Abstract
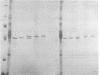
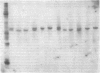
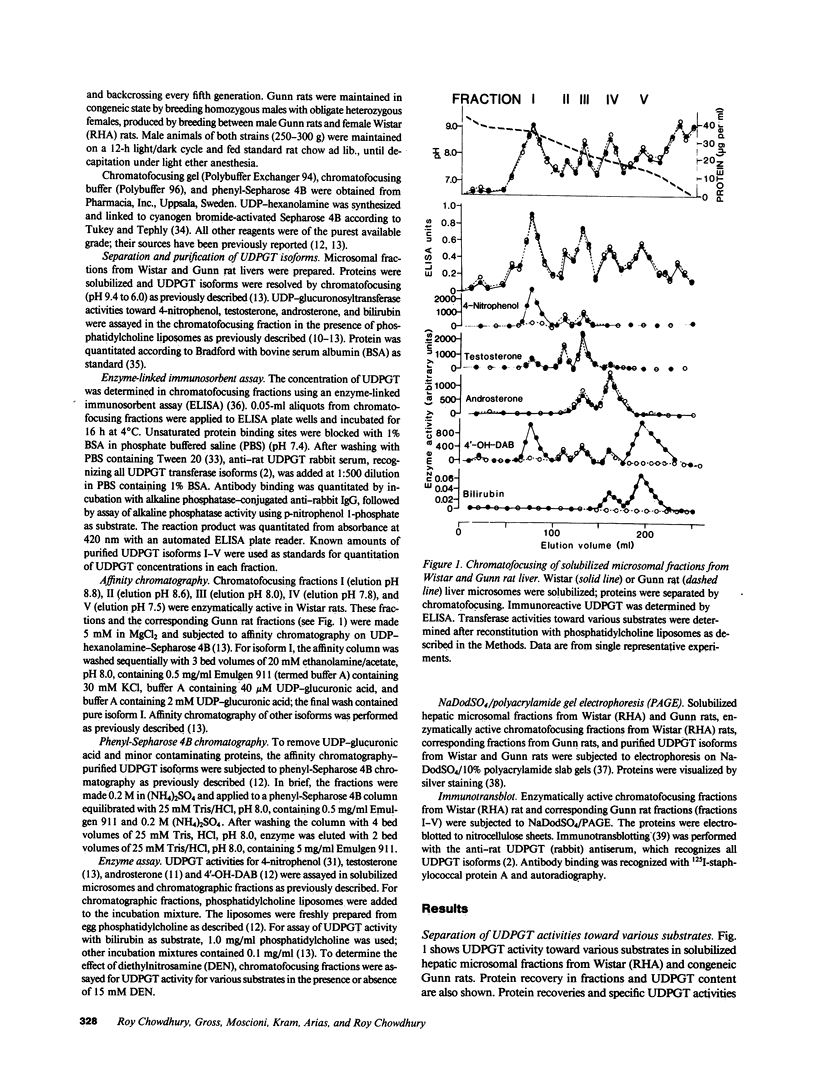
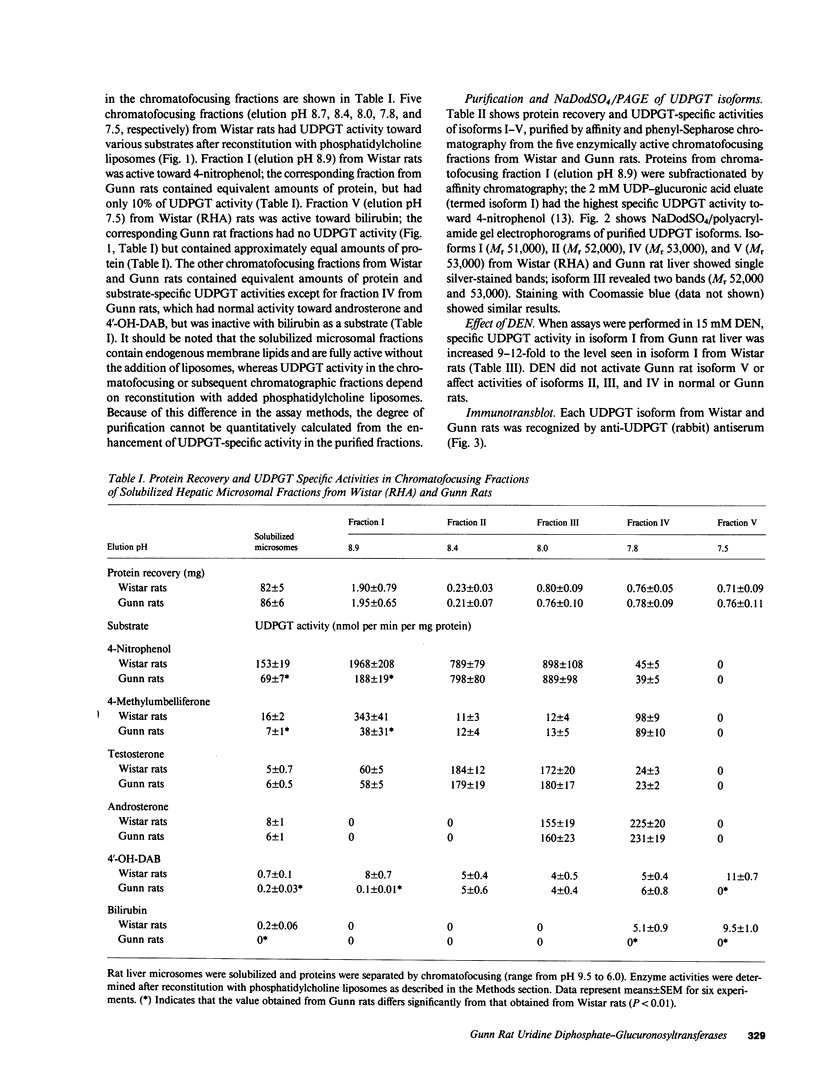
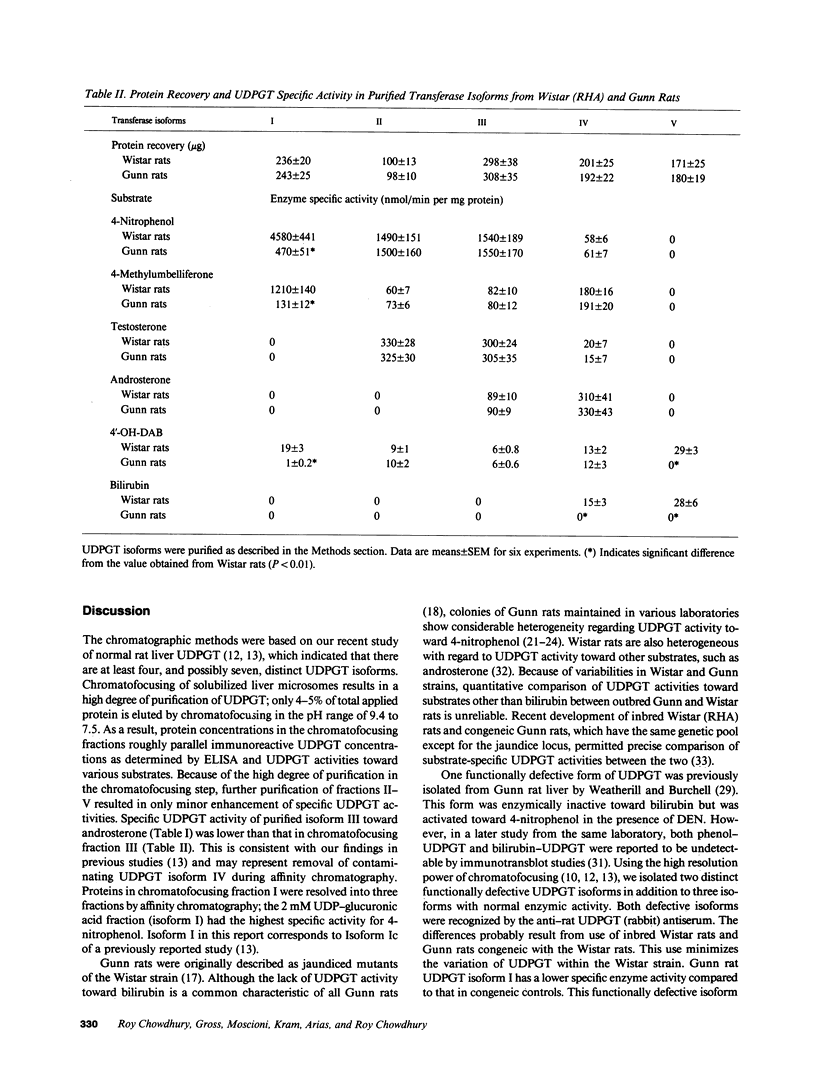
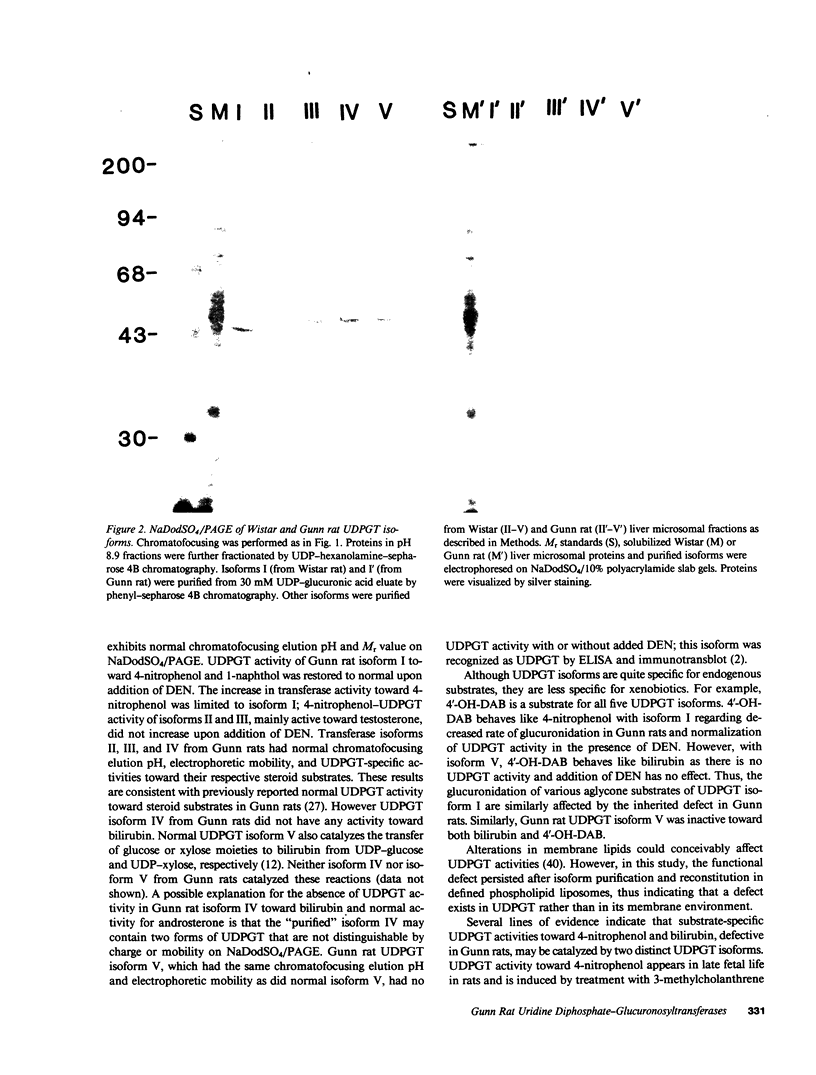
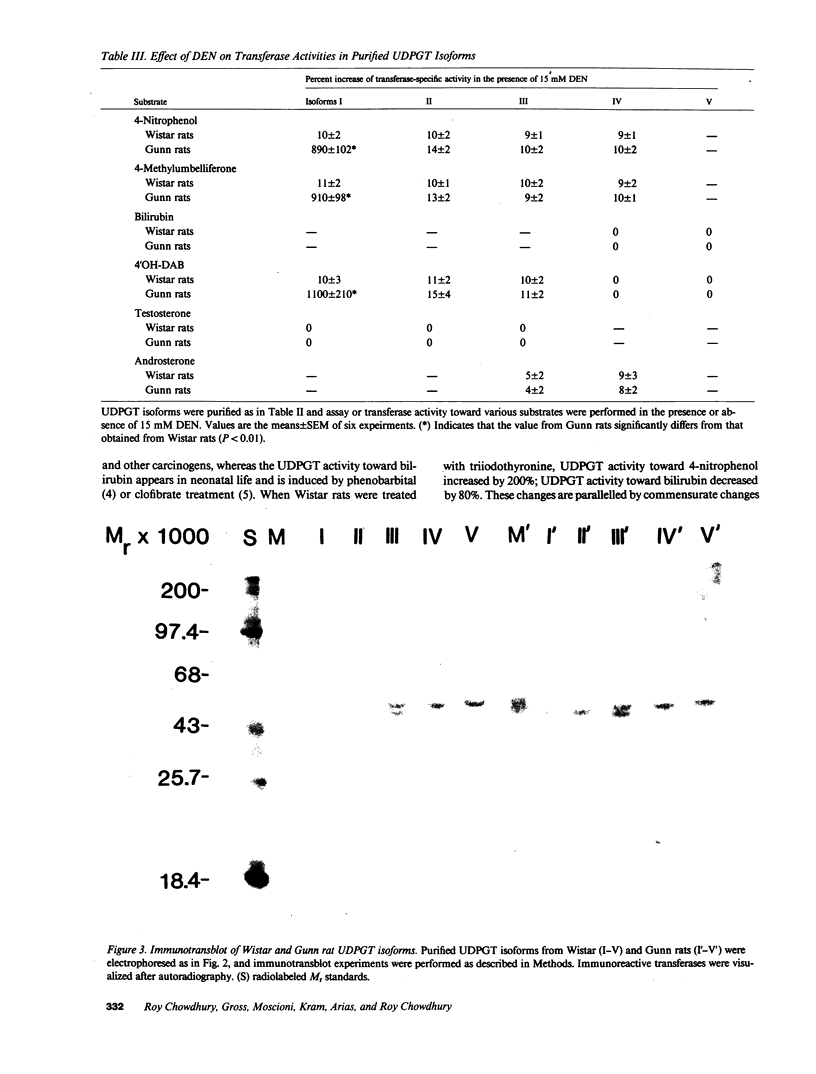
Gunn rats are a mutant strain of Wistar rats that have unconjugated hyperbilirubinemia due to absence of hepatic uridine diphosphate-glucuronosyltransferase (UDPGT; EC. 2.4.1.17) activity toward bilirubin. We isolated five UDPGT isoforms from solubilized microsomal fractions from liver of inbred Wistar (RHA) rats and congeneic Gunn rats. UDPGT isoform V (elution pH 7.5) from Wistar (RHA) rats is active toward bilirubin and 4'-hydroxydimethylaminoazobenzene. The corresponding isoform from Gunn rat liver was enzymically inactive but exhibited normal elution pH and mobility on NaDodSO4/polyacrylamide gel electrophoresis (Mr 53,000), and was recognized by a UDPGT-specific antiserum. UDPGT isoform I (elution pH 8.7) from Wistar (RHA) and Gunn rats was active toward 4-nitrophenol. The isoform from Gunn rat liver had only 10% of normal UDPGT activity, however UDPGT activity increased to normal upon addition of 15 mM diethylnitrosamine in vitro. Isoforms II (elution pH 8.4), III (elution pH 8.0), and IV (elution pH 7.8) from Gunn rats had normal UDPGT activities, except that Isoform IV was inactive toward bilirubin.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARIAS I. M. Ethereal and N-linked glucuronide formation by normal and Gunn rats in vitro and in vivo. Biochem Biophys Res Commun. 1961 Nov 1;6:81–84. doi: 10.1016/0006-291x(61)90388-6. [DOI] [PubMed] [Google Scholar]
- Blanckaert N., Fevery J., Heirwegh K. P., Compernolle F. Characterization of the major diazo-positive pigments in bile of homozygous Gunn rats. Biochem J. 1977 Apr 15;164(1):237–249. doi: 10.1042/bj1640237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Chowdhury J. R., Chowdhury N. R. Conjugation and excretion of bilirubin. Semin Liver Dis. 1983 Feb;3(1):11–23. doi: 10.1055/s-2008-1040667. [DOI] [PubMed] [Google Scholar]
- Chowdhury J. R., Chowdhury N. R., Moscioni A. D., Tukey R., Tephly T., Arias I. M. Differential regulation by triiodothyronine of substrate-specific uridinediphosphoglucuronate glucuronosyl transferases in rat liver. Biochim Biophys Acta. 1983 Nov 22;761(1):58–65. doi: 10.1016/0304-4165(83)90362-8. [DOI] [PubMed] [Google Scholar]
- Chowdhury J. R., Novikoff P. M., Chowdhury N. R., Novikoff A. B. Distribution of UDPglucuronosyltransferase in rat tissue. Proc Natl Acad Sci U S A. 1985 May;82(9):2990–2994. doi: 10.1073/pnas.82.9.2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury N. R., Arias I. M., Lederstein M., Chowdhury J. R. Substrates and products of purified rat liver bilirubin UDP-glucuronosyltransferase. Hepatology. 1986 Jan-Feb;6(1):123–128. doi: 10.1002/hep.1840060124. [DOI] [PubMed] [Google Scholar]
- Chowdhury N. R., Arias I. M., Lederstein M., Chowdhury J. R. Substrates and products of purified rat liver bilirubin UDP-glucuronosyltransferase. Hepatology. 1986 Jan-Feb;6(1):123–128. doi: 10.1002/hep.1840060124. [DOI] [PubMed] [Google Scholar]
- Drucker W. D. Glucuronic acid conjugation of tetrahydrocortisone and p-nitrophenol in the homozygous Gunn rat. Proc Soc Exp Biol Med. 1968 Oct;129(1):308–311. doi: 10.3181/00379727-129-33310. [DOI] [PubMed] [Google Scholar]
- Falany C. N., Chowdhury J. R., Chowdhury N. R., Tephly T. R. Steroid 3- and 17-OH UDP-glucuronosyltransferase activities in rat and rabbit liver microsomes. Drug Metab Dispos. 1983 Sep-Oct;11(5):426–432. [PubMed] [Google Scholar]
- Falany C. N., Tephly T. R. Separation, purification and characterization of three isoenzymes of UDP-glucuronyltransferase from rat liver microsomes. Arch Biochem Biophys. 1983 Nov;227(1):248–258. doi: 10.1016/0003-9861(83)90368-5. [DOI] [PubMed] [Google Scholar]
- Flock E. V., Bollman J. L., Owen C. A., Jr, Zollman P. E. Conjugation of thyroid hormones and analogs by the Gunn rat. Endocrinology. 1965 Aug;77(2):303–314. doi: 10.1210/endo-77-2-303. [DOI] [PubMed] [Google Scholar]
- Jansen P. L., Arias I. M. Delipidation and reactivation of UDPglucuronosyltransferase from rat liver. Biochim Biophys Acta. 1975 May 23;391(1):23–38. [PubMed] [Google Scholar]
- Javitt N. B. Ethereal and acyl glucuronide formation in the homozygous Gunn rat. Am J Physiol. 1966 Aug;211(2):424–428. doi: 10.1152/ajplegacy.1966.211.2.424. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lilienblum W., Walli A. K., Bock K. W. Differential induction of rat liver microsomal UDP-glucuronosyltransferase activites by various inducing agents. Biochem Pharmacol. 1982 Mar 15;31(6):907–913. doi: 10.1016/0006-2952(82)90319-7. [DOI] [PubMed] [Google Scholar]
- Mackenzie P. I., Owens I. S. Differences in UDP-glucuronosyltransferase activities in congenic inbred rats homozygous and heterozygous for the jaundice locus. Biochem Pharmacol. 1983 Dec 15;32(24):3777–3781. doi: 10.1016/0006-2952(83)90149-1. [DOI] [PubMed] [Google Scholar]
- Matsui M., Watanabe H. K. Classification and genetic expression of Wistar rats with high and low hepatic microsomal UDP-glucuronosyltransferase activity towards androsterone. Biochem J. 1982 Jan 15;202(1):171–174. doi: 10.1042/bj2020171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowat A. P., Arias I. M. Observations of the effect of diethylnitrosamine on glucuronide formation. Biochim Biophys Acta. 1970 Jul 15;212(1):175–178. doi: 10.1016/0005-2744(70)90192-0. [DOI] [PubMed] [Google Scholar]
- Nakata D., Zakim D., Vessey D. A. Defective function of a microsomal UDP-glucuronyltransferase in Gunn rats. Proc Natl Acad Sci U S A. 1976 Feb;73(2):289–292. doi: 10.1073/pnas.73.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Peters W. H., Jansen P. L., Nauta H. The molecular weights of UDP-glucuronyltransferase determined with radiation-inactivation analysis. A molecular model of bilirubin UDP-glucuronyltransferase. J Biol Chem. 1984 Oct 10;259(19):11701–11705. [PubMed] [Google Scholar]
- Puukka R., Tanner P., Hänninen O. Enzymes of the glucuronic acid pathway in wistar and gunn rats and their heterozygotes. Biochem Genet. 1973 Aug;9(4):343–349. doi: 10.1007/BF00486069. [DOI] [PubMed] [Google Scholar]
- Roy Chowdhury J., Roy Chowdhury N., Falany C. N., Tephly T. R., Arias I. M. Isolation and characterization of multiple forms of rat liver UDP-glucuronate glucuronosyltransferase. Biochem J. 1986 Feb 1;233(3):827–837. doi: 10.1042/bj2330827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy Chowdhury J., Roy Chowdhury N., Falany C. N., Tephly T. R., Arias I. M. Isolation and characterization of multiple forms of rat liver UDP-glucuronate glucuronosyltransferase. Biochem J. 1986 Feb 1;233(3):827–837. doi: 10.1042/bj2330827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHMID R., AXELROD J., HAMMAKER L., SWARM R. L. Congenital jaundice in rats, due to a defect in glucuronide formation. J Clin Invest. 1958 Aug;37(8):1123–1130. doi: 10.1172/JCI103702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson I., Greenwood D., McEwen J. Hepatic UDP-glucuronyltransferase in Wistar and Gunn rats--in vitro activation by diethylnitrosamine. Biochem Biophys Res Commun. 1968 Sep 6;32(5):866–872. doi: 10.1016/0006-291x(68)90321-5. [DOI] [PubMed] [Google Scholar]
- Tukey R. H., Billings R. E., Tephly T. R. Separation of oestrone UDP-glucuronyltransferase and p-nitrophenol UDP-glucuronyltransferase activities. Biochem J. 1978 Jun 1;171(3):659–663. doi: 10.1042/bj1710659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weatherill P. J., Burchell B. Reactivation of a pure defective UDP-glucuronyltransferase from homozygous Gunn rat liver. FEBS Lett. 1978 Mar 15;87(2):207–211. doi: 10.1016/0014-5793(78)80333-0. [DOI] [PubMed] [Google Scholar]
- Weatherill P. J., Burchell B. The separation and purification of rat liver UDP-glucuronyltransferase activities towards testosterone and oestrone. Biochem J. 1980 Aug 1;189(2):377–380. doi: 10.1042/bj1890377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wishart G. J. Functional heterogeneity of UDP-glucuronosyltransferase as indicated by its differential development and inducibility by glucocorticoids. Demonstration of two groups within the enzyme's activity towards twelve substrates. Biochem J. 1978 Aug 15;174(2):485–489. doi: 10.1042/bj1740485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolkoff A. W., Chowdhury J. R., Gartner L. A., Rose A. L., Biempica L., Giblin D. R., Fink D., Arias I. M. Clinical conference. Crigler-Najjar syndrome (type I) in an adult male. Gastroenterology. 1979 Apr;76(4):840–848. [PubMed] [Google Scholar]
- Zakim D. Biochemical foundations of preventive medicine: the study of abnormal enzymes. Horiz Biochem Biophys. 1974;1:97–137. [PubMed] [Google Scholar]