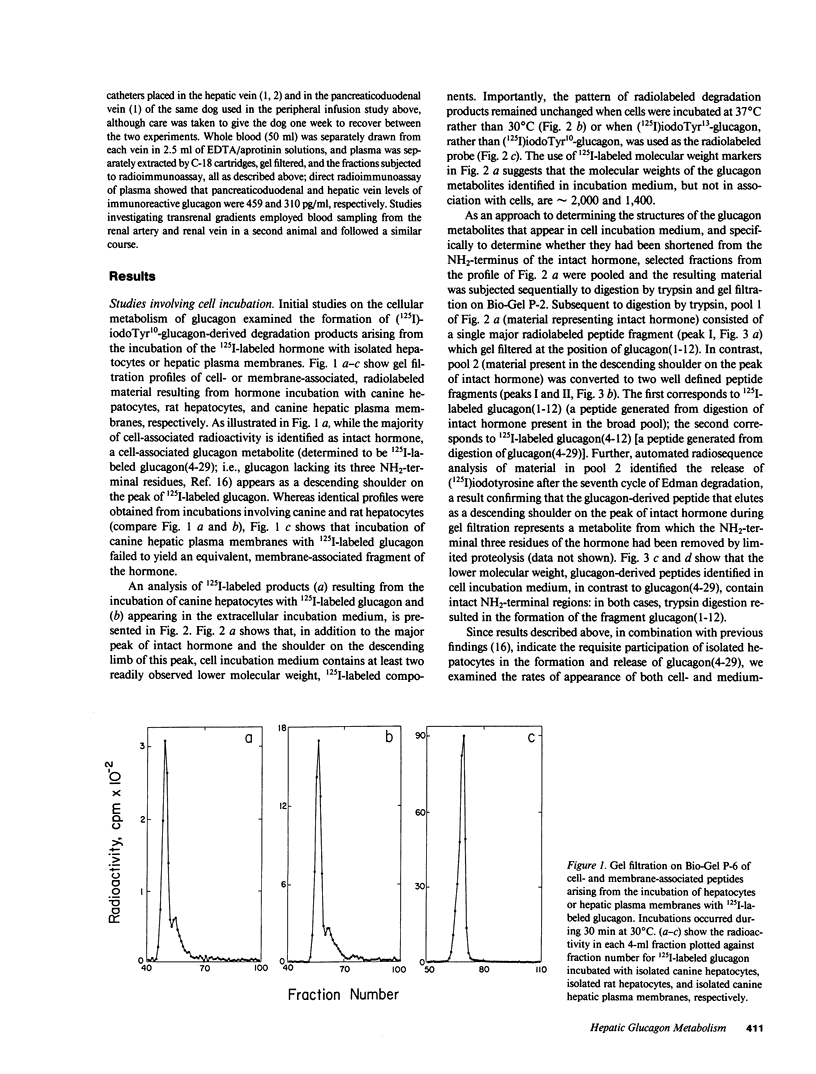
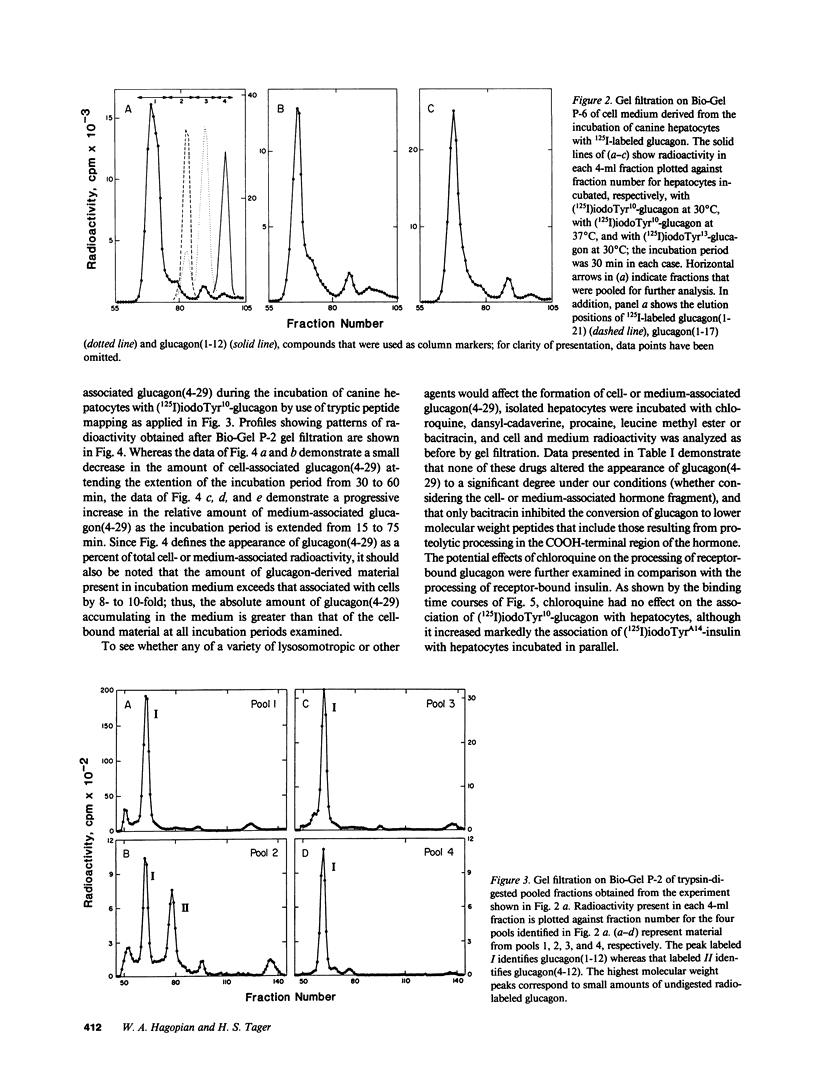
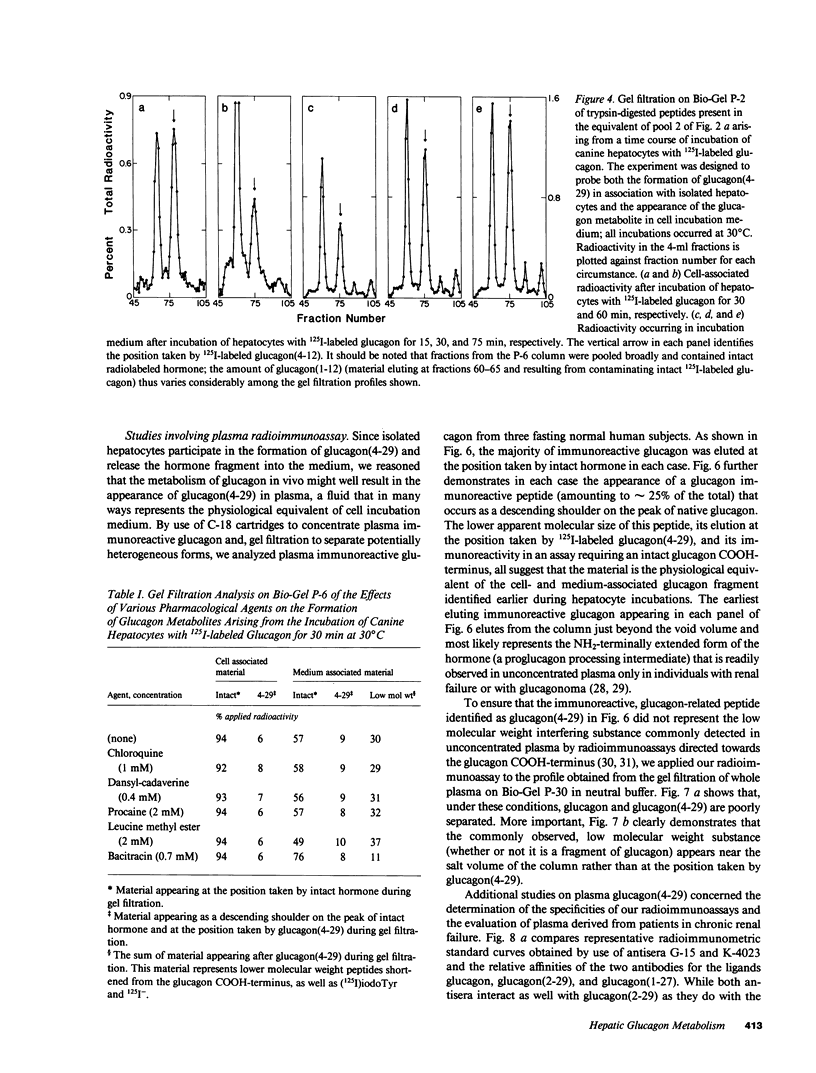
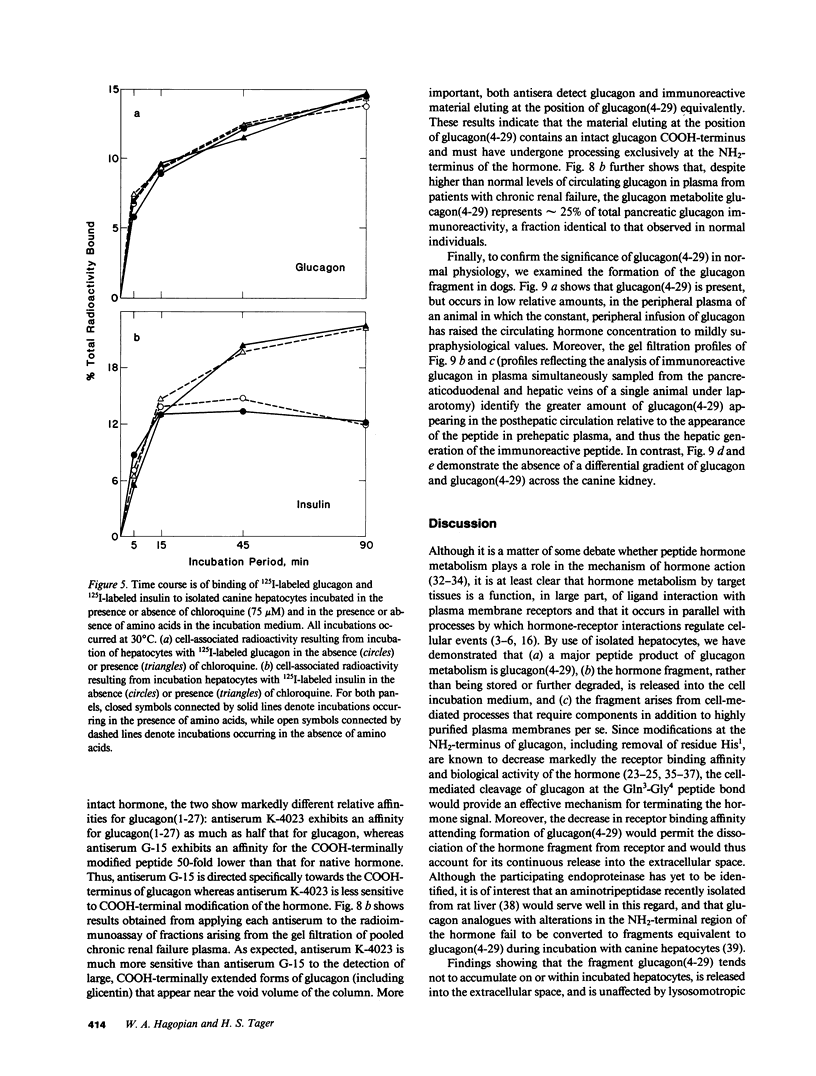
Abstract
We have found that canine and rat hepatocytes convert (125I)iodoTyr10-glucagon to a peptide metabolite lacking the NH2-terminal three residues of the hormone. The peptide is released into the cell incubation medium and its formation is unaffected by a variety of lysosomotropic or other agents. Use of specific radioimmunoassays and gel filtration demonstrated in both normal subjects and in chronic renal failure patients a plasma peptide having the properties of the hormone fragment identified by cell studies. Studies of the dog revealed a positive gradient of the fragment across the liver and no differential gradient of the fragment and glucagon across the kidney. We conclude that the glucagon fragment arises from the cell-mediated processing of the hormone on a superficial aspect of the hepatocyte, the glucagon fragment identified during experiments in vitro represents the cognate of a peptide formed during the hepatic metabolism of glucagon in vivo, and measurement of the fragment by COOH-terminal radioimmunoassays could lead to an understimulation of hepatic glucagon extraction.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assoian R. K., Tager H. S. Peptide intermediates in the cellular metabolism of insulin. J Biol Chem. 1982 Aug 10;257(15):9078–9085. [PubMed] [Google Scholar]
- Assoian R. K., Tager H. S. [(125I]IodotyrosylB1]insulin. Semisynthesis, receptor binding, and cell-mediated degradation of a B chain-labeled insulin. J Biol Chem. 1981 Apr 25;256(8):4042–4049. [PubMed] [Google Scholar]
- Barazzone P., Gorden P., Carpentier J. L., Orci L., Freychet P., Canivet B. Binding, internalization, and lysosomal association of 125I-glucagon in isolated rat hepatocytes. A quantitative electron microscope autoradiographic study. J Clin Invest. 1980 Nov;66(5):1081–1093. doi: 10.1172/JCI109937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnevie-Nielsen V., Polonsky K. S., Jaspan J. J., Rubenstein A. H., Schwartz T. W., Tager H. S. Surface receptors for pancreatic hormones in dog and rat hepatocytes: qualitative and quantitative differences in hormone-target cell interactions. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2167–2171. doi: 10.1073/pnas.79.7.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnevie-Nielsen V., Tager H. S. Glucagon receptors on isolated hepatocytes and hepatocyte membrane vesicles. Discrete populations with ligand- and environment-dependent affinities. J Biol Chem. 1983 Sep 25;258(18):11313–11320. [PubMed] [Google Scholar]
- Bregman M. D., Trivedi D., Hruby V. J. Glucagon amino groups. Evaluation of modifications leading to antagonism and agonism. J Biol Chem. 1980 Dec 25;255(24):11725–11731. [PubMed] [Google Scholar]
- Burghen G. A., Kitabchi A. E., Brush J. S. Characterization of a rat liver protease with specificity for insulin. Endocrinology. 1972 Sep;91(3):633–642. doi: 10.1210/endo-91-3-633. [DOI] [PubMed] [Google Scholar]
- Bålöw R. M., Ragnarsson U., Zetterqvist O. Tripeptidyl aminopeptidase in the extralysosomal fraction of rat liver. J Biol Chem. 1983 Oct 10;258(19):11622–11628. [PubMed] [Google Scholar]
- Carpentier J. L., Gorden P., Barazzone P., Freychet P., Le Cam A., Orci L. Intracellular localization of 125I-labeled insulin in hepatocytes from intact rat liver. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2803–2807. doi: 10.1073/pnas.76.6.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentier J. L., Gorden P., Freychet P., Le Cam A., Orci L. Lysosomal association of internalized 125I-insulin in isolated rat hepatocytes. Direct demonstration by quantitative electron microscopic autoradiography. J Clin Invest. 1979 Jun;63(6):1249–1261. doi: 10.1172/JCI109420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frandsen E. K., Grønvald F. C., Heding L. G., Johansen N. L., Lundt B. F., Moody A. J., Markussen J., Vølund A. Glucagon: structure-function relationships investigated by sequence deletions. Hoppe Seylers Z Physiol Chem. 1981 Jun;362(6):665–677. doi: 10.1515/bchm2.1981.362.1.665. [DOI] [PubMed] [Google Scholar]
- Hagopian W. A., Tager H. S. Receptor binding and cell-mediated metabolism of [125I]monoiodoglucagon by isolated canine hepatocytes. J Biol Chem. 1984 Jul 25;259(14):8986–8993. [PubMed] [Google Scholar]
- Hagopian W., Lever E. G., Cohen D., Emmanouel D., Polonsky K. S., Pugh W., Moossa A., Jaspan J. B. Predominance of renal and absence of hepatic metabolism of pancreatic polypeptide in the dog. Am J Physiol. 1983 Aug;245(2):E171–E177. doi: 10.1152/ajpendo.1983.245.2.E171. [DOI] [PubMed] [Google Scholar]
- Hammons G. T., Smith R. M., Jarett L. Inhibition by bacitracin of rat adipocyte plasma membrane degradation of 125I-insulin is associated with an increase in plasma membrane bound insulin and a potentiation of glucose oxidation by adipocytes. J Biol Chem. 1982 Oct 10;257(19):11563–11570. [PubMed] [Google Scholar]
- Hofmann C., Marsh J. W., Miller B., Steiner D. F. Cultured hepatoma cells as a model system for studying insulin processing and biologic responsiveness. Diabetes. 1980 Nov;29(11):865–874. doi: 10.2337/diab.29.11.865. [DOI] [PubMed] [Google Scholar]
- Hruby V. J. Structure-conformation-activity studies of glucagon and semi-synthetic glucagon analogs. Mol Cell Biochem. 1982 Apr 16;44(1):49–64. doi: 10.1007/BF00573846. [DOI] [PubMed] [Google Scholar]
- Hruska K. A., Blondin J., Bass R., Santiago J., Thomas L., Altsheler P., Martin K., Klahr S. Effect of intact parathyroid hormone on hepatic glucose release in the dog. J Clin Invest. 1979 Oct;64(4):1016–1023. doi: 10.1172/JCI109538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs S., Cuatrecasas P. Insulin receptor: structure and function. Endocr Rev. 1981 Summer;2(3):251–263. doi: 10.1210/edrv-2-3-251. [DOI] [PubMed] [Google Scholar]
- Jaspan J. B., Polonsky K. S., Lewis M., Pensler J., Pugh W., Moossa A. R., Rubenstein A. H. Hepatic metabolism of glucagon in the dog: contribution of the liver to overall metabolic disposal of glucagon. Am J Physiol. 1981 Mar;240(3):E233–E244. doi: 10.1152/ajpendo.1981.240.3.E233. [DOI] [PubMed] [Google Scholar]
- Jaspan J. B., Rubenstein A. H. Circulating glucagon. Plasma profiles and metabolism in health and disease. Diabetes. 1977 Sep;26(9):887–904. doi: 10.2337/diab.26.9.887. [DOI] [PubMed] [Google Scholar]
- Khan M. N., Posner B. I., Khan R. J., Bergeron J. J. Internalization of insulin into rat liver Golgi elements. Evidence for vesicle heterogeneity and the path of intracellular processing. J Biol Chem. 1982 May 25;257(10):5969–5976. [PubMed] [Google Scholar]
- Marshall S. Degradative processing of internalized insulin in isolated adipocytes. J Biol Chem. 1985 Nov 5;260(25):13517–13523. [PubMed] [Google Scholar]
- Marshall S. Dual pathways for the intracellular processing of insulin. Relationship between retroendocytosis of intact hormone and the recycling of insulin receptors. J Biol Chem. 1985 Nov 5;260(25):13524–13531. [PubMed] [Google Scholar]
- McDonald J. K., Callahan P. X., Zeitman B. B., Ellis S. Inactivation and degradation of glucagon by dipeptidyl aminopeptidase I (cathepsin C) of rat liver. J Biol Chem. 1969 Nov 25;244(22):6199–6208. [PubMed] [Google Scholar]
- Misbin R. I., Almira E. C., Buynitzky S. J. Insulin metabolism in rat hepatocytes. Evidence for generation of an insulin fragment missing a portion of the B chain involved in receptor binding. J Biol Chem. 1983 Feb 25;258(4):2157–2162. [PubMed] [Google Scholar]
- Neville D. M., Jr Isolation of an organ specific protein antigen from cell-surface membrane of rat liver. Biochim Biophys Acta. 1968 Apr 9;154(3):540–552. doi: 10.1016/0005-2795(68)90014-7. [DOI] [PubMed] [Google Scholar]
- Plas C., Desbuquois B. Receptor-mediated insulin degradation and insulin-stimulated glycogenesis in cultured foetal hepatocytes. Biochem J. 1982 Feb 15;202(2):333–341. doi: 10.1042/bj2020333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl S. L., Krans H. M., Birnbaumer L., Rodbell M. Inactivation of glucagon by plasma membranes of rat liver. J Biol Chem. 1972 Apr 25;247(8):2295–2301. [PubMed] [Google Scholar]
- Recant L., Perrino P. V., Bhathena S. J., Danforth D. N., Jr, Lavine R. L. Plasma immunoreactive glucagon fractions in four cases of glucagonoma: increased "large glucagon-immunoreactivity". Diabetologia. 1976 Aug;12(4):319–326. doi: 10.1007/BF00420975. [DOI] [PubMed] [Google Scholar]
- Robbins D. C., Shoelson S. E., Tager H. S., Mead P. M., Gaynor D. H. Products of therapeutic insulins in the blood of insulin-dependent (type I) diabetic patients. Diabetes. 1985 May;34(5):510–519. doi: 10.2337/diab.34.5.510. [DOI] [PubMed] [Google Scholar]
- Ross E. M., Gilman A. G. Biochemical properties of hormone-sensitive adenylate cyclase. Annu Rev Biochem. 1980;49:533–564. doi: 10.1146/annurev.bi.49.070180.002533. [DOI] [PubMed] [Google Scholar]
- Schlegel W., Kempner E. S., Rodbell M. Activation of adenylate cyclase in hepatic membranes involves interactions of the catalytic unit with multimeric complexes of regulatory proteins. J Biol Chem. 1979 Jun 25;254(12):5168–5176. [PubMed] [Google Scholar]
- Shoelson S., Haneda M., Blix P., Nanjo A., Sanke T., Inouye K., Steiner D., Rubenstein A., Tager H. Three mutant insulins in man. Nature. 1983 Apr 7;302(5908):540–543. doi: 10.1038/302540a0. [DOI] [PubMed] [Google Scholar]
- Sonne O., Berg T., Christoffersen T. Binding of 125I-labeled glucagon and glucagon-stimulated accumulation of adenosine 3':5'-monophosphate in isolated intact rat hepatocytes. Evidence for receptor heterogeneity. J Biol Chem. 1978 May 10;253(9):3203–3210. [PubMed] [Google Scholar]
- Soybel D., Jaspan J., Polonsky K., Goldberg I., Rayfield E., Tager H. Differential immunoreactivity of plasma glucagon components in man: studies with different glucagon antibodies. J Clin Endocrinol Metab. 1983 Mar;56(3):612–618. doi: 10.1210/jcem-56-3-612. [DOI] [PubMed] [Google Scholar]
- Terris S., Steiner D. F. Binding and degradation of 125I-insulin by rat hepatocytes. J Biol Chem. 1975 Nov 10;250(21):8389–8398. [PubMed] [Google Scholar]
- Terris S., Steiner D. F. Retention and degradation of 125I-insulin by perfused livers from diabetic rats. J Clin Invest. 1976 Apr;57(4):885–896. doi: 10.1172/JCI108365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda M., Robinson F. W., Smith M. M., Kono T. Effects of monensin on insulin processing in adipocytes. Evidence that the internalized insulin-receptor complex has some physiological activities. J Biol Chem. 1985 Apr 10;260(7):3941–3946. [PubMed] [Google Scholar]
- Welton A. F., Lad P. M., Newby A. C., Yamamura H., Nicosia S., Rodbell M. Solubilization and separation of the glucagon receptor and adenylate cyclase in guanine nucleotide-sensitive states. J Biol Chem. 1977 Sep 10;252(17):5947–5950. [PubMed] [Google Scholar]
- Wright D. E., Rodbell M. Glucagon1-6 binds to the glucagon receptor and activates hepatic adenylate cyclase. J Biol Chem. 1979 Jan 25;254(2):268–269. [PubMed] [Google Scholar]


