Abstract
Predator–prey relationships and trophic levels are indicators of community structure, and are important for monitoring ecosystem changes. Mammals colonized the marine environment on seven separate occasions, which resulted in differences in species' physiology, morphology and behaviour. It is likely that these changes have had a major effect upon predator–prey relationships and trophic position; however, the effect of environment is yet to be clarified. We compiled a dataset, based on the literature, to explore the relationship between body mass, trophic level and predator–prey ratio across terrestrial (n = 51) and marine (n = 56) mammals. We did not find the expected positive relationship between trophic level and body mass, but we did find that marine carnivores sit 1.3 trophic levels higher than terrestrial carnivores. Also, marine mammals are largely carnivorous and have significantly larger predator–prey ratios compared with their terrestrial counterparts. We propose that primary productivity, and its availability, is important for mammalian trophic structure and body size. Also, energy flow and community structure in the marine environment are influenced by differences in energy efficiency and increased food web stability. Enhancing our knowledge of feeding ecology in mammals has the potential to provide insights into the structure and functioning of marine and terrestrial communities.
Keywords: predator–prey ratio, phylogenetic comparative analysis, macroecology, carnivore
1. Introduction
Mammals are a diverse group of organisms spanning eight orders of magnitude in body mass, exploiting a variety of habitats and niches, and they encompass a range of feeding ecologies [1,2]. These characteristics make mammals ideal to investigate patterns in trophic level. Mammals have re-entered the marine environment on seven separate occasions, and there are five extant clades: Cetacea, Sirenia, Pinnipedia, Ursus maritimus and Enhydra lutris [3]. This provides a unique opportunity to explore the possible changes that have occurred as mammals moved into an environment where not only physiological and morphological modifications have taken place, but also additional behavioural changes associated with foraging ecology.
Two relationships used to investigate the feeding ecology of carnivorous species include the association between trophic level and body mass, as well as the relationship between trophic level and predator–prey body mass ratios. Depending on the complexity of the ecosystem, carnivores are not always secondary consumers. For example, a carnivore from a complex food web with more than five trophic levels will sit higher in the food chain than a carnivore in a simple food web with just three trophic levels [4]. Also, as large mammalian carnivores tend to feed on larger prey, due to their high energetic requirements [5], larger carnivores also tend to have higher trophic positions. This is linked with the idea that food webs are size structured; for example, a large carnivore targeting larger prey (e.g. fish) will have a higher trophic level than a carnivore feeding upon smaller prey (e.g. zooplankton).
Productivity differs between the marine and terrestrial environments. In the ocean, primary producers represent approximately 0.2% of the global primary-producer biomass; however, turnover rate (i.e. carbon productivity) is greater in the marine environment (up to 1000 times higher [6,7]). In combination with dominance of single-celled plants such as phytoplankton, energy flow is faster and more easily accessible to consumers within the marine environment. Where terrestrial primary producers represent a higher proportion of the earth's primary-producer biomass (approx. 99.8%), their net turnover rate is much slower than the oceanic primary producers (e.g. carbon turnover 19 years for terrestrial versus 2–6 days for marine [8,9]). Compared with single-celled species, the multicellular plants that are dominant on land are more difficult for consumers to process and extract energy from. In the marine environment, as the majority of primary production is driven by small single-celled organisms, aquatic systems tend to be heavily size structured. Trophic interactions are driven by large consumers feeding on smaller species [10].
The relationship between trophic level and body mass across mammals has three possible patterns that we aim to test. (i) Differences in the environmental characteristics are driving trophic level patterns across mammals, with a positive relationship expected between trophic level and body mass in both marine and terrestrial species, as demonstrated by Riede et al. [11]. This would mean that differences in the food web structure and the number of trophic levels [12] would result in marine mammals having a higher intercept for trophic level than terrestrial mammals. (ii) Body mass, regardless of environment, is driving trophic level patterns. If body mass is the key driver for trophic level patterns, then the relationship should remain the same with the addition of marine species. (iii) The trophic-level–body-mass relationship would be quadratic (i.e. hump shaped) because the addition of the marine species complicates the relationship. The positive relationship between trophic level and body mass ‘holds up’ to a maximum threshold, where the relationship then shifts and becomes negative due to the largest mammals (the mysticete whales) feeding upon small invertebrates situated at low trophic levels [13]. In this scenario, the terrestrial carnivores contribute to the initial positive relationship between trophic level and body size [11].
Predator–prey body mass ratios (PP ratios) provide information on food web complexity, ecosystem stability and community structure [14,15]. Food webs are usually more stable when predators are larger than their prey [16,17], as this minimizes the chance that new predators invade and outcompete current predatory species [15,18]. There are exceptions, however, such as pack-hunting mammalian carnivores (e.g. wolves) and large cats (e.g. tigers). It has been demonstrated across whole food webs that PP ratios vary with the trophic position of the predator, where the PP ratio approaches one with increasing trophic level [11] (i.e. the predators with high trophic positions consume prey more similar to their own body size).
Using diet information and trophic-level data for terrestrial and marine mammals, we investigate how living within the marine or the terrestrial environment has impacted the relationship between body size, trophic level and PP ratio across mammals. To achieve this we (i) examine how the differences between consumers within terrestrial and marine environments influence the trophic and food web structure of mammals, and (ii) test whether the negative relationship between the PP ratio and the predator trophic level is a general rule across mammals by examining this relationship with the addition of marine mammals.
2. Material and methods
(a). Database
(i). Trophic level
We analysed trophic level and species' body masses from 107 carnivorous mammal species across marine (n = 56) and terrestrial (n = 51) environments. We chose these 107 species based upon the availability of detailed dietary information within the literature, which also means that the data are skewed towards species that have been well studied. Carnivores were defined as those species with diets comprising at least 90% meat, with insectivores also classified as carnivores [19]. Trophic level positions for marine mammals are readily available in the literature [13]; however, this information is more difficult to obtain for terrestrial mammals and had to be calculated. To achieve this, terrestrial prey preference data were collected from the literature (i.e. the proportion of prey species consumed by that carnivore), and these data included all species preyed upon by the carnivore species. Each prey species was assigned a trophic position, where herbivorous prey species were assigned a trophic level of 2, omnivorous prey species were assigned 2.5 and carnivorous prey species were assigned 3. Combining the information on the carnivore prey preference and the trophic level of the prey, the carnivore trophic level was then calculated using the following equation from Pauly et al. [13]:
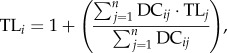 |
2.1 |
where DCij is the diet composition with the proportion of prey (j) in the diet of species (i), TLj is the trophic level of prey (j) and n is the number of groups in the system. Body mass data were log10-transformed prior to analysis.
(ii). Predator–prey body mass
Predator and prey body masses were extracted from the literature (n = 107). Where predators consume more than one prey item, we calculated the mean prey size that represented the common prey items consumed by that species. PP ratio was calculated for the carnivorous species by dividing the average mass of each predator species by their average prey mass.
(b). Phylogeny construction
Owing to the absence of a single phylogeny with all species of interest, a composite tree was created by combining information from several sources (see electronic supplementary material, figure S1). The majority of the phylogenetic information was based on the mammalian supertree of Fritz et al. [20], in which branch lengths were proportional to time since divergence. Divergence times were based on molecular clock analysis, and the tree included fossil data for calibration [21]. Two species were positioned within the pruned trees based on the topologies of the following sources: Sciurus aberti [22] and Sotalia guianensis [23]. Carnivora and Cetacea positioning were updated using the recently published trees by Slater et al. [24] and Nyakatura & Bininda-Emonds [25], respectively. All tree manipulations were performed using Mesquite v. 2.74 [26].
(c). Analysis
A model selection approach was applied to test the level of support for alternative models of trophic level patterns in carnivorous mammals: (i) a body mass and environment model (β0 + βmass + βenvironment), where both body mass and whether species occupied a terrestrial or marine environment explained differences in trophic-level position and environment was coded in a binary fashion as living in either the terrestrial (0) or marine (1) environment; (ii) a body mass and environment model with an interaction term (β0 + βmass + βenvironment + βmass × βenvironment), which is the same as the previous model but with an interaction term (i.e. βmass × βenvironment) to test for differences in allometry in relation to the physical environment; (iii) a quadratic model (β0 + βmass
+
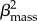 ), where a quadratic term was added (
), where a quadratic term was added (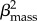 ) to explained differences in trophic-level position and body mass across all species as a quadratic relationship; (iv) a body mass model (β0 + βmass), which predicted differences in trophic level among species was exclusively explained by body size; and (v) a null model (β0), where no predictor variable was included and the variance in species trophic level was modelled as the outcome of Brownian evolution and stochastic factors associated with evolutionary differentiation.
) to explained differences in trophic-level position and body mass across all species as a quadratic relationship; (iv) a body mass model (β0 + βmass), which predicted differences in trophic level among species was exclusively explained by body size; and (v) a null model (β0), where no predictor variable was included and the variance in species trophic level was modelled as the outcome of Brownian evolution and stochastic factors associated with evolutionary differentiation.
The likelihood that a given model explained species differences in trophic level was assessed via second-order Akaike's information criterion with a correction for sample size (AICc) [27,28]. The model with the lowest AICc value reflects the model with the highest support, with any other models within two units of this lowest model also considered to be likely candidates (i.e. ΔAIC < 2.0 [29]). To compute AICc values, we applied each model as a phylogenetic generalized least squares (PGLS) regression using compare v. 4.6b [30]. Log-likelihood estimates are produced for each model, which are then converted into AICc values following equations presented by Burnham & Anderson [31]. Output from these analyses also included an estimate of α, which is the maximum-likelihood estimate of the strength of evolutionary constraint in phenotypic diversification (trophic level evolution). When α is close to 0, evolutionary diversification has been strongly correlated with phylogeny, while values close to 15.50 suggest little correlation with phylogeny [32]. We also reported the phylogenetic effect size, r, computed for each model. Finally, for the model that received the most support, we extracted the 95% confidence intervals (CIs) of the slope values and assessed the allometric effects associated with predictor variables.
The relationship between trophic level and PP ratio was investigated using PGLS regression. For all PGLS regression results, significance was deemed when the CIs did not overlap 0. Differences in body mass associated with diet across the two environments were examined using ANOVA and R v. 2.13.2 [33], respectively. Sample sizes for the diet categories in each environment were as follows: marine carnivores (n = 56), marine herbivores (n = 5), terrestrial carnivores (n = 51) and terrestrial herbivores (n = 99).
3. Results
(a). Trophic level patterns
A model including body mass and physical environment (marine versus terrestrial) was the best-supported model for predicting the evolution of trophic level in carnivorous mammals. This model explained 46% of the variance in trophic level among species (table 1). The second model with the most support included the interaction term of βmass × βenvironment. This model was within 2 units of the best model and was therefore considered to be equally supported (table 1). The remaining models, with ΔAICc greater than 20, had virtually no support. Examining the parameters of the β0 + βmass + βenvironment + βmass × βenvironment model, the interaction term was not significant (CIs −0.24, 0.14), which indicated that the slope of the relationship between trophic level and body mass is the same across the two environments. This means that the results of the two best-supported models (β0 + βmass + βenvironment and β0 + βmass + βenvironment + βmass × βenvironment) are essentially identical.
Table 1.
Level of support for explanatory models of trophic level evolution in mammals. Results are from PGLS regressions computed for the mammalian phylogeny.
| model | ΔAICc | PGLS α | effect size (r) |
|---|---|---|---|
| β0 + βmass + βenvironment | 0.00 | 8.55 | 0.69 |
| β0 + βmass + βenvironment + βmass × βenvironment | 1.68 | 8.71 | 0.69 |
| β0 + βmass | 27.30 | 2.65 | 0.11 |
β0 + βmass
+
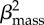
|
32.07 | 2.00 | 0.08 |
| β0 | 44.36 | 0.78 | — |
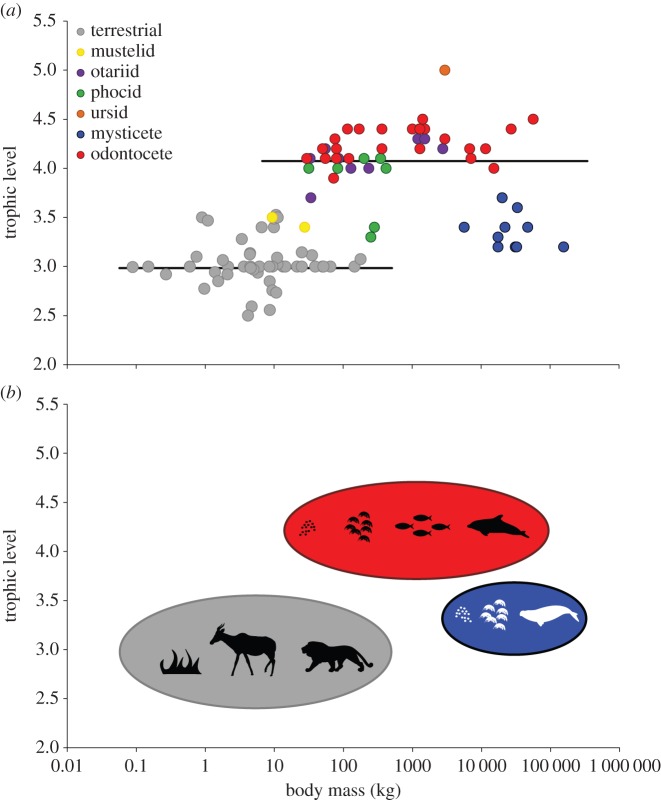
There is a significant difference (CIs 0.71, 1.39; figure 1a) in the intercept of trophic level between marine and terrestrial species. The mean trophic level for terrestrial carnivores was lower (2.7) than that of marine carnivores (3.9). The relationship between mass and trophic level was not significant (CIs −0.08, 0.06), indicating that there is no relationship between mass and trophic level in either environment. For marine species, there is a slight negative (slope = −0.02) relationship between trophic level and increasing mass; however, this is not significant.
Figure 1.
(a) Trophic level as a function of species body mass compared for species occupying terrestrial (grey circles) and marine (coloured circles) environments. Each datum represents a species mean value (n = 107 species). The solid black lines are the PGLS regression lines for terrestrial mammals and marine mammals: log y = 0.01(log x) + 2.98 and log y = −0.02(log x) + 4.03, respectively. River otter (9 kg) and blue whale (155 000 kg) are the smallest and largest marine mammal, respectively, whereas the collared pika (120 g) and brown bear (217 kg) are the smallest and largest terrestrial mammals, respectively. Note that body mass is on a log10 scale. (b) Schematic of general trophic level patterns for terrestrial mammals (grey), odontocetes (red) and mysticetes (blue). Images: Chris Huh (marine mammals), Hans Hillewaert (Amphipoda), Lukasiniho (Alcelaphus) and uncredited (Panthera) were downloaded from http://phylopic.org. Fish and grass silhouettes by Marlee Tucker.
On the basis of data included in this study there is a difference in the maximum and minimum body size across the two environments (figure 1a). The offset between marine and terrestrial mammals demonstrates that terrestrial mammals reach smaller body sizes while marine mammals can obtain much larger body sizes.
(b). Predator–prey ratios
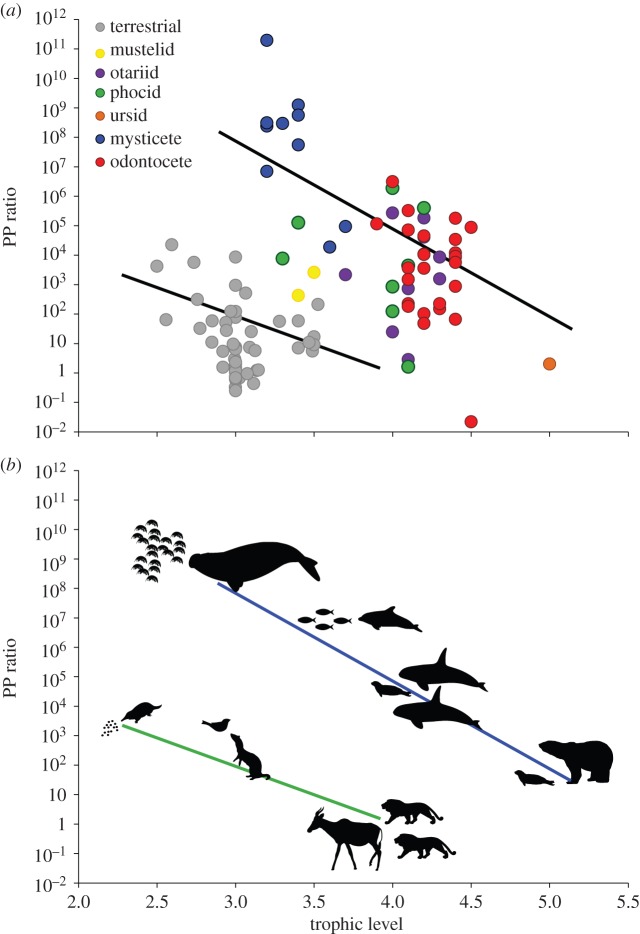
The PP ratio decreases with increasing trophic level in both marine and terrestrial environments (figure 2a). There was no significant (CIs −34.13, 0.61) interaction between trophic level and environment, suggesting that the scaling of the relationship between the PP ratio and the trophic level is the same in both environments. The intercept values, however, were significantly different (CIs 5.16, 23.06) for marine and terrestrial mammals—marine species had larger PP ratios than terrestrial species of a similar mass.
Figure 2.
(a) The relationship between log10 predator–prey ratio as a function of predator trophic level across marine (coloured circles) and terrestrial (grey circles) mammals (n = 107 species). The solid black lines are the PGLS regression line for terrestrial mammals and marine mammals: log y = −13.08(log x) + 7.83 and log y = −26.83(log x) + 20.54, respectively. (b) Examples of feeding strategies used by marine (blue) and terrestrial (green) carnivorous mammals. Marine (left to right): bulk feeders (mysticetes), small vertebrate feeders (odontocetes) and large vertebrate feeders (orcas and polar bears). Terrestrial (left to right): small insectivores (echidnas), small vertebrate feeders (mustelids) and large vertebrate feeders (lions). Images: Chris Huh (marine mammals), Hans Hillewaert (Amphipoda), Lukasiniho (Alcelaphus), Tracy Heath (Phocidae and U. maritimus), Nobu Tamura/T. Michael Keesey (Tachyglossus) and uncredited (Mustelid and Panthera) were downloaded from http://phylopic.org. Fish and bird silhouettes by Marlee Tucker.
(c). Diet and body size
There was a significant difference between the average mass of herbivores (F1,102 = 10.66, p < 0.01) and carnivores (F1,105 = 134.12, 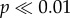 ) between the marine and terrestrial environments (electronic supplementary material, figure S2), with marine mammals having larger body masses in both diet categories. A caveat of this analysis is the dietary bias within terrestrial and marine mammals. In the marine environment, mammals are predominantly carnivorous and there are fewer than five herbivorous mammals. This limits the number of species we can compare across diet categories, specifically for marine mammals.
) between the marine and terrestrial environments (electronic supplementary material, figure S2), with marine mammals having larger body masses in both diet categories. A caveat of this analysis is the dietary bias within terrestrial and marine mammals. In the marine environment, mammals are predominantly carnivorous and there are fewer than five herbivorous mammals. This limits the number of species we can compare across diet categories, specifically for marine mammals.
When investigating the relationship between diet niche and body mass, there is a broader range of mass values across terrestrial herbivores (0.03–3981 kg) compared with terrestrial carnivores (0.09–177 kg; electronic supplementary material, figure S2). Conversely, the opposite is found in the marine system, with marine carnivores ranging from 9 to 155 000 kg, and marine herbivores ranging from 195 to 19 200 kg (electronic supplementary material, figure S2).
4. Discussion
(a). The effects of body mass on trophic position
The evolution of trophic position in mammalian carnivores appears to be driven by the environment in which they live. Marine carnivores sit on average 1.3 trophic levels higher than mammals that live on land. This trophic shift is driven by the more complex nature of marine food webs where there are higher numbers of trophic levels (figure 1a,b) as well as interactions between these trophic levels [11,12]. There was no relationship between changes in body mass and trophic level for terrestrial nor for marine mammals. Our results contradict previous studies that combined data from endothermic vertebrates (i.e. mammals and birds) and showed a strong positive relationship between body mass and trophic level in vertebrates [11]. Our study examined the relationship between body mass and trophic level within mammals only, and we used different methodologies (e.g. data normalization; see [11]). Ideally, we would like to examine trophic level patterns in mammals across food webs more broadly; however, at present data using complete food webs are limited.
We were surprised that we found no positive relationship between body mass and trophic level for terrestrial mammals. The strong negative body mass and trophic level relationship described previously appears to be seen only when using data from whole food webs and a smaller sample size of mammals [11]. Similar to our study, a weak relationship between body mass and trophic level is seen in fish [34]. By including the largest species, the marine mammals, we demonstrate that the trophic-level–body-mass relationship may not be uniform across animals. The mysticete whales cluster towards the bottom right of the trophic-level–body-mass relationship (having large body mass and yet feeding at low trophic levels; figure 1b). As previous studies did not include the largest mammals (e.g. mysticete whales), the species that feed on prey within the lower trophic levels (approx. 3–3.5), important information on trophic level patterns and the drivers behind these patterns have been missed.
(b). Environmental effects on herbivory
Productivity appears to drive the differences in trophic level patterns and food web structures across the two environments. Marine productivity is driven by high quantities of small, single-celled primary producers, which is the reverse of the trophic level patterns and food web structures found on land. Vegetation on land (typically composed of complex long-chain chemical structures such as lignin) is generally multicellular, with a high proportion of structural tissues and chemical defences to protect against herbivory [6,35]. Terrestrial vegetation has to compete for sunlight and nutrients, and against the effects of gravity, resulting in a diverse array of vegetation types, from fast (i.e. grasses) to slow (i.e. woody plants) growth-rate plant types [36]. This provides terrestrial herbivores with a number of niches from browsers (woody plant foliage specialists), granivores (grain specialists) and frugivores (fruit specialists). The presence of numerous herbivorous niches has also driven the diversification and abundance of herbivorous mammals on land.
Although the primary productivity in the ocean supports complex food webs and a greater range of trophic levels [36,37], herbivory is rare in marine mammals. Only one group of mammals, the sirenians (including dugongs, manatees and sea cows) are truly herbivorous. Marine mammal herbivores have restricted food resources on which they can feed including seagrass, multicellular algae or phytoplankton. The sirenians feed mostly on seagrasses but use multicellular algae (of marine origin) and also mangrove leaves (of terrestrial origin). Seagrasses are marine flowering plants that grow in meadows under restricted conditions, as they require shallow and sheltered coastal waters with a sandy or soft-mud substrate (restricted to temperate and tropical coastlines across the globe) [38]. The limited availability of seagrass habitat, and consequently seagrass, may have restricted herbivory in marine mammals. In comparison, the typically single-celled phytoplankton lack well-developed chemical defences, and have fast growth rates and a high proportion of photosynthetic tissues that are high in phosphorus and nitrogen [6]. The differences in structure and chemical stoichiometry of terrestrial and marine primary production means that marine herbivores feed on an abundant, nutrient-rich (high in phosphorus and nitrogen) and easily digested resource compared with terrestrial herbivores. Mammals have not specialized to use phytoplankton or multicellular algae as a primary food resource, although phytoplankton may be ingested incidentally while filter feeding.
(c). Environmental effects on carnivory
The abundance of protein-rich (composed of amino acids) resources in the ocean supports a greater number of carnivorous marine mammals. The great quantities of phytoplankton in the ocean and aquatic bodies support large communities of small zooplankton (passively moving aquatic organisms, e.g. protozoans including foraminiferans, radiolarians and dinoflagellates). These tiny primary consumers eat the phytoplankton, along with bacterioplankton (the bacterial component of the plankton), detritus and other zooplankton. The zooplankton themselves become a source of energy for larger metazoan zooplankton (e.g. cnidarians such as jellyfish; crustaceans such as copepods and larval krill; molluscs such as pteropods; and chordates such as salps and juvenile fish). The metazoan zooplankton are in turn eaten by larger metazoan zooplankton and/or nektonic species (actively swimming aquatic organisms, e.g. molluscs such as squid and octopus; crustaceans such as krill and amphipods; and vertebrates such as fish, turtles, seals and whales). The small size and fast generation times of zooplanktonic species means that their communities can reproduce and grow rapidly to exploit increases in phytoplankton abundance, harnessing rapid spikes in primary productivity typical of phytoplanktonic blooms. This high abundance of small primary producers and primary consumers supports a high diversity of small carnivorous species within the metazoan zooplanktonic communities, and in turn in the nektonic communities. The high abundance of small organisms forming the base of marine food webs facilitates the presence of numerous steps within marine food chains, as larger species feed upon smaller species [36,37]. The abundance of protein-rich resources spanning a range of body sizes from extremely small (30 mg) to large (13 000 kg) results in a wide range of available niches for carnivorous marine mammals, from invertebrate feeders (krill, amphipods, squid, shellfish, etc.) to vertebrate consumers (fish, birds, mammals).
(d). Body size, diet and environment
For mammals, changes in body mass have followed reverse patterns of diet dominance in the marine and terrestrial environments. Theory predicts that for mammals with increasing body mass, the number of prey items they consume should decrease [11]. This is based on the imbalance between the increase in resources that larger carnivorous mammals need against the finite resources that are available to meet their requirements [11]. This is the case for mammals living on land, where the majority of large mammals are herbivorous (electronic supplementary material, figure S2). In the terrestrial environment, where vegetation is an abundant resource, most of the net primary productivity is lignocellulose, which is difficult for mammals to digest, and there is relatively little easily digestible material [36]. Land mammals have long, complex digestive systems to digest and assimilate nutrients from large quantities of poor-quality vegetation. They have symbiotic relationships with microbes and protozoa in their gut, which assists in the further extraction of nutrients from their poor-quality diet [36,39,40]. Herbivores retain vegetation within their digestive systems for long periods (up to 92 h [41]) to allow enzymatic and microbial action to maximize the breakdown and then assimilation of nutrients [40]. A large body size is required to accommodate these long and complex digestive systems, as well as the large quantities of poor-quality vegetation that need to be processed over long periods of time. This has resulted in the trend towards large body mass in terrestrial herbivorous mammals.
By contrast, there has been an increase in carnivore body size in the marine environment (electronic supplementary material, figure S2). For marine carnivorous mammals, the combination of the thermal advantages, prey availability and hunting efficiency have resulted in large body size. Using dense aggregations of prey (up to 770 000 m−3 [42]) has aided the evolution and maintenance of extreme body size in mysticete (approx. 0.9 to 5 cm swarming prey) and odontocete (approx. 1 to more than 37 cm cephalopod prey) whales [43]. Large marine carnivores are more efficient at hunting than their terrestrial counterparts. Terrestrial carnivores spend long periods of time foraging (up to several hours) and expend large amounts of energy hunting (including capture and prey consumption [44]). Marine carnivores, particularly those above 100 kg, have a higher hunting efficiency [44]. They have evolved physiological (e.g. increased levels of globins for more efficient oxygen transfer [45]), morphological (e.g. feeding apparatus such as baleen) and behavioural traits (e.g. alternate forms of locomotion during diving [46]) to become more energy-efficient hunters. Marine vegetation contains small amounts of lignin and cellulose so that higher amounts of nutrients are available to marine consumers (including zooplankton, fish and sirenians), which pass up through the food web [6,36]. This greater productivity provides support for large populations of consumers, resulting in the reverse pattern to that seen on land [11]. Where marine clades (cetaceans, pinnipeds and sirenians) tend to stick to a single diet type (i.e. either carnivory or herbivory), some terrestrial carnivorous mammals switch between diets depending on food availability (e.g. bears shift between omnivory and carnivory [2]).
Not only have there been changes in body mass across different diets, we also see changes in minimum and maximum body size trends across marine and terrestrial mammals. This is driven by the combined effects of environmental characteristics, physiology and vegetation/prey availability. Terrestrial mammals, compared with marine mammals, can reach smaller body sizes (approx. 0.001 kg versus approx. 10 kg [1]). Mammals living in the water experience high thermoregulation costs, and this constrains how small a marine mammal can be. This is accentuated at birth, when the surface area to body volume is high, resulting in an increased rate of heat loss and energy spent on maintaining body temperature [1,47]. Four of the six marine mammal lineages have retained giving birth and raising their young out of the water, either on land or ice (mustelids, otariids, phocids and ursids). For both young and adults, it is a thermal advantage to have larger body size in the marine environment. Marine mammals reach much larger body sizes than terrestrial mammals (approx. 200 tonnes versus approx. 15 tonnes [1]).
On land, resource availability constrains how large mammals can become, because having a large body size has high energetic costs, and requires abundant and reliable resources. In particular, the interaction between resource availability, land mass area and the cost of gathering sufficient resources limits how large a terrestrial mammal can become [48]. In the ocean, similar constraints are present, but take effect at a much larger body size. For example, the size of the blue whale is likely to be constrained by biomechanical limitations (e.g. gape size and lunge feeding costs) and prey availability (e.g. prey density [43]).
(e). Trophic level and predator–prey ratio relationships
A general rule for the relationship between the PP ratio and the trophic level of carnivorous mammals appears to apply to both the marine and terrestrial environments. With increasing trophic level, carnivores shift to feeding on prey that have a body mass more similar to that of the carnivore's own (i.e. PP ratio approaching greater than or equal to 1). The underlying drivers behind this negative relationship are believed to be the combined effects of increasing body mass with trophic level for endotherms, and increasing prey mass with increasing carnivore body mass [11]. However, our study suggests that there are issues with this reasoning as (i) we did not find a relationship between trophic level and carnivore body mass, and (ii) while terrestrial carnivores demonstrate a positive relationship between carnivore body size and prey body size [49], this is not the case for many marine carnivores (e.g. mysticete whales feeding on krill).
The large PP ratios found in the marine system are not plausible on land, as small prey (excluding invertebrates) do not form large swarms or groups that are easily accessible as they do in the ocean. In the marine system, small prey such as plankton or fish form large aggregations (schools or swarms) as protection from predation. Large marine mammals (such as the mysticetes) and smaller marine mammals (such as seals) have morphological adaptations for bulk feeding (e.g. baleen instead of teeth in mysticete whales and specialized multi-cuspidate teeth in pinnipeds) and behavioural (bubble netting, group feeding, lunge diving) specializations to capture large quantities of small prey in a single feeding event. Harvesting small swarming prey requires minimal capture (individual pursuit and capture) and processing (e.g. butchering) time. There are invertebrate-feeding specialists on land, yet this type of diet can support animals only up to the restricted weight range of under 20 kg owing to the increasing costs associated with larger body mass [5]. For terrestrial mammalian carnivores, the abundance, distribution and energy content of terrestrial invertebrates are not sufficient to support body masses above 20 kg [5].
An implication arising from the combination of a mean trophic level position of 3.9 for marine carnivores and the abundance of higher PP ratios in the marine environment is its effect on trophic efficiency. Trophic efficiency includes the exchange of energy between trophic levels based on predator–prey interactions, but excludes growth (i.e. somatic) [10]. When PP ratios are large, trophic efficiency decreases [10]. For example, a marine organism with a PP ratio of 1000 has an efficiency of approximately 13%, compared with a marine organism with a PP ratio of 10 that has an efficiency of 50% (irrespective of size or trophic position [10]). This has follow-on effects for the energy flow through an ecosystem, as well as community structure and dynamics. However, this is less of an issue in the marine environment, where large PP ratios (i.e. predators are larger than their prey) increase the stability of marine food webs [18,50,51]. Large PP ratios cause a reduction in the interaction strengths between predators and their prey (per unit biomass [52,53]). In addition, there are benefits for carnivores that feed from lower trophic levels. The similarity between the tissue composition of carnivores and their prey (amino acids) has been shown to result in higher energy transfer efficiencies [54,55]. When herbivores are included in a food chain this can limit the energy efficiency between trophic levels. This is because of the decreased assimilation efficiency of herbivores that have greater difficulty extracting energy from plants with high carbon–nitrogen ratios [56].
Our study provides insights into the drivers behind mammalian PP ratios, trophic evel positions and body mass. We caution that our approach is simplistic, yet ecosystems are often complex. Collecting prey preference and trophic level information from the literature has limitations. Historical studies may have over simplified food webs [57]. While we attempted to select studies that did not oversimplify food web data, as we examined patterns across mammals and from a wide range of food webs with different structures, this may not have always been the case. In the future, we propose using equations that incorporate complete food-web data to calculate trophic levels [58].
At present, we are restricted by the limited amount of detailed food web data available. To gather information across 107 species, we were reliant upon published dietary studies, and this biased data toward well-studied species. Unfortunately, this cannot be avoided at this point in time and can only be overcome by further research into less well-known species, which are often difficult to study (e.g. beaked whales). Our study provides a framework for exploring how environment impacts upon the broad patterns in ecology across the marine and terrestrial systems. As more information on trophic levels and food webs that incorporate mammals are compiled, future studies can then examine these patterns using more specific and detailed data.
Our results demonstrate that primary productivity, and its availability, is important for the mammalian trophic structure of food webs, body size, and prevalence of carnivory and herbivory. Marine and terrestrial mammals share the same relationship between trophic level and mass, with the trophic level of marine species shifting 1.3 levels higher. Interestingly, carnivorous mammals do not follow strictly the expected patterns for the scaling relationship between trophic level and body mass (i.e. positive or quadratic). While there is a distinct shift in trophic position between marine and terrestrial carnivores (figure 1a), we did not find a positive relationship or a quadratic relationship. The marine environment has a higher abundance of carnivorous mammals and a shift towards larger minimal and maximal body size. The terrestrial system has greater diversification of herbivores and a general trend towards a smaller minimal and maximal body size. The relationship between body mass and diet on land has been reversed in the marine system, where the mean mass of carnivores is higher than the mean mass of herbivores, the opposite of terrestrial mammals. The patterns in trophic level and PP ratio are stronger within each environment than across mammals in general, further suggesting the importance of environment.
When mammals colonized the marine environment, the shift from a plant-dominated landscape to a habitat rich in protein-rich resources, with little competition, resulted in changes to the trophic level relationships, dietary niches and foraging ecology. Large body mass, the shift in available resources and altered physiology have driven marine mammals to be largely carnivorous. Marine carnivores consume highly mobile prey that sit within a wide range of trophic levels and have smaller body masses than those of terrestrial carnivores, which are locked into consuming large prey to meet their metabolic requirements. This study illustrates the influence this shift in environment has had on mammalian ecology, and the importance of using this information to examine the structure and function of marine and terrestrial communities.
Supplementary Material
Acknowledgements
We thank A. Poore and J. Meade for their feedback, which has improved the manuscript and is greatly appreciated. Thanks also to T. Ord for statistical assistance. All silhouettes in figures 1 and 2 were downloaded from http://phylopic.org and are available for re-use under the Creative Commons Attribution-ShareAlike 3.0 Unported licence (marine mammals, Amphipoda, Alcelaphus); the Creative Commons Attribution 3.0 Unported licence (Tachyglossus); and the Public Domain Mark 1.0 licence (Panthera, Mustelid, Phocid and Ursus maritimus).
Data accessibility
The data used in this publication can be accessed from figshare: http://dx.doi.org/10.6084/m9.figshare.988696.
Funding statement
This research was conducted under the Australian Research Council Program LP0989933 to T.L.R.
References
- 1.Smith FA, Lyons SK. 2011. How big should a mammal be? A macroecological look at mammalian body size over space and time. Phil. Trans. R. Soc. B 366, 2364–2378. ( 10.1098/rstb.2011.0067) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Price SA, Hopkins SSB, Smith KK, Roth VL. 2012. Tempo of trophic evolution and its impact on mammalian diversification. Proc. Natl Acad. Sci. USA 109, 7008–7012. ( 10.1073/pnas.1117133109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uhen M. 2007. Evolution of marine mammals: back to the sea after 300 million years. Anat. Rec. 290, 514–522. ( 10.1002/ar.20545) [DOI] [PubMed] [Google Scholar]
- 4.Pauly D, Christensen V. 1995. Primary production required to sustain global fisheries. Nature 374, 255–257. ( 10.1038/374255a0) [DOI] [Google Scholar]
- 5.Carbone C, Teacher A, Rowcliffe J. 2007. The costs of carnivory. PLoS Biol. 5, 363–368. ( 10.1371/journal.pbio.0050022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shurin JB, Gruner DS, Hillebrand H. 2006. All wet or dried up? Real differences between aquatic and terrestrial food webs. Proc. R. Soc. B 273, 1–9. ( 10.1098/rspb.2005.3377) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Field CB, Behrenfeld MJ, Randerson JT, Falkowski P. 1998. Primary production of the biosphere: integrating terrestrial and oceanic components. Science 281, 237–240. ( 10.1126/science.281.5374.237) [DOI] [PubMed] [Google Scholar]
- 8.Thompson MV, Randerson JT. 1999. Impulse response functions of terrestrial carbon cycle models: method and application. Glob. Change Biol. 5, 371–394. ( 10.1046/j.1365-2486.1999.00235.x) [DOI] [Google Scholar]
- 9.Falkowski PG, Raven JA. 1997. Aquatic photosynthesis. Oxford, UK: Blackwell. [Google Scholar]
- 10.Andersen KH, Beyer JE, Lundberg P. 2009. Trophic and individual efficiencies of size-structured communities. Proc. R. Soc. B 276, 109–114. ( 10.1098/rspb.2008.0951) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riede JO, Brose U, Ebenman B, Jacob U, Thompson R, Townsend CR, Jonsson T. 2011. Stepping in Elton's footprints: a general scaling model for body masses and trophic levels across ecosystems. Ecol. Lett. 14, 169–178. ( 10.1111/j.1461-0248.2010.01568.x) [DOI] [PubMed] [Google Scholar]
- 12.Brose U. 2010. Body-mass constraints on foraging behaviour determine population and food-web dynamics. Funct. Ecol. 24, 28–34. ( 10.1111/j.1365-2435.2009.01618.x) [DOI] [Google Scholar]
- 13.Pauly D, Trites AW, Capuli E, Christensen V. 1998. Diet composition and trophic levels of marine mammals. ICES J. Mar. Sci. 55, 467–481. ( 10.1006/jmsc.1997.0280) [DOI] [Google Scholar]
- 14.Petchey OL, Beckerman AP, Riede JO, Warren PH. 2008. Size, foraging, and food web structure. Proc. Natl Acad. Sci. USA 105, 4191–4196. ( 10.1073/pnas.0710672105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weitz JS, Levin SA. 2006. Size and scaling of predator–prey dynamics. Ecol. Lett. 9, 548–557. ( 10.1111/j.1461-0248.2006.00900.x) [DOI] [PubMed] [Google Scholar]
- 16.Brose U, et al. 2006. Consumer–resource body-size relationships in natural food webs. Ecology 87, 2411–2417. ( 10.1890/0012-9658(2006)87[2411:cbrinf]2.0.co;2) [DOI] [PubMed] [Google Scholar]
- 17.Jennings S, Mackinson S. 2003. Abundance–body mass relationships in size-structured food webs. Ecol. Lett. 6, 971–974. ( 10.1046/j.1461-0248.2003.00529.x) [DOI] [Google Scholar]
- 18.Kartascheff B, Heckmann L, Drossel B, Guill C. 2010. Why allometric scaling enhances stability in food web models. Theor. Ecol. 3, 195–208. ( 10.1007/s12080-009-0063-3) [DOI] [Google Scholar]
- 19.Kelt DA, van Vuren DH. 2001. The ecology and macroecology of mammalian home range area. Am. Nat. 157, 637–645. ( 10.1086/320621) [DOI] [PubMed] [Google Scholar]
- 20.Fritz SA, Bininda-Emonds ORP, Purvis A. 2009. Geographical variation in predictors of mammalian extinction risk: big is bad, but only in the tropics. Ecol. Lett. 12, 538–549. ( 10.1111/j.1461-0248.2009.01307.x) [DOI] [PubMed] [Google Scholar]
- 21.Bininda-Emonds OR, et al. 2007. The delayed rise of present-day mammals. Nature 446, 507–512. ( 10.1038/nature05634) [DOI] [PubMed] [Google Scholar]
- 22.Grill A, Amori G, Aloise G, Lisi I, Tosi G, Wauters LA, Randi E. 2009. Molecular phylogeography of European Sciurus vulgaris: refuge within refugia?. Mol. Ecol. 18, 2687–2699. ( 10.1111/j.1365-294X.2009.04215.x) [DOI] [PubMed] [Google Scholar]
- 23.Caballero S, et al. 2008. Molecular systematics of South American dolphins Sotalia: sister taxa determination and phylogenetic relationships, with insights into a multi-locus phylogeny of the Delphinidae. Mol. Phylogenet. Evol. 46, 252–268. ( 10.1016/j.ympev.2007.10.015) [DOI] [PubMed] [Google Scholar]
- 24.Slater GJ, Price SA, Santini F, Alfaro ME. 2010. Diversity versus disparity and the radiation of modern cetaceans. Proc. R. Soc. B 277, 3097–3104. ( 10.1098/rspb.2010.0408) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nyakatura K, Bininda-Emonds ORP. 2012. Updating the evolutionary history of Carnivora (Mammalia): a new species-level supertree complete with divergence time estimates. BMC Biol. 10, 12 ( 10.1186/1741-7007-10-12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maddison WP, Maddison DR. 2010. Mesquite: a modular system for evolutionary analysis. Version 2.74 See http://mesquiteproject.org/mesquite/mesquite.html.
- 27.Field A. 2005. Discovering statistics using SPSS. London, UK: Sage. [Google Scholar]
- 28.Johnson JB, Omland KS. 2004. Model selection in ecology and evolution. Trends Ecol. Evol. 19, 101–108. ( 10.1016/j.tree.2003.10.013) [DOI] [PubMed] [Google Scholar]
- 29.Burnham KP, Anderson DR. 2002. Model selection and multimodal inference: a practical information-theoretical approach. New York, NY: Springer. [Google Scholar]
- 30.Martins EP. 2004. COMPARE, version 46b: computer programs for the statistical analysis of comparative data. See http://compare.bio.indiana.edu. [Google Scholar]
- 31.Burnham KP, Anderson DR. 2004. Multimodal inference understanding AIC and BIC in model selection. Sociol. Meth. Res. 33, 261–304. ( 10.1177/0049124104268644) [DOI] [Google Scholar]
- 32.Martins EP, Hansen TF. 1997. Phylogenies and the comparative method: a general approach to incorporating phylogenetic information into analysis of interspecific data. Am. Nat. 149, 646–667. ( 10.1086/286013) [DOI] [Google Scholar]
- 33.Team RDC. 2011. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing: (http://www.R-project.org/) [Google Scholar]
- 34.Jennings S, Pinnegar JK, Polunin NVC, Boon TW. 2001. Weak cross-species relationships between body size and trophic level belie powerful size-based trophic structuring in fish communities. J. Anim. Ecol. 70, 934–944. ( 10.1046/j.0021-8790.2001.00552.x) [DOI] [Google Scholar]
- 35.Cebrian J, Shurin JB, Borer ET, Cardinale BJ, Ngai JT, Smith MD, Fagan WF. 2009. Producer nutritional quality controls ecosystem trophic structure. PLoS ONE 4, e4929 ( 10.1371/journal.pone.0004929) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Polis GA, Strong DR. 1996. Food web complexity and community dynamics. Am. Nat. 147, 813–846. ( 10.1086/285880) [DOI] [Google Scholar]
- 37.Takimoto G, Post D, Spiller D, Holt R. 2012. Effects of productivity, disturbance, and ecosystem size on food-chain length: insights from a metacommunity model of intraguild predation. Ecol. Res. 27, 481–493. ( 10.1007/s11284-012-0929-5) [DOI] [Google Scholar]
- 38.Short F, Carruthers T, Dennison W, Waycott M. 2007. Global seagrass distribution and diversity: a bioregional model. J. Exp. Mar. Biol. Ecol. 350, 3–20. ( 10.1016/j.jembe.2007.06.012) [DOI] [Google Scholar]
- 39.Smith FA, et al. 2010. The evolution of maximum body size of terrestrial mammals. Science 330, 1216–1219. ( 10.1126/science.1194830) [DOI] [PubMed] [Google Scholar]
- 40.Demment MW, Soest PJV. 1985. A nutritional explanation for body-size patterns of ruminant and nonruminant herbivores. Am. Nat. 125, 641–672. ( 10.2307/2461476) [DOI] [Google Scholar]
- 41.Clauss M, Nunn C, Fritz J, Hummel J. 2009. Evidence for a tradeoff between retention time and chewing efficiency in large mammalian herbivores. Comp. Biochem. Physiol. A 154, 376–382. ( 10.1016/j.cbpa.2009.07.016) [DOI] [PubMed] [Google Scholar]
- 42.Nicol S. 1986. Shape, size and density of daytime surface swarms of the euphausiid Meganyctiphanes norvegica in the Bay of Fundy. J. Plankt. Res. 8, 29–39. ( 10.1093/plankt/8.1.29) [DOI] [Google Scholar]
- 43.Goldbogen J, Calambokidis J, Oleson E, Potvin J, Pyenson ND, Schorr G, Shadwick R. 2011. Mechanics, hydrodynamics and energetics of blue whale lunge feeding: efficiency dependence on krill density. J. Exp. Biol. 214, 131–146. ( 10.1242/jeb.048157) [DOI] [PubMed] [Google Scholar]
- 44.Williams TM, Yeates L. 2004. The energetics of foraging in large mammals: a comparison of marine and terrestrial predators. Int. Congr. Ser. 1275, 351–358. ( 10.1016/j.ics.2004.08.069) [DOI] [Google Scholar]
- 45.Williams TM, et al. 2008. Running, swimming and diving modifies neuroprotecting globins in the mammalian brain. Proc. R. Soc. B 275, 751–758. ( 10.1098/rspb.2007.1484) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Williams TM. 1999. The evolution of cost efficient swimming in marine mammals: limits to energetic optimization. Phil. Trans. R. Soc. Lond. B 354, 193–201. ( 10.1098/rstb.1999.0371) [DOI] [Google Scholar]
- 47.Downhower JF, Blumer LS. 1988. Calculating just how small a whale can be. Nature 335, 675 ( 10.1038/335675b0)3173490 [DOI] [Google Scholar]
- 48.Burness GP, Diamond J, Flannery T. 2001. Dinosaurs, dragons, and dwarfs: the evolution of maximal body size. Proc. Natl Acad. Sci. USA 98, 14 518–14 523. ( 10.1073/pnas.251548698) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carbone C, Mace GM, Roberts SC, Macdonald DW. 1999. Energetic constraints on the diet of terrestrial carnivores. Nature 402, 286–288. ( 10.1038/46266) [DOI] [PubMed] [Google Scholar]
- 50.Brose U, Williams RJ, Martinez ND. 2006. Allometric scaling enhances stability in complex food webs. Ecol. Lett. 9, 1228–1236. ( 10.1111/j.1461-0248.2006.00978.x) [DOI] [PubMed] [Google Scholar]
- 51.Heckmann L, Drossel B, Brose U, Guill C. 2012. Interactive effects of body-size structure and adaptive foraging on food-web stability. Ecol. Lett. 15, 243–250. ( 10.1111/j.1461-0248.2011.01733.x) [DOI] [PubMed] [Google Scholar]
- 52.Brose U, Berlow EL, Martinez ND. 2005. Scaling up keystone effects from simple to complex ecological networks. Ecol. Lett. 8, 1317–1325. ( 10.1111/j.1461-0248.2005.00838.x) [DOI] [Google Scholar]
- 53.Yodzis P, Innes S. 1992. Body size and consumer-resource dynamics. Am. Nat. 139, 1151–1175. ( 10.1086/285380) [DOI] [Google Scholar]
- 54.Dickman EM, Newell JM, González MJ, Vanni MJ. 2008. Light, nutrients, and food-chain length constrain planktonic energy transfer efficiency across multiple trophic levels. Proc. Natl Acad. Sci. USA 105, 18 408–18 412. ( 10.1073/pnas.0805566105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sterner RW, Elser JJ. 2002. Ecological stoichiometry: the biology of elements from molecules to the biosphere. Princeton, NJ: Princeton University Press. [Google Scholar]
- 56.Frost PC, Benstead JP, Cross WF, Hillebrand H, Larson JH, Xenopoulos MA, Yoshida T. 2006. Threshold elemental ratios of carbon and phosphorus in aquatic consumers. Ecol. Lett. 9, 774–779. ( 10.1111/j.1461-0248.2006.00919.x) [DOI] [PubMed] [Google Scholar]
- 57.Polis GA. 1991. Complex trophic interactions in deserts: an empirical critique of food-web theory. Am. Nat. 138, 123–155. ( 10.2307/2462536) [DOI] [Google Scholar]
- 58.Williams Richard J, Martinez Neo D. 2004. Limits to trophic levels and omnivory in complex food webs: theory and data. Am. Nat. 163, 458–468. ( 10.1086/381964) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used in this publication can be accessed from figshare: http://dx.doi.org/10.6084/m9.figshare.988696.