Abstract
Clinical prognosis of metastasized colorectal carcinoma (CRC) is still not at desired levels and novel drugs are needed. Here, we focused on the multi-tyrosine kinase inhibitor E7080 (Lenvatinib) and assessed its therapeutic efficacy against human CRC cell lines in vitro and human CRC xenografts in vivo. The effect of E7080 on cell viability was examined on 10 human CRC cell lines and human endothelial cells (HUVEC). The inhibitory effect of E7080 on VEGF-induced angiogenesis was studied in an ex vivo mouse aortic ring angiogenesis assay. In addition, the efficacy of E7080 against xenografts derived from CRC cell lines and CRC patient resection specimens with mutated KRAS was investigated in vivo. A relatively low cytotoxic effect of E7080 on CRC cell viability was observed in vitro. Endothelial cells (HUVEC) were more susceptible to the incubation with E7080. This is in line with the observation that E7080 demonstrated an anti-angiogenic effect in a three-dimensional ex vivo mouse aortic ring angiogenesis assay. E7080 effectively disrupted CRC cell-mediated VEGF-stimulated growth of HUVEC in vitro. Daily in vivo treatment with E7080 (5 mg/kg) significantly delayed the growth of KRAS mutated CRC xenografts with decreased density of tumor-associated vessel formations and without tumor regression. This observation is in line with results that E7080 did not significantly reduce the number of Ki67-positive cells in CRC xenografts. The results suggest antiangiogenic activity of E7080 at a dosage that was well tolerated by nude mice. E7080 may provide therapeutic benefits in the treatment of CRC with mutated KRAS.
Introduction
Colorectal carcinoma (CRC) is the most common malignancy of the gastrointestinal tract and constitutes approximately 15% of all cases of cancer. Despite multiple advances in diagnosis and treatment of CRC, approximately 45% of patients with CRC experience local recurrence and/or metastases with a consequent dramatic decline in prognosis. In the industrialized West, CRC is therefore, the third most common cause of death from cancer [1].
Metastases of CRC are localized in the liver in 40% to 80% of patients. The principal curative treatment option is surgical resection, although only one fourth of patients with colorectal liver metastases are primary operable [2]. Due to this fact, in daily clinical situations, patients are stratified into three groups: patients with resectable liver metastases who are treated by curative surgery, patients with resectable liver metastases after a neoadjuvant therapy undergoing surgical resection at a later date, and patients with wide-spread and unresectable metastases even after downsizing chemotherapy. In recent years marked improvements have been made in the medial treatment of patients with CRC metastasis. Angiogenesis is essential for solid tumor growth and anti-angiogenic therapy may offer an additional treatment option at this stage [3]. New cytostatic agents and antibodies targeting epidermal growth factor receptor (EGFR) on the cancer cell surface and vascular epithelial growth factor (VEGF) released by cancer cells [4] have increased median survival of patients with advanced CRC to more than 2 years, almost doubling the survival time of the 5-Fluorouracil (5-FU) era [5], [6].
One promising mechanism to inhibit tumor growth or induce tumor shrinking in combination with classical chemotherapy is the use of VEGF signaling inhibitors. The recombinant humanized monoclonal antibody bevacizumab is directed against the pro-angiogenic VEGF subgroup A. It has been approved, in combination with chemotherapy, for the treatment of colorectal liver metastasis with mutations in the Kirsten Ras (KRAS) oncogene, coding for a cytoplasmic GTP-binding protein [7], [8]. The KRAS oncogene is mutated in approximately 35% to 45% of CRC [9]. Bevacizumab binds to VEGF and inhibits VEGF receptor binding, thereby preventing the growth and maintenance of tumor blood vessels. Bevacizumab in combination with 5-FU based chemotherapy has shown a survival benefit compared to control groups in several trials [10], whereas bevacizumab alone does not improve patient survival [11] and its application is limited by adverse drug effects [12]. One explanation for the failure of bevacizumab monotherapy to induce enduring clinical responses may be the fact that bevacizumab targets VEGF-A exclusively, thus only one member of a large family of pro-angiogenic growth factors inducing angiogenesis is targeted. This would suggest that the blockage of the interaction of multiple growth factors with their receptors might improve therapeutic efficacy [13]. For example, regorafenib, a novel oral multi-kinase inhibitor targeting VEGF receptor (VEGFR) and a broad range of other receptor tyrosine kinases (RTK) including BRAF, demonstrates superior effects over monoclonal antibodies targeting VEGF only [14]. A regorafenib monotherapy was recently approved for an international, multicenter, randomized, placebo-controlled, phase 3 trial. Regorafenib increased the survival of patients with chemorefractory metastatic colorectal cancer to 1.4 months compared to a placebo group with progressive cancer after all standard therapies [15]. In conclusion, targeting a broad range of RTK seems to have advantages over targeting RTK ligands only. Lenvatinib (E7080) is an orally active multi-kinase inhibitor of VEGFR2 and VEGFR3 with IC50 values of 4 nmol/l and 5.2 nmol/l, respectively. In addition, E7080 also inhibits VEGFR1, fibroblast growth factor receptor (FGFR1), and platelet-derived growth factor receptor (PDGFR) with IC50 of 22 nmol/l, 46 nmol/l and 51 nmol/l [16]. Lenvatinib is also reported to have inhibitory activity to RET (rearranged during transfection) kinase [17], a receptor for growth factors of the glial-derived neurotrophic factor family. Lenvatinib is currently in a phase 2 study with KIF5B-RET-positive adenocarcinoma of the lung (Clinical Trials.gov; NCT01877083). E7080 shows potent antitumor effects in xenograft models of various cancer types, such as breast and lung cancer, pleura mesothelioma or sarcoma [18], [19], [20].
The aim of the present study was to evaluate a) the efficacy of E7080 (Lenvatinib) on a broad range of human CRC cell lines and primary human endothelial cells (HUVEC) in vitro, b) the anti-angiogenic effect of E7080 in a three-dimensional ex vivo mouse aortic ring angiogenesis assay, and c) the therapeutic efficacy of E7080 against xenografts derived from CRC cell lines and CRC patient resection specimens with mutated KRAS in vivo.
Materials and Methods
Cell Lines
Human CRC cells (Table 1) were routinely cultured in their recommended media supplemented with 10% (v/v) heat-inactivated fetal calf serum (FCS), 2 mmol/l glutamine (Invitrogen), 100 U/ml penicillin, and 100 μg/ml streptomycin. Cancer cell lines were obtained from the American Type Culture Collection (www.atcc.org) with exception of HCT116 and HT29 (Leibniz Institute, German Collection of Microorganisms and Cell Cultures, Braunschweig, Germany), SW620 (European Collection of Cell Cultures, Salisbury, UK) and WiDr (Cell Lines Service, Eppenheim, Germany). Human umbilical vein endothelial cells (HUVEC) were obtained from PromoCell (Heidelberg, Germany) and cultured according to the manufacturer's recommendations in endothelial growth medium (PromoCell).
Table 1.
Effect of E7080 on cell viability of human CRC cells and human umbilical vein endothelial cells (HUVEC).
IC50 values were determined following E7080 treatment for 72 hours in media with low serum (1% (v/v) FCS). Cell lines are arranged according to increasing IC50 values. Shown are the results as mean and 95% confidence interval (CI) of IC50 of at least three independent proliferation assays with hexaplicates. IC50 values and 95% CI were determined using GraphPad Prism software. See also Fig. 1A.Wild-type (wt) KRAS and mutated (mut) KRAS were determined with Sanger Catalogue of somatic mutations in cancer (COSMIC) database.
| Cell lines | KRAS status | IC50 (μmol/l) | 95% CI (μmol/l) |
|---|---|---|---|
| CaCo2 | wt | > 1000 | ¯¯¯¯ |
| Colo741 | wt | > 1000 | ¯¯¯¯ |
| SW620 | mut | 548 | 273-111 |
| HT29 | mut | 431 | 299-620 |
| HCT116 | mut | 399 | 296-538 |
| T84 | mut | 276 | 179-427 |
| SW480 | mut | 240 | 183-313 |
| Colo678 | wt | 222 | 175-283 |
| LS174T | mut | 144 | 96-215 |
| HCT15 | mut | 120 | 91-158 |
| HUVEC | wt | 22 | 14-36 |
Human CRC Xenografts from Patient Primary Resection Specimens
Low-passage CRC xenografts HROC71 (T2M1) were established from primary resection specimens of a patient with Lynch syndrome [21]. For this, tumor samples were subcutaneously transplanted into nude mice. Established xenografts were removed, cut in small pieces (3 × 3 × 3 mm) and stored in liquid nitrogen.
Reagents
Lenvatinib (E7080) was obtained from Selleckchem (Boston, USA). Stock solutions for in vitro (1 mmol/l) and in vivo (5,9 mmol/l) studies were prepared with phosphate buffered saline (PBS; Gibco, Life Technologies GmbH, Darmstadt, Germany) with 10% DMSO (final concentration; Sigma Aldrich) and aliquots were stored at − 20 °C. The stock solution was further diluted for in vitro and in vivo studies. The final concentration of DMSO was ≤ 1% in all assays, with and without E7080.
Western Blotting
Cultured cells were rinsed three times with ice-cold PBS, harvested, and lysed directly in RIPA buffer (Pierce, Thermo Scientific, Rockford, USA) for immunoblot analysis. Cell debris was removed by centrifugation at 15,000g for 10 minutes at 4°C. The supernatant was used as total protein lysate. For each sample, 10 μg of total protein lysate was subjected to 10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS page), followed by immunoblot analysis. Immunoblots were probed with antibodies against MYC (Y69, #ab32072; Abcam, Cambridge, UK), GAPDH (#G9295, Sigma Aldrich GmbH, München, Germany), Vinculin (hVIN-1, #9131, Sigma Aldrich GmbH), pERK1/2T202/T204 (#9101, Cell Signaling Technology, Danvers, MA, USA), and ERK1/2 (C14, #SC-154, Santa Cruz Biotechnology, USA). All antibodies were used according to the manufacturer’s instructions. The results were visualized with secondary antibodies (GE Healthcare Life Sciences Europe, Freiburg, Germany) against mouse (NA9310) or rabbit (NA9340) primary antibodies combined with enhanced chemiluminescence (ECL) western blotting substrates (Pierce, Thermo Scientific).
Immunostaining
Following excision, tumors were snap frozen in liquid nitrogen and subsequently stored at − 80°C. Sections were used for staining towards CD31, CD34, Ki67 and CAIX using standard procedures [22]. The following antibodies, diluted in a commercial antibody diluent (DAKO, Hamburg, Germany), were used: anti-Ki-67 antigen (clone MIB-1, DAKO), final dilution 1:25; anti-CD31 (clone SZ31, DAKO), final dilution 1:100; anti-CD34 (clone MEC14.7, Linaris, Dossenheim, Germany), final dilution 1:200 and anti-carbonic anhydrase 9 (CA9, CAIX, clone GT12, GeneTex, Irvine, USA), final dilution 1:500. After incubation with the primary antibody for 1 hour at room temperature, the slides were washed in PBS and incubated with the following secondary antibodies: goat anti-mouse-HRP (DAKO), final dilution 1:100 or rabbit anti-rat-HRP (DAKO), final dilution 1:100. After development in 5% 3,3′-diaminobenzidine (DAKO) and counterstaining with haematoxilin, the sections were dehydrated in graded ethanol and embedded in Vitro Clud (Langenbrinck, Emmendingen, Germany). Stained slides were photographed at 20x magnification with a Keyence Biorevo BZ-9000 Microscope (Keyence Corporation, Osaka, Japan) applying Z-stack technology to improve the quality of images. The number of stained cells per section was quantified by using measurement module BZ-H3C (Hybrid Cell Count Vers.1.1, Keyence).
Aortic Ring Assay
The thoracic aortae from C57BL/6 mice (8 weeks) were isolated and subsequently cut into 1 mm thick rings after removal of adventitial fat. In two steps the aortic rings were then embedded in 48-well plates between two layers of 200 μl collagen gel, containing collagen type 1 (Purecol, CellSystems, Germany), sodium hydroxide, L-glutamine, sodium pyruvate, sodium bicarbonate, MEM (10 ×), penicillin-streptomycin and dH2O. First, the aortic rings were set on the first layer after polymerization of the gel. Then, the rings were covered with the second gel layer and after polymerization cultivated in 300 μl DMEM (10% FCS, 1% penicillin-streptomycin) at 37°C and 5% CO2. Some of the rings were treated with VEGF (50 ng/ml) or E7080 (1 μmol/l and 10 μmol/l), or with a combination of both. The medium was changed every third day. A phase contrast microscope (Leica, Germany) equipped with a digital camera (Leica, Germany) was used for evaluation and documentation of the aortic rings.
CRC Xenograft Studies
To generate CRC xenografts, 2.5 × 106 HCT116 cells, or LS174T cells resuspended in 100 μl phosphate-buffered saline were injected subcutaneously into the left hind flank of athymic female NMRI-Foxn1nu (nude) mice aged 8 to 10 weeks with 24 to 27 g body weight (Janvier, Le Genest-Saint-Isle, France). Subcutaneous CRC xenograft implantation was performed as described previously [23]. Tumors were measured using calipers and tumor volume VT (mm3) was calculated using the ellipsoid formula A2 x B x π/6, where A represents the smaller diameter [22]. Endpoint for the experiments was attainment of a tumour volume between 600 and 700 mm3 (target tumor volume) or within 6 weeks, when arrest of tumor growth was observed. When tumors reached a volume of 40 to 60 mm3, mice were randomized into therapy and control groups. Mice in therapy groups were treated daily with 5 mg/kg E7080 by oral gavage until tumors of control group reached target tumor volume. Tumour size and body weight of all animals were measured every day. All animal experiments were authorized by the local ethics committee, and mice were treated according to institutional and European Union guidelines.
Blood Count and Clinical Chemistry
Blood counts (erythrocytes, leukocytes, thrombocytes), the amount of hemoglobin, and the activity of liver enzymes alanine transaminase (ALT) and aspartate transaminase (AST) were measured at the central laboratory of the University Hospital of Würzburg.
Detection of VEGF Production
One million CRC cells and HUVEC were cultured in 6-well plates with serum-reduced (1%) RPMI 1640 medium for 48 hours. Afterwards, supernatant were harvested, centrifuged and stored at -80 °C. VEGF concentration was assayed by ELISA according to manufacturer’s instructions (R&D Systems GmbH, Germany). All samples were run in triplicate and each assay was conducted 2 times independently.
Proliferation Assay
WST-8 is reduced by cellular dehydrogenases to an orange formazan product that is soluble in tissue culture medium. The amount of formazan produced is directly proportional to the number of living cells. Cells (CRC cell lines and HUVEC) were seeded in 96-well flat-bottom plates at 5 × 103 cells per well with 100 μl RPMI 1640 medium supplemented with 1% (v/v) FCS, 2 mmol/l glutamine (Invitrogen, Darmstadt, Germany) and antibiotics (100 IU/ml penicillin, and 1 μg/ml streptomycin). The assays were cultured at 37 °C in a humidified atmosphere of 5% CO2 in air. The cells were cultured to reach the 50%-60% confluence on the second day when treated with serial dilutions of E7080 as indicated or supernatant. The assays were cultured at 37 °C in a humidified atmosphere of 5% CO2 in air for 3 days. Subsequently, cell viability was determined by WST-8 assay according to manufacturer’s instructions (PromoCell GmbH, Heidelberg, Germany).
Statistical Analysis
All graphs and statistical analyses were made using Prism 5 statistical software (GraphPad Software, Inc, La Jolla, CA, USA). IC50 values were calculated with nonlinear regression fit to a sigmoidal dose-response curve (variable slope). Values for tumor volume and body weight were compared with two-way ANOVA with Bonferroni post hoc tests. One-way ANOVA was performed for comparison between different groups. Differences were considered statistically significant at a P value of less than 0.05.
Results
Human Endothelial Cells are More Susceptible to E7080 than CRC Cells
The effect of E7080 on cell viability was examined on 10 human CRC cell lines harboring wildtype or mutated KRAS (Table 1) and human umbilical endothelial cells (HUVEC) using the cell viability test WST8. Dose response analysis showed that VEGF stimulated HUVEC were 5 times more susceptible to the incubation with E7080 (IC50: 22 μmol/l) than CRC cell line HCT15 (KRAS mutated) with IC50 value of 120 μmol/l, the lowest of the tested CRC cell lines (Figure 1A, Table 1). E7080 had less effect on cell viability of CaCo2 (KRAS wildtype), Colo741 (KRAS wildtype), SW620 (KRAS mutated) and HT29 (KRAS mutated) cells. The IC50 values ranged between > 1000 μmol/l for CaCo2 and Colo741 and 431 μmol/l for HT29. Colo678 with wildtype KRAS was more susceptible to E7080 (IC50: 222 μmol/l) than CaCo2 and Colo741 (both with wildtype KRAS). It seems that E7080 did not demonstrate a strong cytotoxic effect on human CRC cells in comparison to HUVEC.
Figure 1.
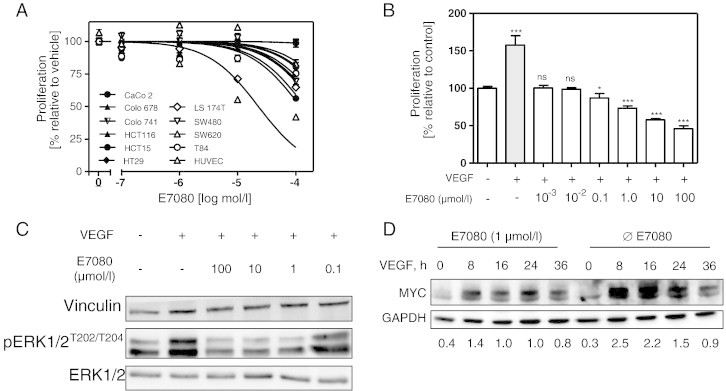
Effects of E7080 on cell viability and cell signalling.
(A) Dose-response effects of E7080 on cell viability of human colorectal carcinoma (CRC) cells and endothelial cells (HUVEC). IC50 values were determined in serum reduced (1%) RPMI 1640 medium following treatment with different concentrations of E7080 for 72 hours. The final concentration of DMSO (vehicle) was ≤ 1%. Results are expressed as the mean ± S.E.M. of at least three independent proliferation assays with hexaplicates. IC50 values were determined using GraphPad Prism software. See also Table 1.
(B) E7080 inhibits VEGF-induced HUVEC proliferation. HUVEC (1.5 × 104 cells) were seeded in 96-well plates coated with attachment factor (#S-006-100, Gibco Life Technologies) for 24 hours. The culture medium was changed and HUVEC were incubated in serum reduced (1%) RPMI 1640 medium in the presence of VEGF (20 ng/ml) and different concentrations of E7080 as indicated. Cell viability was determined with WST-8 assay after 48 hours. Shown are the results of one representative experiment with hexaplicates. *P < 0.05, **P < 0.01, ***P < 0.001 to HUVEC control proliferation (without VEGF and E7080).
(C) E7080 inhibits VEGF-induced phosphorylation of ERK1/2. Serum starved HUVEC were incubated with indicated amounts of E7080 for 4 hours and then stimulated with VEGF (20 ng/ml) for 5 minutes. Cells were harvested immediately on ice and immunoblotted with indicated antibodies against ERK1/2 and pERK1/2 as described in Materials and Methods.
(D) VEGF-stimulated MYC expression is suppressed by E7080. HUVEC were incubated in serum reduced (1%) RPMI 1640 medium with E7080 (1 μmol/l) for 4 hours and afterwards with VEGF (20 ng/ml) until indicated time points; subsequently cells were harvested for western blot analysis of MYC. Detection of MYC and GAPDH was performed using enzyme-linked chemiluminescence. GAPDH was used as loading control. The intensity of the signals corresponding to MYC and GAPDH was quantified by densitometry using ImageJ software (National Institutes of Health). The level of MYC protein is expressed as the ratio of the MYC band to that of GAPDH of a representative experiment.
HUVEC are very responsive to stimulation by VEGF. Since E7080 is supposed to inhibit VEGF signaling, we analyzed the effect of E7080 on cell viability of VEGF-stimulated HUVEC. For this, serum starved HUVEC were stimulated with VEGF (20 ng/ml) and treated with increased levels of E7080 (0.001-100 μmol/l). VEGF-induced cell growth was prevented already at 0.001 μmol/l E7080 and cytotoxic effects were observed at levels of 1.0 μmol/l E7080 (Figure 1B).
It has been hypothesized that the multi-kinase inhibitor E7080 inhibits several receptor tyrosine kinases (RTK) such as VEGF-, PDGF receptors and cKIT. One of the major downstream signaling pathways of these RTK is supposed to be the mitogen-activated protein kinase (MAPK) pathway. HUVEC were serum starved overnight and then stimulated with VEGF (20 ng/ml) in combination with different concentrations of E7080 (0.1 to 100 μmol/l). Addition of VEGF to starved HUVEC led to a pronounced induction of MAPK activity after 5 minutes, shown by induced phosphorylation of extracellular signal regulated kinase (ERK1/2). E7080 inhibits VEGF-induced stimulation of MAPK signaling pathway (Figure 1C). Since MAPK up-regulates the cell proliferation promoting transcription factor MYC, we analyzed the effect of E7080 on MYC expression in VEGF-stimulated HUVEC. For this, we stimulated serum starved HUVEC with VEGF for 8, 16, 24 and 36 hours in the presence or absence of E7080. VEGF leads to an up-regulation of MYC after 8 hours, which was nearly completely abrogated by addition of E7080 (Figure 1D).
E7080 Inhibits Micro Vessel Sprouting in a Three-Dimensional Ex Vivo Mouse Aortic Ring Angiogenesis Assay
The inhibitory effect of E7080 on VEGF-induced angiogenesis was studied in a three-dimensional ex vivo mouse aortic ring angiogenesis assay. The micro vessel sprouting from aortic rings embedded in collagen was stimulated by VEGF (50 ng/ml), leading to a network of vessels around the aortic rings after 11 days. In contrast, the presence of E7080 (1 μmol/l and 10 μmol/l) reduced the VEGF-induced sprouting in a dose-dependent manner (Figure 2).
Figure 2.
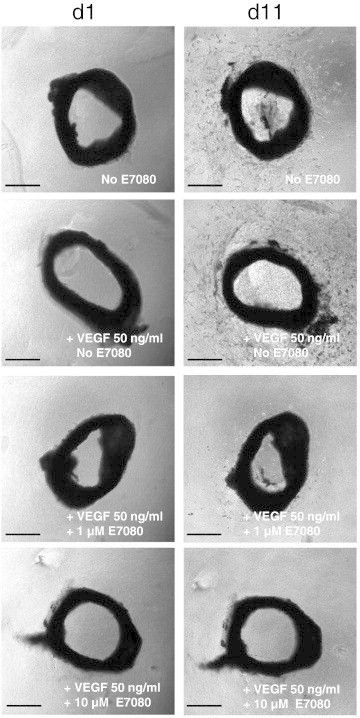
E7080 inhibits VEGF-induced micro vessel sprouting in three-dimensional ex vivo mouse aortic ring angiogenesis assay.
Angiogenesis was induced with 50 ng/ml VEGF and E7080 was used at the indicated final concentrations. Photographs were taken at day 1 and day 11. Representative photographs are shown. Scale bar = 250 μm.
CRC Cells Produce High Levels of VEGF and Express VEGF Receptors
VEGF receptor (VEGFR) expression of CRC cell lines and HUVEC were proved on both mRNA and protein levels. HUVEC and CRC cell lines expressed mRNA of all three VEGFR (Supplementary Figure 1A). In addition, VEGFR 1 and 3 protein expression in all CRC cell lines and HUVEC were demonstrated by immunohistochemical staining (Supplementary Figure 1B). VEGF protein expression was determined in supernatant of CRC cells cultured for 24 hours. All CRC cells secreted large amounts of VEGF without any stimulation. In comparison to CRC cells, no VEGF protein could be determined in supernatant of HUVEC cultures (Figure 3A). To check if E7080 could interrupt the VEGF-mediated interaction between CRC cells and HUVEC, serum starved HUVEC were stimulated with CRC cell supernatant, either with or without E7080 (1 μmol/l) for 24 hours. CRC cell supernatant stimulated HUVEC proliferation, whereas the presence of E7080 prevented the growth induction of HUVEC (Figure 3B, Supplementary Figure 2).
Figure 3.
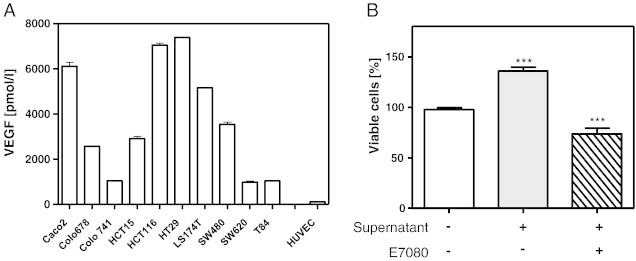
Colorectal carcinoma (CRC) cell-secreted VEGF induces human endothelial cell (HUVEC) proliferation.
(A) Presence of VEGF in the supernatant of CRC cell cultures. VEGF was detected with specific ELISA according to manufacturer’s instructions (R&D Systems GmbH, Germany). For this, 1 million CRC cells and HUVEC were cultured in 6-well plates with serum reduced (1%) RPMI 1640 medium for 24 hours. Levels of detected VEGF ranged from 1000 pmol/l for T84 to over 7000 pmol/l for HT29. In supernatant of HUVEC, detected VEGF levels ranged below 100 pmol/l.
(B) E7080 suppresses HUVEC proliferation in the presence of CRC cell-secreted VEGF. Representative results shown for CaCo2 cells with CRC cell supernatant and/or E7080 (1 μmol/l). See also Supplementary Figure 2. HUVEC (1.5 × 104 cells) were seeded in 96-well plates coated with attachment factor (#S-006-100, Gibco Life Technologies) for 24 hours. The cells were washed with PBS and incubated with 100 μl CRC cell line supernatant, 100 μl fresh serum-reduced (1%) RPMI 1640 medium and E7080 (0.1 μmol/l) for 72 hours. Cell viability was determined with WST-8 assay. ***P < 0.001 to HUVEC control proliferation (without CRC cell supernatant and E7080).
(B) E7080 suppresses HUVEC proliferation in the presence of CRC cell-secreted VEGF. Representative results shown for CaCo2 cells with CRC cell supernatant and/or E7080 (1 μmol/l). See also Supplementary Figure 2. HUVEC (1.5 × 104 cells) were seeded in 96-well plates coated with attachment factor (#S-006-100, Gibco Life Technologies) for 24 hours. The cells were washed with PBS and incubated with 100 μl CRC cell line supernatant, 100 μl fresh serum-reduced (1%) RPMI 1640 medium and E7080 (0.1 μmol/l) for 72 hours. Cell viability was determined with WST-8 assay. ***P < 0.001 to HUVEC control proliferation (without CRC cell supernatant and E7080).
E7080 Delays Growth of CRC Xenografts
The antitumor effect of E7080 was analyzed for human CRC xenografts in nude mice. Subcutaneous implantation of 2.5 × 106 HCT116 or LS174T cells into nude mice has been shown to induce reproducible tumor development within 7 to 10 days. Mice were randomized into a control group treated with saline and an intervention group treated with E7080 when tumors reached a volume of 40-60 mm3. The intervention group received a daily dosage of 5 mg/kg E7080. After 8 to 10 days, tumors of E7080 treated animals demonstrated a significant delay in tumor growth (Figure 4, A and B). E7080 slowed tumor growth but did not cause tumor regression. The experiments were stopped when tumors of control mice reached a tumor volume of 600-800 mm3. Then, tumors were explanted for in vitro evaluation. The volume of E7080 treated HCT116 and LS174T tumors at the end of experiment demonstrated a growth reduction (difference in mean tumor volume between E7080 treated and untreated tumor) between 66 and 72% in comparison to untreated tumors of control animals.
Figure 4.
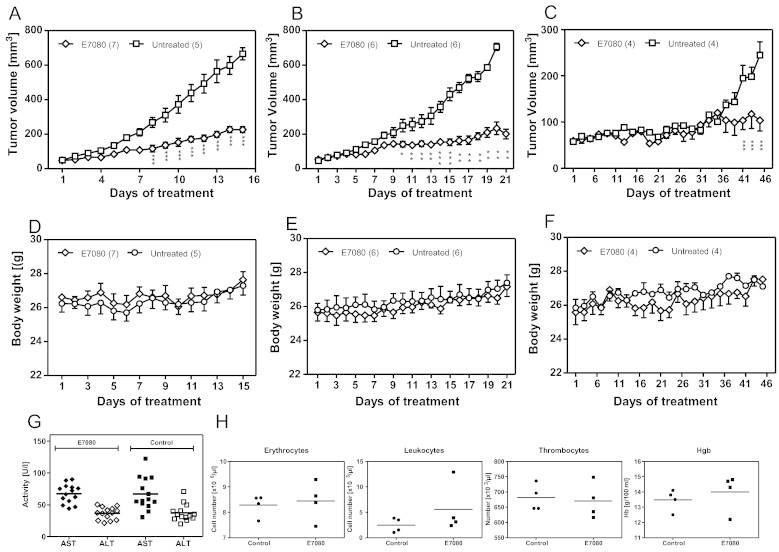
E7080 retards tumor growth in vivo without adverse events.
Xenografts of LS174T (A), HCT116 (B) and patient resection specimens (C). Nude mice received daily 5 mg/kg E7080 orally or vehicle control. (D-F) Body weight was monitored throughout the indicated period. At the end of experiment the activity of liver enzymes alanine transaminase, AST, and aspartate transaminase, ALT (G) and blood parameters (H) were estimated. Values represent means with bars indicating standard errors. *P < 0.05, **P < 0.01, ***P < 0.001 to untreated control.
The effect of E7080 on human CRC xenografts established from primary resection specimens with mutated KRAS was also analyzed in nude mice. Equal amounts of primary CRC samples (one xenograft cube with 3 × 3 × 3 mm) were implanted subcutaneously into nude mice. The growth rate of CRC xenograft samples was much lower compared to xenografts established by HCT116 and LS174T cells. After 36 days of E7080 treatment, a significant difference in tumor growth between E7080 treated and untreated animals was observed. The E7080 induced growth reduction at the end of experiment was 62% (Figure 4C).
The daily application of 5 mg/kg E7080 was well tolerated by the animals without treatment-associated lethality and adverse events such as loss of body weight (Figure 4, D–F). In addition, no differences were observed in liver enzyme activity (Figure 4G), white and red blood count, thrombocytes, and hemoglobin (Figure 4H).
E7080 induces hypoxic conditions in CRC tumors by inhibition of angiogenesis without reduction of Ki67-positive tumor cells
Control and E7080 treated tumors showed central necrosis areas without significant differences in size (data not shown). There was no reduction in the number of Ki67-positive cells in the viable part of tumors in both E7080 treated mice and untreated mice (Figure 5A). E7080 is supposed to inhibit VEGF signaling between tumor cells and endothelial cells within stromal microenvironment and therefore, we evaluated the effect of E7080 on angiogenesis. The CD31 and CD34 staining results showed that E7080 significantly decreased micro vessel density (Figure 5, B and C). A qualitative reduction in vascularity was also observed in primary tumor samples of E7080 treated mice (Supplementary Figure 3). The decreased micro vessel density leads to an insufficient supply with oxygen and, increasing hypoxic conditions within the tumor. Staining with the hypoxia marker carbonic anhydrase 9 (CA9, CAIX) revealed increased areas of hypoxia in tumors of E7080 treated mice (Figure 5, B and C; Supplementary Figure 3). These results indicate that E7080 effectively inhibits angiogenesis in vivo and therefore, delays the growth of CRC tumors.
Figure 5.
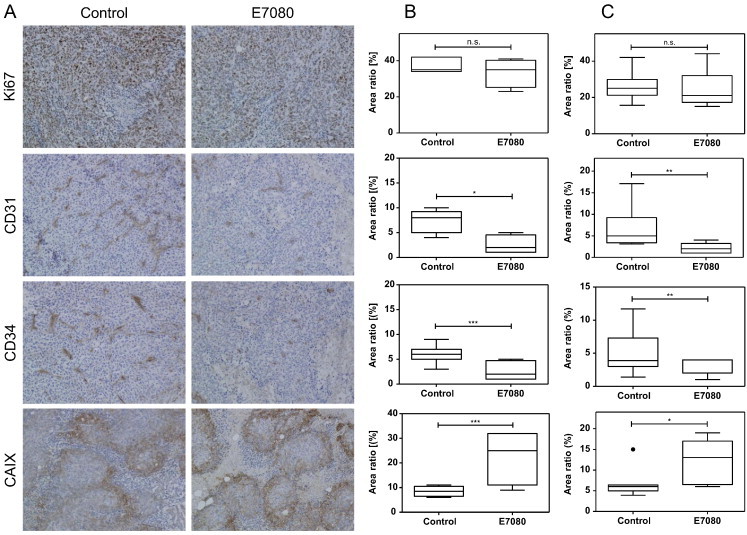
The E7080-mediated influence of microvascularization induces tumor hypoxia.
(A) Representative immunohistochemical staining of untreated and E7080 treated tumors (LS174T). Quantitative analysis of LS174T derived xenografts (B) and HCT116 derived xenografts (C), both KRAS mutated. Tumor sections were stained towards Ki67, CD31, CD34, and CAIX (carbonic anhydrase 9) using standard immunohistochemical procedures as described in Material and Methods. Results are shown as Tukey boxplot with first, second (the median) and third quartiles of four to seven animals (Figure 4) per group. The lower whisker represents the 1.5 interquartile range (IQR) of the lower quartile, and the top whisker represents the 1.5 IQR of the upper quartile. The point indicates an outlier. *P < 0.05, **P < 0.01, ***P < 0.001 to control tumors. For the results of CRC xenografts derived from patient resection specimens with KRAS mutation see Supplementary Figure 3.
(A) Representative immunohistochemical staining of untreated and E7080 treated tumors (LS174T). Quantitative analysis of LS174T derived xenografts (B) and HCT116 derived xenografts (C), both KRAS mutated. Tumor sections were stained towards Ki67, CD31, CD34, and CAIX (carbonic anhydrase 9) using standard immunohistochemical procedures as described in Material and Methods. Results are shown as Tukey boxplot with first, second (the median) and third quartiles of four to seven animals (Figure 4) per group. The lower whisker represents the 1.5 interquartile range (IQR) of the lower quartile, and the top whisker represents the 1.5 IQR of the upper quartile. The point indicates an outlier. *P < 0.05, **P < 0.01, ***P < 0.001 to control tumors. For the results of CRC xenografts derived from patient resection specimens with KRAS mutation see Supplementary Figure 3.
Discussion
In this study we show that the multiple kinase inhibitor, E7080, effectively delays the growth of human CRC xenografts from both HCT116 and LS174T cells (KRAS mutated), respectively, and from primary resection specimens with mutated KRAS in nude mice. The presented in vivo and in vitro data suggest that E7080 suppresses capillary sprouting and inhibits endothelial cell proliferation. The anti-angiogenic effect of E7080 was also observed in a three-dimensional ex vivo mouse aortic ring angiogenesis assay. In addition, the therapy with E7080 in vivo decreased the density of tumor-associated vessel formations that leads to an increase of hypoxic areas within CRC xenografts. Conclusively, these data suggest that the major mechanism by which E7080 interferes with solid tumor growth is apparently the inhibition of angiogenesis, essential for supplying the tumor with oxygen and nutrients.
In the present study we also show that E7080 demonstrates different cytotoxic effects on human CRC cells in vitro. The IC50 values for the 10 CRC cell lines tested ranged between 120 μmol/l und > 1000 μmol/l (Table 1). It is important to emphasize that in vitro assays to determine IC50 values depend to a significant degree on a number of factors, including cell viability, cell proliferation rates, cell confluency at the time of drug exposure and method used to determine cell viability (type of assay). This may explain why IC50 values for certain tumor cell lines can differ [24], [25]. Nevertheless, with this panel of CRC cell lines we demonstrate a relatively low cytotoxic effect of E7080 on CRC cell viability in vitro. In addition, a direct influence of E7080 on the viability of endothelial cells described here was also shown by others [26].
The ability to induce and sustain tumor vascularization by angiogenesis is a hallmark of cancer [27] that correlates with advanced-stage disease and poor prognosis [28]. Solid tumors go through a prolonged state of avascularity in which they are supplied with oxygen and nutrients by simple passive diffusion up to a size of 2 to 4 mm in diameter [29]. Starting from this size, growth and survival of solid tumors require vascularization through angiogenesis and postnatal vasculogenesis [30]. The new blood vessels secure the supply of oxygen and nutrition [29], [31]. Tumor cells secrete numerous growth factors such as VEGF, which is known to be one of the major pro-angiogenic factors [4], [32]. VEGF stimulates both the proliferation and migration of endothelial cells leading to new vessel formation [33]. In tumor cells, VEGF signalling affects tumor function independently of angiogenesis [4]. Our data show that the cells lines used here produce VEGF, which induces the growth of human endothelial cells including HUVEC. The VEGF-induced growth of HUVEC was inhibited by low dose E7080 (0.1 μmol/l). CRC cell lines were relatively resistant to the treatment with E7080 in vitro and the E7080 concentration, which was toxic for HUVEC (IC50: 22 μmol/l), did not influence CRC cell viability. HUVEC treated with low dose E7080 (10 μmol/l) demonstrated a dramatic inhibition of cell viability by blocking the induction of VEGFR-mediated downstream MAPK signaling. In this respect, our results are in line with previous reports [26]. Furthermore, our data show a dramatic reduction of MYC up-regulation in HUVEC by E7080 after VEGF stimulation. MYC is a helix-loop-helix leucine zipper transcription factor and is one of the major downstream targets of the active MAPK-signaling pathway. VEGF has been demonstrated to induce MYC expression in human endothelial cells [34], [35] and up-regulation of MYC in turn has been shown to lead to cell proliferation [36].
The present results of CD31 and CD34 staining show that E7080 significantly decreases the tumor micro vessel density, which might prevent exponential tumor growth by limiting the tumor supply with essential oxygen and nutrients. The undersupply with oxygen in turn increases hypoxic conditions within the tumor. Staining with the hypoxia marker carbonic anhydrase 9 [37] revealed an increase in hypoxic areas within solid tumors grown in E7080-treated mice. Carbonic anhydrase 9 is a hypoxia-inducible protein that regulates cellular pH to promote cancer cell survival and invasion in hypoxic microenvironments. It is also a biomarker of poor prognosis for breast cancer metastasis and patient survival [38]. It is well known, that tumor cells perfectly adapt to hypoxic conditions by constitutive up-regulation of glycolysis with excessive oxygen-independent glucose degradation [39], [40]. The basis of this adaption is the up-regulation of glycolytic enzymes mediated by hypoxia-inducible factor 1α (HIF-1α) and proto-oncogenes like KRAS and MYC or tumor suppressor genes like p53 [39], [41].
CRC is one of the most common cancers and accounts for about 500,000 deaths worldwide every year [1]. Whereas a five-year survival rate of 90% for local limited CRC without lymph node metastasis is relatively good, prognosis drops to less than 10% in the case of distant metastasis. A combination of chemotherapy and a targeted therapy directed against VEGF or EGFR is presently used for metastatic CRC, either as palliative or neoadjuvant therapy [42]. Currently, KRAS mutation status of CRC is used to predict the outcome for treatment with monoclonal antibodies. Patients with CRC harboring wild-type KRAS may benefit from anti-EGFR antibody therapy with Cetuximab [43], whereas patients with KRAS mutated CRC are currently treated with the anti-VEGF antibody Bevacizumab in combination with chemotherapy. Nevertheless, the therapeutic options for patients with KRAS mutated CRC are limited. First, the treatment duration for classic chemotherapeutics is restricted due to cumulative dosage toxicity. Second, addition of Bevacizumab to classic chemotherapy has only a small positive effect. Third, Bevacizumab has to be administered intravenously, making it impractical for maintenance therapy. Therefore, new therapeutic agents are needed to improve the survival of this patient group.
E7080 is currently under evaluation in several clinical trials [16], [44], [45], [46]. Recently, the FDA approved the multi-kinase receptor inhibitor Regorafenib for metastatic colorectal cancer [14]. In comparison to Regorafenib, E7080 shows a more selective inhibition profile for VEGFR2 and VEGFR3, whereas Regorafinib also inhibits several members of the MAPK-signaling pathway, including bRAFV600. With this ability, Regorafenib may also demonstrate a direct inhibition of tumor growth rather than interfering with the interaction between cancer cells and endothelial cells. The application of Regorafenib is, however, limited due to adverse drug effects.
In summary, we have shown that E7080 (Lenvatinib), a multi-tyrosine kinase inhibitor, suppresses in vivo angiogenesis at a dosage of 5 mg/kg and delays the growth of xenografts with KRAS mutation. These findings suggest a therapeutic potential of E7080 for treatment of patients with KRAS mutated CRC that have no benefit from a first line treatment with Cetuximab [43]. In addition, our findings implicate the need to combine E7080 with chemotherapeutics such as FOLFOX in future studies to further enhance its efficacy.
The following are the Supplementary data related to this article.
Expression of VEGF receptor (VEGFR) families in human colorectal carcinoma (CRC) cells and endothelial cells (HUVEC).
A) Representative RT-PCR results of CRC cells and HUVEC for VEGFR1, VEGFR2 and VEGFR3. The following primer pairs were used: VEGFR1 (access No. NM_002019.4), forward: CCC GAG CCT CAG ATC ACT TG, reverse: GTG CTG CTT CCT GGT CCT AA (83 bp); VEGFR2 (access No. AF063658.1), forward: ATG GAG AGC AAG GTG CTG C; reverse: GAG CCT GGG CAG ATC AAG AG (102 bp); VEGFR3 (access No. AB209637.1), forward: CTG AAA GGT GTG GGG TCA GC, reverse: CAG TCT CCC TTC CCG AGT TG (100 bp). The cycler protocol was 5 min at 95°C, 40 cycles of 15 sec at 95°C, 60 sec at 60°C, and 5 min at 72°C. B) Representative immunohistochemical staining of CRC cells and HUVEC for VEGFR1 and VEGFR3. Cytospins were stained with anti-VEGFR1 (#AF321; 1:40; R&D), anti-VEGFR3 (#AF349; 1:40; R&D Systems GmbH, Germany), and rabbit-anti-goat HRP (#0449; 1:100; DAKO, Hamburg, Germany). Stained slides were photographed at 20x magnification with a Keyence Biorevo BZ-9000 Microscope (Keyence Corporation, Osaka, Japan) applying Z-stack technology to improve the quality of images.
E7080 suppresses human endothelial (HUVEC) proliferation in the presence of colorectal carcinoma (CRC) cell-secreted VEGF.
All tested CRC cell lines secreted VEGF detected with specific ELISA according to manufacturer’s instructions (R&D Systems GmbH, Germany). CRC cell supernatant stimulate HUVEC proliferation, whereas the presence of E7080 (1 μmol/l) prevented the growth induction of HUVEC. See also Figure 3. ***P < 0.001 to HUVEC control proliferation (without CRC cell supernatant and E7080).
Immunohistochemical evaluation of human colorectal carcinoma (CRC) xenografts established from patient primary resection specimens in nude mice treated with E7080.
Tumor sections were stained towards Ki67, CD34, and CAIX (carbonic anhydrase 9) using standard immunohistochemical procedures as described in Material and Methods. Stained slides were photographed at 20x magnification with a Keyence Biorevo BZ-9000 Microscope (Keyence Corporation, Osaka, Japan) applying Z-stack technology. The number of stained cells per section was quantified by using measurement module BZ-H3C (Hybrid Cell Count Vers.1.1, Keyence). Results are shown as Tukey boxplot with first, second (the median) and third quartiles of 4 animals Figure 4) per group. The lower whisker represents the 1.5 interquartile range (IQR) of the lower quartile, and the top whisker represents the 1.5 IQR of the upper quartile. *P < 0.05, **P < 0.01 to control tumors.
Acknowledgments
The authors are grateful to Dr. Irina Chodnevskaja, Sabine Gahn, Veronika Heimbach, Michaela Kapp, Monika Koospal, Bettina Mühling, and Manuela Schneider for their skilful assistance with the experiments; and to Dr S. Leo, KEYENCE INTERNATIONAL (Belgium). The work was supported by the Deutsche Forschungsgemeinschaft (DFG), Grant 1516/2-1 (TH), and by funds from the Interdisciplinary Centre for Clinical Research (IZKF) of the University of Würzburg (B-186 to AW, B-121 to AT, and D-150 to CO). The authors assume full responsibility for the contents of the research. This publication was funded by the German Research Foundation (DFG), and the University of Würzburg is in the funding program “Open Access Publishing”.
Contributor Information
Armin Wiegering, Email: otto_c@ukw.de.
Christoph Otto, Email: Otto_c@ukw.de.
References
- 1.Siegel R., Desantis C., Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64(2):104–117. doi: 10.3322/caac.21220. [DOI] [PubMed] [Google Scholar]
- 2.Lorenz M., Staib-Sebler E., Hochmuth K., Heinrich S., Gog C., Vetter G., Encke A., Muller H.H. Surgical Resection of Liver Metastases of Colorectal Carcinoma: Short and Long-Term Results. Semin Oncol. 2000;27(5 Suppl. 10):112–119. [PubMed] [Google Scholar]
- 3.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1(1):27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 4.Goel H.L., Mercurio A.M. VEGF targets the tumour cell. Nat Rev Cancer. 2013;13(12):871–882. doi: 10.1038/nrc3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cunningham D., Humblet Y., Siena S., Khayat D., Bleiberg H., Santoro A., Bets D., Mueser M., Harstrick A., Verslype C. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;4:337–345. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 6.Hurwitz H., Fehrenbacher L., Novotny W., Cartwright T., Hainsworth J., Heim W., Berlin J., Baron A., Griffing S., Holmgren E. Bevacizumab Plus Irinotecan, Fluorouracil, and Leucovorin for Metastatic Colorectal Cancer. N Engl J Med. 2004;23:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 7.Hubbard J., Grothey A. Antiangiogenesis agents in colorectal cancer. Curr Opin Oncol. 2010;22:374–380. doi: 10.1097/CCO.0b013e328339524e. [DOI] [PubMed] [Google Scholar]
- 8.Saltz L.B., Clarke S., Díaz-Rubio E., Scheithauer W., Figer A., Wong R., Koski S., Lichinitser M., Yang T.S., Rivera F. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26:2013–2019. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- 9.Bos J.L., Fearon E.R., Hamilton S.R. Prevalence of ras gene mutations in human colorectal cancers. Nature. 1987;327:293–297. doi: 10.1038/327293a0. [DOI] [PubMed] [Google Scholar]
- 10.Mulder K., Scarfe A., Chua N., Spratlin J. The role of bevacizumab in colorectal cancer: understanding its benefits and limitations. Expert Opin Biol Ther. 2011;11:405–413. doi: 10.1517/14712598.2011.557657. [DOI] [PubMed] [Google Scholar]
- 11.Marques I., Araújo A., de Mello R.A. Anti-angiogenic therapies for metastatic colorectal cancer: current and future perspectives. World J Gastroenterol. 2013;19(44):7955–7971. doi: 10.3748/wjg.v19.i44.7955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shamloo B.K., Chhabra P., Freedman A.N., Potosky A., Malin J., Weiss Smith S. Novel adverse events of bevacizumab in the US FDA adverse event reporting system database: a disproportionality analysis. Drug Saf. 2012;35:507–518. doi: 10.2165/11597600-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 13.Yancopoulos G.D., Davis S., Gale N.W., Rudge J.S., Wiegand S.J., Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 14.Abou-Elkacem L., Arns S., Brix G., Gremse F., Zopf D., Kiessling F., Lederle W. Regorafenib inhibits growth, angiogenesis, and metastasis in a highly aggressive, orthotopic colon cancer model. Mol Cancer Ther. 2013;12:1322–1331. doi: 10.1158/1535-7163.MCT-12-1162. [DOI] [PubMed] [Google Scholar]
- 15.Grothey A., Van Cutsem E., Sobrero A., Siena S., Falcone A., Ychou M., Humblet Y., Bouché O., Mineur L., Barone C. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:303–312. doi: 10.1016/S0140-6736(12)61900-X. [DOI] [PubMed] [Google Scholar]
- 16.Matsui J., Yamamoto Y., Funahashi Y., Tsuruoka A., Watanabe T., Wakabayashi T., Uenaka T., Asada M. E7080, a novel inhibitor that targets multiple kinases, has potent antitumor activities against stem cell factor producing human small cell lung cancer H146, based on angiogenesis inhibition. Int J Cancer. 2008;122(3):664–671. doi: 10.1002/ijc.23131. [DOI] [PubMed] [Google Scholar]
- 17.Okamoto K., Kodama K., Takase K., Sugi N.H., Yamamoto Y., Iwata M., Tsuruoka A. Antitumor activities of the targeted multi-tyrosine kinase inhibitor lenvatinib (E7080) against RET gene fusion-driven tumor models. Cancer Lett. 2013;340(1):97–103. doi: 10.1016/j.canlet.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 18.Matsui J., Funahashi Y., Uenaka T., Watanabe T., Tsuruoka A., Asada M. Multi-kinase inhibitor E7080 suppresses lymph node and lung metastases of human mammary breast tumor MDA-MB-231 via inhibition of vascular endothelial growth factor-receptor (VEGF-R) 2 and VEGF-R3 kinase. Clin Cancer Res. 2008;14:5459–5465. doi: 10.1158/1078-0432.CCR-07-5270. [DOI] [PubMed] [Google Scholar]
- 19.Ikuta K., Yano S., Trung V.T., Hanibuchi M., Goto H., Li Q., Wang W., Yamada T., Ogino H., Kakiuchi S. E7080, a multi-tyrosine kinase inhibitor, suppresses the progression of malignant pleural mesothelioma with different proangiogenic cytokine production profiles. Clin Cancer Res. 2009;15:7229–7237. doi: 10.1158/1078-0432.CCR-09-1980. [DOI] [PubMed] [Google Scholar]
- 20.Glen H., Mason S., Patel H., Macleod K., Brunton V.G. E7080, a multi-targeted tyrosine kinase inhibitor suppresses tumor cell migration and invasion. BMC Cancer. 2011;22(11):309. doi: 10.1186/1471-2407-11-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maletzki C., Stier S., Gruenert U., Gock M., Ostwald C., Prall F., Linnebacher M. Establishment, characterization and chemosensitivity of three mismatch repair deficient cell lines from sporadic and inherited colorectal carcinomas. PLoS One. 2012;7(12):e52485. doi: 10.1371/journal.pone.0052485. [Epub 2012 Dec 31] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Otto C., Kämmerer U., Illert B., Mühling M., Pfetzer N., Wittig R., Ulrich Völker H.U., Thiede A., Coy J.F. Growth of human gastric cancer cells in nude mice is delayed by a ketogenic diet supplemented with omega-3 fatty acids and medium-chain triglycerides. BMC Cancer. 2008;30(8):122. doi: 10.1186/1471-2407-8-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Illert B., Otto C., Thiede A., Timmermann W. Detection of disseminated tumor cells in nude mice with human gastric cancer. Clin Exp Metastasis. 2003;20(6):549–554. doi: 10.1023/a:1025862800798. [DOI] [PubMed] [Google Scholar]
- 24.Altun A., Temiz T.K., Balcı E., Polat Z.A., Turan M. Effects of tyrosine kinase inhibitor E7080 and eNOS inhibitor L-NIO on colorectal cancer alone and in combination. Chin J Cancer Res. 2013;25(5):572–584. doi: 10.3978/j.issn.1000-9604.2013.10.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Altun A., Turgut N.H., Kaya T.T. Anticancer effect of COX-2 inhibitor DuP-697 alone and in combination with tyrosine kinase inhibitor (E7080) on colon cancer cell lines. Asian Pac J Cancer Prev. 2014;15(7):3113–3121. doi: 10.7314/apjcp.2014.15.7.3113. [DOI] [PubMed] [Google Scholar]
- 26.Ogino H., Hanibuchi M., Kakiuchi S., Trung V.T., Goto H., Ikuta K., Yamada T., Uehara H., Tsuruoka A., Uenaka T. E7080 suppresses hematogenous multiple organ metastases of lung cancer cells with nonmutated epidermal growth factor receptor. Mol Cancer Ther. 2011;10:1218–1228. doi: 10.1158/1535-7163.MCT-10-0707. [DOI] [PubMed] [Google Scholar]
- 27.Hanahan D., Weinberg R.A. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 28.Bergers G., Benjamin L.E. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–410. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- 29.Folkman J., Merler E., Abernathy C., Williams G. Isolation of a tumor factor responsible for angiogenesis. J Exp Med. 1971;133(2):275–288. doi: 10.1084/jem.133.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Albini A., Tosetti F., Li V.W., Noonan D.M., Li W.W. Cancer prevention by targeting angiogenesis. Nat Rev Clin Oncol. 2012;9(9):498–509. doi: 10.1038/nrclinonc.2012.120. [DOI] [PubMed] [Google Scholar]
- 31.Hanahan D., Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86(3):353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 32.Kim K.J., Li B., Winer J., Armanini M., Gillett N., Phillips H.S., Ferrara N. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;362(6423):841–844. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- 33.Chen C.T., Hung M.C. Beyond anti-VEGF: dual-targeting antiangiogenic and antiproliferative therapy. Am J Transl Res. 2013;5:393–403. [PMC free article] [PubMed] [Google Scholar]
- 34.Funovics P., Brostjan C., Nigisch A., Fila A., Grochot A., Mleczko K., Was H., Weigel G., Dulak J., Jozkowicz A. Effects of 15d-PGJ(2) on VEGF-induced angiogenic activities and expression of VEGF receptors in endothelial cells. Prostaglandins Other Lipid Mediat. 2006;79:230–244. doi: 10.1016/j.prostaglandins.2006.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hurley N.E., Schildmeyer L.A., Bosworth K.A., Sakurai Y., Eskin S.G., Hurley L.H., McIntire L.V. Modulating the functional contributions of c-Myc to the human endothelial cell cyclic strain response. J Vasc Res. 2010;47(1):80–90. doi: 10.1159/000235928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adhikary S., Eilers M. Transcriptional regulation and transformation by Myc proteins. Nat Rev Mol Cell Biol. 2005;6(8):635–645. doi: 10.1038/nrm1703. [DOI] [PubMed] [Google Scholar]
- 37.Olive P.L., Aquino-Parsons C., MacPhail S.H., Liao S.Y., Raleigh J.A., Lerman M.I., Stanbridge E.J. Carbonic anhydrase 9 as an endogenous marker for hypoxic cells in cervical cancer. Cancer Res. 2001;61(24):8924–8929. [PubMed] [Google Scholar]
- 38.Lock F.E., McDonald P.C., Lou Y., Serrano I., Chafe S.C., Ostlund C., Aparicio S., Winum J.Y., Supuran C.T., Dedhar S. Targeting carbonic anhydrase IX depletes breast cancer stem cells within the hypoxic niche. Oncogene. 2013;32(44):5210–5219. doi: 10.1038/onc.2012.550. [DOI] [PubMed] [Google Scholar]
- 39.Gatenby R.A., Gillies R.J. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004;4(11):891–899. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- 40.Cairns R.A., Harris I.S., Mak T.W. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11(2):85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- 41.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 42.Winder T., Lenz H.J. Vascular endothelial growth factor and epidermal growth factor signaling pathways as therapeutic targets for colorectal cancer. Gastroenterology. 2010;138:2163–2176. doi: 10.1053/j.gastro.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 43.Amado R.G., Wolf M., Peeters M., Van Cutsem E., Siena S., Freeman D.J., Juan T., Sikorski R., Suggs S., Radinsky R. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26(10):1626–1634. doi: 10.1200/JCO.2007.14.7116. [DOI] [PubMed] [Google Scholar]
- 44.Yamada K., Yamamoto N., Yamada Y., Nokihara H., Fujiwara Y., Hirata T., Koizumi F., Nishio K., Koyama N., Tamura T. Phase I dose-escalation study and biomarker analysis of E7080 in patients with advanced solid tumors. Clin Cancer Res. 2011;17:2528–2537. doi: 10.1158/1078-0432.CCR-10-2638. [DOI] [PubMed] [Google Scholar]
- 45.Boss D.S., Glen H., Beijnen J.H., Keesen M., Morrison R., Tait B., Copalu W., Mazur A., Wanders J., O'Brien J.P. A phase I study of E7080, a multitargeted tyrosine kinase inhibitor, in patients with advanced solid tumours. Br J Cancer. 2012;106:1598–1604. doi: 10.1038/bjc.2012.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Molina A.M., Hutson T.E., Larkin J., Gold A.M., Wood K., Carter D., Motzer R., Michaelson M.D. A phase 1b clinical trial of the multi-targeted tyrosine kinase inhibitor lenvatinib (E7080) in combination with everolimus for treatment of metastatic renal cell carcinoma (RCC) Cancer Chemother Pharmacol. 2014;73:181–189. doi: 10.1007/s00280-013-2339-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Expression of VEGF receptor (VEGFR) families in human colorectal carcinoma (CRC) cells and endothelial cells (HUVEC).
A) Representative RT-PCR results of CRC cells and HUVEC for VEGFR1, VEGFR2 and VEGFR3. The following primer pairs were used: VEGFR1 (access No. NM_002019.4), forward: CCC GAG CCT CAG ATC ACT TG, reverse: GTG CTG CTT CCT GGT CCT AA (83 bp); VEGFR2 (access No. AF063658.1), forward: ATG GAG AGC AAG GTG CTG C; reverse: GAG CCT GGG CAG ATC AAG AG (102 bp); VEGFR3 (access No. AB209637.1), forward: CTG AAA GGT GTG GGG TCA GC, reverse: CAG TCT CCC TTC CCG AGT TG (100 bp). The cycler protocol was 5 min at 95°C, 40 cycles of 15 sec at 95°C, 60 sec at 60°C, and 5 min at 72°C. B) Representative immunohistochemical staining of CRC cells and HUVEC for VEGFR1 and VEGFR3. Cytospins were stained with anti-VEGFR1 (#AF321; 1:40; R&D), anti-VEGFR3 (#AF349; 1:40; R&D Systems GmbH, Germany), and rabbit-anti-goat HRP (#0449; 1:100; DAKO, Hamburg, Germany). Stained slides were photographed at 20x magnification with a Keyence Biorevo BZ-9000 Microscope (Keyence Corporation, Osaka, Japan) applying Z-stack technology to improve the quality of images.
E7080 suppresses human endothelial (HUVEC) proliferation in the presence of colorectal carcinoma (CRC) cell-secreted VEGF.
All tested CRC cell lines secreted VEGF detected with specific ELISA according to manufacturer’s instructions (R&D Systems GmbH, Germany). CRC cell supernatant stimulate HUVEC proliferation, whereas the presence of E7080 (1 μmol/l) prevented the growth induction of HUVEC. See also Figure 3. ***P < 0.001 to HUVEC control proliferation (without CRC cell supernatant and E7080).
Immunohistochemical evaluation of human colorectal carcinoma (CRC) xenografts established from patient primary resection specimens in nude mice treated with E7080.
Tumor sections were stained towards Ki67, CD34, and CAIX (carbonic anhydrase 9) using standard immunohistochemical procedures as described in Material and Methods. Stained slides were photographed at 20x magnification with a Keyence Biorevo BZ-9000 Microscope (Keyence Corporation, Osaka, Japan) applying Z-stack technology. The number of stained cells per section was quantified by using measurement module BZ-H3C (Hybrid Cell Count Vers.1.1, Keyence). Results are shown as Tukey boxplot with first, second (the median) and third quartiles of 4 animals Figure 4) per group. The lower whisker represents the 1.5 interquartile range (IQR) of the lower quartile, and the top whisker represents the 1.5 IQR of the upper quartile. *P < 0.05, **P < 0.01 to control tumors.


