Abstract
Two approaches were used to demonstrate that reduction in serum opsonization of Streptococcus pneumoniae via the alternative complement pathway in children with sickle cell disease is related to a deficiency of antibodies to pneumococcal capsular polysaccharide. First, opsonization of S. pneumoniae mediated by the alternative pathway in patients' sera was restored to normal by addition of the purified IgG or IgM fraction of goat antiserum to capsular polysaccharide of the homologous serotype. Secondly, IgG antibody titers to capsular polysaccharide in patients' sera correlated significantly with alternative pathway-mediated opsonization; the correlation between titers of IgM anticapsular antibodies and opsonization approached statistical significance. The sum of the IgG and IgM anticapsular antibody titers correlated most significantly with opsonization. Our results suggest that reduction in alternative pathway-mediated opsonization in sera from children with sickle cell disease is related to low levels of both IgG and IgM anticapsular antibodies.
Full text
PDF


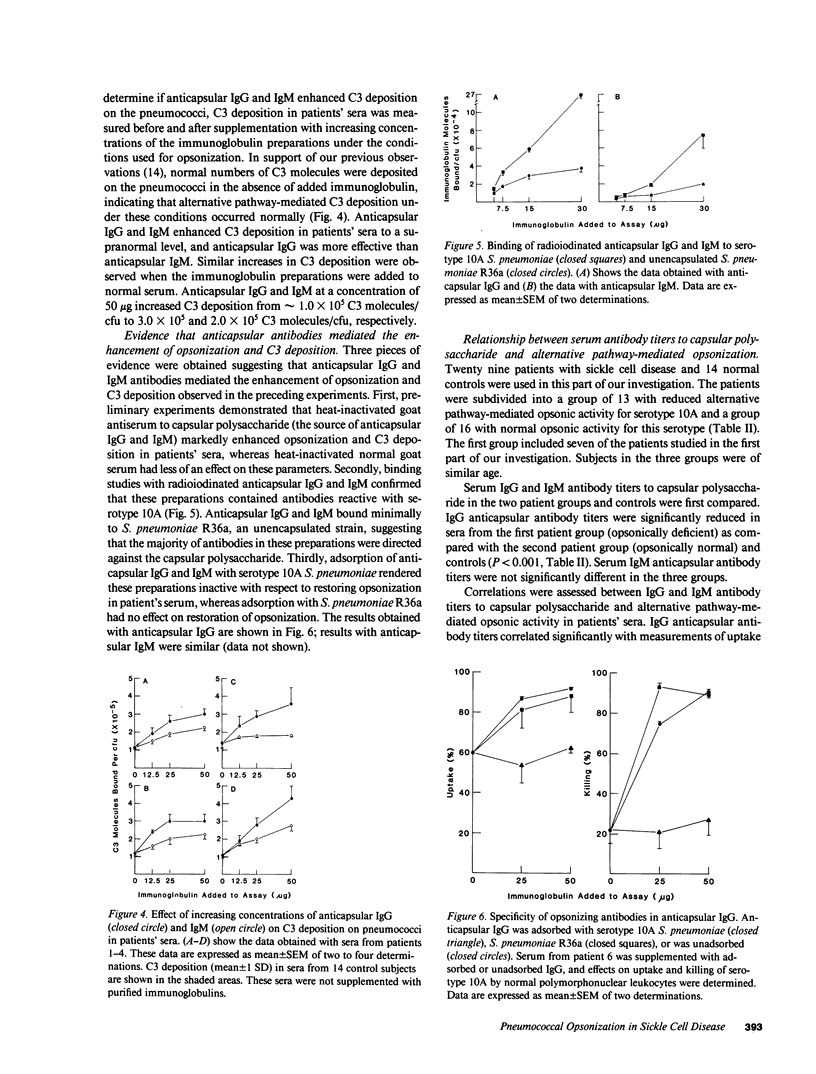
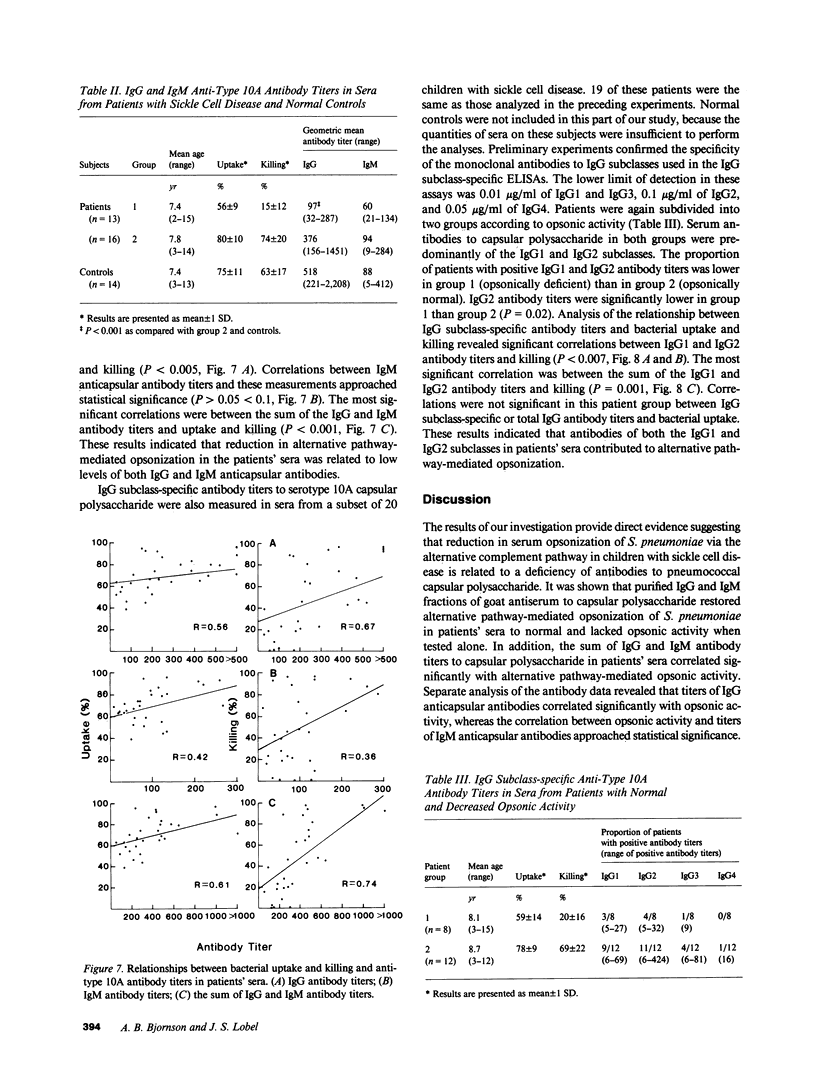
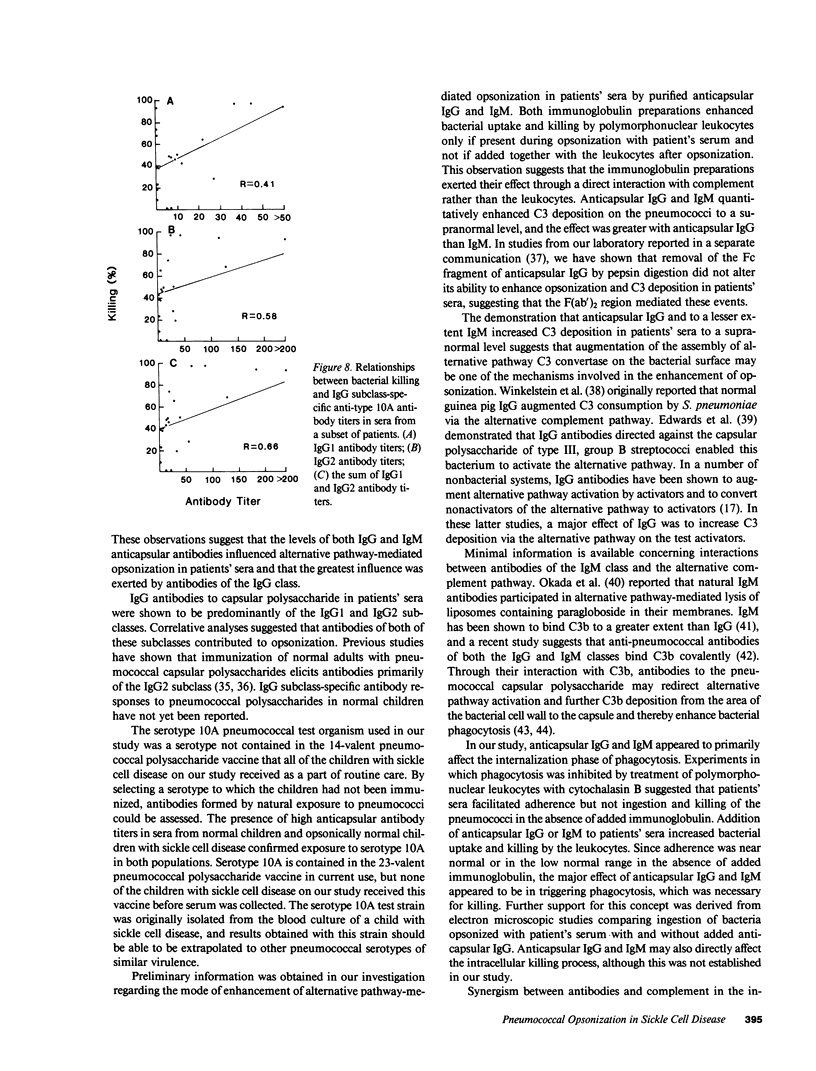



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaberge I. S., Heier H. E., Hem E., Giercksky K. E., Groeng E. C. IgM and IgG response to pneumococcal polysaccharide vaccine in normal individuals and individuals splenectomized due to trauma. Acta Pathol Microbiol Immunol Scand C. 1984 Feb;92(1):11–16. doi: 10.1111/j.1699-0463.1984.tb00046.x. [DOI] [PubMed] [Google Scholar]
- Ambrosino D. M., Schiffman G., Gotschlich E. C., Schur P. H., Rosenberg G. A., DeLange G. G., van Loghem E., Siber G. R. Correlation between G2m(n) immunoglobulin allotype and human antibody response and susceptibility to polysaccharide encapsulated bacteria. J Clin Invest. 1985 Jun;75(6):1935–1942. doi: 10.1172/JCI111909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett-Connor E. Bacterial infection and sickle cell anemia. An analysis of 250 infections in 166 patients and a review of the literature. Medicine (Baltimore) 1971 Mar;50(2):97–112. [PubMed] [Google Scholar]
- Barrett D. J., Ammann A. J. Pneumococcal vaccine in sickle cell disease: IgG and IgM antibody response. Rev Infect Dis. 1981 Mar-Apr;3 (Suppl):S179–S182. doi: 10.1093/clinids/3.supplement_1.s179. [DOI] [PubMed] [Google Scholar]
- Barrett D. J., Ammann A. J., Stenmark S., Wara D. W. Immunoglobulin G and M antibodies to pneumococcal polysaccharides detected by enzyme-linked immunosorbent assay. Infect Immun. 1980 Feb;27(2):411–417. doi: 10.1128/iai.27.2.411-417.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett D. J., Ayoub E. M. IgG2 subclass restriction of antibody to pneumococcal polysaccharides. Clin Exp Immunol. 1986 Jan;63(1):127–134. [PMC free article] [PubMed] [Google Scholar]
- Barrett D. J., Lee C. G., Ammann A. J., Ayoub E. M. IgG and IgM pneumococcal polysaccharide antibody responses in infants. Pediatr Res. 1984 Nov;18(11):1067–1071. doi: 10.1203/00006450-198411000-00001. [DOI] [PubMed] [Google Scholar]
- Bjornson A. B., Gaston M. H., Zellner C. L. Decreased opsonization for Streptococcus pneumoniae in sickle cell disease: studies on selected complement components and immunoglobulins. J Pediatr. 1977 Sep;91(3):371–378. doi: 10.1016/s0022-3476(77)81303-6. [DOI] [PubMed] [Google Scholar]
- Bjornson A. B., Lobel J. S., Harr K. S. Relation between serum opsonic activity for Streptococcus pneumoniae and complement function in sickle cell disease. J Infect Dis. 1985 Oct;152(4):701–709. doi: 10.1093/infdis/152.4.701. [DOI] [PubMed] [Google Scholar]
- Bjornson A. B., Lobel J. S. Lack of a requirement for the Fc region of IgG in restoring pneumococcal opsonization via the alternative complement pathway in sickle cell disease. J Infect Dis. 1986 Nov;154(5):760–769. doi: 10.1093/infdis/154.5.760. [DOI] [PubMed] [Google Scholar]
- Bjornson A. B., Lobel J. S., Lampkin B. C. Humoral components of host defense in sickle cell disease during painful crisis and asymptomatic periods. J Pediatr. 1980 Feb;96(2):259–262. doi: 10.1016/s0022-3476(80)80818-3. [DOI] [PubMed] [Google Scholar]
- Bjornson A. B., Lobel J. S., Magnafichi P. I., Lampkin B. C. Restoration by normal human immunoglobulin G of deficient serum opsonization for Streptococcus pneumoniae in sickle cell disease. Infect Immun. 1981 Aug;33(2):636–640. doi: 10.1128/iai.33.2.636-640.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch E. F., Castro O., Gregory J. E., Okoh C. Opsonization of pneumococci by whole serum from sickle cell disease patients. J Natl Med Assoc. 1984 Feb;76(2):179–182. [PMC free article] [PubMed] [Google Scholar]
- Brown E. J., Berger M., Joiner K. A., Frank M. M. Classical complement pathway activation by antipneumococcal antibodies leads to covalent binding of C3b to antibody molecules. Infect Immun. 1983 Nov;42(2):594–598. doi: 10.1128/iai.42.2.594-598.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E. J., Hosea S. W., Hammer C. H., Burch C. G., Frank M. M. A quantitative analysis of the interactions of antipneumococcal antibody and complement in experimental pneumococcal bacteremia. J Clin Invest. 1982 Jan;69(1):85–98. doi: 10.1172/JCI110444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E. J., Joiner K. A., Cole R. M., Berger M. Localization of complement component 3 on Streptococcus pneumoniae: anti-capsular antibody causes complement deposition on the pneumococcal capsule. Infect Immun. 1983 Jan;39(1):403–409. doi: 10.1128/iai.39.1.403-409.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capel P. J., Groeneboer O., Grosveld G., Pondman K. W. The binding of activated C3 to polysaccharides and immunoglobulins. J Immunol. 1978 Dec;121(6):2566–2572. [PubMed] [Google Scholar]
- Chudwin D. S., Artrip S. G., Korenblit A., Schiffman G., Rao S. Correlation of serum opsonins with in vitro phagocytosis of Streptococcus pneumoniae. Infect Immun. 1985 Oct;50(1):213–217. doi: 10.1128/iai.50.1.213-217.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cripps A. W., Neoh S. H., Smart I. J. Isolation of human IgA and IgM from normal serum using polyethylene glycol precipitation and affinity chromatography. J Immunol Methods. 1983 Feb 25;57(1-3):197–204. doi: 10.1016/0022-1759(83)90078-9. [DOI] [PubMed] [Google Scholar]
- Di Padova F., Dürig M., Wadström J., Harder F. Role of spleen in immune response to polyvalent pneumococcal vaccine. Br Med J (Clin Res Ed) 1983 Dec 17;287(6408):1829–1832. doi: 10.1136/bmj.287.6408.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew P. A., Kiroff G. K., Ferrante A., Cohen R. C. Alterations in immunoglobulin synthesis by peripheral blood mononuclear cells from splenectomized patients with and without splenic regrowth. J Immunol. 1984 Jan;132(1):191–196. [PubMed] [Google Scholar]
- Edwards M. S., Nicholson-Weller A., Baker C. J., Kasper D. L. The role of specific antibody in alternative complement pathway-mediated opsonophagocytosis of type III, group B Streptococcus. J Exp Med. 1980 May 1;151(5):1275–1287. doi: 10.1084/jem.151.5.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlenberger A. G., Nussenzweig V. The role of membrane receptors for C3b and C3d in phagocytosis. J Exp Med. 1977 Feb 1;145(2):357–371. doi: 10.1084/jem.145.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon D. T., Kaneko I., Thomson G. G. Membrane distribution and adsorptive endocytosis by C3b receptors on human polymorphonuclear leukocytes. J Exp Med. 1981 Jun 1;153(6):1615–1628. doi: 10.1084/jem.153.6.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrante A., Thong Y. H. Separation of mononuclear and polymorphonuclear leucocytes from human blood by the one-step Hypaque-Ficoll method is dependent on blood column height. J Immunol Methods. 1982;48(1):81–85. doi: 10.1016/0022-1759(82)90212-5. [DOI] [PubMed] [Google Scholar]
- Giebink G. S., Foker J. E., Kim Y., Schiffman G. Serum antibody and opsonic responses to vaccination with pneumococcal capsular polysaccharide in normal and splenectomized children. J Infect Dis. 1980 Mar;141(3):404–412. doi: 10.1093/infdis/141.3.404. [DOI] [PubMed] [Google Scholar]
- Giebink G. S., Le C. T., Schiffman G. Decline of serum antibody in splenectomized children after vaccination with pneumococcal capsular polysaccharides. J Pediatr. 1984 Oct;105(4):576–582. doi: 10.1016/s0022-3476(84)80422-9. [DOI] [PubMed] [Google Scholar]
- Gray B. M. ELISA methodology for polysaccharide antigens: protein coupling of polysaccharides for adsorption to plastic tubes. J Immunol Methods. 1979;28(1-2):187–192. doi: 10.1016/0022-1759(79)90340-5. [DOI] [PubMed] [Google Scholar]
- Henson P. M., Johnson H. B., Spiegelberg H. L. The release of granule enzymes from human neutrophils stimulated by aggregated immunoglobulins of different classes and subclasses. J Immunol. 1972 Dec;109(6):1182–1192. [PubMed] [Google Scholar]
- Hernandez D. E., Gonzalez N., Rios R., Merchan L., Wuani H. Phagocytosis in patients with sickle cell disease. J Clin Lab Immunol. 1983 Nov;12(3):137–140. [PubMed] [Google Scholar]
- Horwitz M. A. The roles of the Fc and C3 receptors in the phagocytosis and killing of bacteria by human phagocytes. J Reticuloendothel Soc. 1980 Dec;28(Suppl):17s–26s. [PubMed] [Google Scholar]
- Hosea S. W., Burch C. G., Brown E. J., Berg R. A., Frank M. M. Impaired immune response of splenectomised patients to polyvalent pneumococcal vaccine. Lancet. 1981 Apr 11;1(8224):804–807. doi: 10.1016/s0140-6736(81)92681-7. [DOI] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Klemperer M. R., Alper C. A., Rosen F. S. The enhancement of bacterial phagocytosis by serum. The role of complement components and two cofactors. J Exp Med. 1969 Jun 1;129(6):1275–1290. doi: 10.1084/jem.129.6.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Newman S. L., Struth A. G. An abnormality of the alternate pathway of complement activation in sickle-cell disease. N Engl J Med. 1973 Apr 19;288(16):803–808. doi: 10.1056/NEJM197304192881601. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lanng Nielsen J., Tauris P., Johnsen H. E., Ellegaard J. The cellular immune response after splenectomy in humans. Impaired immunoglobulin synthesis in vitro. Scand J Haematol. 1983 Jul;31(1):85–95. doi: 10.1111/j.1600-0609.1983.tb02141.x. [DOI] [PubMed] [Google Scholar]
- Lawrence D. A., Weigle W. O., Spiegelberg H. L. Immunoglobulins cytophilic for human lymphocytes, monocytes, and neutrophils. J Clin Invest. 1975 Feb;55(2):368–376. doi: 10.1172/JCI107940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leist-Welsh P., Bjornson A. B. Immunoglobulin-independent utilization of the classical complement pathway in opsonophagocytosis of Escherichia coli by human peripheral leukocytes. J Immunol. 1982 Jun;128(6):2643–2651. [PubMed] [Google Scholar]
- Luo N. K., Rowland H. A. The bactericidal and opsonic effects of serum from patients with sickle cell anaemia. Bull Soc Pathol Exot Filiales. 1983 Nov;76(5):657–667. [PubMed] [Google Scholar]
- March S. C., Parikh I., Cuatrecasas P. A simplified method for cyanogen bromide activation of agarose for affinity chromatography. Anal Biochem. 1974 Jul;60(1):149–152. doi: 10.1016/0003-2697(74)90139-0. [DOI] [PubMed] [Google Scholar]
- Müller C., Mannhalter J. W., Ahmad R., Zlabinger G., Wurnig P., Eibl M. M. Peripheral blood mononuclear cells of splenectomized patients are unable to differentiate into immunoglobulin-secreting cells after pokeweed mitogen stimulation. Clin Immunol Immunopathol. 1984 Apr;31(1):118–123. doi: 10.1016/0090-1229(84)90196-x. [DOI] [PubMed] [Google Scholar]
- Newman S. L., Johnston R. B., Jr Role of binding through C3b and IgG in polymorphonuclear neutrophil function: studies with trypsin-generated C3b. J Immunol. 1979 Oct;123(4):1839–1846. [PubMed] [Google Scholar]
- Okada H., Okada N., Yasuda T. Activation of the alternative complement pathway by IgM antibody reacted on paragloboside incorporated into liposome membrane. Mol Immunol. 1983 Apr;20(4):499–500. doi: 10.1016/0161-5890(83)90031-7. [DOI] [PubMed] [Google Scholar]
- Overturf G. D., Powars D., Baraff L. J. Bacterial meningitis and septicemia in sickle cell disease. Am J Dis Child. 1977 Jul;131(7):784–787. doi: 10.1001/archpedi.1977.02120200066014. [DOI] [PubMed] [Google Scholar]
- Pearson H. A., Spencer R. P., Cornelius E. A. Functional asplenia in sickle-cell anemia. N Engl J Med. 1969 Oct 23;281(17):923–926. doi: 10.1056/NEJM196910232811703. [DOI] [PubMed] [Google Scholar]
- Pedersen F. K., Henrichsen J., Schiffman G. Antibody response to vaccination with pneumococcal capsular polysaccharides in splenectomized children. Acta Paediatr Scand. 1982 May;71(3):451–455. doi: 10.1111/j.1651-2227.1982.tb09451.x. [DOI] [PubMed] [Google Scholar]
- Powars D., Overturf G., Weiss J., Lee S., Chan L. Pneumococcal septicemia in children with sickle cell anemia. Changing trend of survival. JAMA. 1981 May 8;245(18):1839–1842. [PubMed] [Google Scholar]
- Ratnoff W. D., Fearon D. T., Austen K. F. The role of antibody in the activation of the alternative complement pathway. Springer Semin Immunopathol. 1983;6(4):361–371. doi: 10.1007/BF02116280. [DOI] [PubMed] [Google Scholar]
- Rogers D. W., Serjeant B. E., Serjeant G. R. Early rise in the "pitted" red cell count as a guide to susceptibility to infection in childhood sickle cell anaemia. Arch Dis Child. 1982 May;57(5):338–342. doi: 10.1136/adc.57.5.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers D. W., Vaidya S., Serjeant G. R. Early splenomegaly in homozygous sickle-cell disease: An indicator of susceptibility to infection. Lancet. 1978 Nov 4;2(8097):963–965. doi: 10.1016/s0140-6736(78)92527-8. [DOI] [PubMed] [Google Scholar]
- Schreiber R. D., Pangburn M. K., Bjornson A. B., Brothers M. A., Müller-Eberhard H. J. The role of C3 fragments in endocytosis and extracellular cytotoxic reactions by polymorphonuclear leukocytes. Clin Immunol Immunopathol. 1982 May;23(2):335–357. doi: 10.1016/0090-1229(82)90119-2. [DOI] [PubMed] [Google Scholar]
- Siber G. R., Schur P. H., Aisenberg A. C., Weitzman S. A., Schiffman G. Correlation between serum IgG-2 concentrations and the antibody response to bacterial polysaccharide antigens. N Engl J Med. 1980 Jul 24;303(4):178–182. doi: 10.1056/NEJM198007243030402. [DOI] [PubMed] [Google Scholar]
- Sieber G., Breyer H. G., Herrmann F., Rühl H. Abnormalities of B-cell activation and immunoregulation in splenectomized patients. Immunobiology. 1985 Apr;169(3):263–271. doi: 10.1016/s0171-2985(85)80038-3. [DOI] [PubMed] [Google Scholar]
- Tack B. F., Harrison R. A., Janatova J., Thomas M. L., Prahl J. W. Evidence for presence of an internal thiolester bond in third component of human complement. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5764–5768. doi: 10.1073/pnas.77.10.5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vroon D. H., Schultz D. R., Zarco R. M. The separation of nine components and two inactivators of components of complement in humansserum. Immunochemistry. 1970 Jan;7(1):43–61. doi: 10.1016/0019-2791(70)90029-7. [DOI] [PubMed] [Google Scholar]
- Walsh G. M., Kay A. B. Binding of immunoglobulin classes and subclasses to human neutrophils and eosinophils. Clin Exp Immunol. 1986 Feb;63(2):466–472. [PMC free article] [PubMed] [Google Scholar]
- Ward H. K., Enders J. F. AN ANALYSIS OF THE OPSONIC AND TROPIC ACTION OF NORMAL AND IMMUNE SERA BASED ON EXPERIMENTS WITH THE PNEUMOCOCCUS. J Exp Med. 1933 Mar 31;57(4):527–547. doi: 10.1084/jem.57.4.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkelstein J. A., Drachman R. H. Deficiency of pneumococcal serum opsonizing activity in sickle-cell disease. N Engl J Med. 1968 Aug 29;279(9):459–466. doi: 10.1056/NEJM196808292790904. [DOI] [PubMed] [Google Scholar]
- Winkelstein J. A., Shin H. S. The role of immunoglobulin in the interaction of pneumococci and the properdin pathway: evidence for its specificity and lack of requirement for the Fc portion of the molecule. J Immunol. 1974 May;112(5):1635–1642. [PubMed] [Google Scholar]