Abstract
Zoonotic pathogens cause an estimated 70% of emerging and re-emerging infectious diseases in humans. In sub-Saharan Africa, bushmeat hunting and butchering is considered the primary risk factor for human-wildlife contact and zoonotic disease transmission, particularly for the transmission of simian retroviruses. However, hunting is only one of many activities in sub-Saharan Africa that bring people and wildlife into contact. Here, we examine human-animal interaction in western Uganda, identifying patterns of injuries from animals and contact with nonhuman primates. Additionally, we identify individual-level risk factors associated with contact. Nearly 20% (246/ 1,240) of participants reported either being injured by an animal or having contact with a primate over their lifetimes. The majority (51.7%) of injuries were dog bites that healed with no long term medical consequences. The majority (76.8%) of 125 total primate contacts involved touching a carcass; however, butchering (20%), hunting (10%), and touching a live primate (10%) were also reported. Red colobus (Piliocolobus rufomitratus tephrosceles) accounted for most primate contact events. Multivariate logistic regression indicated that men who live adjacent to forest fragments are at elevated risk of animal contact and specifically primate contact. Our results provide a useful comparison to West and Central Africa where “bushmeat hunting” is the predominant paradigm for human-wildlife contact and zoonotic disease transmission.
Introduction
Emerging infectious diseases from animals pose significant threats to human health on a global scale. Zoonotic agents cause an estimated 70% of emerging and re-emerging diseases in humans, with RNA viruses being particularly important (Jones et al., 2008). As humans and wildlife come into increasing contact, the risks of pathogen transmission increase in concert. In sub-Saharan Africa, bushmeat hunting and butchering is widely considered to be the primary risk factor for human-wildlife contact and zoonotic viral transmission (Peeters et al., 2002; Wolfe et al., 2004a; Locatelli and Peeters, 2012). Human immunodeficiency virus, the cause of AIDS, evolved from related viruses of nonhuman primates (“primates,” hereafter) that entered human populations through multiple zoonotic events as a result of bushmeat hunting and butchering in West and Central Africa (Sharp and Hahn, 2010; Locatelli and Peeters, 2012). In addition, other retroviruses have crossed into persons with primate contact in Africa, including simian foamy virus (SFV) and simian T-cell lymphotropic virus (Wolfe and Switzer, 2009). However, bushmeat hunting and butchering are part of a broader spectrum of activities in sub-Saharan Africa that bring people and animals into direct and potentially risky contact. For example, it has been recently shown that persons simply entering forests in the Democratic Republic of Congo, without known primate contact, are at increased risk for SFV infection (Switzer et al., 2012).
Although abundant data exist on levels of animal exposure and bushmeat hunting and consumption in West and Central Africa, including the potential for associated zoonotic pathogen transmission, very little is known about animal exposures and cross-species transmission risks in East Africa. To address this knowledge gap, we performed a community-based cross-sectional study to determine animal exposure risks in people surrounding Kibale National Park in western Uganda and nearby communities. The objective of our study was to identify and describe human-animal contact in the region and to assess individual risk factors associated with different types of animal contact.
Methods
Ethics statement
All research activities were approved by the Uganda Wildlife Authority and the Uganda National Council of Science and Technology, as well as by the Institutional Review Board (IRB) at the University of Wisconsin-Madison. Site-specific permission was secured through oral consent by local leaders. Local field staff obtained oral informed consent for voluntary individual participation using IRB-approved consent processes.
Study site
Kibale National Park (KNP) (0°13′–0°41′N, 30°19′–30°32′E) in western Uganda is 795-km2 with an altitude ranging from 1,110–1,590 meters. KNP is noteworthy for its high primate diversity and biomass (Struhsaker, 1997), as well as conflict between people living outside KNP and crop-raiding wildlife (Naughton-Treves, 1996; Hartter, 2009; MacKenzie and Ahabyona, 2012). The landscape surrounding KNP is a matrix of gardens, crop lands, tea fields, households, wetlands, and forest fragments (Naughton-Treves, 1997; Chapman et al., 2007; Goldberg et al., 2008a; Hartter and Southworth, 2009). In this environment, people and primates overlap and interact frequently (Goldberg et al., 2008a; Naughton-Treves, 1998, 1999; Onderdonk and Chapman, 2000). Cross-species transmission of infectious agents is frequent in this context (Salzer et al., 2007; Goldberg et al., 2008b; Johnston et al., 2010; Salyer et al., 2012; Hamer et al., 2013; Thurber et al., 2013). Given this situation, the recent identification of a surprising diversity of novel simian RNA viruses in primates in KNP (Goldberg et al., 2008c; Lauck et al., 2011, 2013a,b) is potentially of great concern.
Study sites were chosen to represent different primate habitats in the north of KNP. Forest fragment communities were located near small (0.5–3 km2) patches of remnant forest from which local residents extract forest resources, such as building materials, fuel wood, herbal medicines, and other forest products. Fragments also provide habitat for up to three primate species; (red colobus [Piliocolobus rufomitratus tephrosceles], black and white colobus [Colobus guerza], and red-tailed guenons [Cercopithecus ascanius]). Forest edge communities included households nearest to the boundary of KNP and/or whose domestic animals lived at the border. Control communities were 2–3 km from the park edge and thus served as a comparison group.
Participants
Recruitment and enrollment occurred at the household level. Once heads of household agreed to household participation, all household members were individually consented and enrolled. Responsible adults responded on behalf of children. Data were then collected at the level of the individual.
Within fragment communities, participants were identified by randomly selecting households across spatial strata. This process involved enumerating, mapping and censusing (to determine the number of persons per household) the residents of all households within 0.5 km of each forest fragment. Next, this set of eligible households was quartered by cardinal direction and household numbers were randomly selected. With each random selection, individuals were tallied. The random selection process was terminated when a minimum of 30 individuals per quarter were identified.
Identification and sampling of participants living in forest edge communities was also conducted at the household level. In each community along the north-western border of KNP, households nearest to the park boundary were recruited. Households were enumerated and mapped; five households per community were randomly selected and all individuals within the household were invited to participate.
For every five forest edge communities, one community 2–3 km from the KNP edge at the corresponding latitude was identified as a control community. Control communities were far from forest habitats and therefore served as a comparison group. Within each control community, five households were randomly selected and again, all individuals within selected households were invited to participate.
Data collection
Data collection included a household information survey and an animal contact survey. Household information included age, sex, cultural group and occupation of each household resident, as well as questions regarding domestic animal ownership. The animal contact survey was administered to for each participant who reported an injury from an animal or reported any primate contact. Animal contact surveys generated details of the event(s) primarily through open-ended questions, including: the age of the participant when the event occurred; the species involved; and a brief description of the event. In the case of a reported injury, questions were asked about the extent and bodily location of the injury; what treatment was sought, if any; and any long-term medical consequences.
Interviews were conducted in the local language (Rutooro) by trained, local field assistants. Data were manually recorded on paper forms. Participant interviews were conducted by one male and one female assistant. At the time of enrollment, subjects were informed that they would receive gifts for participation in the form of soap, water purification tablets, or ectoparasite treatment for their domestic animals, regardless of their survey responses.
Data Analysis
Data were coded into five categorical variables (sex, community type, cultural group, occupation, and dog ownership) and one continuous variable (age). Three binary response variables represented (1) any animal contact; (2) animal injury; and, (3) primate contact. ‘Any animal contact’ was an aggregated variable representing either animal injury or primate contact. We controlled for individuals with multiple reports by compiling all the cases of animal injury or primate contact and then removing multiple reports by individual participants. We repeated this process for animal injury and primate contact, so that we could generate a set of cases comprised of unique participants and their corresponding demographic information for each outcome.
Summary statistics were calculated for overall participant characteristics, for participant characteristics by type of contact, and for types of contact by species. We employed hierarchical logistic regression to identify individual-level risk factors for each of the three response variables. We controlled for within-community correlations by including community as a random effect. Predictor variables included age, sex, community type, cultural group, occupation, and dog ownership. Occupation was grouped into eight categories to reflect local, culturally-relevant classes of socioeconomic status. For example, “high social status” included retired elected leaders and esteemed elders but not necessarily people with high incomes, and “high wage” included participants who had management or leadership titles along with relatively high incomes. We included dog ownership as a predictor because our previous work suggested an important role of dogs in animal injuries and because dogs in the region often guard crops and hunt primates (Goldberg et al., 2008a 2012).
Due to low variance at the community level, we subsequently collapsed the hierarchical model to a simple fixed effects logistic regression model without the community-level random effect. Low variance at the community level indicates that differences in individual-level factors within each community are not extreme, thus justifying this more parsimonious model. We assessed goodness-of-fit for each model using area under the receiver operating characteristic (ROC) curve and the Hosmer-Lemeshow test. Important predictor variables were confirmed using the Akaike Information Criterion (AIC). We also fitted the logistic regression using penalized likelihood (glmnet package in R; Friedman et al. 2010); important variables are those present in the most heavily penalized models. Summary statistics were calculated in Excel (Redmond, WA) and all model calculations were conducted using R (R Core Development Team, 2013).
Results
Between 2008 and 2011, 1240 individuals from 38 communities were surveyed (Supplemental Table 1). The majority of participants lived in forest fragment communities (51.4%) while 37.6% lived along the edge of KNP and 13.2% lived in control communities. Approximately 48.1% were female, 85.9% were younger than 45 years, and 68.9% self-identified as Mutooro. Of the 1240 participants, 38.7% were in school and 30.1% were subsistence farmers. Unemployed residents comprised 21.4% of participants (the majority of which were age 5 years old or under). A slight majority of participants did not own dogs (51.8%).
High wage and high social status occupations were held by 2.2% of the sample population. These included salaried positions such as research assistants, tea factory managers, and teachers, as well as positions that convey high social status such as Local Council Chairmen (who are elected) and retired teachers or factory managers. Most people in these positions were male (74%) and were on average 42 years old. The majority were Mutooro (81.2%).
Within the control communities, a slight majority were male (53.7%) and the mean age was 19 years. Participants overwhelmingly identified as Mutooro (94.1%) and were primarily students (48.5%) and farmers (16.9%). High wage/high social status occupations were held by 2.9% of participants.
In forest edge communities, the slight majority of participants again were male (52.8%) and the mean age was 21 years old. Mutooro participants comprised 55.8% of respondents, and Mukiga followed at 38.2%. Similar to control communities, forest edge participants were often in school (45.3%) or were subsistence farmers (29.8%). The fewest high wage/high social status participants (1.9%) lived along the forest edge.
In forest fragment communities, 50.8% of participants were male. The mean age was 22 years. The majority of participants were Mutooro (73%) followed by Mukiga (17.1%). Compared to control and forest edge communities, fewer participants in forest fragment communities were in school (32.3%) and more participants were subsistence farmers (33.1%). 2.2% of participants with high wage/high social status occupations lived in forest fragment communities.
Summary of Participant Characteristics by Type of Animal Contact
Of the 1,240 participants, 13.2% reported an animal injury and 8.9% reported direct contact with primates. Forty-seven participants reported more than one injury or contact: 41 individuals reported two injuries/contacts, five individuals reported three injuries/contacts, and one individual reported five injuries/contacts. Overall, a total of 303 injury/contact events were reported.
The mean age for animal injury was 23 and the mean age for primate contact was 24 years. Male participants reported the majority (67.5%) of animal contacts, including both injuries and primate contact. Participants living in forest fragment communities reported the highest rates of all categories of animal contact. For example, 23.7% of participants living in forest fragment communities reported any animal contact, compared to 17.6% in forest edge communities and 9.6% in control communities. Participants with high social tier occupations reported the highest rate of animal injury (50%) and primate contact (33.3%). Participants who reported an occupation as “none” or “student” reported the lowest rates of animal injury (5.9% and 14.8% respectively) and primate contact (3.1% and 7.8% respectively) (Supplemental Table 2).
Different types of animal contact were associated with different demographic characteristics. For example, the modal age for “any animal contact” and “animal injury” was 10 years, but the modal age for “primate contact” was 20 years. Mukiga participants reported a slightly higher rate of any animal contact at 21.2%, followed by Munyankole at 20.4%. While Mutooro participants comprised the majority of our sample, only 19.4% reported any animal contact. Further, Mukiga reported the highest rate of primate contact (12.5%) along with the lowest rate of animal injury (10.8%). The rate for animal injury was slightly higher for participants who did not own dogs (13.6% as opposed to 12.9%) (Supplemental Table 2).
Summary of Types of Animal Contact
Participants also shared details of injuries from animals occurring at any point in their lifetimes. Dogs (Canis lupis familiaris) accounted for the majority (51.7%) of reported injuries, followed by cows (Bos taurus and Bos indicus and hybrids) at 14.4%. The most commonly reported animal injuries reported were bites (72.9%) and scratches (23.2%) (Supplemental Table 3). Of the 132 bites, dogs accounted for 62.9%, snakes accounted for 11.1%, and primates accounted for 2.3% (Fig. 1a).
Figure 1.
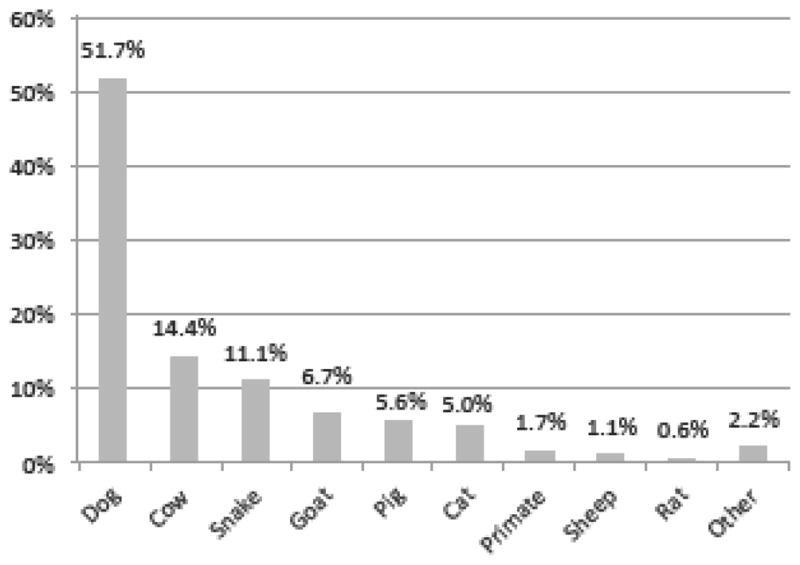
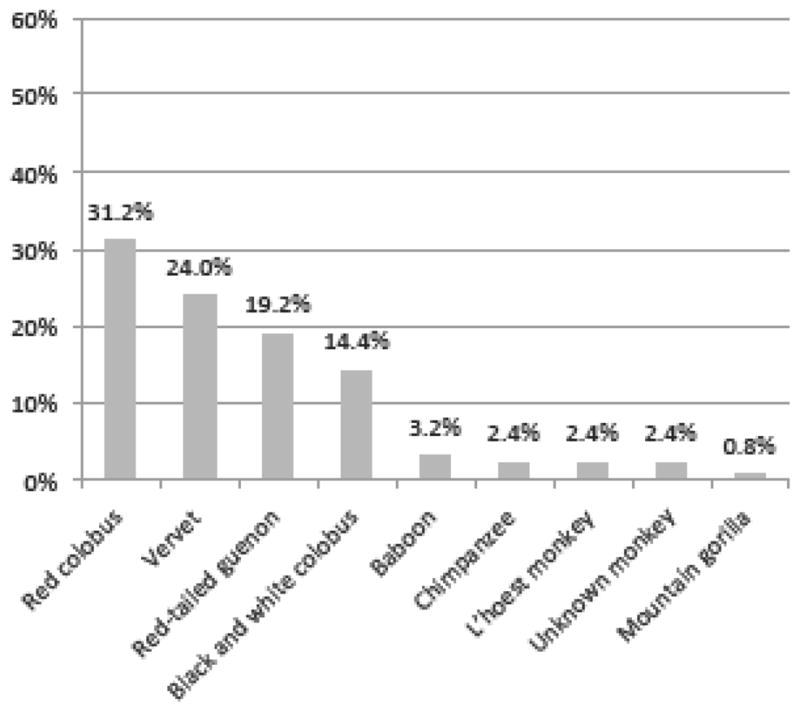
Figure 1a. Frequency of animals causing injuries around Kibale National Park. Timeframe covered participants’ lifetimes. Denominator is the number of injuries reported. (n=181)
Figure 1b. Frequency of primates involved in human-primate contact around Kibale National Park. Timeframe covered participants’ lifetimes. Denominator is the number of contacts reported. (n=125)
The majority of participants who reported an injury received medical treatment (57.8%) Treatment included: anti-rabies injections, antibiotics, pain medicines, and many ‘unknown’ medications and treatments from local hospitals and clinics. Local medicines were also used to treat injuries and included “blackstone,” tobacco, and sweet potato leaves. Although the majority (61%) of injuries healed, long term medical complaints included dizziness, impaired mobility, swelling, and chest pain.
Participants also reported species information for primate contacts during their lifetimes (Fig. 1b). Red colobus comprised the majority (31%) of the 125 reported contacts, followed by vervets (Chlorocebus aethiops, 24%), red-tailed guenons (19%), and black and white colobus (14%) (Fig. 1b). Rare contact with baboons (Papio anubis), chimpanzees (Pan troglodytes schweinfurthii), L’hoest monkeys (Cercopithecus lhoestii), and one mountain gorilla (Gorilla gorilla beringei) were also reported. Gorillas do not occur in KNP, but gorilla contact was reported by a former Uganda Wildlife Authority employee who had previously worked in Bwindi Impenetrable National Park in southwestern Uganda where habituated gorillas do occur.
The most common type of contact reported was touching a dead primate (60.8% of cases). Red colobus (31.6%), red-tailed guenon (26.3%), and vervet (23.7%) were the most frequently touched carcasses. Primate carcass butchering followed at 16% of cases, which is notable because carcass preparation is considered a high-risk activity for zoonotic pathogen exposure and transmission (Peeters et al., 2002; Wolfe et al., 2005; Wilkie, 2006). Vervets (40.0%) were the most frequently butchered primate. Only one female participant (out of 20) reported primate carcass preparation. Hunting was involved in 10.4% of contacts and 9.6% of contacts occurred with a live primate. Red colobus and black and white colobus were hunted with equal frequency (46.2% each); and red colobus (41.7%) and vervets (25%) were the most frequently primate touched while alive. The remaining 3.2% of “other” contacts (Table 4) included bites and being touched by a primate.
Individual Level Risk Factors
Logistic regression indicated that the odds of any animal contact were statistically significantly higher for males than females (OR = 2.53; 95% confidence interval 1.82–3.53). Residents of fragment communities had statistically higher odds of any animal contact relative to control communities (OR= 3.08; 95% confidence interval 1.67–6.12; (Table 1). Age was also statistically significant, with a one-year increase in age increasing the odds of an animal contact by 2% (95% confidence interval 1.02–1.04). Occupation was also significant; subsistence farmers had higher odds of any animal contact relative to participants with “no occupation” (OR = 4.54; 95% confidence interval 2.50–9.09). Age, sex, occupation, and community type were confirmed as important variables by AIC and glmnet analysis. Cultural group and dog ownership were not significantly associated with contact in univariate or multivariate analyses.
Table 1.
Multiple logistic regression results identifying individual-level risk factors for animal contact, animal injury and primate contact in communities around Kibale National Park, Uganda.
| Any Animal Contact | Animal Injury | Primate Contact | ||||
|---|---|---|---|---|---|---|
| Predictor Variable | Odds Ratio (CI) | p-value | Odds Ratio (CI) | p-value | Odds Ratio (CI) | p-value |
| Intercept | 0.04 (0.02–0.09) | <0.0001 | 0.07 (0.03–0.15) | <0.0001 | 0.01 (0.001–0.02) | <0.0001 |
| Current Age | ||||||
| Age in years | 1.02 (1.02–1.04) | <0.0001 | 1.02 (1.02–1.04) | <0.0001 | 1.01 (1.0–1.02) | 0.12184 |
| Sex | ||||||
| Female | ref | ref | ref | |||
| Male | 2.53 (1.82–3.53) | <0.0001 | 1.95 (1.34–2.86) | 0.000517 | 3.57 (2.22–5.96) | <0.0001 |
| Community | ||||||
| Control | ref | ref | ref | |||
| Forest Edge | 1.87 (0.99–3.81) | 0.06628 | 1.38 (0.70–2.90) | 0.370523 | 2.55 (0.84–11.10) | 0.14148 |
| Forest Fragment | 3.08 (1.67–6.12) | 0.00062 | 1.71 (0.99–3.50) | 0.120771 | 6.53 (2.28–27.73) | 0.00235 |
| Cultural Group | ||||||
| Mutooro | ref | ref | ref | |||
| Mukiga | 1.01 (0.69–1.46) | 0.97134 | 0.60 (0.37–0.95) | 0.032420 | 1.93 (1.18–3.13) | 0.00781 |
| Munyankole | 1.07 (0.46–2.34) | 0.86538 | 1.23 (0.50–2.78) | 0.628027 | 1.56 (0.44–4.38) | 0.43627 |
| Other | 1.41 (0.58–3.14) | 0.42439 | 1.23 (0.43–3.02) | 0.67445 | 1.56 (0.43–4.35) | 0.44181 |
| Occupation | ||||||
| Farmer | ref | ref | ref | |||
| Student | 0.74 (0.47–1.16) | 0.19377 | 0.52 (0.31–0.88) | 0.015699 | 0.98 (0.52–1.84) | 0.93794 |
| Low Wage | 1.43 (0.68–2.97) | 0.33871 | 0.97 (0.42–2.11) | 0.940889 | 1.55 (0.56–3.84) | 0.36390 |
| None | 0.22 (0.11–0.40) | <0.0001 | 0.18 (0.08–0.37) | <0.0001 | 0.30 (0.12–0.69) | 0.00747 |
| Tradesman | 0.57 (0.26–1.21) | 0.15868 | 0.40 (0.14–0.96) | 0.056794 | 0.97 (0.34–2.42) | 0.94625 |
| Business | 1.37 (0.52–3.48) | 0.51467 | 0.91 (0.31–2.43) | 0.862077 | 2.22 (0.58–7.03) | 0.20014 |
| High Wage | 1.33 (0.48–3.52) | 0.56716 | 0.65 (0.18–1.88) | 0.458534 | 2.78 (0.80–8.49) | 0.08405 |
| High Social Tier | 1.55 (0.28–11.85) | 0.62962 | 1.28 (0.22–7.60) | 0.773898 | 1.71 (0.22–9.59) | 0.55682 |
| Dog Ownership | ||||||
| No | ref | ref | ref | |||
| Yes | 1.06 (0.78–1.45) | 0.69635 | 0.92 (0.64–1.32) | 0.656638 | 1.54 (1.01–2.36) | 0.04696 |
Statistically significant individual risk factors for animal injury were sex, age, cultural group, and occupation (Table 1). The odds of males reporting an animal injury were significantly higher than for females (OR = 1.95; 95% confidence interval 1.34 – 2.86). A one-year increase in age increased the odds of an animal contact by 2% (95% confidence interval 1.02 – 1.04). The odds of Mutooro participants reporting animal injury were significantly higher relative to Mukiga participants, (OR = 1.67; 95% confidence interval 1.05–2.7). Subsistence farmers had significantly higher odds of injury relative to those who reported “no occupation” (OR 5.56, 95% confidence interval (OR = 5.46; 95% confidence interval 2.7–12.38).
Sex, community, cultural group, and occupation were significantly associated with primate contact (Table 1). The odds of a male having contact with a primate was more than three times higher than that for a female (OR = 3.57; 95% confidence interval 2.22–5.96). For residents of forest fragment communities, the odds of reporting primate contact was over six times higher than for residents in control communities (OR= 6.53; 95% confidence interval 2.28–27.73). The odds of a Mukiga participant reporting primate contact was significantly higher than for a Mutooro participant (OR = 1.93; 95% confidence interval1.18–3.13). Subsistence farmers had significantly higher odds of primate contact relative to participants with “no occupation” (OR = 3.33; 95% confidence interval 1.45–0.69).
All three models were evaluated for goodness-of-fit and to confirm significant variables, without adjustment for multiple testing. Penalized likelihood methods resulted in the same model as AIC in which age, sex, and community type were the most important predictor variables for each response. The regression model that calculated odds of primate contact had the smallest ROC value at 0.7498, indicating that the primate contact model was the strongest of all three models. In each model, the Hosmer-Lemeshow goodness-of-fit test resulted in non-significant p-values, indicating that the models were adequate fits for the observed data.
Discussion
We identified a variety of contact scenarios that could result in exposure to infectious agents from a wide range of animals, including several primate species known to harbor potentially zoonotic pathogens. These findings have implications for zoonotic disease surveillance. Human populations traditionally characterized as “at risk” have been those in such places as West Africa, where hunting and pet-keeping are widespread and highly visible activities (Daszak, 2006). Results from our study challenge current assumptions about where, when, and under what conditions these types of activities occur. Specifically, our results expand the “bushmeat paradigm” by demonstrating that “risky contact” occurs even in settings where bushmeat hunting does not commonly occur, such as where people and wildlife occupy mosaic landscapes of farms and fragmented forests and where human-wildlife interaction is generally indirect or incidental (Goldberg et al., 2012)
Further, our results highlight characteristics of individuals in the region who are at greatest risk of contact, and presumably exposure to zoonoses. For example, both location and social status were significant predictors of risky contact. Overall, males living near forest habitats were at highest risk, and this was especially true for participants who self-identified as Mukiga. Local prevention programs should therefore include Mukiga males living at the forest edge or near forest fragments. Such individuals would presumably represent the most likely points of entry for zoonotic pathogens into the local human population.
Our results were surprising in that nearly one fifth of the study population experienced an animal injury or reported primate contact. As previously described, male participants living in forest fragment communities consistently had the highest rates of animal injury and primate contact. Being male, increasing age, and living in a forest fragment community were significant risk factors for animal and primate contact, including percutaneous animal injuries, by several analyses. It is notable that the dominant, resident cultural group, Mutooro, had the lowest rate of primate contact while the second most common cultural group, Mukiga (recent migrants to the area from the southwest region of Uganda) had the highest rate of primate contact. This finding could reflect cultural differences between the groups, or livelihood pressures faced by recent immigrants.
Most animal injuries were dog bites without serious long-term medical consequences. However, given the widespread lack of rabies vaccination, we cannot discount the possibility that fatal dog bites were not reported and that our data were biased in this regard. Snakebite was also a commonly reported animal injury, which is not surprising considering the occurrence of several venomous snakes in the region (Vonesh, 2001). Most primate contact occurred by touching and/or butchering carcasses, but contact with live primates was also reported.
Our results provide an informative comparison to those from previous studies of human-animal contact in West and Central Africa. For example, Wolfe et al. (2004a) found that up to 90% of individuals in “high risk” populations in Cameroon reported primate contact, including hunting, butchering, pet ownership, and consumption. Butchering was the most common activity associated with direct contact with primate blood and bodily fluids and was found to place women at greatest risk of zoonoses (Wolfe et al. 2004a,b). LeBreton et al. (2007) further explored animal exposure in Cameroon by focusing on individuals who tested positive for HIV. Almost all participants reported consuming wildlife, with 80% consuming primates (LeBreton et al. 2007). Butchering practices were also common; 80% reported butchering wildlife and 60% reported butchering primates.
A recent study in the Democratic Republic of Congo by Switzer et al. (2012) also investigated associations between human-animal contact and the risk of zoonotic infections. Instead of focusing on high-risk populations who hunted and butchered primates, persons from two health zones in central Democratic Republic of Congo were randomly sampled and tested for SFV infection. Participants reported high rates of direct contact with primates, primarily through consumption (79%) and/or butchering (42%). Zoonotic SFV infection was detected in 0.5% (n=16) of the study population. Interestingly, two of the 16 SFV-positive individuals (12.5%) reported no direct contact with primates, but instead reported simply entering nearby forests where primates live. These results suggest that contact with body fluids deposited in the forest may also place persons at risk for exposure and infection with simian retroviruses.
Herein, we document far lower rates of butchering (1.6%) and hunting (1.1%) of primates than the aforementioned studies. Unlike in West and Central Africa, participants in our study did not report eating primates; rather, primates are primarily butchered to feed to dogs (Goldberg et al, 2012). Furthermore, in contrast to studies in West and Central Africa, women reported infrequent butchering of primate carcasses. However, our results nevertheless demonstrated an abundance of “risky” animal contacts unrelated to hunting, in this setting dominated by men. Although overall rates of primate exposure in Uganda were lower than those found in West and Central Africa, the high human and primate population density in the area may be cause for concern, especially given findings such as those by Switzer et al. (2012) suggesting that merely entering primate habitats can facilitate zoonotic retroviral transmission. The potential for zoonotic infection may thus impact residents living near primate habitats who do not engage in practices that would be considered risky according to the “bushmeat paradigm.” People living in fragment communities rely on resources collected from inside the fragment to support their subsistence livelihoods and as a buffer for economic uncertainty (Naughton-Treves 2011). Therefore, the role of the forest fragment is critical for their well-being and livelihood, but may simultaneously place individuals at risk for zoonotic infections.
Overall, our findings expand our understanding of risky human-animal contact beyond the “bushmeat paradigm” that has emerged as a result of research focused on West and Central Africa. Forest fragmentation that characterizes our study site is a rapidly increasing global phenomenon (Marsh and Chapman, 2013). Our results indicate that mosaic landscapes of forest fragments and agricultural fields are important settings for human-animal contact and zoonotic disease transmission risk. Future assessments of zoonotic disease risk from directly-transmitted pathogens such as simian retroviruses should consider these increasingly common ecological environments.
Conclusion
We document human-animal contact in one-fifth of a study population in western Uganda, with men at higher risk for animal injury and primate contact than women, with people living near forest habitats more likely to report primate contact than those living away from forest habitats, and with people living near forest fragments at highest overall risk. Our findings differ from similar studies in West and Central Africa, in that persons in our study reported more frequent primate contact by touching a carcass than by butchering a carcass or consuming primates. Our data also show that cultural factors such as social status and cultural group membership can significantly affect the risk of animal contact, primate contact, and injury. Future assessments of zoonotic disease risk and future public health intervention programs should consider that “risky” contact with wildlife occurs not only in forests where bushmeat hunting routinely occurs, but also in mosaic landscapes of forest fragments and agricultural fields, which are becoming increasingly common worldwide.
Supplementary Material
Contributor Information
Sarah B. Paige, University of Wisconsin – Madison, Department of Pathobiological Sciences
Simon D.W. Frost, University of Cambridge, Department of Veterinary Medicine
Mhairi A. Gibson, University of Bristol, Department of Archaeology and Anthropology
James Holland, Jones Stanford University, Department of Anthropology, Woods Institute for the Environment.
Anupama Shankar, Division of HIV/AIDS Prevention, Centers for Disease Control and Prevention.
William M. Switzer, Division of HIV/AIDS Prevention, Centers for Disease Control and Prevention
Nelson Ting, Department of Anthropology, University of Oregon.
References
- Chapman C, Naughton-Treves L, Lawes M, Wasserman M, Gillespie T. Population Declines of Colobus in Western Uganda and Conservation Value of Forest Fragments. International Journal of Primatology. 2007;28:513–528. [Google Scholar]
- Daszak P. Risky behavior in the Ebola zone. Animal Conservation. 2006;9:366–367. doi: 10.1111/j.1469-1795.2006.00070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman J, Hastie T, Tibshirani R. Regularization Paths for Generalized Linear Models via Coordinate Descent. Journal of Statistical Software. 2010;33:1–22. [PMC free article] [PubMed] [Google Scholar]
- Goldberg TL, Gillespie TR, Rwego IB. Health and disease in the people, primates and domestic animals of Kibale National Park: Implications for conservation. In: Wrangham R, Ross E, editors. Science and Conservation in African Forests: The Benefits of Long-Term Research. Cambridge: Cambridge University Press; 2008a. pp. 75–87. [Google Scholar]
- Goldberg TL, Gillespie TR, Rwego IB, Estoff EL, Chapman CA. Forest fragmentation as cause of bacterial transmission among nonhuman primates, humans, and livestock, Uganda. Emerging Infectious Diseases. 2008b;14:1375–1382. doi: 10.3201/eid1409.071196. [Online September 2008] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg TL, Chapman CA, Cameron K, Saj T, Karesh WB, Wolfe N, et al. Serologic evidence for novel poxvius in endangered red colobus monkeys, western Uganda. Emerging Infectious Diseases. 2008c;14:801–803. doi: 10.3201/eid1405.071686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg TL, Paige S, Chapman CA. The Kibale EcoHealth Project: exploring connections among human health, animal health, and landscape dynamics in western Uganda. In: Aguirre AA, Daszak P, Ostfeld RS, editors. Conservation Medicine: Applied Cases of Ecosystem Health. New York: Oxford University Press; 2012. pp. 452–465. [Google Scholar]
- Hamer SA, Bernard AB, Donovan RM, Hartel JA, Wrangham RW, Otali E, et al. Coincident tick infestations in the nostrils of wild chimpanzees and a human in Uganda. American Journal of Tropical Medicine and Hygiene. 2013;89:924–927. doi: 10.4269/ajtmh.13-0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartter J. Attitudes of rural communities toward wetlands and forest fragments around Kibale National Park, Uganda. Human Dimensions of Wildlife. 2009;14:433–447. [Google Scholar]
- Hartter J, Southworth J. Dwindling resources and fragmentation of landscapes around parks: wetlands and forest patches around Kibale National Park, Uganda. Landscape Ecology. 2009;24:643–656. [Google Scholar]
- Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL, et al. Global trends in emerging infectious diseases. Nature. 2008;451:990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston AR, Gillespie TR, Rwego IB, Tranby McLachlan TL, Kent AD, Goldberg TL. Molecular epidemiology of cross-species Giardia duodenalis transmission in western Uganda. PLoS Neglected Tropical Diseases. 2010;4:e683. doi: 10.1371/journal.pntd.0000683. [Online May 11, 2010] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauck M, Hyeroba D, Tumukunde A, Weny G, Lank SM, Chapman CA, et al. Novel, divergent simian hemorrhagic fever viruses in a wild Ugandan red colobus monkey discovered using direct pyrosequencing. PLoS ONE. 2011;6:e19056. doi: 10.1371/journal.pone.0019056. [Online April 22, 2011] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauck M, Switzer WM, Sibley SD, Hyeroba D, Tumukunde A, Weny G, et al. Discovery and full genome characterization of two highly divergent simian immunodeficiency viruses infecting black-and-white colobus monkeys (Colobus guereza) in Kibale National Park, Uganda. Retrovirology. 2013a doi: 10.1186/1742-4690-10-107. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauck M, Sibley SD, Lara J, Purdy MA, Khudyakov Y, Hyeroba D, et al. A novel hepacivirus with an unusually long and intrinsically disordered NS5A protein in a wild Old World primate. Journal of Virolology. 2013b;87:8971–8981. doi: 10.1128/JVI.00888-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebreton M, Prosser AT, Tamoufe U, Sateren W, Mpoudi-Ngole E, Diffo JLD, et al. Patterns of bushmeat hunting and perceptions of disease risk among central African communities. Animal Conservation. 2006;9:495–495. [Google Scholar]
- Locatelli S, Peeters M. Cross-species transmission of simian retroviruses: how and why they could lead to the emergence of new diseases in the human population. AIDS. 2012;26:659–73. doi: 10.1097/QAD.0b013e328350fb68. [DOI] [PubMed] [Google Scholar]
- MacKenzie CA, Ahabyona P. Elephants in the garden: Financial and social costs of crop raiding. Ecological Economics. 2012;75:72–82. [Google Scholar]
- Marsh L, Chapman C. Primates in fragments: Complexity and resilience. New York: Springer; 2013. [Google Scholar]
- Naughton-Treves L. Dissertation Thesis. University of Florida; Gainesville: 1996. Uneasy neighbors: wildlife and farmers around Kibale National Park, Uganda. [Google Scholar]
- Naughton-Treves L. Farming the forest edge: Vulnerable places and people around Kibale National Park, Uganda. Geographical Review. 1997;87:27–46. [Google Scholar]
- Naughton-Treves L. Predicting patterns of crop damage by wildlife around Kibale National Park, Uganda. Conservation Biology. 1998;12:156–168. [Google Scholar]
- Naughton-Treves L. Whose Animals? A history of property rights to wildlife in Toro, Western Uganda. Land Degradation and Development. 1999;10:311–328. [Google Scholar]
- Naughton-Treves N, Alix-Garcia J, Chapman CA. Lessons about parks and poverty from a decade of forest loss and economic growth around Kibale National Park, Uganda. Proceedings of the National Academy of Sciences. 2011;108:13919–13924. doi: 10.1073/pnas.1013332108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onderdonk DA, Chapman CA. Coping with forest fragmentation: The primates of Kibale National Park, Uganda. International Journal of Primatology. 2000;21:587–612. [Google Scholar]
- Peeters M, Courgnaud V, Abela B, Auzel P, Pourrut X, Bibollet-Ruche F, et al. Risk to human health from a plethora of simian immunodeficiency viruses in primate bushmeat. Emerging Infectious Diseases. 2002;8:451–457. doi: 10.3201/eid0805.01-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Development Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2013. http://www.R-project.org. [Google Scholar]
- Salyer SJ, Gillespie TR, Rwego IB, Chapman CA, Goldberg TL. Epidemiology and molecular relationships of Cryptosporidium spp. in people, primates, and livestock from Western Uganda. PLoS Neglected Tropical Diseases. 2012;6:e1597. doi: 10.1371/journal.pntd.0001597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzer JS, Rwego IB, Goldberg TL, Kuhlenschmidt MS, Gillespie TR. Giardia sp. and Cryptosporidium sp. infections in primates in fragmented and undisturbed forest in Western Uganda. Journal of Parasitology. 2007;93:439–440. doi: 10.1645/GE-970R1.1. [DOI] [PubMed] [Google Scholar]
- Sharp P, Hahn B. Origins of HIV and the AIDS pandemic. Cold Springs Harbor Perspectives in Medicine. 2011;1:1–22. doi: 10.1101/cshperspect.a006841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhsaker TT. Ecology of an African rain forest : logging in Kibale and the conflict between conservation and exploitation. Gainesville: University Press of Florida; 1997. [Google Scholar]
- Switzer WM, Tang S, Ahuka-Mundeke S, Shankar A, Hanson DL, Zheng H, et al. Novel simian foamy virus infections from multiple monkey species in women from the Democratic Republic of Congo. Retrovirology. 2012;9:100. doi: 10.1186/1742-4690-9-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurber MI, Ghai RR, Hyeroba D, Weny G, Tumukunde A, Chapman CA, et al. Co-infection and cross-species transmission of divergent Hepatocystis lineages in a wild African primate community. International Journal of Parasitology. 2013;43:613–619. doi: 10.1016/j.ijpara.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonesh JR. Natural history and biogeography of the amphibians and reptiles of Kibale National Park, Uganda. [accessed December 10, 2013];Contemporary Herpetology. 2001 :4. Available from http://www.cnah.org/ch/ch/2001/2004/index.htm.
- Wolfe ND, Prosser AT, Carr JK, Tamoufe U, Mpoudi-Ngole E, Torimiro JN, et al. Exposure to nonhuman primates in rural Cameroon. Emerging Infectious Diseases. 2004a;10:2094–2099. doi: 10.3201/eid1012.040062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe ND, Switzer WM, Carr JK, Bhullar VB, Shanmugam V, Tamoufe U, et al. Naturally-acquired simian retrovirus infections in central African hunters. Lancet. 2004b;363:932–937. doi: 10.1016/S0140-6736(04)15787-5. [DOI] [PubMed] [Google Scholar]
- Wolfe ND, Switzer WM. Primate Exposure and the Emergence of Novel Retroviruses. In: Huffman MA, Chapman CA, editors. Primate Parasite Ecology: The Dynamics and Study of Host-Parasite Relationships. Cambridge: Cambridge University Press; 2009. pp. 353–70. Cambridge Studies in Biological and Evolutionary Anthropology. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


