Abstract
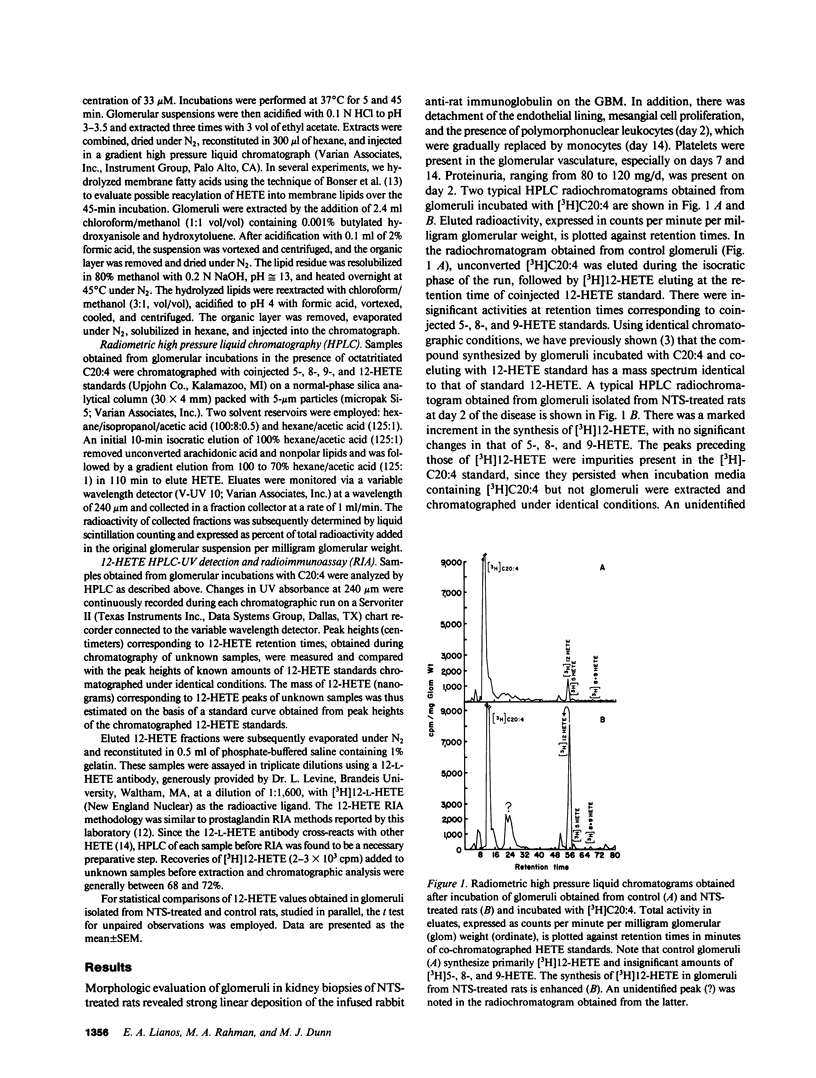
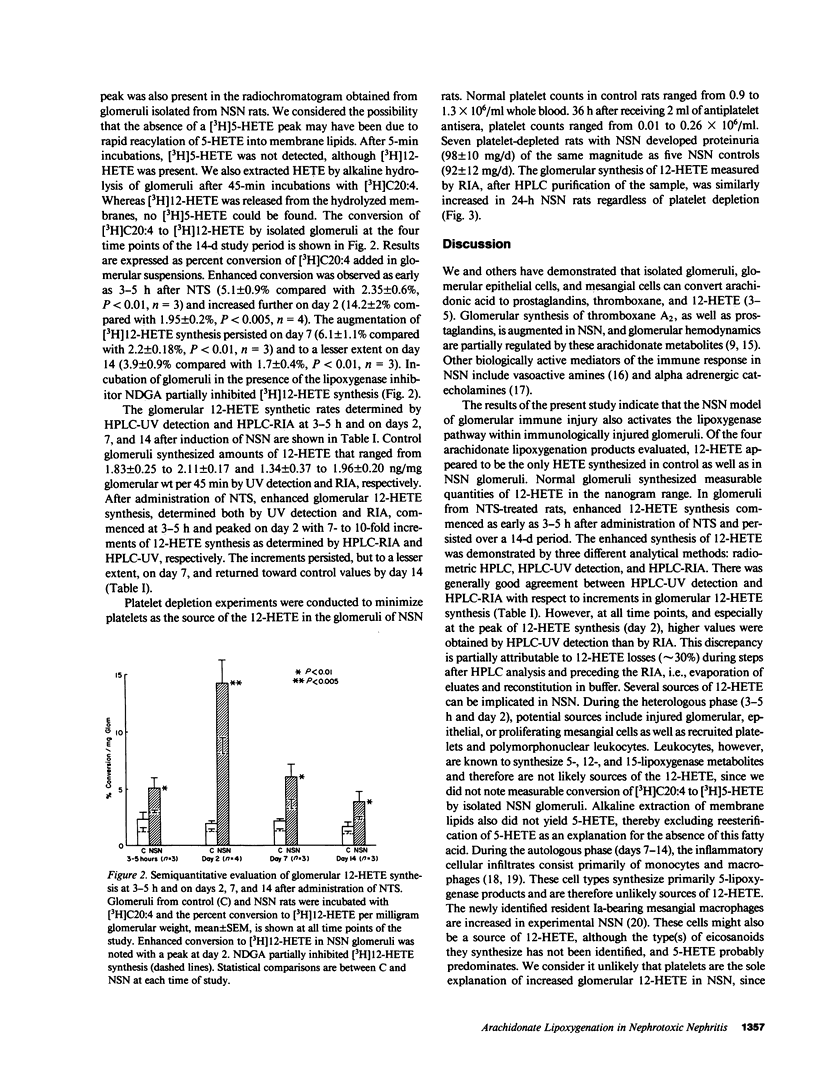
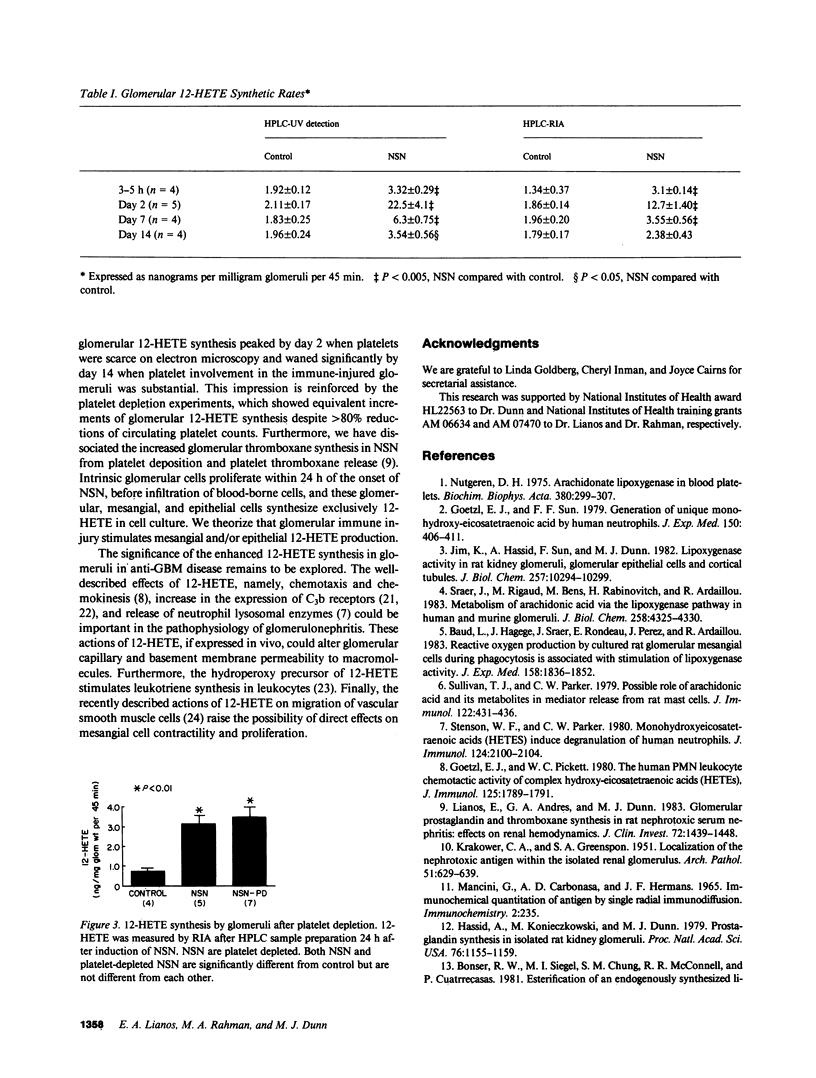
Arachidonate lipoxygenation to monohydroxylated eicosatetraenoic acids (HETE) was studied in rat nephrotoxic serum nephritis (NSN). A single infusion of nephrotoxic serum enhanced conversion of [3H]arachidonic acid ([3H]C20:4) to [3H]12-HETE in glomeruli isolated from nephritic rats compared with controls. The percent conversion of [3H]arachidonic acid was 1.95 +/- 0.2% in control glomeruli and 14.2 +/- 2% in nephritic glomeruli 2 d after induction of disease. No significant changes in the conversion of [3H]C20:4 to [3H]5-, 8-, and 9-HETE were noted. Extraction of glomerular HETE by alkaline hydrolysis, to evaluate possible reacylation of HETE after their production, confirmed the presence of 12-HETE and did not provide evidence of 5-HETE synthesis. Increased glomerular 12-HETE synthesis in nephritic rats was also demonstrated by high pressure liquid chromatography-UV detection and by 12-HETE radioimmunoassay. The enhanced glomerular 12-HETE synthesis commenced as early as 3-5 h after administration of nephrotoxic serum and peaked at day 2 with 10-fold enhancement of 12-HETE production. Increments of glomerular 12-HETE persisted on day 7 and returned toward control levels by day 14. Platelet depletion, induced by antiplatelet antisera, did not decrease glomerular 12-HETE synthesis in NSN, thereby eliminating platelets as the cellular origin of 12-HETE. Glomerular epithelial and mesangial cells are the most likely sources of enhanced 12-lipoxygenase activity. The enhanced arachidonate 12-lipoxygenation in glomerular immune injury could have important proinflammatory effects in the evolution of glomerulonephritis since 12-HETE has important effects on leukocyte function.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baud L., Hagege J., Sraer J., Rondeau E., Perez J., Ardaillou R. Reactive oxygen production by cultured rat glomerular mesangial cells during phagocytosis is associated with stimulation of lipoxygenase activity. J Exp Med. 1983 Dec 1;158(6):1836–1852. doi: 10.1084/jem.158.6.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blantz R. C., Tucker B. J., Gushwa L. C., Peterson O. W., Wilson C. B. Glomerular immune injury in the rat: the influence of angiotensin II and alpha-adrenergic inhibitors. Kidney Int. 1981 Oct;20(4):452–461. doi: 10.1038/ki.1981.161. [DOI] [PubMed] [Google Scholar]
- Bonser R. W., Siegel M. I., Chung S. M., McConnell R. T., Cuatrecasas P. Esterification of an endogenously synthesized lipoxygenase product into granulocyte cellular lipids. Biochemistry. 1981 Sep 1;20(18):5297–5301. doi: 10.1021/bi00521a032. [DOI] [PubMed] [Google Scholar]
- Dworkin L. D., Ichikawa I., Brenner B. M. Hormonal modulation of glomerular function. Am J Physiol. 1983 Feb;244(2):F95–104. doi: 10.1152/ajprenal.1983.244.2.F95. [DOI] [PubMed] [Google Scholar]
- Goetzl E. J., Pickett W. C. The human PMN leukocyte chemotactic activity of complex hydroxy-eicosatetraenoic acids (HETEs). J Immunol. 1980 Oct;125(4):1789–1791. [PubMed] [Google Scholar]
- Goetzl E. J., Sun F. F. Generation of unique mono-hydroxy-eicosatetraenoic acids from arachidonic acid by human neutrophils. J Exp Med. 1979 Aug 1;150(2):406–411. doi: 10.1084/jem.150.2.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J. G., Hopkins J., Reynolds J. Studies of efferent lymph cells from nodes stimulated with oxazolone. Immunology. 1980 Feb;39(2):141–149. [PMC free article] [PubMed] [Google Scholar]
- Hassid A., Konieczkowski M., Dunn M. J. Prostaglandin synthesis in isolated rat kidney glomeruli. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1155–1159. doi: 10.1073/pnas.76.3.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jim K., Hassid A., Sun F., Dunn M. J. Lipoxygenase activity in rat kidney glomeruli, glomerular epithelial cells, and cortical tubules. J Biol Chem. 1982 Sep 10;257(17):10294–10299. [PubMed] [Google Scholar]
- KRAKOWER C. A., GREENSPON S. A. Localization of the nephrotoxic antigen within the isolated renal glomerulus. AMA Arch Pathol. 1951 Jun;51(6):629–639. [PubMed] [Google Scholar]
- Kniker W. T., Cochrane C. G. The localization of circulating immune complexes in experimental serum sickness. The role of vasoactive amines and hydrodynamic forces. J Exp Med. 1968 Jan 1;127(1):119–136. doi: 10.1084/jem.127.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine L., Alam I., Gjika H., Carty T. J., Goetzl E. J. The development of a radioimmunoassay for 12-L-hydroxyeicosatetraenoic acid. Prostaglandins. 1980 Nov;20(5):923–934. doi: 10.1016/0090-6980(80)90142-2. [DOI] [PubMed] [Google Scholar]
- Lianos E. A., Andres G. A., Dunn M. J. Glomerular prostaglandin and thromboxane synthesis in rat nephrotoxic serum nephritis. Effects on renal hemodynamics. J Clin Invest. 1983 Oct;72(4):1439–1448. doi: 10.1172/JCI111100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maclouf J., de Laclos B. F., Borgeat P. Stimulation of leukotriene biosynthesis in human blood leukocytes by platelet-derived 12-hydroperoxy-icosatetraenoic acid. Proc Natl Acad Sci U S A. 1982 Oct;79(19):6042–6046. doi: 10.1073/pnas.79.19.6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Nakao J., Ooyama T., Chang W. C., Murota S., Orimo H. Platelets stimulate aortic smooth muscle cell migration in vitro. Involvement of 12-L-hydroxy-5,8,10,14-eicosatetraenoic acid. Atherosclerosis. 1982 Jun;43(2-3):143–150. doi: 10.1016/0021-9150(82)90018-1. [DOI] [PubMed] [Google Scholar]
- Nugteren D. H. Arachidonate lipoxygenase in blood platelets. Biochim Biophys Acta. 1975 Feb 20;380(2):299–307. doi: 10.1016/0005-2760(75)90016-8. [DOI] [PubMed] [Google Scholar]
- Schreiner G. F., Cotran R. S., Pardo V., Unanue E. R. A mononuclear cell component in experimental immunological glomerulonephritis. J Exp Med. 1978 Feb 1;147(2):369–384. doi: 10.1084/jem.147.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sraer J., Rigaud M., Bens M., Rabinovitch H., Ardaillou R. Metabolism of arachidonic acid via the lipoxygenase pathway in human and murine glomeruli. J Biol Chem. 1983 Apr 10;258(7):4325–4330. [PubMed] [Google Scholar]
- Stenson W. F., Parker C. W. Monohydroxyeicosatetraenoic acids (HETEs) induce degranulation of human neutrophils. J Immunol. 1980 May;124(5):2100–2104. [PubMed] [Google Scholar]
- Sterzel R. B., Pabst R. The temporal relationship between glomerular cell proliferation and monocyte infiltration in experimental glomerulonephritis. Virchows Arch B Cell Pathol Incl Mol Pathol. 1982;38(3):337–350. doi: 10.1007/BF02892829. [DOI] [PubMed] [Google Scholar]
- Sullivan T. J., Parker C. W. Possible role of arachidonic acid and its metabolites in mediator release from rat mast cells. J Immunol. 1979 Feb;122(2):431–436. [PubMed] [Google Scholar]


