenteA, Andolfi A, Vurro M, Zonno MC, Motta A.2000. Trans‐4‐aminoproline, a phytotoxic metabolite with herbicidal activity produced by Ascochyta caulina.Phytochemistry 53: 231–237. [DOI] [PubMed] [Google Scholar]
- GahanPB.1984. Carbohydrates. In: Plant histochemistry London: Academic Press, 236–244. [Google Scholar]
- GresselJ.2001. Potential failsafe mechanisms against spread and introgression of transgenic hypervirulent biocontrol fungi. Trends in Biotechnology 19: 149–154. [DOI] [PubMed] [Google Scholar]
- GresselJ.2002. Molecular biology of weed control. London: Taylor and Francis. [DOI] [PubMed] [Google Scholar]
- GresselJ, Vered Y, Bar‐Lev S, Milstein O, Flowers HM.1983. Partial suppression of cellulase action by artificial lignification of cellulose. Plant Science Letters 32: 349–353. [Google Scholar]
- HeitefussR, Stahmann MA, Walker JC.1960. Production of pectolytic enzymes and fusaric acid by Fusarium oxysporum f. sp. conglutinans in relation to cabbage yellows. Phytopathology 50: 367–370. [Google Scholar]
- HirschbergK, Rodger J, Futerman AH.1993. The long‐chain sphingoid base of sphingolipids is acylated at the cytosolic surface of the endoplasmic reticulum in rat liver. Biochemistry 290: 751–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JacksonMB, Parker C.1991. Induction of germination by a strigol analog requires ethylene action in Striga hermonthica but not in S. forbesii. Journal of Plant Physiology 138: 383–386. [Google Scholar]
- JensenWA.1962. Histological procedures. In: Botanical histochemistry. San Francisco: Freeman and Company, 55–99. [Google Scholar]
- KaussH.1992. Callose and callose synthase. In: Bowles DJ, Gurr S, McPherson M, eds. Practical approaches to molecular plant pathology Oxford: Oxford University Press, 1–8. [Google Scholar]
- MaorR, Puyesky M, Horwitz BA, Sharon A.1998. Use of green fluorescent protein (GFP) for studying development and fungal‐plant interaction in Cochliobolus heterostrophus.Mycological Research 102: 491–496. [Google Scholar]
- MaternU, Kneusel RE.1988. Phenolic compounds in plant disease resistance. Phytoparasitica 16: 153–170. [Google Scholar]
- MitchellHJ, Hall SA, Stratford R, Hall JL, Barber MS.1999. Differential induction of cinnamyl alcohol dehydrogenase during defencive lignification in wheat (Triticum aestivum L.): char acterization of the major inducible form. Planta 208: 31–37. [Google Scholar]
- NorredWP, Bacon CW, Riley RT, Voss KA, Meredith FI.1999. Screening of fungal species for fumonisin production and fumonisin‐like disruption of sphingolipid biosynthesis. Mycopathologia 146: 91–98. [DOI] [PubMed] [Google Scholar]
- ParkerC, Dixon N.1983. The use of polyethylene bags in the culture and study of Striga spp. and other organisms on crop roots. Annals of Applied Biology 103: 485–488. [Google Scholar]
- ParkerC, Riches CR.1993. Orobanche spp: the broomrapes. In: Parker C, Riches CR, eds. Parasitic weeds of the world. Wallingford, UK: CAB International, 111–164. [Google Scholar]
- RobbJ, Powell DA, Street PFS.1989. Vascular coating: a barrier to colonization by the pathogen in Verticillium wilt of tomato. Canadian Journal of Botany 67: 600–607. [Google Scholar]
- SharonA, Gressel J.1991. Elicitation of flavonoid phytoalexin accumulation in Cassia obtusifolia by a mycoherbicide: estimation by AlCl3‐spectrofluorimetry. Pesticide Biochemistry and Physiology 41: 142–149. [Google Scholar]
- SharonA, Amsellem Z, Gressel J.1992. Glyphosate suppression of an elicited defence response. Plant Physiology 98: 654–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SchneiderH.1981. Plant anatomy and general botany. In: Clark G, ed. Staining procedures Baltimore: Williams and Wilkins, 315–334. [Google Scholar]
- SiegristJ, Jeblick W, Kauss H.1994. Defence responses in infected and elicited cucumber (Cucumis sativus L.) hypocotyl segments exhibiting acquired resistance. Plant Physiology 105: 1365–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SkovgaardK, Rosendahl S.1998. Comparison of intra‐ and extracellular isozyme banding patterns of Fusarium oxysporum. Mycological Research 102: 1077–1084. [Google Scholar]
- StoneBA, Evans NA, Bonig I, Clarke AE.1984. The application of sirofluor, a chemically defined fluorochrome from aniline blue for the histochemical detection of callose. Protoplasma 122: 191–195. [Google Scholar]
- ThomasH, Heller A, Sauerborn J, Muller‐Stover D.1999. Fusarium oxysporum f. sp. orthoceras, a potential mycoherbicide, parasitizes seeds of Orobanche cumana (sunflower broomrape): a cytological study. Annals of Botany 83: 453–458. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.