Abstract
The transcription factor MYC and its related family members MYCN and MYCL have been implicated in the etiology of a wide spectrum of human cancers. Compared to other oncoproteins, such as RAS or SRC, MYC is unique because its protein coding region is rarely mutated. Instead, MYC’s oncogenic properties are unleashed by regulatory mutations leading to unconstrained high levels of expression. Under both normal and pathological conditions MYC regulates multiple aspects of cellular physiology including proliferation, differentiation, apoptosis, growth and metabolism by controlling the expression of thousands of genes. How a single transcription factor exerts such broad effects remains a fascinating puzzle. Notably, MYC is part of a network of bHLHLZ proteins centered on the MYC heterodimeric partner MAX and its counterpart, the MAX-like protein MLX. This network includes MXD1-4, MNT, MGA, MONDOA and MONDOB proteins. With some exceptions, MXD proteins have been functionally linked to cell cycle arrest and differentiation, while MONDO proteins control cellular metabolism. Although the temporal expression patterns of many of these proteins can differ markedly they are frequently expressed simultaneously in the same cellular context, and potentially bind to the same, or similar DNA consensus sequence. Here we review the activities and interactions among these proteins and propose that the broad spectrum of phenotypes elicited by MYC deregulation is intimately connected to the functions and regulation of the other network members. Furthermore, we provide a meta-analysis of TCGA data suggesting that the coordinate regulation of the network is important in MYC driven tumorigenesis.
1. The MAX/MLX network: identification, evolution and organization of the network members
1.1. From the discovery of v-myc to the MAX/MLX network
Since its identification in the late 1970s the MYC gene has been the subject of intense research that has linked it to all major hallmarks of cancer. The ability of MYC to influence such a broad spectrum of cellular activities may be explained by the fact that its protein product, MYC, belongs to an extended network of transcription factors (referred to here as the MAX/MLX network) with diverse activities, which in turn regulate many biological processes (Fig. 1).
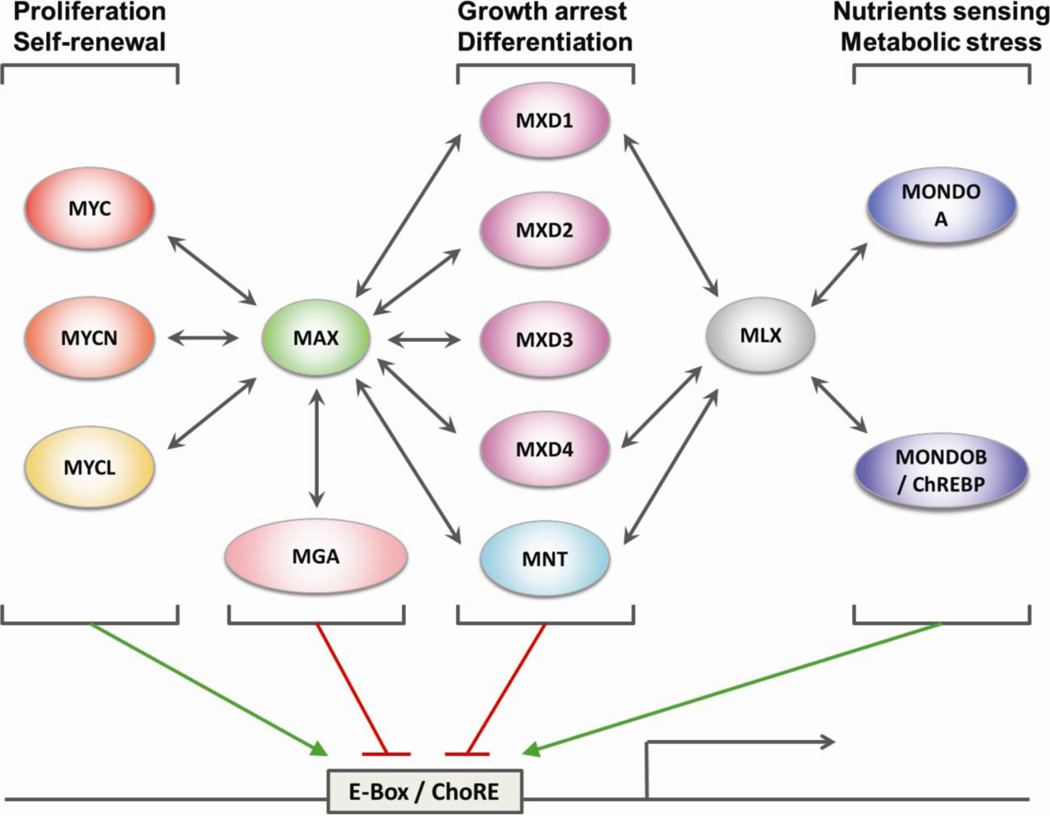
Figure 1. Diagram of the MAX/MLX network.
All members of the MAX/MLX network and their reciprocal heterodimerization partners (indicated by the two-headed arrows) are represented. Green arrows and red lines indicate transcriptional activation and repression respectively. E-Box, Enhancer-box; ChoRE, Carbohydrate response element.
1.1.1. MYC and MAX: enabling MYC activity
The oncogene v-myc was among the first retroviral oncogenes described. Initially identified as the common transforming genetic element shared by the retroviruses MC29, CMII, MH2, and OK10, it was later shown to be derived from a cellular oncogene, termed c-MYC, (from now on referred to as MYC) [1–3]. The prominent role of MYC in human cancer etiology was revealed by the findings that MYC overexpression, due to chromosomal translocation or gene amplification, was a common event in Burkitt’s lymphoma [4, 5] and myeloid leukemia, respectively [6]. Amplification of the subsequently identified MYC paralogs MYCN and MYCL were also reported in neuroblastoma and small cell lung carcinoma, respectively, firmly linking MYC family proteins to the etiology of human cancers [7–10].
Initial sequence comparisons of MYC family genes revealed they share a predicted basic Helix-Loop-Helix Leucine Zipper (bHLHLZ) domain also found in several transcription factors and known to be involved in dimerization and DNA binding [11], suggesting the possibility that MYC proteins may homo or heterodimerize. Initial experiments failed to establish homodimerization of MYC protein at physiological expression levels [12–17]. However, the search for a MYC heterodimerization partner led to the discovery of a novel bHLHZ protein, MAX (MYC interacting factor X) [18]. Importantly, MAX also recognizes and heterodimerizes with MYCN and MYCL, implicating MAX in mediating the function of the three MYC family proteins, which have distinct and largely mutually exclusive spatiotemporal expression profiles during development [18, 19]. In contrast with MYC family members, Max is an abundant and stable protein, expressed in both proliferating and in resting cells, regardless of MYC levels [20]. These properties of MAX, together with its ability to bind DNA and repress transcription as a homodimer, led to the first model in which gene expression of MYC targets was controlled by the shift between repressive MAX-MAX homodimers and activating MYC-MAX heterodimers [21–24].
1.1.2. MXD proteins: MYC antagonists
Because MAX was found to be present under conditions in which MYC proteins are not expressed, and because MAX homodimerizes poorly, if at all, in vivo [25] it was considered that MAX may bind to other bHLHLZ containing proteins. Indeed, two MAX interacting proteins MXD1 and MXD2 (formerly known as MAD1 and MXI1) were soon identified [26, 27]. MXD1 and MXD2 are structurally similar, sharing 43% of their overall protein sequence, and are evolutionarily related. Moreover, with the exception of the bHLHLZ domain, which mediates their specific interaction with MAX, they are distinct from MYC. In vitro DNA binding and protein interaction assays confirmed that both MXD1 and MXD2 are unable to homodimerize, yet efficiently compete with MYC for heterodimerization with MAX. More importantly, both MXD1-MAX and MXD2-MAX heterodimers recognize the same E-box containing DNA consensus sequence as MYC-MAX heterodimers, but function as transcriptional repressors (MXD1-MAX) [26] or are unable to activate transcription (MXD2-MAX) [27]. The discovery of MXD1 and MXD2 added a new level of complexity to the earlier model by showing that MAX acts to mediate the activity of both MYC and MXD proteins which appeared to antagonize each other. In addition, the data suggested that it is the relative abundance and regulation of MYC and MXD proteins that define the transcriptional and biological outcome of network activity [26].
Shortly after the discovery of MXD1 and MXD2, four additional MXD-like proteins members were added to the network (MXD3, MXD4, MNT and MGA) [28–30]. While MXD3 and MXD4 are highly homologous with the previously identified MXD1 and MXD2 proteins, MNT and MGA differ significantly. However, apart from MGA, which contains an additional T-box domain and can both activate and repress transcription, the similar functional properties of MXD1-4 and MNT proteins (as all included in the MXD family proteins), which act as transcriptional repressors, further strengthen the model in which MXD proteins antagonize MYC in regulating many aspects of cell biology.
1.1.3 The MLX module: the metabolite sensing arm of the network
The existence of a parallel network which resembles the MYC/MAX/MXD transcriptional network was proposed after the discovery of a MAX-like protein called MLX. Two research groups independently identified MLX as the binding partner of MXD1, MLX4, and MNT proteins , and showed that MXD/MLX complexes mediate transcriptional repression of E-box containing promoters [31, 32]. Subsequently two large bHLHZ proteins were found to heterodimerize with MLX and regulate transcription by binding to E-box containing promoters [33, 34]. MONDOA was identified as an MLX heterodimeric partner by a two hybrid screen, while MONDOB was identified by sequence homology analysis. MONDOB, which had previously been identified as CHREBP (Carbohydrate Response Element Binding Protein) was also known as the WBSCR14 gene, since it maps to chromosome 7q11.23, a region usually deleted in Williams-Beuren Syndrome (WBS) [33]. CHREBP was subsequently confirmed to be an MLX interacting protein [34]. In contrast with the nuclear localized MYC, MAX and MXD proteins, MLX and MONDO proteins localize principally in the cytoplasm and can shuttle between the two compartments in response to glucose and other metabolic stimuli [35–38]. Because most of the literature refers to MONDOB as CHREBP, we will also refer to it as CHREBP in this review.
1.2. Evolutionary conservation of the MAX/MLX network
Underscoring the fundamental importance of the MAX/MLX transcriptional network in regulating cell biology is the fact that it has been conserved throughout evolution. Orthologs of human MAX MYC, MXD, MLX, and MONDO genes have been identified in the Placazoan Trichoplax adhaerens, considered the simplest metazoan [39]. Moreover, MYC and MAX orthologs also exist in Capsaspora owczarzaki, and in the choanoflagellate Monosiga brevicollis [39–41]. The presence of MYC and MAX orthologs in these two unicellular organisms, which are the closest living organisms to metazoa, indicates the emergence of the MAX module of the network even predates the evolution of multicellularity and metazoans.
A comprehensive analysis of the evolutionary conservation of the MAX/MLX network in metazoa identified or predicted the existence of network members in almost all surveyed species (Fig. 2A) [42]. Although the composition and sequence of network members has changed considerably during evolution, a core network consisting of MAX, MYC, MXD, MLX, MNT, and MONDO orthologs predominates and is the basis from which other networks emerged. The MAX/MLX network in vertebrates has acquired additional complexity due to the presence of multiple paralogous genes, which have distinct heterodimerization preference, gene expression profiles and functions. Meanwhile, lower order organisms usually have fewer members and, in the case of nematodes and flies, the network appears to have a distinct, yet balanced structure (Fig. 2B).
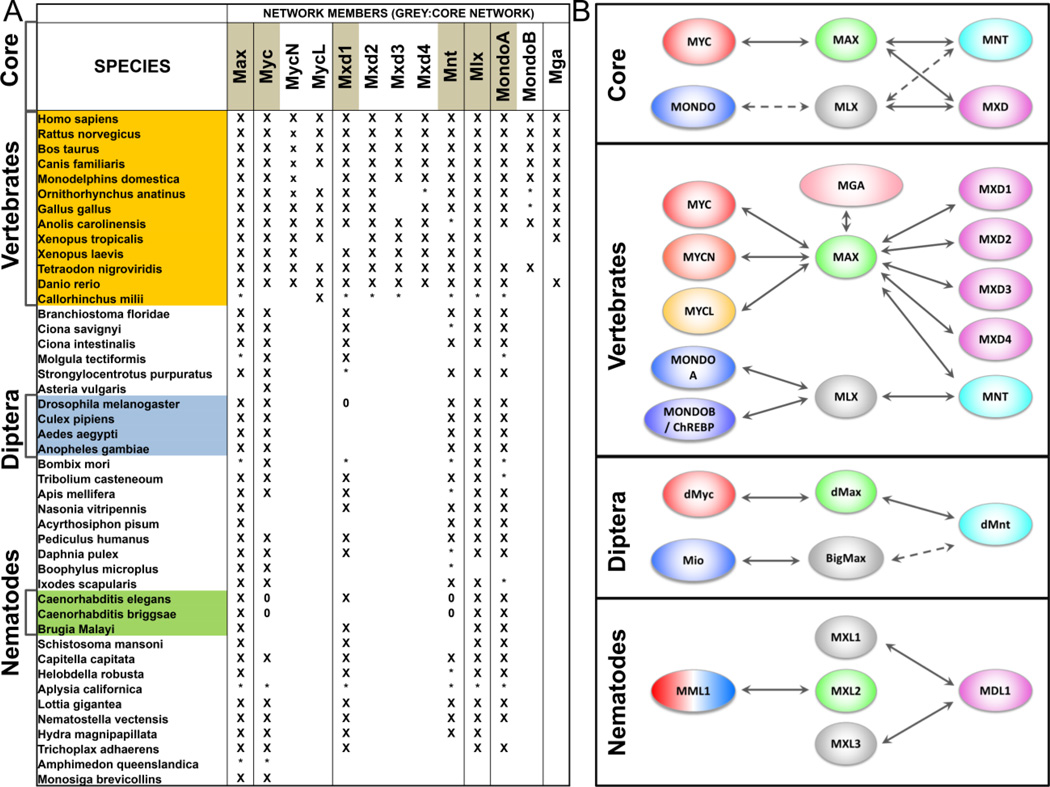
Figure 2. The MAX/MLX network in the animal kingdom.
A) Species specific distribution of MAx/MLX network members. “X”, the bHLHZ was found within a protein or expressed sequence tag; “*”, part or all of the sequence was found within a genetic region; “0”, the protein is known to be absent. B) Schematic representation of the four main network topologies. Solid arrows indicate experimentally verified interactions, whereas dotted arrows indicate debated or unknown interactions. Figure created from data in McFerrin and Atchley (c) 2011, with permission from the authors.
The Diptera lineage possesses a minimal network consisting of single orthologs of human MYC, MAX, MNT, MLX and MONDO genes (Fig. 2b) [36, 43–45]. Drosophila myc (dmyc), previously known as diminutive (dm), and max (dmax) were the first members of the network identified in Drosophila [43, 46]. Since then, many laboratories have confirmed the high functional redundancy between human and Drosophila myc, max and mnt orthologs [for review 47, 48]. Moreover a recent report demonstrated Drosophila mlx (known as bigmax) and its Mondo-like dimerization partner mio are involved in sugar sensing and utilization. This suggests the parallel and balancing functions of the MAX/MLX network that control cell proliferation and metabolism, have been also maintained during evolution [49, 50].
1.3. Organization and Structure of MAX/MLX network members
All members of the MAX/MLX network are defined by the presence of a bHLHLZ domain which mediates heterodimerization between family members and sequence-specific DNA binding (Fig. 3) [42, 51–53]. MYC family members are characterized by having the bHLHLZ domain localized at the C-terminus of the protein, and a transcriptional activation domain (TAD) located near the N-terminus. Within the TAD, MYC family members contain two well defined and conserved domains termed MYC Box I and MYC Box II (MBI and MBII) both of which are functionally important. While MBI serves as phosphodegron and is mainly involved in the regulation of MYC protein stability [54]; MBII mediates the interaction of MYC with multiple proteins such as TRRAP, GCN5, TIP60 and TIP48 [55–58], all of which have been identified as components of histone acetyltransferase complexes [59, 60]. Deletion of MBII inhibits most MYC functions, consistent with the importance of histone acetyltransferase complexes recruitment in MYC’s normal and pathological activities [61]. Moreover, the TAD region of MYC also mediates its interaction with other effectors of MYC activity such as the bromodomain protein BRD4 and the P-TEFb transcriptional anti-pause complex [62–64]. Recent studies have proposed that MYC-mediated augmentation of RNA polymerase II (RNA Pol II) dependent elongation is the principal mechanism through which MYC increases expression of its target genes [65–67]. Two other conserved domains MBIII [68] and MBIV [69] have also been identified within MYC proteins. MYC mutants lacking these domains are partially defective in their transcriptional activity and have a reduced tumorigenic potential.
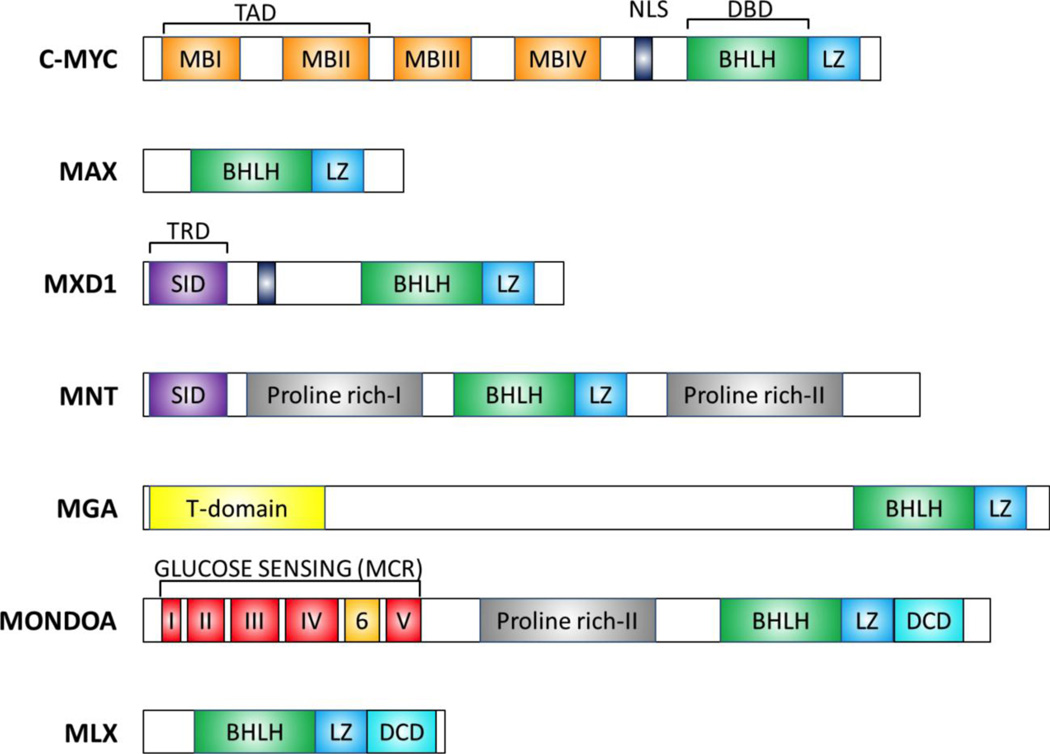
Figure 3. Domain organization of the MAX/MLX network members.
A representative member of each subfamily is included. Colored box indicates functional domains. MBI-IV, MYC box domains; NLS, nuclear localization signal; BHLH, Basic helix loop helix; LZ, leucine zipper; SID, SID3-interacting domain; DCD, dimerization and cytoplasmic localization domain; TAD, transcriptional activation domain; TRD, transcriptional repression domain. The Glucose sensing domain contains six conserved regions named MONDO Conserved Region (MCR). Proteins size is not represented to scale.
While MYC proteins possess a TAD which acts as binding sites for factors involved in transcriptional activation, MXD proteins possess a repression domain known as the SIN3 interaction domain (SID) [70]. The SID domain is localized near the N-termini of MXD proteins and mediates interaction with the paired amphipathic helix 2 (PAH2) domain of SIN3 proteins, which in turn recruits histone deacetylase containing complexes [71–73]. The loss of repression activity resulting from the inhibition of MXD-SIN3 interaction suggests that histone deacetylation is the main function contributing to transcriptional repression by MXD proteins [70]. Hence, in addition to competition for MAX and DNA binding, another level of MYC and MXD protein biological antagonism occurs as a consequence of molecular antagonism manifested by histone acetylation and deacetylation.
The antagonistic roles of these proteins also seem to be manifested at the level of histone methylation. High levels of trimethyl Histone H3 lysine 4 (H3K4me3) are associated with active promoters [74, 75]. MXD dependent recruitment of histone demethylase RBP2/KDM5a induces histone H3K4me3 demethylation at the TERT promoter and correlates with decreased expression of TERT during DMSO-induced differentiation of HL-60 Human promyelocytic leukemia cells [76]. Interestingly, Drosophila Myc interacts directly with the fly ortholog of RBP2, known as Lid, but this binding directly inhibits the demethylase activity of Lid [77, 78]. Moreover, MYC has been reported to interact with MEN1 [79], a subunit of the mixed-lineage leukemia-1 (MLL-1) methylation complex, which mediates H3K4 methylation. Consistent with this, MYC’s genomic binding profile strongly correlates with H3K4me3 levels [80, 81]. Several reports have shown that changes in global levels of histone H3K4me3 correspond with increasing or decreasing MYC expression [82, 83]. In the MYC inducible lymphoma cell line p493-6, however, activation of MYC was found to promote H3K4me3 methylation at only a subset of MYC target genes [84]. Overall, these data strongly suggest that the MYC and MXD proteins antagonize each other by promoting and inhibiting, respectively, both histone acetylation and methylation.
MAX and MLX apparently possess neither TAD nor SID domains and therefore are considered transcriptionally inert. However, MLX differs from MAX because, like MONDO proteins, it contains a dimerization and cytoplasmic localization domain (DCD domain) at its C-terminus [33–35]. MONDOA and CHREBP also contain five domains named Mondo Conserved Regions (MCRI-V) within their N-termini, which mediate glucose sensing and transcriptional activity [see reviews 36, 85]. Moreover the existence of a MCR6 domain in MONDOA protein, which represents a potential binding site for glucose-6-phosphate (G6P), has recently been proposed [86]. MONDOA associates to the outer mitochondrial membrane, where it can also potentially sense other metabolites such as glutamine (through α-ketoglutarate), adenine nucleotides and non-glucose sugars [37, 87–91]. Thus, MONDO proteins sense multiple metabolites to directly link MONDO transcriptional activity to the metabolic state of the cell. While the exact mechanism through which MONDO proteins regulates transcription is still not completely clear, it has been shown that ChREBP associates, through its TAD domain, with p300 and mediates histone acetylation [92].
MGA differs from all other members of the network because it contains an additional T-loop DNA binding domain and can either act as activator or repressor depending on its interaction with MAX. Apart from MGA belonging to a repressive complex that binds to MYC and E2F responsive genes in G0, very little is known about its biological functions [30, 93]. Very recently MGA, together with MAX and SMAD4, has been shown to be involved in BMP signaling during gastrulation in Xenopus embryonic development [94]. Interestingly MGA is recurrently inactivated in high risk chronic lymphocytic leukemia [95] and in lung cancer [96], suggesting that it may function as a tumor suppressor gene.
2. Functional interactions among network members
Many reviews have previously discussed the role of MYC in controlling multiple aspects of cell biology [97–104]. Here we will focus on how the activities of the two arms of the MAX/MLX network are functionally interconnected. In particular, we will discuss how a broad range of common MYC activities (Fig. 4A) are shaped by other network members (Fig. 4B) and the manner in which these biological functions are affected when deregulated MYC drives cell transformation. For example, it is becoming evident that MXD members oppose MYC pro-apoptotic functions, suggesting that some aspects of an intact MAX/MLX network may be required for MYC induced tumorigenesis.
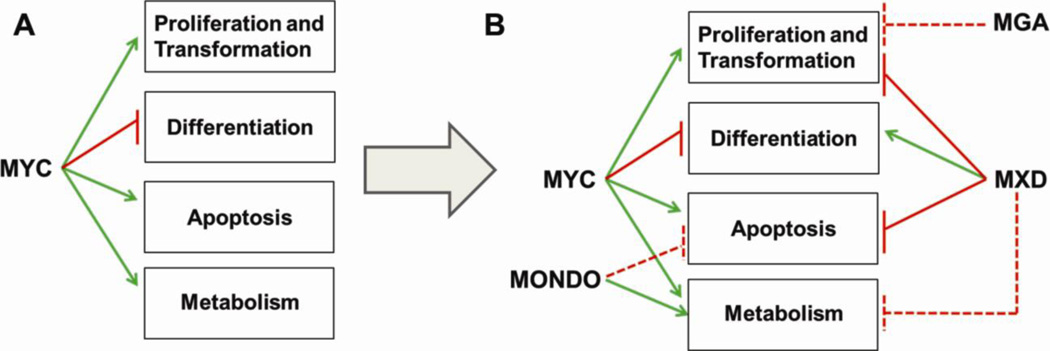
Figure 4. Schematic representation of the biological activities regulated by the MAX/MLX network members.
Green arrows and red lines indicate positive and negative regulation respectively. Dotted red lines indicate a functional connection that has not been directly investigated or for which only a limited amount of data is available. A) MYC centered view. B) MAX/MLX network centered view.
2.1. Proliferation and transformation: in vitro and in vivo experimental models
2.1.1 MXD Proteins: inhibiting MYC induced proliferation and transformation
MYC proteins promote proliferation by interacting with MAX and altering the expression of thousands of genes. However, all MXD proteins, MXD1-4 and MNT, also heterodimerize with Max, bind to the canonical MYC/MAX E-Box consensus sequence and repress transcription from an E-Box containing promoter [26–29]. Competition between MYC and MXD proteins on each of these levels suggests that these transcriptional regulators act as functional antagonists.
Overexpression of all MXD proteins inhibits MYC/RAS induced co-transformation, confirming that MXD proteins can antagonistically inhibit MYC activities in vitro [28, 105–111]. Moreover, overexpression of MXD1 and MXD2 proteins inhibits proliferation and induces accumulation of cells in the G0/G1 cell cycle phase in multiple in vitro experimental systems [109, 110, 112–122]. The ability of MXD proteins to block MYC induced transformation requires the HLHLZ domain, the basic region and the SID domain, indicating that dimerizing with MAX, binding DNA, and interacting with the SIN3 containing histone deacetylase complex are required for inhibition of MYC activity [107, 108, 111, 123].
Consistent with MXD1 inhibition of proliferation in vitro, transgenic mice expressing MXD1 under the control of a constitutive and ubiquitous β-actin promoter have reduced body size and weight compared to their wild type littermates. Both embryonic fibroblast and hematopoietic progenitor cells derived from Mxd1 transgenic animals show reduced proliferation [120]. Specifically, mouse embryonic fibroblasts (MEF) overexpressing MXD1 were more sensitive to contact inhibition and underwent proliferative arrest at lower density [120]. However, overexpression of MXD1 under control of the β-actin promoter is not sufficient to completely inhibit proliferation as it does not preclude embryonic development [120]. Similar results were obtained when MXD1 was specifically overexpressed in T and B lymphocytes. Although in both cases mature T and B cells were obtained, neither cell types were able to efficiently proliferate in response to multiple mitogenic stimuli in vitro [124, 125].
These results are consistent with the phenotype of Mxd1−/− mice where Mxd1 deletion delays cell cycle exit in granulocytes but does not completely block cell cycle arrest and differentiation [126]. Overall the analysis of Mxd1 transgenic and Mxd1−/− mice suggests that additional pathways, besides those directly affected by Mxd1 overexpression, are likely required to fully promote cell cycle arrest in collaboration with Mxd1. Indeed, Mxd1/p27Kip1 double knockout mice show a much stronger phenotype than ether Mxd1−/− or p27Kip1−/− mice, with markedly impaired cell cycle arrest and differentiation of double null granulocytes. [127]. Finally, Mxd1−/− mice appear normal and are not prone to tumor development, indicating Mxd1 is involved in, but not absolutely required for cell cycle arrest.
The involvement of MXD proteins in the regulation of cell cycle in vivo has been further strengthened by the analysis of Mxd2 knockout mice. Mice carrying homozygous deletion of Mxd2 gene show several tissue abnormalities, including prostate and spleen hyperplasia/dysplasia [128]. Moreover, Mxd2−/− T lymphocytes show increased proliferation in response to anti-CD3 plus anti-CD28 antibody stimulation and Mxd2−/−mice develop B cell lymphoma at low frequency and long latency. These data indicate that Mxd2 may be a potential tumor suppressor, although additional events may be required for tumorigenesis. A formal proof of this hypothesis came from experiments showing that Mxd2−/− mice are more susceptible to developing squamous cell carcinoma of the skin and malignant lymphoma when treated with weekly topical application of the carcinogen 9,10-dimethyl-1,2-benzanthracene (DMBA). Consistently, loss of Mxd2 also accelerates the development of fibrosarcoma and lymphoma in an Ink4−/− background [128].
2.1.2. MNT: the major MYC antagonist
In contrast with Mxd1, overexpression or deletion of Mnt in mice results in very strong phenotypes. Mnt transgenic mice die during embryonic development and are significantly smaller than their wildtype littermates [108]. Preliminary analysis of the nervous system indicated defective proliferation and differentiation. Mnt deficient mice develop normally, yet are subviable, as more than 95% of the Mnt−/− animals die within a few days of birth [129, 130]. These broad organismal effects most likely reflect the ubiquitous expression of Mnt during development. [108, 129, 131–134].
Several lines of evidence point to MNT as a major MYC antagonist. First, primary MEFs lacking Mnt phenocopy Myc overexpressing cells. Mnt−/− MEFs proliferate at an accelerated rate, grow to high density and prematurely enter S phase [129, 131]. Analogous results were obtained when Mnt expression was silenced by the use of a Mnt-specific siRNA [131]. Interestingly, the expression cyclin E1 (Ccne1) and cyclin-dependent kinase 4 (Cdk4), both previously identified as Myc responsive genes [129, 135–137], are elevated in Mnt−/− cells. Hence, increased activity of CCNE1/CDK2 and CCND1/CDK4 complexes probably drives the hyper-proliferative phenotype of Mnt null cells. Moreover, Mnt−/− MEFs are more sensitive to apoptosis, display reduced senescence, and are more susceptible to RAS induced transformation both in vitro and in vivo [129, 131].
The close functional antagonism between MYC and MNT is further underscored by the observation that, in Mnt deficient fibroblasts, MYC expression is drastically reduced, suggesting that cells compensate for the lack of MNT by decreasing the levels of MYC. Nevertheless, such cells are still capable of proliferation [129]. Notably, silencing of Mnt in rat Myc−/− fibroblasts promotes both proliferation and apoptosis under conditions of serum starvation. These results suggest a model in which MYC, in response to mitogenic stimuli competes for MAX binding and alleviates MNT-mediated repression. The demonstration that MNT/MAX heterodimeric complexes are converted to MYC/MAX complexes when cells enter the cell cycle supports this model [138]. In addition Mnt deficiency rescues the proliferation defect caused by Myc deletion in primary MEFs [138]. This is consistent with data showing that in Drosophila while complete inactivation of dmyc is lethal due to arrested development at the early larval stage, double mutants flies lacking both dmyc and dmnt grow larger and advance further in development [139, 140]. Overall, the data obtained in both mammalian and Drosophila experimental systems provide a strong argument for considering MNT as the principal MYC antagonist.
In this regard it is not surprising that conditional disruption of Mnt in mouse mammary epithelium and T cells promotes the development of mammary adenocarcinoma and T cell lymphomas, respectively [129, 132, 141]. However, in both cases, the latency of tumor formation, ranging from 6 to 22 months, indicates that secondary mutations are likely to be cooperating with loss of Mnt in tumorigenesis, as proposed for Mxd2 knockout mice. In the case of Eε-Myc induced B-cell lymphomas such secondary mutations generally act to disable the pro-apoptotic machinery and are required for the development of the B-cell lymphoma in this mouse model [142, 143].However, recent reports demonstrate that loss of Mnt inhibits Myc driven tumorigenesis by promoting apoptosis. Therefore MNT may promote or inhibit tumorigenesis depending on the context [144] (see apoptosis section).
2.1.3. The MLX module: an underappreciated player in tumorigenesis
While much research on the functions of the MYC/MAX/MXD module has been carried out, and, as described above, the metabolic functions of MLX and MONDO proteins have been well defined [36, 145–148], comparatively little is known concerning the role of MONDO proteins in tumorigenesis and the possible interactions between the MAX and the MLX branches of the network, specifically in relationship to MYC activity.
It is important to note that, in addition to MONDOA and CHREBP, MXD1, MXD4, and MNT are also able to form heterodimers with MLX [31, 32]. In vitro assays show that both MXD1 and MNT maintain their function as transcriptional repressors when bound to either MAX or MLX. [31, 32]. Therefore, some aspects of the phenotypes observed when expression of MXD1 and MNT are altered, may be mediated by the heterodimerization with MLX. MONDO/MLX heterodimers have been considered the positive transcriptional arm of the MONDO/MLX/MXD module. Interestingly, initial experiments investigating the transcriptional capacity of MONDOA or CHREBP suggested they may possess both transcriptional activation and repression functions. MONDOA/MLX heterodimers activated an E-box driven artificial luciferase reporter, while CHREBP repressed the same promoter either when expressed alone or in combination with MLX [33, 34]. However, both MONDOA and CHREBP transcriptionally activate many genes involved in glucose and lipid metabolism [85]. Moreover, MONDOA may act either as an activator or as a repressor depending on nutrient availability [87]. Therefore, the ability of MONDO proteins to activate or repress gene expression may be context specific.
Considerable experimental evidence indicates that modulation of MONDO protein expression can affect tumor cell growth. MONDOA knockdown in HA1ER transformed embryonic human kidney epithelial cells increases both proliferation and soft agar colony formation compared to control cells [87]. CHREBP overexpression has also been shown to reduce the ability of U2OS cells to form colonies [34], suggesting that loss of MONDO proteins may promote tumorigenesis. However, silencing of MONDOA in Acute Lymphoblastic Leukemia (AML) and CHREBP in pancreatic beta cells and human colon carcinoma HCT116 or HepG2 hepatoblastoma cells, decreases cell proliferation and promotes apoptosis [149–151]. These contradictory results may simply be due to the use of overexpression versus silencing experimental strategies to investigate the role of the MONDO proteins. Alternatively, they may reflect cell type intrinsic functions of MONDO proteins. A recent review discussing MONDOA and MLX expression, as derived from available databases, showed that high MONDOA expression can correlate with either good or poor cancer prognosis [147]. It is important to note that because MONDOA transcriptional function depends on availability of metabolites and heterodimerization with MLX, the mRNA levels of MONDOA alone may not reflect its activity. Moreover, it is possible that MONDOA may act either as an oncogene or a tumor suppressor depending on the cellular context.
To date, the ability of MONDO/MLX heterodimers to promote or antagonize MYC dependent transformation in a defined mammalian system has not been fully investigated. However, dmyc and mio genetically interact in Drosophila, where double homozygosity for a hypomorphic dmyc diminutive allele (dm) and a mio mutant (dmon1) resulted in a synthetic lethal interaction [36]. This result implies that the two modules of the MAX/MLX network functionally interact in invertebrates. Overall these results point toward functional cross-talk between the two modules of the network and suggest a collaborative relationship between MYC/MAX and MONDO/MLX heterodimers.
2.2. Role of the network in differentiation
The expression patterns of individual network proteins are distinct and cell context dependent. In general, MAX and MNT are mainly unchanged during the cell cycle and are broadly expressed throughout development and adulthood. In contrast, MYC expression peaks upon cell cycle entry at the G0-G1 transition and is expressed at a constant level during the proliferating cell cycle [152]. Conversely, MXD1, 2 and 4 expression is increased during cell cycle exit and differentiation [153]. MXD3 differs from the other MXD proteins because it is expressed during S phase in proliferating cells [154, 155]. While CHREBP and MLX have both been shown to be induced after growth factor or serum stimulation [32, 150], MONDOA expression profiles have not been extensively studied during cell cycle, development or differentiation.
High levels of expression of MYC inhibit differentiation of many cell types and promote the reprogramming of somatic cells to induced pluripotent stem cells (iPS cells) [156–158]. In hematopoietic cells induced to differentiate, MYC levels decrease while MXD1 expression levels increase [159–162]. Analysis of hematopoiesis in Mxd1−/− mice revealed a delay in granulocyte differentiation [126] and overexpression of MXD1 has been shown to be sufficient to induce erythroid differentiation of murine and human erythroleukemia cells [163, 164]. This suggests MXD1 plays an active role during differentiation. There is evidence indicating that the contribution of MXD1 to the differentiation process may primarily be due to its role as a MYC antagonist. Indeed, expression of a chimeric protein containing the MYC bHLHLZ domain fused to the MXD1 transcriptional repression domain represses MYC target genes and efficiently induces differentiation of the erythroleukemia cell line K562 [163].
Furthermore, Mxd1 knockout mice, as well as Mxd2, Mxd3 null mice develop properly and do not show any major differentiation defect [28, 126, 128]. While compensation among MXD proteins may partly explain the lack of a developmental defect in Mxd deficient mice, these results strongly suggest that, while MXD proteins may participate in the differentiation process, they are not individually necessary.
Interestingly, rat PC12 pheochromocytoma cells, which lack the Max gene [165] and therefore would be expected to be lacking any MYC or MXD dependent activities, are capable of differentiating in response to nerve growth factor (NGF) and RAS [166–168]. In addition, Max-null murine embryonic stem cells expressing an inducible Max gene cease proliferating, differentiate, and eventually undergo apoptosis when Max expression is repressed [169]. However, cell viability and proliferation can be rescued by treating these cells with MAPK and GSK3β inhibitors (2i condition) or overexpressing OCT4 [169]. The fact that both PC12 and max null mECS can undergo differentiation, strongly suggests that MXD proteins are not directly responsible for induction of differentiation, at least as heterodimers with MAX. Instead, these results imply that activation of MAPK and GSK3β signaling pathways are required for mESC differentiation. Interestingly in the 2i condition, MYC expression is strongly diminished; suggesting that in absence of cell cycle arrest or differentiation signals MYC expression can be reduced without impacting proliferation. Genetic studies in Drosophila have demonstrated that dmax loss of function mutations are not as severe as dmyc deletion, suggesting that dmyc possesses functions that are independent of dmax [170, 171]. The finding that loss of human MAX gene is strongly associated with heritable pheochromocytomas is consistent with MAX independent function of Myc in mammalian cells [172, 173]. Interestingly, MYC overexpression in PC12 cells inhibits Ras-mediated differentiation in the absence of MAX [174].
Both MLX and MONDO protein expression are developmentally regulated but their specific involvement in cellular differentiation has not been fully evaluated. However, Mlx (Carroll unpublished), MondoA (Don Ayer, personal communication) and Chrebp [175] knockout mice develop to adulthood without major developmental defects, indicating that the MLX module is not required for either cell viability or differentiation during embryonic development.
2.3. Apoptosis: a matter of balance
The induction of apoptosis by the deregulated expression of MYC has been well documented [for reviews see 97, 98, 176–179]. Deregulated expression of MYC elicits the activation of an intrinsic tumor suppressor program, which induces apoptosis. Therefore, the establishment of MYC driven tumors requires the inactivation of the apoptotic machinery, which can result from the loss of tumor suppressor genes such as TP53, or gain of function of anti-apoptotic genes [142, 143, 180, 181]. Moreover, high levels of MYC also sensitize cells to many different extrinsic apoptotic stimuli such as UV, ionizing irradiation, pro-apoptotic signals and nutrient or serum withdrawal [182–187].
Among the network members, MXD1, MXD4 and MNT have been shown to inhibit apoptosis in several different experimental systems. The first observation that MXD proteins are involved in apoptosis came from the analysis of Mxd1−/− mice. Mxd1−/−granulocytes are more sensitive to cytokine withdrawal and other apoptotic stimuli such as γ-irradiation and doxorubicin [126]. Conversely, overexpression of Mxd1 protects against apoptosis induced by tumor necrosis factor-related apoptosis inducing ligand (TRAIL), Fas ligand, serum withdrawal, UV and cysplatin treatments [113, 117]. Moreover, MXD1 is induced by hypoxia and mediates hypoxia-induced resistance to doxorubicin [188]. Importantly, MXD1 overexpression also specifically inhibits MYC dependent apoptosis upon serum withdrawal [113]. The mechanistic basis for this may be at least in part due to MXD1 mediated repression of PTEN expression and promotion of the PI3K/AKT pathway. Both MYC and MXD1 bind to the PTEN promoter, indicating that MXD may block MYC induced apoptosis at least in part by repression of a subset of MYC target genes [189]. However, in a set of experiments designed to evaluate the functional equivalence of MYC and MXD1 bHLHLZ domains it was shown that a chimeric protein containing the N-terminal domain of MYC and the bHLHLZ of MXD1 was unable to induce apoptosis upon serum withdrawal [190]. An interpretation of this result is that the bHLHLZ of MYC and MXD1 differ in their ability to interact with additional factors involved in apoptosis. Notably the MYC, but not the MXD1 bHLHZ domain mediates the interaction with the transcription factor MIZ1. Binding of MYC to MIZ1 inhibits expression of the MIZ1 targets p21Cip1 and p15ink4b. [191–193] and is required for MYC induced apoptosis of rat cells upon serum withdrawal [194].
Similarly, overexpression of MNT inhibits MYC transcriptional activity and oncogenic properties, while loss of MNT promotes proliferation but also sensitizes cells to apoptosis [108, 129, 131]. Surprisingly, RNAi mediated silencing of MNT effectively sensitizes primary MEFs, 3T3 immortalized mouse fibroblasts, and Rat Myc −/− fibroblasts to serum withdrawal induced apoptosis. This Indicates that loss of MNT mediated apoptosis is not solely MYC dependent [131]. In agreement with this interpretation, MYC inactivation only partially rescues MNT dependent apoptosis in MEFs [138]; hence inactivation of MNT promotes apoptosis, which is further exacerbated by activation of MYC.
Analysis of developing T-cells in a mouse model harboring a conditional deletion allele of Mnt confirmed that loss of Mnt promotes apoptosis in single (CD4+ or CD8+) and double positive (CD4+/CD8+) T-cells and also when MYC is ectopically expressed either in T cells or in primary and immortalized MEFs, suggesting that MNT provides a pro-survival signal and protects cells against MYC mediated apoptosis [144].
The precise molecular mechanisms through which loss of MNT sensitizes cells to apoptosis remain unclear. Nonetheless there are some clues. Inappropriate and increased levels of TP53 and ARF19 have been reported in primary Mnt −/− MEFs and Mnt KD cells compared to control cells [129, 131]. However, acute CRE-mediated deletion of Mnt in primary MEFs failed to confirm these results [138]. Moreover, Mnt-deficient thymocytes do not show abnormal TP53 expression levels. Notably, while BCL2 can rescue cells from MYC induced apoptosis, overexpression of BCL2 in either the presence or absence of MYC fails to rescue the Mnt −/− apoptotic phenotype, suggesting that MYC overexpression and loss of MNT promote apoptosis, at least in part, through two different pathways [144] .
Another possible mechanism related to loss of MNT induced apoptosis comes from a recent study reporting a high level of reactive oxygen species (ROS) in cells overexpressing MYC and lacking MNT. Furthermore treatment of Mnt null cells with antioxidants rescues cell viability, indicating that increased levels of ROS might be responsible for the reduced viability of Mnt null cells [144]. The fact that Mnt −/− cells also have reduced oxygen consumption indicates that such cells have impaired mitochondrial metabolism. Activation of MYC has been shown to promote mitochondria activity and ROS production [144]. Consistent with this, MYC overexpressing Mnt−/− cells have higher ROS than either Mnt −/− or MYC overexpressing wild type cells. Similar to MNT, MXD4 has also been shown to protect cells from apoptosis during OX40 mediated activation of T cells [195].
Underscoring the close connection between oxidative metabolism and the MAX/MXD network, MXD2 has been reported to be a target of Hypoxia Inducible Factor-1 (HIF-1) [196]. HIF-1 is a heterodimer comprised of the constitutively expressed HIF1b subunit (also known as ARNT) and an oxygen regulated HIF-1a or HIF2a (commonly known as EPAS1) subunit. HIF1 regulates glucose metabolism and has been linked to tumor growth, metastasis and angiogenesis. In renal carcinoma cells, HIF1 is hyper-activated because the tumor suppressor Von Hippel-Lindau (VHL), which mediates its degradation, is lost [197]. In both renal cell cancer cell lines and primary tumors, MXD is highly expressed and antagonizes MYC transcriptional activity [198]. Surprisingly cells with reduced expression of MXD2 proliferate faster in vitro, yet require MXD2 to efficiently form tumors in vivo [198]. A possible explanation is that MXD2 expression partially antagonizes MYC activity and prevents MYC induced apoptosis under conditions of limited oxygen availability. Moreover, MXD2-mediated, HIF-dependent downregulation of mitochondrial metabolism may provide a survival advantage by reducing ROS levels [196, 198]. Indeed, MXD1 is also induced by hypoxia in colon cancer cells and mediates resistance to doxorubicin under hypoxia [188].
Overall MXD proteins can be considered to promote survival under stress conditions particularly when MYC is highly expressed. Therefore, although decreased MXD protein expression may be beneficial for tumor growth when expression of MYC is not deregulated, low levels or complete loss of MXD proteins becomes counterproductive and may promote apoptosis when MYC is overexpressed.
Very little is known about the role of MONDOA and CHREBP in apoptosis and more specifically in MYC mediated apoptosis. Silencing of MONDOA in acute lymphoblastic leukemia and MYCN amplified neuroblastoma cells promotes apoptosis suggesting that MONDOA may cooperate with MYC in tumorigenesis [151](Carroll, Diolaiti, McFerrin and Eisenman, unpublished).
CHREBP by contrast has been shown to mediate pancreatic beta cell sensitivity to high glucose (glucotoxicity). In response to sustained high levels of glucose, CHREBP induces oxidative stress and apoptosis through its target gene TXNIP which promotes oxidative stress by inhibition of thioredoxin [199]. CHREBP is also required for HCT116 colon cancer and HepG2 liver cancer cells survival [150], however how CHREBP activity affects MYC dependent apoptosis remains to be understood.
2.4. Growth and metabolism: does the MLX module enable nutrient addiction in Myc transformed cells?
Early studies defining MYC transcriptional targets provided a list of genes involved in several metabolic processes, indicating that MYC not only drives proliferation but also promotes the activity of both catabolic and anabolic metabolism [200–204] Recently the role of MYC as a master regulator of growth and metabolism has become widely accepted [102, 104, 205] MYC not only promotes the activity of all three RNA polymerases [206–208], but also directly promotes the expression of many genes involved in glucose and glutamine metabolism [209–216] as well as lipid and nucleotide biosynthesis and mitochondrial activity [104, 217–219] Moreover MYC regulates ribosome biogenesis and protein synthesis [220]. Overall, MYC activation promotes an extensive reprogramming of cellular metabolism in order to fuel anabolic pathways and provide the building blocks for cellular growth and thus, proliferation.
In contrast to the large amount of data available on MYC, the role of MXD proteins in regulating metabolism has not been systematically addressed. Interestingly MNT, as well as MXD1 and MXD4, can bind not only to MAX but also to MLX. Therefore, MXD proteins can potentially control cellular metabolism by influencing the transcriptional activity of both MAX and MLX modules. However, to date only MNT has been linked, albeit indirectly, to metabolism. CRE-mediated deletion of Mnt in T cells results in diminished oxygen consumption rate (OCR) and increased ROS production, implying an imbalance in cellular oxidative metabolism [144]. Moreover, miR-210, a hypoxia inducible miRNA, targets MNT to promote cellular proliferation under hypoxic conditions. Since both miR-210 overexpression and MNT silencing promote expression of many metabolic genes previously identified as MYC targets, it is possible that this effect is mediated indirectly by increased MYC transcriptional activity [221].
Both MONDOA and CHREBP play a crucial role in metabolism [36, 85, 222]. Both the subcellular localization and transcriptional activity of MONDO proteins are regulated by glucose. In general, upon glucose stimulation, MONDO/MLX heterodimers accumulate in the nucleus and activate transcription of their target genes. However, additional mechanisms control MONDO expression and transcriptional activity [223–225]. CHREBP and MONDOA are expressed in most tissues, but while CHREBP is enriched in liver [175], MONDOA is highly expressed in skeletal muscle [33]. This differential expression profile also appears to reflect the major biological functions of these proteins. Glucose induced CHREBP activation in the liver promotes the conversion of glucose to lipid. Indeed Chrebp−/− mice exhibit hyperglycemia and diminished serum triglycerides [175]. Gene expression analysis and chromatin immunoprecipitation experiments have shown that CHREBP activates and directly binds to many glycolytic genes and to key regulators of lipogenesis such as Acetyl coA carboxylase (ACC), fatty acid synthase (FAS) and sterol-coA desaturase (SCD) [226–230]. MONDOA instead has been shown to regulate mainly glycolytic genes [37, 91]. Moreover both CHREBP and MONDOA regulate the common target gene TXNIP implying that they can mediate, at least in part, similar biological processes [37, 92].
As discussed in the next section, both MYC and MONDO proteins have been detected bound to the promoters of specific metabolic genes indicating that these proteins functionally cooperate to orchestrate cellular metabolism. [91, 231–233]. While MYC has been shown to be required for CHREBP and glucose-dependent activation of glycolytic genes [231, 232], it is not known whether MYC requires MONDO activity to properly reprogram metabolic pathways and drive proliferation. It is possible that MYC and MONDO may transcriptionally regulate common target genes; although it cannot be excluded that MONDO proteins may affect MYC biological activity by regulating a complementary subset of genes. MYC driven tumor cells have been shown to be dependent on high level of either glutamine or glucose [185, 186, 234] and inactivation of metabolic genes such as LDH-A (a MYC and MONDOA common transcriptional target) inhibits MYC dependent tumorigenesis [204, 235]. Interestingly MONDOA responds to both glucose and glutamine and has been proposed to coordinate glycolysis with glutaminolysis by directly sensing TCA cycle function to regulate TXNIP expression and modulate glucose uptake and glycolytic rate [87, 146]. Therefore, it is possible that altering MONDO function would negatively impact MYC oncogenic activity.
2.5. The target gene enigma
Based on the biochemical, transcriptional, and functional studies described above, it has been suggested that MAX/MLX network members may bind to common target genes. This model is also supported by the fact that MXD1 and MXD2 specifically inhibit MYC transforming activity but are less effective in blocking E1A mediated transformation. [105, 109]. Hence MXD proteins do not behave as general inhibitors of transformation, but specifically antagonize MYC activity. Indeed, there is compelling evidence that MXD1 and MNT bind to MYC target genes in vivo. However, very little genomic binding data are available for the other network members. MYC and MXD1 have been shown to directly bind and respectively activate and repress relevant genes such as TERT, CCND2 and PTEN [189, 236–243]. In each of these cases MYC/MAX heterodimers could be replaced by MXD1/MAX heterodimers, resulting in histone deacetylation and transcriptional repression through recruitment of an HDAC containing complex. In addition, MXD1 recruits RBP2 to the TERT promoter, which mediates histone H3k4me3 demethylation [76]. Other studies have reported an antagonistic role for MYC and MXD1 in controlling the expression of other important genes such as CDC25A [244, 245] and the three EIF4F complex subunits EIF4EF, EIF4EAI, EIF4EGI [246, 247]. However the direct binding of MXD1 to the promoters of these genes has not been examined.
Interestingly, the most comprehensive analysis of MYC and MXD target gene overlap has been performed in vivo. Overexpression of MXD1 in lymphocytes inhibits expansion, maturation, and growth of T cells. Gene expression analysis showed that over 80% of the genes repressed by MXD1 have previously been found to be induced by MYC [124]. These results strongly support the hypothesis that both MYC and MXD1 control the expression of a common set of target genes. Similar results were obtained by comparing the expression profiles of mammary adenocarcinomas resulting from either the overexpression of MYC or the conditional deletion of Mnt in murine mammary gland epithelial cells [132]. In this case the tumor gene expression profiles were basically indistinguishable and MYC and MNT genomic binding profiles were highly overlapping, strongly arguing that MYC and MNT regulate the same target genes in vivo.
While there is evidence showing that both MYC and MXD proteins reciprocally control the expression of a large set of common genes, it is not known whether MXD/MLX heterodimers share target genes with MXD/MAX or MONDO/MLX heterodimers. So far, the specific biological functions of MXD/MLX heterodimers, if any, are completely unknown. By contrast, the role of MONDOA and CHREBP in the regulation of glycolysis and lipogenesis has been well established [49, 85, 248, 249]. Surprisingly, MONDOA and CHREBP seem to bind to a much smaller number of genes than MYC. The fact that CHREBP binds to a tandem E-box spaced by 6 nucleotides (Carbohydrate response element or ChoRE) may in part explain this difference [250–252]. Moreover, while MYC target genes regulate many biological processes, most of the MONDO/MLX target genes are involved in metabolism. Specifically, when a constitutively transcriptionally active version of MONDOA definedΔN237NLS-MondoA was overexpressed in C2C12 myoblasts, only 81 genes were activated more than 1.8 fold. Among these, 53% and 29% of the MONDOA targets genes were respectively involved in metabolism and cell growth [91]. Among these genes, MONDOA was shown to directly bind and regulate the expression of LDHA and HKII, two previously reported MYC target genes, and PFK3FB [204, 213, 253]. Importantly, both MYC and MONDOA bind to the promoter of all three genes in C2C12 cells [91]. Although these results suggest a coordinate regulation of metabolic genes by MYC and MONDOA, a detailed analysis of the specific and relative contribution of MYC and MONDOA to the expression of these genes has not been evaluated.
MYC and CHREBP are both required for the glucose dependent activation of the Liver-type pyruvate kinase gene (PKLR). MYC and CHREBP bind to the PKLR promoter in presence of glucose. Silencing of MYC expression or inhibition of MYC/MAX heterodimerization decreases the binding of CHREBP/MLX to the PKLR gene promoter and its expression [233].These data suggest that MYC, through an unknown mechanism, facilitates the recruitment of CHREBP to the PKLR promoter. However the reciprocal analysis was not performed leaving it an open question as to whether CHREBP affects MYC transcriptional activity or how general this cooperation may be.
Overall, these data strongly argue for a crosstalk between MAX and MLX modules; however the full extent of the transcriptional and functional overlap between them has not been rigorously established. This will require detailed analyses of genomic binding, epigenetic changes, and gene expression for all network members in specific biological contexts.
3. The balance among network components determines distinct cellular states: a comprehensive model
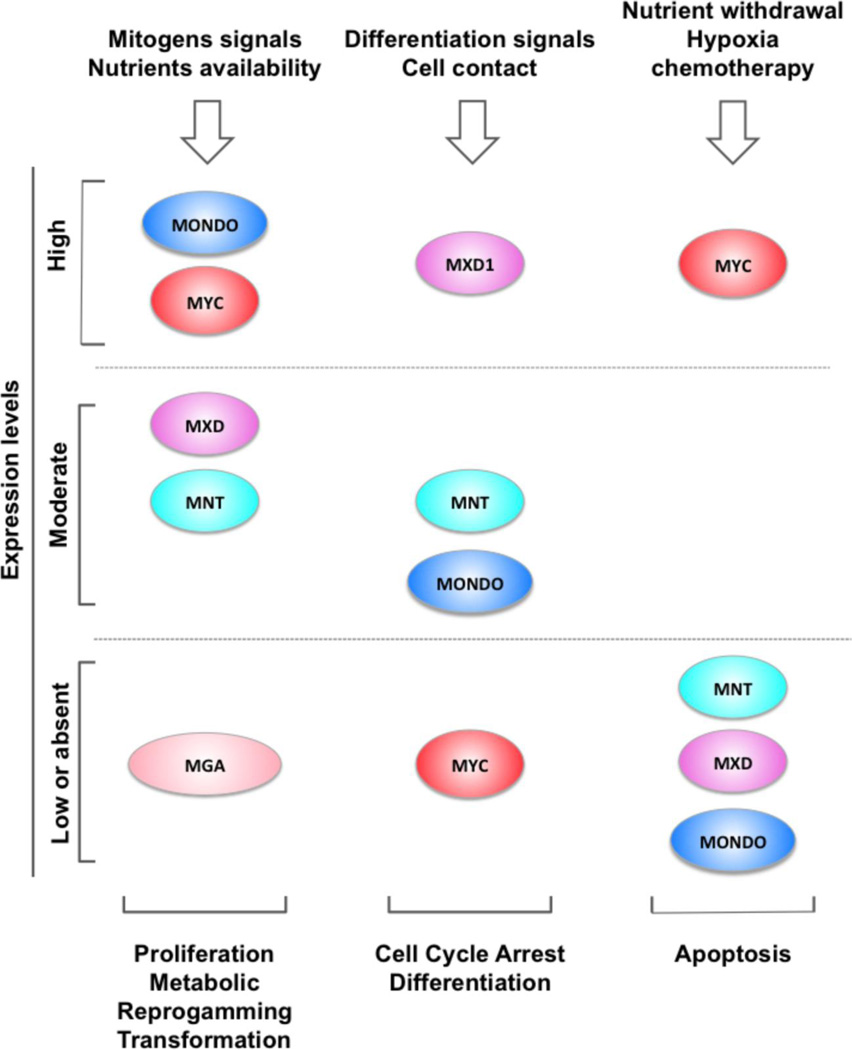
Overall the results discussed so far can be summarized by the model shown in figure 5, where external signals define the balance between MYC, MXD and MONDO proteins and promote distinct cellular fates. High levels of MYC drive growth and proliferation while the switch from MYC to MXD proteins promotes cell cycle arrest and differentiation. However MXD family expression alone is not sufficient to completely arrest proliferation. Therefore, additional signals are necessary to inhibit MYC expression or promote MYC degradation in order to allow MXD proteins to efficiently counteract MYC activity. An apoptotic response occurs in situations with extremely high levels of MYC activity. MYC driven apoptosis may be further facilitated by loss of MXD, MNT or MONDO activities or under stress conditions such as fluctuation in nutrient availability, hypoxia or chemotherapy.
Figure 5. Comprehensive model of the MAX/MLX network and its role in determining cellular fates.
External signals (top) determine the expression levels of the network members, which in turn promote alternative cellular state (bottom). High levels of MYCc sensitize cells to pro-apoptotic stimuli. Loss of function of MXD1, MNT or MONDOA further promotes apoptosis in MYC driven tumor models. MGA has been reported recurrently lost in leukemia and lung cancers. Because MAX and MLX are transcriptionally inert and have multiple heterodimerization partners, they have been excluded from the representation for simplicity.
In order to support proliferation, MYC promotes the metabolic reprogramming of the cell. Therefore coordinate regulation of metabolic genes by MYC and MONDO proteins may be required to sustain tumor cell viability. In support of this model, MYC binds to the promoter and appears to regulate the expression of both CHREBP and MONDOA, and inhibiting MONDO protein expression promotes apoptosis in several experimental system [83, 146, 150, 151].
The prevailing view of MYC function has been that MYC/MAX heterodimers bind genomic DNA and recruit different transcriptional co-regulator complexes to modulate gene expression – in essence considering MYC as a standard transcription factor albeit with a very large number of gene targets [103]. However two recent reports have taken another view, namely that MYC acts as a universal amplifier of gene expression [66, 67]. The authors proposed a new model in which MYC, instead of acting as a molecular ON/OFF switch for a specific subset of target genes, globally amplifies the pre-existing gene expression program already in place in in a given cell. This general augmentation of gene expression occurs through MYC recruitment of P-TEFb, a complex that alleviates transcriptional pausing by RNA Pol II, and therefore promotes transcript elongation. While the “universal amplifier” model accounts for some properties of MYC, such as the wide variability of gene targets identified in different studies, its general validity is being hotly debated [254–257]. Moreover, whether and how the activities of the other members of the MAX/MLX network relate to this model remains an open question. Nonetheless, it is possible to imagine that, by competing with MYC for its genomic targets, MXD proteins may also globally counteract MYC’s anti-pausing activity. Alternatively MXD proteins may recruit pausing factors and inhibit RNA Pol II elongation. Thus, in the context of the “universal amplifier” model MXD proteins may be required to attenuate MYC pro-proliferative transcriptional activity, thereby weakening the ongoing transcriptional program and allowing other factors to promote cell cycle arrest or differentiation.
Interestingly, the amplifier model can also be considered in the context of cooperative regulation of genes involved in metabolism. MYC has been shown to regulate the expression of many metabolic genes, a subset of which are also targets of MONDOA and CHREBP. Perhaps MONDO proteins act as specific selectors of critical genes, with their expression mainly dependent on MONDO activity, while MYC binding would further amplify their expression through its RNA Pol II anti-pausing activity. This is consistent with the need to coordinate nutrient availably with mitogenic signals and with the ability of MYC to promote proliferation and cellular metabolic reprogramming. In this model, activation of MYC in the presence of nutrients would promote cellular metabolic reprogramming by co-opting and enhancing the MONDO-dependent transcriptional program. On the other hand, the absence of nutrients such as glucose would turn off MONDO transcriptional activity and prevent MYC driven metabolic reprogramming and proliferation, thus triggering apoptosis in a deregulated MYC state.
4. The MAX/MLX network in human cancer
Accumulating evidence indicates that the biological outcome of MYC deregulation is dependent on the expression and function of the other members of the network, which either antagonize or collaborate with MYC. Based on the organization of the network, it is reasonable to consider that changes in the expression of one network member may have a significant biological impact by modifying the relative abundance and activities of other functionally distinct network members. This notion is based on several considerations. First, MYC, MXD, or MONDO family proteins are expected to compete for binding to limiting amounts of MAX or MLX. Second, alternative heterodimers will bind the same or related DNA sequences and thus compete for binding sites, and third, network members impose different consequences in terms of gene target expression.
The importance of maintaining a homeostatic balance among network components is also suggested by their extremely tight regulation of expression. For example, MYC represses itself in a negative feedback loop which is often inactivated in tumor cells [258]. Also, MYC family members exhibit transcriptional crosstalk. For example overexpression of MYCN leads to downregulation of MYC in neuroblastoma and B cell lymphoma [259, 260]. In addition, loss of MNT is balanced by a decrease in MYC and MYCN expression [129, 130]. Moreover, MYC represses MXD4 expression by its interaction with MIZ1 [261, 262]. By repressing MXD4 expression MYC may not only promote its own association with MAX, but potentially influence the balance between MXD/MLX and MONDO/MLX heterodimers. Finally, MYC can also influence expression of the MONDO factors. For example MYC binds to the CHREBP gene promoter [ENCODE data and 83] and both MYC and MYCN overexpression enhances MONDOA expression and nuclear localization in several tumor cell lines (Carroll, Diolaiti, McFerrin and Eisenman, unpublished).
Interestingly, although MXD proteins antagonize MYC activity in many experimental systems, there are contradictory evidence that MXD family members are lost in human cancers [172, 173, 263–275]. As discussed above, it is generally accepted that MXD proteins are not frequently lost during oncogenesis because they are mainly expressed in differentiated tissues where MYC expression is low or absent. However, another explanation may be that MXD and MONDO proteins may be required to suppress MYC pro-apoptotic activity [276]. If correct it would be expected that MXD proteins should be widely expressed in tumor cells. Consistent with this hypothesis, MXD genes expression has been detected in several hematopoietic and solid tumors [263, 264, 266, 274, 277, 278]. However, a comprehensive analysis of MXD expression in human tumors has not been reported.
Even less is known about MONDO protein expression in human cancers. By sensing nutrient availability and regulating metabolic processes such as glycolysis and lipogenesis, Mondo proteins may profoundly impact tumor growth. MONDOA is overexpressed in acute lymphoblastic leukemia and its silencing promoted differentiation and apoptosis in leukemic cells [151] Recent data from our laboratory show that MONDOA silencing promotes apoptosis in cells expressing high level of either MYC or MYCN, suggesting that MONDOA may be required by MYC for properly reprogram cellular metabolism and for tumorigenesis (Carroll, Diolaiti, McFerrin, and Eisenman, unpublished).
4.1. The MAX/MLX Network as viewed from the TCGA
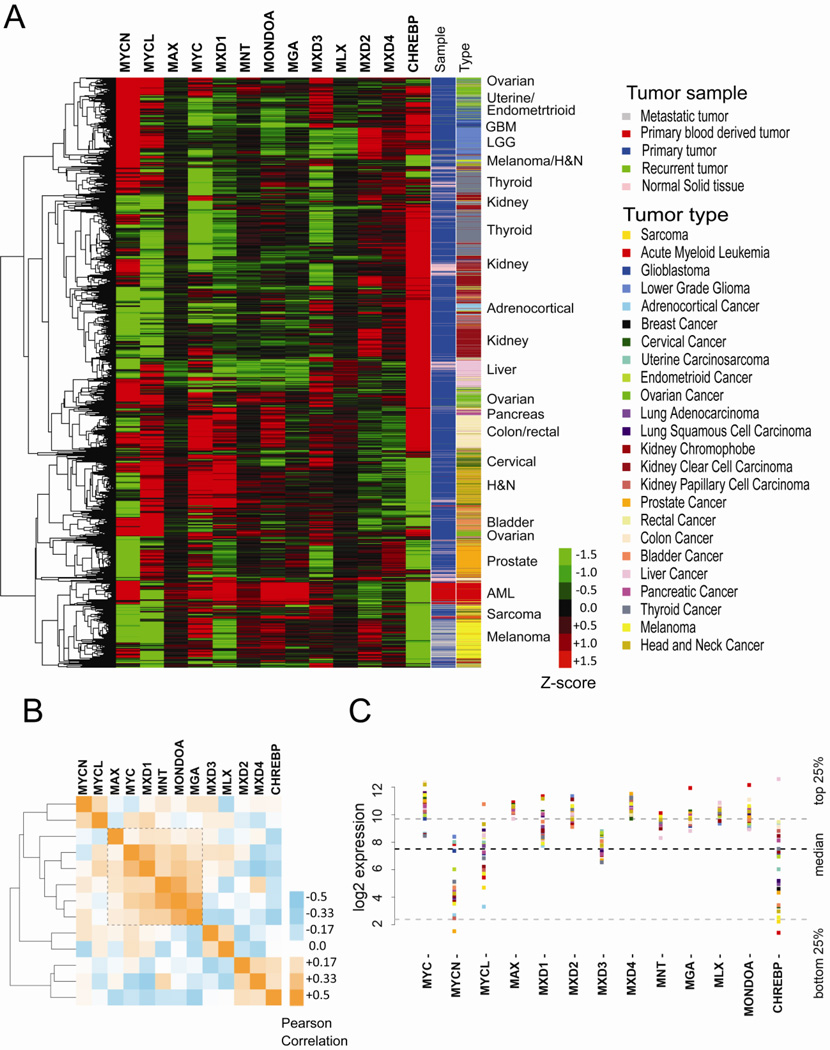
To evaluate the profile of the MAX/MLX network in human tumors, we analyzed the relative expression levels of the network members using the Pan-Cancer data from The Cancer Genome Atlas (TCGA) database (http://cancergenome.nih.gov/). This publically available dataset is mean normalized to reflect the variability of gene expression across 6609 samples representing twenty-four diverse tumor types, including both primary tumor and normal tissue where available. Using spearman correlation as a distance measure during hierarchical clustering [279], samples were largely clustered according to tissue and tumor type, with clusters of normal tissue being grouped with the corresponding primary tumor (Fig. 6A). Interestingly, it appears that most tumor types can be distinguished according to a specific expression pattern of MAX/MLX network members suggesting that each tissue has a distinct balance among members of the network. Lung and breast cancers are the exception, as the expression pattern of network members within these tissues is highly heterogeneous causing them to be broadly interspersed with the other tumor types and not distinctly clustered. For this reason, lung and breast samples were excluded from Figure 6A to avoid obfuscating the other tumor type clusters. The reason for such difference is not known, but it may in part reflect the great heterogeneity of lung and breast cancers.
Figure 6. Analysis of the MAX/MLX network in human cancer.
A) Relative distribution of the network members across TCGA tissue types, each represented by a different color (“tumor type column”). “Sample” column: Pink normal tissue; Blue, Primary Tumor; Red, Acute Myeloid Leukemia; Grey, Metastatic tumor; Green Recurrent tumor. Due to the heterogeneity in expression, breast and lung samples have been excluded from this representation to more readily identify the distinct expression patterns of the other tumors. B) Pearson correlation of network member expression levels among all samples (including lung and breast). Orange: positive correlation, blue: negative correlation. C) Median of expression values within each tumor type (colored coded squares) are shown for each network member. Black dashed line, median expression of all genes across tumor types; Grey dashed lines, top and bottom 25% of genes.
In general, MAX, MLX and MNT genes expression show minimal variability among tissues including either normal or tumor samples, as indicated by the almost homogeneous black color. This is consistent with the fact that they are expressed both widely and constitutively. In contrast, MYC family members show a variable expression profile being enriched or depleted dependent upon the tumor type. In particular, while MYC is expressed at high levels in a broad spectrum of cancers, MYCN and MYCL are highly expressed in a smaller number of tumors consistent with their more restricted tissue expression profile. Surprisingly, many cancers express high levels of multiple MYC family members (e.g. Ovarian cancer, Acute Myeloid Leukemia and Lower Grade Glioma).
MXD genes also show variable expression profiles. MXD2 has higher relative expression in kidney where it has been shown to be required for tumor growth [196, 198] Interestingly MXD3 seems to be more highly expressed in the same samples as MYC family members, consistent with the reported connection between MXD3 expression, proliferation and protection from DNA damage induced apoptosis [154]. Unexpectedly MXD1 is present at higher levels in a subset of tissues where MYC is also more highly expressed. Finally, both MONDOA and CHREBP show a tissue specific profile perhaps reflecting alternative metabolic functions and requirements of different tissues and related tumors. The observation that the network expression profile is tissue specific is important because it implies different tissues may have diverse thresholds of susceptibility to MYC deregulation and variable biological outcomes depending on the specific network structure.
Moreover, because it is known that the expression levels of the network members influence each other’s expression profile, we asked if there is any relationship between the expression levels of each network member by calculating the Pearson correlation between network genes using all available samples, including those from lung and breast cancer (Fig 6B). Intriguingly, five of the core network members (MAX, MYC, MXD1, MNT, MONDOA) as well as MGA are all positively correlated with each other and form a distinct clade (within dashed line). This again suggests their expression patterns are potentially linked and supports a model in which MXD proteins may be required to balance MYC pro-apoptotic activity, while MONDOA would collaborate with MYC to sustain the increased metabolic demand of cancer cells.
Interestingly, MYC drives the formation of tumors possessing different metabolic profiles when expressed in cells of different origins [280]. Conceivably, MYC driven metabolic reprogramming can vary depending on the cell type specific expression profile of MONDOA, CHREBP and other metabolic genes. Moreover, although MONDO proteins seem to mediate at least partially overlapping metabolic pathways, it is possible that they possess specific functions. As shown by figure 6A–C their expression, especially CHREBP, is highly variable among samples, therefore potentially influencing MYC-induced metabolic reprogramming in a tissue specific manner.
Overall, the analysis of TCGA data represented in fig. 6A,B suggests that MXD genes expression is not globally downregulated during oncogenesis. However, while the analysis highlights the tissue-specific distribution of the network members across tumor types, it does not provide comparative information regarding the relative expression level among network members within tumor types.
Figure 6C shows the relative log (base 2) gene expression profile of all network members within each tumor type. Although the median expression level is a simplified statistic and does not reflect the heterogeneity associated with each tumor type, the data shows that most network members have comparable ranges of expression and that their expression is, in general, greater than the overall median gene expression. The exceptions being MYCN, MYCL and ChREBP, which have more tissue specific expression, and MXD3, which is expressed at a lower level in all tissues. These data, together with the fact that loss of MXD or MONDO genes promotes MYC driven apoptosis supports a model where extreme network imbalance, as a result of the loss of a network member, may not be beneficial for tumor cells and suggests an intact and functional MAX/MLX network may be required for tumorigenesis.
5. Concluding remarks
In conclusion, while MYC undoubtedly is the major factor in driving tumorigenesis, the presence and activities of the other members of the network is likely to modulate the biological consequences of MYC deregulation. Understanding the specific biological functions of each network member in both normal and pathological conditions will lead to a deeper understanding of how deregulated MYC alters cellular behavior and potentially provide new opportunities for inhibiting tumorigenesis.
Highlights.
A comprehensive model of the MAX/MLX intra-network interaction is proposed
MAX/MLX network members are widely expressed in both normal and malignant tissues
The MAX and MLX modules coordinate proliferation and metabolism
Balanced expression among network members contributes to cell fate determination
Different human cancers possess a distinct MAX/MLX network organization
Acknowledgments
We are thankful to Don Ayer for sharing unpublished data and Alexandre Neves for a critical reading of the manuscript. We apologize to colleagues whose work was not cited because of limited space. Work from the laboratory of R.N.E. described in this review was supported by grants RO1-CA20525 and R37-CA57138 from NIH/NCI.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lee CM, Reddy EP. The v-myc oncogene. Oncogene. 1999;18:2997–3003. doi: 10.1038/sj.onc.1202786. [DOI] [PubMed] [Google Scholar]
- 2.Vennstrom B, Sheiness D, Zabielski J, Bishop JM. Isolation and characterization of c-myc, a cellular homolog of the oncogene (v-myc) of avian myelocytomatosis virus strain 29. J Virol. 1982;42:773–779. doi: 10.1128/jvi.42.3.773-779.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sheiness D, Bishop JM. DNA and RNA from uninfected vertebrate cells contain nucleotide sequences related to the putative transforming gene of avian myelocytomatosis virus. J Virol. 1979;31:514–521. doi: 10.1128/jvi.31.2.514-521.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taub R, Kirsch I, Morton C, Lenoir G, Swan D, Tronick S, Aaronson S, Leder P. Translocation of the c-myc gene into the immunoglobulin heavy chain locus in human Burkitt lymphoma and murine plasmacytoma cells. Proc Natl Acad Sci U S A. 1982;79:7837–7841. doi: 10.1073/pnas.79.24.7837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dalla-Favera R, Bregni M, Erikson J, Patterson D, Gallo RC, Croce CM. Human c-myc onc gene is located on the region of chromosome 8 that is translocated in Burkitt lymphoma cells. Proc Natl Acad Sci U S A. 1982;79:7824–7827. doi: 10.1073/pnas.79.24.7824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collins S, Groudine M. Amplification of endogenous myc-related DNA sequences in a human myeloid leukaemia cell line. Nature. 1982;298:679–681. doi: 10.1038/298679a0. [DOI] [PubMed] [Google Scholar]
- 7.Schwab M, Alitalo K, Klempnauer KH, Varmus HE, Bishop JM, Gilbert F, Brodeur G, Goldstein M, Trent J. Amplified DNA with limited homology to myc cellular oncogene is shared by human neuroblastoma cell lines and a neuroblastoma tumour. Nature. 1983;305:245–248. doi: 10.1038/305245a0. [DOI] [PubMed] [Google Scholar]
- 8.Kohl NE, Kanda N, Schreck RR, Bruns G, Latt SA, Gilbert F, Alt FW. Transposition and amplification of oncogene-related sequences in human neuroblastomas. Cell. 1983;35:359–367. doi: 10.1016/0092-8674(83)90169-1. [DOI] [PubMed] [Google Scholar]
- 9.Brodeur GM, Seeger RC, Schwab M, Varmus HE, Bishop JM. Amplification of N-myc in untreated human neuroblastomas correlates with advanced disease stage. Science. 1984;224:1121–1124. doi: 10.1126/science.6719137. [DOI] [PubMed] [Google Scholar]
- 10.Nau MM, Brooks BJ, Battey J, Sausville E, Gazdar AF, Kirsch IR, McBride OW, Bertness V, Hollis GF, Minna JD. L-myc, a new myc-related gene amplified and expressed in human small cell lung cancer. Nature. 1985;318:69–73. doi: 10.1038/318069a0. [DOI] [PubMed] [Google Scholar]
- 11.Murre C, McCaw PS, Baltimore D. A new DNA binding and dimerization motif in immunoglobulin enhancer binding, daughterless, MyoD, and myc proteins. Cell. 1989;56:777–783. doi: 10.1016/0092-8674(89)90682-x. [DOI] [PubMed] [Google Scholar]
- 12.Dang CV, Barrett J, Villa-Garcia M, Resar LM, Kato GJ, Fearon ER. Intracellular leucine zipper interactions suggest c-Myc hetero-oligomerization. Mol Cell Biol. 1991;11:954–962. doi: 10.1128/mcb.11.2.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blackwell TK, Kretzner L, Blackwood EM, Eisenman RN, Weintraub H. Sequence-specific DNA binding by the c-Myc protein. Science. 1990;250:1149–1151. doi: 10.1126/science.2251503. [DOI] [PubMed] [Google Scholar]
- 14.Penn LJ, Laufer EM, Land H. C-MYC: evidence for multiple regulatory functions. Semin Cancer Biol. 1990;1:69–80. [PubMed] [Google Scholar]
- 15.Luscher B, Eisenman RN. New light on Myc and Myb. Part I. Myc. Genes Dev. 1990;4:2025–2035. doi: 10.1101/gad.4.12a.2025. [DOI] [PubMed] [Google Scholar]
- 16.Luscher B, Eisenman RN. New light on Myc and Myb. Part II. Myb. Genes Dev. 1990;4:2235–2241. doi: 10.1101/gad.4.12b.2235. [DOI] [PubMed] [Google Scholar]
- 17.Prendergast GC, Ziff EB. Methylation-sensitive sequence-specific DNA binding by the c-Myc basic region. Science. 1991;251:186–189. doi: 10.1126/science.1987636. [DOI] [PubMed] [Google Scholar]
- 18.Blackwood EM, Eisenman RN. Max: a helix-loop-helix zipper protein that forms a sequence-specific DNA-binding complex with Myc. Science. 1991;251:1211–1217. doi: 10.1126/science.2006410. [DOI] [PubMed] [Google Scholar]
- 19.Pirity M, Blanck JK, Schreiber-Agus N. Lessons learned from Myc/Max/Mad knockout mice. Curr Top Microbiol Immunol. 2006;302:205–234. doi: 10.1007/3-540-32952-8_8. [DOI] [PubMed] [Google Scholar]
- 20.Blackwood EM, Luscher B, Eisenman RN. Myc and Max associate in vivo. Genes Dev. 1992;6:71–80. doi: 10.1101/gad.6.1.71. [DOI] [PubMed] [Google Scholar]
- 21.Blackwood EM, Eisenman RN. Regulation of Myc: Max complex formation and its potential role in cell proliferation. Tohoku J Exp Med. 1992;168:195–202. doi: 10.1620/tjem.168.195. [DOI] [PubMed] [Google Scholar]
- 22.Kretzner L, Blackwood EM, Eisenman RN. Myc and Max proteins possess distinct transcriptional activities. Nature. 1992;359:426–429. doi: 10.1038/359426a0. [DOI] [PubMed] [Google Scholar]
- 23.Blackwood EM, Kretzner L, Eisenman RN. Myc and Max function as a nucleoprotein complex. Curr Opin Genet Dev. 1992;2:227–235. doi: 10.1016/s0959-437x(05)80278-3. [DOI] [PubMed] [Google Scholar]
- 24.Amati B, Brooks MW, Levy N, Littlewood TD, Evan GI, Land H. Oncogenic activity of the c-Myc protein requires dimerization with Max. Cell. 1993;72:233–245. doi: 10.1016/0092-8674(93)90663-b. [DOI] [PubMed] [Google Scholar]
- 25.Berberich SJ, Cole MD. Casein kinase II inhibits the DNA-binding activity of Max homodimers but not Myc/Max heterodimers. Genes Dev. 1992;6:166–176. doi: 10.1101/gad.6.2.166. [DOI] [PubMed] [Google Scholar]
- 26.Ayer DE, Kretzner L, Eisenman RN. Mad: a heterodimeric partner for Max that antagonizes Myc transcriptional activity. Cell. 1993;72:211–222. doi: 10.1016/0092-8674(93)90661-9. [DOI] [PubMed] [Google Scholar]
- 27.Zervos AS, Gyuris J, Brent R. Mxi1, a protein that specifically interacts with Max to bind Myc-Max recognition sites. Cell. 1993;72:223–232. doi: 10.1016/0092-8674(93)90662-a. [DOI] [PubMed] [Google Scholar]
- 28.Hurlin PJ, Queva C, Koskinen PJ, Steingrimsson E, Ayer DE, Copeland NG, Jenkins NA, Eisenman RN. Mad3 and Mad4: novel Max-interacting transcriptional repressors that suppress c-myc dependent transformation and are expressed during neural and epidermal differentiation. EMBO J. 1995;14:5646–5659. doi: 10.1002/j.1460-2075.1995.tb00252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hurlin PJ, Queva C, Eisenman RN. Mnt: a novel Max-interacting protein and Myc antagonist. Curr Top Microbiol Immunol. 1997;224:115–121. doi: 10.1007/978-3-642-60801-8_11. [DOI] [PubMed] [Google Scholar]
- 30.Hurlin PJ, Steingrimsson E, Copeland NG, Jenkins NA, Eisenman RN. Mga, a dual-specificity transcription factor that interacts with Max and contains a T-domain DNA-binding motif. EMBO J. 1999;18:7019–7028. doi: 10.1093/emboj/18.24.7019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Billin AN, Eilers AL, Queva C, Ayer DE. Mlx, a novel Max-like BHLHZip protein that interacts with the Max network of transcription factors. J Biol Chem. 1999;274:36344–36350. doi: 10.1074/jbc.274.51.36344. [DOI] [PubMed] [Google Scholar]
- 32.Meroni G, Cairo S, Merla G, Messali S, Brent R, Ballabio A, Reymond A. Mlx, a new Max-like bHLHZip family member: the center stage of a novel transcription factors regulatory pathway? Oncogene. 2000;19:3266–3277. doi: 10.1038/sj.onc.1203634. [DOI] [PubMed] [Google Scholar]
- 33.Billin AN, Eilers AL, Coulter KL, Logan JS, Ayer DE. MondoA, a novel basic helix-loop-helix-leucine zipper transcriptional activator that constitutes a positive branch of a max-like network. Mol Cell Biol. 2000;20:8845–8854. doi: 10.1128/mcb.20.23.8845-8854.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cairo S, Merla G, Urbinati F, Ballabio A, Reymond A. WBSCR14, a gene mapping to the Williams--Beuren syndrome deleted region, is a new member of the Mlx transcription factor network. Hum Mol Genet. 2001;10:617–627. doi: 10.1093/hmg/10.6.617. [DOI] [PubMed] [Google Scholar]
- 35.Eilers AL, Sundwall E, Lin M, Sullivan AA, Ayer DE. A novel heterodimerization domain, CRM1, and 14-3-3 control subcellular localization of the MondoA-Mlx heterocomplex. Mol Cell Biol. 2002;22:8514–8526. doi: 10.1128/MCB.22.24.8514-8526.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Billin AN, Ayer DE. The Mlx network: evidence for a parallel Max-like transcriptional network that regulates energy metabolism. Curr Top Microbiol Immunol. 2006;302:255–278. doi: 10.1007/3-540-32952-8_10. [DOI] [PubMed] [Google Scholar]
- 37.Stoltzman CA, Peterson CW, Breen KT, Muoio DM, Billin AN, Ayer DE. Glucose sensing by MondoA:Mlx complexes: a role for hexokinases and direct regulation of thioredoxin-interacting protein expression. Proc Natl Acad Sci U S A. 2008;105:6912–6917. doi: 10.1073/pnas.0712199105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kawaguchi T, Takenoshita M, Kabashima T, Uyeda K. Glucose and cAMP regulate the L-type pyruvate kinase gene by phosphorylation/dephosphorylation of the carbohydrate response element binding protein. Proc Natl Acad Sci U S A. 2001;98:13710–13715. doi: 10.1073/pnas.231370798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Young SL, Diolaiti D, Conacci-Sorrell M, Ruiz-Trillo I, Eisenman RN, King N. Premetazoan ancestry of the Myc-Max network. Mol Biol Evol. 2011;28:2961–2971. doi: 10.1093/molbev/msr132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.King N, Westbrook MJ, Young SL, Kuo A, Abedin M, Chapman J, Fairclough S, Hellsten U, Isogai Y, Letunic I, Marr M, Pincus D, Putnam N, Rokas A, Wright KJ, Zuzow R, Dirks W, Good M, Goodstein D, Lemons D, Li W, Lyons JB, Morris A, Nichols S, Richter DJ, Salamov A, Sequencing JG, Bork P, Lim WA, Manning G, Miller WT, McGinnis W, Shapiro H, Tjian R, Grigoriev IV, Rokhsar D. The genome of the choanoflagellate Monosiga brevicollis and the origin of metazoans. Nature. 2008;451:783–788. doi: 10.1038/nature06617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruiz-Trillo I, Roger AJ, Burger G, Gray MW, Lang BF. A phylogenomic investigation into the origin of metazoa. Mol Biol Evol. 2008;25:664–672. doi: 10.1093/molbev/msn006. [DOI] [PubMed] [Google Scholar]
- 42.McFerrin LG, Atchley WR. Evolution of the Max and Mlx networks in animals. Genome Biol Evol. 2011;3:915–937. doi: 10.1093/gbe/evr082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gallant P, Shiio Y, Cheng PF, Parkhurst SM, Eisenman RN. Myc and Max homologs in Drosophila. Science. 1996;274:1523–1527. doi: 10.1126/science.274.5292.1523. [DOI] [PubMed] [Google Scholar]
- 44.Peyrefitte S, Kahn D, Haenlin M. New members of the Drosophila Myc transcription factor subfamily revealed by a genome-wide examination for basic helix-loop-helix genes. Mech Dev. 2001;104:99–104. doi: 10.1016/s0925-4773(01)00360-4. [DOI] [PubMed] [Google Scholar]
- 45.Loo LW, Secombe J, Little JT, Carlos LS, Yost C, Cheng PF, Flynn EM, Edgar BA, Eisenman RN. The transcriptional repressor dMnt is a regulator of growth in Drosophila melanogaster. Mol Cell Biol. 2005;25:7078–7091. doi: 10.1128/MCB.25.16.7078-7091.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schreiber-Agus N, Stein D, Chen K, Goltz JS, Stevens L, DePinho RA. Drosophila Myc is oncogenic in mammalian cells and plays a role in the diminutive phenotype. Proc Natl Acad Sci U S A. 1997;94:1235–1240. doi: 10.1073/pnas.94.4.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gallant P. Myc/Max/Mad in invertebrates: the evolution of the Max network. Curr Top Microbiol Immunol. 2006;302:235–253. doi: 10.1007/3-540-32952-8_9. [DOI] [PubMed] [Google Scholar]
- 48.Gallant P. Myc function in Drosophila. Cold Spring Harb Perspect Med. 2013;3 doi: 10.1101/cshperspect.a014324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Havula E, Teesalu M, Hyotylainen T, Seppala H, Hasygar K, Auvinen P, Oresic M, Sandmann T, Hietakangas V. Mondo/ChREBP-Mlx-regulated transcriptional network is essential for dietary sugar tolerance in Drosophila. PLoS Genet. 2013;9:e1003438. doi: 10.1371/journal.pgen.1003438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Musselman LP, Fink JL, Ramachandran PV, Patterson BW, Okunade AL, Maier E, Brent MR, Turk J, Baranski TJ. Role of fat body lipogenesis in protection against the effects of caloric overload in Drosophila. J Biol Chem. 2013;288:8028–8042. doi: 10.1074/jbc.M112.371047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nair SK, Burley SK. X-ray structures of Myc-Max and Mad-Max recognizing DNA. Molecular bases of regulation by proto-oncogenic transcription factors. Cell. 2003;112:193–205. doi: 10.1016/s0092-8674(02)01284-9. [DOI] [PubMed] [Google Scholar]
- 52.Montagne M, Naud JF, McDuff FO, Lavigne P. Toward the elucidation of the structural determinants responsible for the molecular recognition between Mad1 and Max. Biochemistry. 2005;44:12860–12869. doi: 10.1021/bi0500731. [DOI] [PubMed] [Google Scholar]
- 53.Ferre-D’Amare AR, Prendergast GC, Ziff EB, Burley SK. Recognition by Max of its cognate DNA through a dimeric b/HLH/Z domain. Nature. 1993;363:38–45. doi: 10.1038/363038a0. [DOI] [PubMed] [Google Scholar]
- 54.Hann SR. Role of post-translational modifications in regulating c-Myc proteolysis, transcriptional activity and biological function. Semin Cancer Biol. 2006;16:288–302. doi: 10.1016/j.semcancer.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 55.McMahon SB, Van Buskirk HA, Dugan KA, Copeland TD, Cole MD. The novel ATM-related protein TRRAP is an essential cofactor for the c-Myc and E2F oncoproteins. Cell. 1998;94:363–374. doi: 10.1016/s0092-8674(00)81479-8. [DOI] [PubMed] [Google Scholar]
- 56.McMahon SB, Wood MA, Cole MD. The essential cofactor TRRAP recruits the histone acetyltransferase hGCN5 to c-Myc. Mol Cell Biol. 2000;20:556–562. doi: 10.1128/mcb.20.2.556-562.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Frank SR, Parisi T, Taubert S, Fernandez P, Fuchs M, Chan HM, Livingston DM, Amati B. MYC recruits the TIP60 histone acetyltransferase complex to chromatin. EMBO Rep. 2003;4:575–580. doi: 10.1038/sj.embor.embor861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wood MA, McMahon SB, Cole MD. An ATPase/helicase complex is an essential cofactor for oncogenic transformation by c-Myc. Mol Cell. 2000;5:321–330. doi: 10.1016/s1097-2765(00)80427-x. [DOI] [PubMed] [Google Scholar]
- 59.Cole MD, McMahon SB. The Myc oncoprotein: a critical evaluation of transactivation and target gene regulation. Oncogene. 1999;18:2916–2924. doi: 10.1038/sj.onc.1202748. [DOI] [PubMed] [Google Scholar]
- 60.Liu X, Tesfai J, Evrard YA, Dent SY, Martinez E. c-Myc transformation domain recruits the human STAGA complex and requires TRRAP and GCN5 acetylase activity for transcription activation. J Biol Chem. 2003;278:20405–20412. doi: 10.1074/jbc.M211795200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cole MD, Nikiforov MA. Transcriptional activation by the Myc oncoprotein. Curr Top Microbiol Immunol. 2006;302:33–50. doi: 10.1007/3-540-32952-8_2. [DOI] [PubMed] [Google Scholar]
- 62.Eberhardy SR, Farnham PJ. Myc recruits P-TEFb to mediate the final step in the transcriptional activation of the cad promoter. J Biol Chem. 2002;277:40156–40162. doi: 10.1074/jbc.M207441200. [DOI] [PubMed] [Google Scholar]
- 63.Gargano B, Amente S, Majello B, Lania L. P-TEFb is a crucial co-factor for Myc transactivation. Cell Cycle. 2007;6:2031–2037. doi: 10.4161/cc.6.16.4554. [DOI] [PubMed] [Google Scholar]
- 64.Zuber J, Shi J, Wang E, Rappaport AR, Herrmann H, Sison EA, Magoon D, Qi J, Blatt K, Wunderlich M, Taylor MJ, Johns C, Chicas A, Mulloy JC, Kogan SC, Brown P, Valent P, Bradner JE, Lowe SW, Vakoc CR. RNAi screen identifies Brd4 as a therapeutic target in acute myeloid leukaemia. Nature. 2011;478:524–528. doi: 10.1038/nature10334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rahl PB, Lin CY, Seila AC, Flynn RA, McCuine S, Burge CB, Sharp PA, Young RA. c-Myc regulates transcriptional pause release. Cell. 2010;141:432–445. doi: 10.1016/j.cell.2010.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lin CY, Loven J, Rahl PB, Paranal RM, Burge CB, Bradner JE, Lee TI, Young RA. Transcriptional amplification in tumor cells with elevated c-Myc. Cell. 2012;151:56–67. doi: 10.1016/j.cell.2012.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nie Z, Hu G, Wei G, Cui K, Yamane A, Resch W, Wang R, Green DR, Tessarollo L, Casellas R, Zhao K, Levens D. c-Myc is a universal amplifier of expressed genes in lymphocytes and embryonic stem cells. Cell. 2012;151:68–79. doi: 10.1016/j.cell.2012.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Herbst A, Hemann MT, Tworkowski KA, Salghetti SE, Lowe SW, Tansey WP. A conserved element in Myc that negatively regulates its proapoptotic activity. EMBO Rep. 2005;6:177–183. doi: 10.1038/sj.embor.7400333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cowling VH, Chandriani S, Whitfield ML, Cole MD. A conserved Myc protein domain, MBIV, regulates DNA binding, apoptosis, transformation, and G2 arrest. Mol Cell Biol. 2006;26:4226–4239. doi: 10.1128/MCB.01959-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ayer DE, Laherty CD, Lawrence QA, Armstrong AP, Eisenman RN. Mad proteins contain a dominant transcription repression domain. Mol Cell Biol. 1996;16:5772–5781. doi: 10.1128/mcb.16.10.5772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Eilers AL, Billin AN, Liu J, Ayer DE. A 13-amino acid amphipathic alpha-helix is required for the functional interaction between the transcriptional repressor Mad1 and mSin3A. J Biol Chem. 1999;274:32750–32756. doi: 10.1074/jbc.274.46.32750. [DOI] [PubMed] [Google Scholar]
- 72.Laherty CD, Yang WM, Sun JM, Davie JR, Seto E, Eisenman RN. Histone deacetylases associated with the mSin3 corepressor mediate mad transcriptional repression. Cell. 1997;89:349–356. doi: 10.1016/s0092-8674(00)80215-9. [DOI] [PubMed] [Google Scholar]
- 73.Brubaker K, Cowley SM, Huang K, Loo L, Yochum GS, Ayer DE, Eisenman RN, Radhakrishnan I. Solution structure of the interacting domains of the Mad-Sin3 complex: implications for recruitment of a chromatin-modifying complex. Cell. 2000;103:655–665. doi: 10.1016/s0092-8674(00)00168-9. [DOI] [PubMed] [Google Scholar]
- 74.Bernstein BE, Humphrey EL, Erlich RL, Schneider R, Bouman P, Liu JS, Kouzarides T, Schreiber SL. Methylation of histone H3 Lys 4 in coding regions of active genes. Proc Natl Acad Sci U S A. 2002;99:8695–8700. doi: 10.1073/pnas.082249499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Santos-Rosa H, Schneider R, Bannister AJ, Sherriff J, Bernstein BE, Emre NC, Schreiber SL, Mellor J, Kouzarides T. Active genes are tri-methylated at K4 of histone H3. Nature. 2002;419:407–411. doi: 10.1038/nature01080. [DOI] [PubMed] [Google Scholar]
- 76.Ge Z, Li W, Wang N, Liu C, Zhu Q, Bjorkholm M, Gruber A, Xu D. Chromatin remodeling: recruitment of histone demethylase RBP2 by Mad1 for transcriptional repression of a Myc target gene, telomerase reverse transcriptase. FASEB J. 2010;24:579–586. doi: 10.1096/fj.09-140087. [DOI] [PubMed] [Google Scholar]
- 77.Secombe J, Eisenman RN. The function and regulation of the JARID1 family of histone H3 lysine 4 demethylases: the Myc connection. Cell Cycle. 2007;6:1324–1328. doi: 10.4161/cc.6.11.4269. [DOI] [PubMed] [Google Scholar]
- 78.Secombe J, Li L, Carlos L, Eisenman RN. The Trithorax group protein Lid is a trimethyl histone H3K4 demethylase required for dMyc-induced cell growth. Genes Dev. 2007;21:537–551. doi: 10.1101/gad.1523007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bres V, Yoshida T, Pickle L, Jones KA. SKIP interacts with c-Myc and Menin to promote HIV-1 Tat transactivation. Mol Cell. 2009;36:75–87. doi: 10.1016/j.molcel.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Guccione E, Martinato F, Finocchiaro G, Luzi L, Tizzoni L, Dall’ Olio V, Zardo G, Nervi C, Bernard L, Amati B. Myc-binding-site recognition in the human genome is determined by chromatin context. Nat Cell Biol. 2006;8:764–770. doi: 10.1038/ncb1434. [DOI] [PubMed] [Google Scholar]
- 81.Kim J, Woo AJ, Chu J, Snow JW, Fujiwara Y, Kim CG, Cantor AB, Orkin SH. A Myc network accounts for similarities between embryonic stem and cancer cell transcription programs. Cell. 2010;143:313–324. doi: 10.1016/j.cell.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Knoepfler PS, Zhang XY, Cheng PF, Gafken PR, McMahon SB, Eisenman RN. Myc influences global chromatin structure. EMBO J. 2006;25:2723–2734. doi: 10.1038/sj.emboj.7601152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lin CH, Lin C, Tanaka H, Fero ML, Eisenman RN. Gene regulation and epigenetic remodeling in murine embryonic stem cells by c-Myc. PLoS One. 2009;4:e7839. doi: 10.1371/journal.pone.0007839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Martinato F, Cesaroni M, Amati B, Guccione E. Analysis of Myc-induced histone modifications on target chromatin. PLoS One. 2008;3:e3650. doi: 10.1371/journal.pone.0003650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Havula E, Hietakangas V. Glucose sensing by ChREBP/MondoA-Mlx transcription factors. Semin Cell Dev Biol. 2012;23:640–647. doi: 10.1016/j.semcdb.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 86.McFerrin LG, Atchley WR. A novel N-terminal domain may dictate the glucose response of Mondo proteins. PLoS One. 2012;7:e34803. doi: 10.1371/journal.pone.0034803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kaadige MR, Looper RE, Kamalanaadhan S, Ayer DE. Glutamine-dependent anapleurosis dictates glucose uptake and cell growth by regulating MondoA transcriptional activity. Proc Natl Acad Sci U S A. 2009;106:14878–14883. doi: 10.1073/pnas.0901221106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen JL, Merl D, Peterson CW, Wu J, Liu PY, Yin H, Muoio DM, Ayer DE, West M, Chi JT. Lactic acidosis triggers starvation response with paradoxical induction of TXNIP through MondoA. PLoS Genet. 2010;6:e1001093. doi: 10.1371/journal.pgen.1001093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Stoltzman CA, Kaadige MR, Peterson CW, Ayer DE. MondoA senses non-glucose sugars: regulation of thioredoxin-interacting protein (TXNIP) and the hexose transport curb. J Biol Chem. 2011;286:38027–38034. doi: 10.1074/jbc.M111.275503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Han KS, Ayer DE. MondoA senses adenine nucleotides: transcriptional induction of thioredoxin-interacting protein. Biochem J. 2013;453:209–218. doi: 10.1042/BJ20121126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sans CL, Satterwhite DJ, Stoltzman CA, Breen KT, Ayer DE. MondoA-Mlx heterodimers are candidate sensors of cellular energy status: mitochondrial localization and direct regulation of glycolysis. Mol Cell Biol. 2006;26:4863–4871. doi: 10.1128/MCB.00657-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cha-Molstad H, Saxena G, Chen J, Shalev A. Glucose-stimulated expression of Txnip is mediated by carbohydrate response element-binding protein, p300, and histone H4 acetylation in pancreatic beta cells. J Biol Chem. 2009;284:16898–16905. doi: 10.1074/jbc.M109.010504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ogawa H, Ishiguro K, Gaubatz S, Livingston DM, Nakatani Y. A complex with chromatin modifiers that occupies E2F- and Myc-responsive genes in G0 cells. Science. 2002;296:1132–1136. doi: 10.1126/science.1069861. [DOI] [PubMed] [Google Scholar]
- 94.Yuhua S, Wei-Chia T, Xiang F, Rebecca B, Scott T D. Extra-embryonic signals under the control of Mga, Max and Smad4 are required for dorsoventral patterning. Developmental Cells. doi: 10.1016/j.devcel.2014.01.003. in press. [DOI] [PubMed] [Google Scholar]
- 95.De Paoli L, Cerri M, Monti S, Rasi S, Spina V, Bruscaggin A, Greco M, Ciardullo C, Fama R, Cresta S, Maffei R, Ladetto M, Martini M, Laurenti L, Forconi F, Marasca R, Larocca LM, Bertoni F, Gaidano G, Rossi D. MGA, a suppressor of MYC, is recurrently inactivated in high risk chronic lymphocytic leukemia. Leuk Lymphoma. 2013;54:1087–1090. doi: 10.3109/10428194.2012.723706. [DOI] [PubMed] [Google Scholar]
- 96.Romero OA, Torres-Diz M, Pros E, Savola S, Gomez A, Moran S, Saez C, Iwakawa R, Villanueva A, Montuenga LM, Kohno T, Yokota J, Sanchez-Cespedes M. MAX inactivation in small-cell lung cancer disrupts the MYC-SWI/SNF programs and is synthetic lethal with BRG1. Cancer Discov. 2013 doi: 10.1158/2159-8290.CD-13-0799. [DOI] [PubMed] [Google Scholar]
- 97.Dang CV. c-Myc target genes involved in cell growth, apoptosis, and metabolism. Mol Cell Biol. 1999;19:1–11. doi: 10.1128/mcb.19.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Meyer N, Kim SS, Penn LZ. The Oscar-worthy role of Myc in apoptosis. Semin Cancer Biol. 2006;16:275–287. doi: 10.1016/j.semcancer.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 99.Eilers M. Control of cell proliferation by Myc family genes. Mol Cells. 1999;9:1–6. [PubMed] [Google Scholar]
- 100.Singh AM, Dalton S. The cell cycle and Myc intersect with mechanisms that regulate pluripotency and reprogramming. Cell Stem Cell. 2009;5:141–149. doi: 10.1016/j.stem.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Amati B, Alevizopoulos K, Vlach J. Myc and the cell cycle. Front Biosci. 1998;3:d250–d268. doi: 10.2741/a239. [DOI] [PubMed] [Google Scholar]
- 102.Dang CV. Therapeutic targeting of Myc-reprogrammed cancer cell metabolism. Cold Spring Harb Symp Quant Biol. 2011;76:369–374. doi: 10.1101/sqb.2011.76.011296. [DOI] [PubMed] [Google Scholar]
- 103.Eilers M, Eisenman RN. Myc’s broad reach. Genes Dev. 2008;22:2755–2766. doi: 10.1101/gad.1712408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dang CV. MYC, metabolism, cell growth, and tumorigenesis. Cold Spring Harb Perspect Med. 2013;3 doi: 10.1101/cshperspect.a014217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lahoz EG, Xu L, Schreiber-Agus N, DePinho RA. Suppression of Myc, but not E1a, transformation activity by Max-associated proteins, Mad and Mxi1. Proc Natl Acad Sci U S A. 1994;91:5503–5507. doi: 10.1073/pnas.91.12.5503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vastrik I, Kaipainen A, Penttila TL, Lymboussakis A, Alitalo R, Parvinen M, Alitalo K. Expression of the mad gene during cell differentiation in vivo and its inhibition of cell growth in vitro. J Cell Biol. 1995;128:1197–1208. doi: 10.1083/jcb.128.6.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Koskinen PJ, Ayer DE, Eisenman RN. Repression of Myc-Ras cotransformation by Mad is mediated by multiple protein-protein interactions. Cell Growth Differ. 1995;6:623–629. [PubMed] [Google Scholar]
- 108.Hurlin PJ, Queva C, Eisenman RN. Mnt, a novel Max-interacting protein is coexpressed with Myc in proliferating cells and mediates repression at Myc binding sites. Genes Dev. 1997;11:44–58. doi: 10.1101/gad.11.1.44. [DOI] [PubMed] [Google Scholar]
- 109.Cerni C, Bousset K, Seelos C, Burkhardt H, Henriksson M, Luscher B. Differential effects by Mad and Max on transformation by cellular and viral oncoproteins. Oncogene. 1995;11:587–596. [PubMed] [Google Scholar]
- 110.Cerni C, Skrzypek B, Popov N, Sasgary S, Schmidt G, Larsson LG, Luscher B, Henriksson M. Repression of in vivo growth of Myc/Ras transformed tumor cells by Mad1. Oncogene. 2002;21:447–459. doi: 10.1038/sj.onc.1205107. [DOI] [PubMed] [Google Scholar]
- 111.Schreiber-Agus N, Chin L, Chen K, Torres R, Rao G, Guida P, Skoultchi AI, DePinho RA. An amino-terminal domain of Mxi1 mediates anti-Myc oncogenic activity and interacts with a homolog of the yeast transcriptional repressor SIN3. Cell. 1995;80:777–786. doi: 10.1016/0092-8674(95)90356-9. [DOI] [PubMed] [Google Scholar]
- 112.Chen J, Willingham T, Margraf LR, Schreiber-Agus N, DePinho RA, Nisen PD. Effects of the MYC oncogene antagonist, MAD, on proliferation, cell cycling and the malignant phenotype of human brain tumour cells. Nat Med. 1995;1:638–643. doi: 10.1038/nm0795-638. [DOI] [PubMed] [Google Scholar]
- 113.Gehring S, Rottmann S, Menkel AR, Mertsching J, Krippner-Heidenreich A, Luscher B. Inhibition of proliferation and apoptosis by the transcriptional repressor Mad1. Repression of Fas-induced caspase-8 activation. J Biol Chem. 2000;275:10413–10420. doi: 10.1074/jbc.275.14.10413. [DOI] [PubMed] [Google Scholar]
- 114.Roussel MF, Ashmun RA, Sherr CJ, Eisenman RN, Ayer DE. Inhibition of cell proliferation by the Mad1 transcriptional repressor. Mol Cell Biol. 1996;16:2796–2801. doi: 10.1128/mcb.16.6.2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sommer A, Hilfenhaus S, Menkel A, Kremmer E, Seiser C, Loidl P, Luscher B. Cell growth inhibition by the Mad/Max complex through recruitment of histone deacetylase activity. Curr Biol. 1997;7:357–365. doi: 10.1016/s0960-9822(06)00183-7. [DOI] [PubMed] [Google Scholar]
- 116.Wechsler DS, Shelly CA, Petroff CA, Dang CV. MXI1, a putative tumor suppressor gene, suppresses growth of human glioblastoma cells. Cancer Res. 1997;57:4905–4912. [PubMed] [Google Scholar]
- 117.Bejarano MT, Albihn A, Cornvik T, Brijker SO, Asker C, Osorio LM, Henriksson M. Inhibition of cell growth and apoptosis by inducible expression of the transcriptional repressor Mad1. Exp Cell Res. 2000;260:61–72. doi: 10.1006/excr.2000.4996. [DOI] [PubMed] [Google Scholar]
- 118.Zou L, Zhang P, Luo C, Tu Z. Mad1 suppresses bladder cancer cell proliferation by inhibiting human telomerase reverse transcriptase transcription and telomerase activity. Urology. 2006;67:1335–1340. doi: 10.1016/j.urology.2005.12.029. [DOI] [PubMed] [Google Scholar]
- 119.Ohta Y, Hamada Y, Saitoh N, Katsuoka K. Effect of the transcriptional repressor Mad1 on proliferation of human melanoma cells. Exp Dermatol. 2002;11:439–447. doi: 10.1034/j.1600-0625.2002.110507.x. [DOI] [PubMed] [Google Scholar]
- 120.Queva C, McArthur GA, Ramos LS, Eisenman RN. Dwarfism and dysregulated proliferation in mice overexpressing the MYC antagonist MAD1. Cell Growth Differ. 1999;10:785–796. [PubMed] [Google Scholar]
- 121.Manni I, Tunici P, Cirenei N, Albarosa R, Colombo BM, Roz L, Sacchi A, Piaggio G, Finocchiaro G. Mxi1 inhibits the proliferation of U87 glioma cells through down-regulation of cyclin B1 gene expression. Br J Cancer. 2002;86:477–484. doi: 10.1038/sj.bjc.6600065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Taj MM, Tawil RJ, Engstrom LD, Zeng Z, Hwang C, Sanda MG, Wechsler DS. Mxi1, a Myc antagonist, suppresses proliferation of DU145 human prostate cells. Prostate. 2001;47:194–204. doi: 10.1002/pros.1063. [DOI] [PubMed] [Google Scholar]
- 123.Ayer DE, Lawrence QA, Eisenman RN. Mad-Max transcriptional repression is mediated by ternary complex formation with mammalian homologs of yeast repressor Sin3. Cell. 1995;80:767–776. doi: 10.1016/0092-8674(95)90355-0. [DOI] [PubMed] [Google Scholar]
- 124.Iritani BM, Delrow J, Grandori C, Gomez I, Klacking M, Carlos LS, Eisenman RN. Modulation of T-lymphocyte development, growth and cell size by the Myc antagonist and transcriptional repressor Mad1. EMBO J. 2002;21:4820–4830. doi: 10.1093/emboj/cdf492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rudolph B, Hueber AO, Evan GI. Expression of Mad1 in T cells leads to reduced thymic cellularity and impaired mitogen-induced proliferation. Oncogene. 2001;20:1164–1175. doi: 10.1038/sj.onc.1204196. [DOI] [PubMed] [Google Scholar]
- 126.Foley KP, McArthur GA, Queva C, Hurlin PJ, Soriano P, Eisenman RN. Targeted disruption of the MYC antagonist MAD1 inhibits cell cycle exit during granulocyte differentiation. EMBO J. 1998;17:774–785. doi: 10.1093/emboj/17.3.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.McArthur GA, Foley KP, Fero ML, Walkley CR, Deans AJ, Roberts JM, Eisenman RN. MAD1 and p27(KIP1) cooperate to promote terminal differentiation of granulocytes and to inhibit Myc expression and cyclin E-CDK2 activity. Mol Cell Biol. 2002;22:3014–3023. doi: 10.1128/MCB.22.9.3014-3023.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Schreiber-Agus N, Meng Y, Hoang T, Hou H, Jr., Chen K, Greenberg R, Cordon-Cardo C, Lee HW, DePinho RA. Role of Mxi1 in ageing organ systems and the regulation of normal and neoplastic growth. Nature. 1998;393:483–487. doi: 10.1038/31008. [DOI] [PubMed] [Google Scholar]
- 129.Hurlin PJ, Zhou ZQ, Toyo-oka K, Ota S, Walker WL, Hirotsune S, Wynshaw-Boris A. Deletion of Mnt leads to disrupted cell cycle control and tumorigenesis. EMBO J. 2003;22:4584–4596. doi: 10.1093/emboj/cdg442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Toyo-oka K, Hirotsune S, Gambello MJ, Zhou ZQ, Olson L, Rosenfeld MG, Eisenman R, Hurlin P, Wynshaw-Boris A. Loss of the Max-interacting protein Mnt in mice results in decreased viability, defective embryonic growth and craniofacial defects: relevance to Miller-Dieker syndrome. Hum Mol Genet. 2004;13:1057–1067. doi: 10.1093/hmg/ddh116. [DOI] [PubMed] [Google Scholar]
- 131.Nilsson JA, Maclean KH, Keller UB, Pendeville H, Baudino TA, Cleveland JL. Mnt loss triggers Myc transcription targets, proliferation, apoptosis, and transformation. Mol Cell Biol. 2004;24:1560–1569. doi: 10.1128/MCB.24.4.1560-1569.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Toyo-oka K, Bowen TJ, Hirotsune S, Li Z, Jain S, Ota S, Escoubet-Lozach L, Garcia-Bassets I, Lozach J, Rosenfeld MG, Glass CK, Eisenman R, Ren B, Hurlin P, Wynshaw-Boris A. Mnt-deficient mammary glands exhibit impaired involution and tumors with characteristics of myc overexpression. Cancer Res. 2006;66:5565–5573. doi: 10.1158/0008-5472.CAN-05-2683. [DOI] [PubMed] [Google Scholar]
- 133.Meroni G, Reymond A, Alcalay M, Borsani G, Tanigami A, Tonlorenzi R, Lo Nigro C, Messali S, Zollo M, Ledbetter DH, Brent R, Ballabio A, Carrozzo R. Rox, a novel bHLHZip protein expressed in quiescent cells that heterodimerizes with Max, binds a non-canonical E box and acts as a transcriptional repressor. EMBO J. 1997;16:2892–2906. doi: 10.1093/emboj/16.10.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Popov N, Wahlstrom T, Hurlin PJ, Henriksson M. Mnt transcriptional repressor is functionally regulated during cell cycle progression. Oncogene. 2005;24:8326–8337. doi: 10.1038/sj.onc.1208961. [DOI] [PubMed] [Google Scholar]
- 135.Berns K, Hijmans EM, Bernards R. Repression of c-Myc responsive genes in cycling cells causes G1 arrest through reduction of cyclin E/CDK2 kinase activity. Oncogene. 1997;15:1347–1356. doi: 10.1038/sj.onc.1201280. [DOI] [PubMed] [Google Scholar]
- 136.Steiner P, Philipp A, Lukas J, Godden-Kent D, Pagano M, Mittnacht S, Bartek J, Eilers M. Identification of a Myc-dependent step during the formation of active G1 cyclin-cdk complexes. EMBO J. 1995;14:4814–4826. doi: 10.1002/j.1460-2075.1995.tb00163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rudolph B, Saffrich R, Zwicker J, Henglein B, Muller R, Ansorge W, Eilers M. Activation of cyclin-dependent kinases by Myc mediates induction of cyclin A, but not apoptosis. EMBO J. 1996;15:3065–3076. [PMC free article] [PubMed] [Google Scholar]
- 138.Walker W, Zhou ZQ, Ota S, Wynshaw-Boris A, Hurlin PJ. Mnt-Max to Myc-Max complex switching regulates cell cycle entry. J Cell Biol. 2005;169:405–413. doi: 10.1083/jcb.200411013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Pierce SB, Yost C, Anderson SA, Flynn EM, Delrow J, Eisenman RN. Drosophila growth and development in the absence of dMyc and dMnt. Dev Biol. 2008;315:303–316. doi: 10.1016/j.ydbio.2007.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Pierce SB, Yost C, Britton JS, Loo LW, Flynn EM, Edgar BA, Eisenman RN. dMyc is required for larval growth and endoreplication in Drosophila. Development. 2004;131:2317–2327. doi: 10.1242/dev.01108. [DOI] [PubMed] [Google Scholar]
- 141.Dezfouli S, Bakke A, Huang J, Wynshaw-Boris A, Hurlin PJ. Inflammatory disease and lymphomagenesis caused by deletion of the Myc antagonist Mnt in T cells. Mol Cell Biol. 2006;26:2080–2092. doi: 10.1128/MCB.26.6.2080-2092.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Eischen CM, Weber JD, Roussel MF, Sherr CJ, Cleveland JL. Disruption of the ARF-Mdm2-p53 tumor suppressor pathway in Myc-induced lymphomagenesis. Genes Dev. 1999;13:2658–2669. doi: 10.1101/gad.13.20.2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zindy F, Eischen CM, Randle DH, Kamijo T, Cleveland JL, Sherr CJ, Roussel MF. Myc signaling via the ARF tumor suppressor regulates p53-dependent apoptosis and immortalization. Genes Dev. 1998;12:2424–2433. doi: 10.1101/gad.12.15.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Link JM, Ota S, Zhou ZQ, Daniel CJ, Sears RC, Hurlin PJ. A critical role for Mnt in Myc-driven T-cell proliferation and oncogenesis. Proc Natl Acad Sci U S A. 2012;109:19685–19690. doi: 10.1073/pnas.1206406109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sloan EJ, Ayer DE. Myc, mondo, and metabolism. Genes Cancer. 2010;1:587–596. doi: 10.1177/1947601910377489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kaadige MR, Elgort MG, Ayer DE. Coordination of glucose and glutamine utilization by an expanded Myc network. Transcription. 2010;1:36–40. doi: 10.4161/trns.1.1.12142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.O’Shea JM, Ayer DE. Coordination of nutrient availability and utilization by MAX- and MLX-centered transcription networks. Cold Spring Harb Perspect Med. 2013),a014258;3 doi: 10.1101/cshperspect.a014258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Peterson CW, Ayer DE. An extended Myc network contributes to glucose homeostasis in cancer and diabetes. Front Biosci (Landmark Ed) 2011;16:2206–2223. doi: 10.2741/3848. [DOI] [PubMed] [Google Scholar]
- 149.Metukuri MR, Zhang P, Basantani MK, Chin C, Stamateris RE, Alonso LC, Takane KK, Gramignoli R, Strom SC, O’Doherty RM, Stewart AF, Vasavada RC, Garcia-Ocana A, Scott DK. ChREBP mediates glucose-stimulated pancreatic beta-cell proliferation. Diabetes. 2012;61:2004–2015. doi: 10.2337/db11-0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tong X, Zhao F, Mancuso A, Gruber JJ, Thompson CB. The glucose-responsive transcription factor ChREBP contributes to glucose-dependent anabolic synthesis and cell proliferation. Proc Natl Acad Sci U S A. 2009;106:21660–21665. doi: 10.1073/pnas.0911316106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wernicke CM, Richter GH, Beinvogl BC, Plehm S, Schlitter AM, Bandapalli OR, Prazeres da Costa O, Hattenhorst UE, Volkmer I, Staege MS, Esposito I, Burdach S, Grunewald TG. MondoA is highly overexpressed in acute lymphoblastic leukemia cells and modulates their metabolism, differentiation and survival. Leuk Res. 2012;36:1185–1192. doi: 10.1016/j.leukres.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 152.Rabbitts PH, Watson JV, Lamond A, Forster A, Stinson MA, Evan G, Fischer W, Atherton E, Sheppard R, Rabbitts TH. Metabolism of c-myc gene products: c-myc mRNA and protein expression in the cell cycle. EMBO J. 1985;4:2009–2015. doi: 10.1002/j.1460-2075.1985.tb03885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Queva C, Hurlin PJ, Foley KP, Eisenman RN. Sequential expression of the MAD family of transcriptional repressors during differentiation and development. Oncogene. 1998;16:967–977. doi: 10.1038/sj.onc.1201611. [DOI] [PubMed] [Google Scholar]
- 154.Queva C, McArthur GA, Iritani BM, Eisenman RN. Targeted deletion of the S-phase-specific Myc antagonist Mad3 sensitizes neuronal and lymphoid cells to radiation-induced apoptosis. Mol Cell Biol. 2001;21:703–712. doi: 10.1128/MCB.21.3.703-712.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Fox EJ, Wright SC. S-phase-specific expression of the Mad3 gene in proliferating and differentiating cells. Biochem J. 2001;359:361–367. doi: 10.1042/0264-6021:3590361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Leon J, Ferrandiz N, Acosta JC, Delgado MD. Inhibition of cell differentiation: a critical mechanism for MYC-mediated carcinogenesis? Cell Cycle. 2009;8:1148–1157. doi: 10.4161/cc.8.8.8126. [DOI] [PubMed] [Google Scholar]
- 157.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 158.Chappell J, Dalton S. Roles for MYC in the Establishment and Maintenance of Pluripotency. Cold Spring Harb Perspect Med. 2013;3 doi: 10.1101/cshperspect.a014381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ayer DE, Eisenman RN. A switch from Myc:Max to Mad:Max heterocomplexes accompanies monocyte/macrophage differentiation. Genes Dev. 1993;7:2110–2119. doi: 10.1101/gad.7.11.2110. [DOI] [PubMed] [Google Scholar]
- 160.Hurlin PJ, Ayer DE, Grandori C, Eisenman RN. The Max transcription factor network: involvement of Mad in differentiation and an approach to identification of target genes. Cold Spring Harb Symp Quant Biol. 1994;59:109–116. doi: 10.1101/sqb.1994.059.01.014. [DOI] [PubMed] [Google Scholar]
- 161.Larsson LG, Pettersson M, Oberg F, Nilsson K, Luscher B. Expression of mad, mxi1, max and c-myc during induced differentiation of hematopoietic cells: opposite regulation of mad and c-myc. Oncogene. 1994;9:1247–1252. [PubMed] [Google Scholar]
- 162.Larsson LG, Bahram F, Burkhardt H, Luscher B. Analysis of the DNA-binding activities of Myc/Max/Mad network complexes during induced differentiation of U-937 monoblasts and F9 teratocarcinoma cells. Oncogene. 1997;15:737–748. doi: 10.1038/sj.onc.1201390. [DOI] [PubMed] [Google Scholar]
- 163.Acosta JC, Ferrandiz N, Bretones G, Torrano V, Blanco R, Richard C, O’Connell B, Sedivy J, Delgado MD, Leon J. Myc inhibits p27-induced erythroid differentiation of leukemia cells by repressing erythroid master genes without reversing p27-mediated cell cycle arrest. Mol Cell Biol. 2008;28:7286–7295. doi: 10.1128/MCB.00752-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Cultraro CM, Bino T, Segal S. Function of the c-Myc antagonist Mad1 during a molecular switch from proliferation to differentiation. Mol Cell Biol. 1997;17:2353–2359. doi: 10.1128/mcb.17.5.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hopewell R, Ziff EB. The nerve growth factor-responsive PC12 cell line does not express the Myc dimerization partner Max. Mol Cell Biol. 1995;15:3470–3478. doi: 10.1128/mcb.15.7.3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Greenberg ME, Greene LA, Ziff EB. Nerve growth factor and epidermal growth factor induce rapid transient changes in proto-oncogene transcription in PC12 cells. J Biol Chem. 1985;260:14101–14110. [PubMed] [Google Scholar]
- 167.Noda M, Ko M, Ogura A, Liu DG, Amano T, Takano T, Ikawa Y. Sarcoma viruses carrying ras oncogenes induce differentiation-associated properties in a neuronal cell line. Nature. 1985;318:73–75. doi: 10.1038/318073a0. [DOI] [PubMed] [Google Scholar]
- 168.Bar-Sagi D, Feramisco JR. Microinjection of the ras oncogene protein into PC12 cells induces morphological differentiation. Cell. 1985;42:841–848. doi: 10.1016/0092-8674(85)90280-6. [DOI] [PubMed] [Google Scholar]
- 169.Hishida T, Nozaki Y, Nakachi Y, Mizuno Y, Okazaki Y, Ema M, Takahashi S, Nishimoto M, Okuda A. Indefinite self-renewal of ESCs through Myc/Max transcriptional complex-independent mechanisms. Cell Stem Cell. 2011;9:37–49. doi: 10.1016/j.stem.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 170.Gallant P, Steiger D. Myc’s secret life without Max. Cell Cycle. 2009;8:3848–3853. doi: 10.4161/cc.8.23.10088. [DOI] [PubMed] [Google Scholar]
- 171.Steiger D, Furrer M, Schwinkendorf D, Gallant P. Max-independent functions of Myc in Drosophila melanogaster. Nat Genet. 2008;40:1084–1091. doi: 10.1038/ng.178. [DOI] [PubMed] [Google Scholar]
- 172.Burnichon N, Cascon A, Schiavi F, Morales NP, Comino-Mendez I, Abermil N, Inglada-Perez L, de Cubas AA, Amar L, Barontini M, de Quiros SB, Bertherat J, Bignon YJ, Blok MJ, Bobisse S, Borrego S, Castellano M, Chanson P, Chiara MD, Corssmit EP, Giacche M, de Krijger RR, Ercolino T, Girerd X, Gomez-Garcia EB, Gomez-Grana A, Guilhem I, Hes FJ, Honrado E, Korpershoek E, Lenders JW, Leton R, Mensenkamp AR, Merlo A, Mori L, Murat A, Pierre P, Plouin PF, Prodanov T, Quesada-Charneco M, Qin N, Rapizzi E, Raymond V, Reisch N, Roncador G, Ruiz-Ferrer M, Schillo F, Stegmann AP, Suarez C, Taschin E, Timmers HJ, Tops CM, Urioste M, Beuschlein F, Pacak K, Mannelli M, Dahia PL, Opocher G, Eisenhofer G, Gimenez-Roqueplo AP, Robledo M. MAX mutations cause hereditary and sporadic pheochromocytoma and paraganglioma. Clin Cancer Res. 2012;18:2828–2837. doi: 10.1158/1078-0432.CCR-12-0160. [DOI] [PubMed] [Google Scholar]
- 173.Comino-Mendez I, Gracia-Aznarez FJ, Schiavi F, Landa I, Leandro-Garcia LJ, Leton R, Honrado E, Ramos-Medina R, Caronia D, Pita G, Gomez-Grana A, de Cubas AA, Inglada-Perez L, Maliszewska A, Taschin E, Bobisse S, Pica G, Loli P, Hernandez-Lavado R, Diaz JA, Gomez-Morales M, Gonzalez-Neira A, Roncador G, Rodriguez-Antona C, Benitez J, Mannelli M, Opocher G, Robledo M, Cascon A. Exome sequencing identifies MAX mutations as a cause of hereditary pheochromocytoma. Nat Genet. 2011;43:663–667. doi: 10.1038/ng.861. [DOI] [PubMed] [Google Scholar]
- 174.Vaque JP, Fernandez-Garcia B, Garcia-Sanz P, Ferrandiz N, Bretones G, Calvo F, Crespo P, Marin MC, Leon J. c-Myc inhibits Ras-mediated differentiation of pheochromocytoma cells by blocking c-Jun up-regulation. Mol Cancer Res. 2008;6:325–339. doi: 10.1158/1541-7786.MCR-07-0180. [DOI] [PubMed] [Google Scholar]
- 175.Iizuka K, Bruick RK, Liang G, Horton JD, Uyeda K. Deficiency of carbohydrate response element-binding protein (ChREBP) reduces lipogenesis as well as glycolysis. Proc Natl Acad Sci U S A. 2004;101:7281–7286. doi: 10.1073/pnas.0401516101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Prendergast GC. Mechanisms of apoptosis by c-Myc. Oncogene. 1999;18:2967–2987. doi: 10.1038/sj.onc.1202727. [DOI] [PubMed] [Google Scholar]
- 177.Pelengaris S, Rudolph B, Littlewood T. Action of Myc in vivo - proliferation and apoptosis. Curr Opin Genet Dev. 2000;10:100–105. doi: 10.1016/s0959-437x(99)00046-5. [DOI] [PubMed] [Google Scholar]
- 178.Nieminen AI, Partanen JI, Klefstrom J. c-Myc blazing a trail of death: coupling of the mitochondrial and death receptor apoptosis pathways by c-Myc. Cell Cycle. 2007;6:2464–2472. doi: 10.4161/cc.6.20.4917. [DOI] [PubMed] [Google Scholar]
- 179.Nilsson JA, Cleveland JL. Myc pathways provoking cell suicide and cancer. Oncogene. 2003;22:9007–9021. doi: 10.1038/sj.onc.1207261. [DOI] [PubMed] [Google Scholar]
- 180.Eischen CM, Roussel MF, Korsmeyer SJ, Cleveland JL. Bax loss impairs Myc-induced apoptosis and circumvents the selection of p53 mutations during Myc-mediated lymphomagenesis. Mol Cell Biol. 2001;21:7653–7662. doi: 10.1128/MCB.21.22.7653-7662.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wagner AJ, Small MB, Hay N. Myc-mediated apoptosis is blocked by ectopic expression of Bcl-2. Mol Cell Biol. 1993;13:2432–2440. doi: 10.1128/mcb.13.4.2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Waikel RL, Wang XJ, Roop DR. Targeted expression of c-Myc in the epidermis alters normal proliferation, differentiation and UV-B induced apoptosis. Oncogene. 1999;18:4870–4878. doi: 10.1038/sj.onc.1203040. [DOI] [PubMed] [Google Scholar]
- 183.Chen CH, Zhang J, Ling CC. Transfected c-myc and c-Ha-ras modulate radiation-induced apoptosis in rat embryo cells. Radiat Res. 1994;139:307–315. [PubMed] [Google Scholar]
- 184.Rupnow BA, Alarcon RM, Giaccia AJ, Knox SJ. p53 mediates apoptosis induced by c-Myc activation in hypoxic or gamma irradiated fibroblasts. Cell Death Differ. 1998;5:141–147. doi: 10.1038/sj.cdd.4400328. [DOI] [PubMed] [Google Scholar]
- 185.Shim H, Chun YS, Lewis BC, Dang CV. A unique glucose-dependent apoptotic pathway induced by c-Myc. Proc Natl Acad Sci U S A. 1998;95:1511–1516. doi: 10.1073/pnas.95.4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Yuneva M, Zamboni N, Oefner P, Sachidanandam R, Lazebnik Y. Deficiency in glutamine but not glucose induces MYC-dependent apoptosis in human cells. J Cell Biol. 2007;178:93–105. doi: 10.1083/jcb.200703099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Nieminen AI, Partanen JI, Hau A, Klefstrom J. c-Myc primed mitochondria determine cellular sensitivity to TRAIL-induced apoptosis. EMBO J. 2007;26:1055–1067. doi: 10.1038/sj.emboj.7601551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Cho K, Shin HW, Kim YI, Cho CH, Chun YS, Kim TY, Park JW. Mad1 mediates hypoxia-induced doxorubicin resistance in colon cancer cells by inhibiting mitochondrial function. Free Radic Biol Med. 2013;60:201–210. doi: 10.1016/j.freeradbiomed.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 189.Rottmann S, Speckgens S, Luscher-Firzlaff J, Luscher B. Inhibition of apoptosis by MAD1 is mediated by repression of the PTEN tumor suppressor gene. FASEB J. 2008;22:1124–1134. doi: 10.1096/fj.07-9627com. [DOI] [PubMed] [Google Scholar]
- 190.James L, Eisenman RN. Myc and Mad bHLHZ domains possess identical DNA-binding specificities but only partially overlapping functions in vivo. Proc Natl Acad Sci U S A. 2002;99:10429–10434. doi: 10.1073/pnas.162369299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Staller P, Peukert K, Kiermaier A, Seoane J, Lukas J, Karsunky H, Moroy T, Bartek J, Massague J, Hanel F, Eilers M. Repression of p15INK4b expression by Myc through association with Miz-1. Nat Cell Biol. 2001;3:392–399. doi: 10.1038/35070076. [DOI] [PubMed] [Google Scholar]
- 192.Seoane J, Pouponnot C, Staller P, Schader M, Eilers M, Massague J. TGFbeta influences Myc, Miz-1 and Smad to control the CDK inhibitor p15INK4b. Nat Cell Biol. 2001;3:400–408. doi: 10.1038/35070086. [DOI] [PubMed] [Google Scholar]
- 193.Seoane J, Le HV, Massague J. Myc suppression of the p21(Cip1) Cdk inhibitor influences the outcome of the p53 response to DNA damage. Nature. 2002;419:729–734. doi: 10.1038/nature01119. [DOI] [PubMed] [Google Scholar]
- 194.Patel JH, McMahon SB. Targeting of Miz-1 is essential for Myc-mediated apoptosis. J Biol Chem. 2006;281:3283–3289. doi: 10.1074/jbc.M513038200. [DOI] [PubMed] [Google Scholar]
- 195.Vasilevsky NA, Ruby CE, Hurlin PJ, Weinberg AD. OX40 engagement stabilizes Mxd4 and Mnt protein levels in antigen-stimulated T cells leading to an increase in cell survival. Eur J Immunol. 2011;41:1024–1034. doi: 10.1002/eji.201040449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Corn PG, Ricci MS, Scata KA, Arsham AM, Simon MC, Dicker DT, El-Deiry WS. Mxi1 is induced by hypoxia in a HIF-1-dependent manner and protects cells from c-Myc-induced apoptosis. Cancer Biol Ther. 2005;4:1285–1294. doi: 10.4161/cbt.4.11.2299. [DOI] [PubMed] [Google Scholar]
- 197.Kaelin WG., Jr. The von Hippel-Lindau tumour suppressor protein: O2 sensing and cancer. Nat Rev Cancer. 2008;8:865–873. doi: 10.1038/nrc2502. [DOI] [PubMed] [Google Scholar]
- 198.Tsao CC, Teh BT, Jonasch E, Shreiber-Agus N, Efstathiou E, Hoang A, Czerniak B, Logothetis C, Corn PG. Inhibition of Mxi1 suppresses HIF-2alpha-dependent renal cancer tumorigenesis. Cancer Biol Ther. 2008;7:1619–1627. doi: 10.4161/cbt.7.10.6583. [DOI] [PubMed] [Google Scholar]
- 199.Poungvarin N, Lee JK, Yechoor VK, Li MV, Assavapokee T, Suksaranjit P, Thepsongwajja JJ, Saha PK, Oka K, Chan L. Carbohydrate response element-binding protein (ChREBP) plays a pivotal role in beta cell glucotoxicity. Diabetologia. 2012;55:1783–1796. doi: 10.1007/s00125-012-2506-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Bello-Fernandez C, Cleveland JL. c-myc transactivates the ornithine decarboxylase gene. Curr Top Microbiol Immunol. 1992;182:445–452. doi: 10.1007/978-3-642-77633-5_56. [DOI] [PubMed] [Google Scholar]
- 201.Miltenberger RJ, Sukow KA, Farnham PJ. An E-box-mediated increase in cad transcription at the G1/S-phase boundary is suppressed by inhibitory c-Myc mutants. Mol Cell Biol. 1995;15:2527–2535. doi: 10.1128/mcb.15.5.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Mai S. Overexpression of c-myc precedes amplification of the gene encoding dihydrofolate reductase. Gene. 1994;148:253–260. doi: 10.1016/0378-1119(94)90696-3. [DOI] [PubMed] [Google Scholar]
- 203.Pusch O, Soucek T, Hengstschlager-Ottnad E, Bernaschek G, Hengstschlager M. Cellular targets for activation by c-Myc include the DNA metabolism enzyme thymidine kinase. DNA Cell Biol. 1997;16:737–747. doi: 10.1089/dna.1997.16.737. [DOI] [PubMed] [Google Scholar]
- 204.Shim H, Dolde C, Lewis BC, Wu CS, Dang G, Jungmann RA, Dalla-Favera R, Dang CV. c-Myc transactivation of LDH-A: implications for tumor metabolism and growth. Proc Natl Acad Sci U S A. 1997;94:6658–6663. doi: 10.1073/pnas.94.13.6658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Dang CV, Le A, Gao P. MYC-induced cancer cell energy metabolism and therapeutic opportunities. Clin Cancer Res. 2009;15:6479–6483. doi: 10.1158/1078-0432.CCR-09-0889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Gomez-Roman N, Grandori C, Eisenman RN, White RJ. Direct activation of RNA polymerase III transcription by c-Myc. Nature. 2003;421:290–294. doi: 10.1038/nature01327. [DOI] [PubMed] [Google Scholar]
- 207.Grandori C, Gomez-Roman N, Felton-Edkins ZA, Ngouenet C, Galloway DA, Eisenman RN, White RJ. c-Myc binds to human ribosomal DNA and stimulates transcription of rRNA genes by RNA polymerase I. Nat Cell Biol. 2005;7:311–318. doi: 10.1038/ncb1224. [DOI] [PubMed] [Google Scholar]
- 208.Gomez-Roman N, Felton-Edkins ZA, Kenneth NS, Goodfellow SJ, Athineos D, Zhang J, Ramsbottom BA, Innes F, Kantidakis T, Kerr ER, Brodie J, Grandori C, White RJ. Activation by c-Myc of transcription by RNA polymerases I, II and III. Biochem Soc Symp. 2006:141–154. doi: 10.1042/bss0730141. [DOI] [PubMed] [Google Scholar]
- 209.Yeung SJ, Pan J, Lee MH. Roles of p53, MYC and HIF-1 in regulating glycolysis - the seventh hallmark of cancer. Cell Mol Life Sci. 2008;65:3981–3999. doi: 10.1007/s00018-008-8224-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Wise DR, DeBerardinis RJ, Mancuso A, Sayed N, Zhang XY, Pfeiffer HK, Nissim I, Daikhin E, Yudkoff M, McMahon SB, Thompson CB. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc Natl Acad Sci U S A. 2008;105:18782–18787. doi: 10.1073/pnas.0810199105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Dang CV. Rethinking the Warburg effect with Myc micromanaging glutamine metabolism. Cancer Res. 2010;70:859–862. doi: 10.1158/0008-5472.CAN-09-3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Wang R, Dillon CP, Shi LZ, Milasta S, Carter R, Finkelstein D, McCormick LL, Fitzgerald P, Chi H, Munger J, Green DR. The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity. 2011;35:871–882. doi: 10.1016/j.immuni.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Kim JW, Zeller KI, Wang Y, Jegga AG, Aronow BJ, O’Donnell KA, Dang CV. Evaluation of myc E-box phylogenetic footprints in glycolytic genes by chromatin immunoprecipitation assays. Mol Cell Biol. 2004;24:5923–5936. doi: 10.1128/MCB.24.13.5923-5936.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Osthus RC, Shim H, Kim S, Li Q, Reddy R, Mukherjee M, Xu Y, Wonsey D, Lee LA, Dang CV. Deregulation of glucose transporter 1 and glycolytic gene expression by c-Myc. J Biol Chem. 2000;275:21797–21800. doi: 10.1074/jbc.C000023200. [DOI] [PubMed] [Google Scholar]
- 215.Hu S, Balakrishnan A, Bok RA, Anderton B, Larson PE, Nelson SJ, Kurhanewicz J, Vigneron DB, Goga A. 13C-pyruvate imaging reveals alterations in glycolysis that precede c-Myc-induced tumor formation and regression. Cell Metab. 2011;14:131–142. doi: 10.1016/j.cmet.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Gao P, Tchernyshyov I, Chang TC, Lee YS, Kita K, Ochi T, Zeller KI, De Marzo AM, Van Eyk JE, Mendell JT, Dang CV. c-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature. 2009;458:762–765. doi: 10.1038/nature07823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Morrish F, Giedt C, Hockenbery D. c-MYC apoptotic function is mediated by NRF-1 target genes. Genes Dev. 2003;17:240–255. doi: 10.1101/gad.1032503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Morrish F, Noonan J, Perez-Olsen C, Gafken PR, Fitzgibbon M, Kelleher J, VanGilst M, Hockenbery D. Myc-dependent mitochondrial generation of acetyl-CoA contributes to fatty acid biosynthesis and histone acetylation during cell cycle entry. J Biol Chem. 2010;285:36267–36274. doi: 10.1074/jbc.M110.141606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Li F, Wang Y, Zeller KI, Potter JJ, Wonsey DR, O’Donnell KA, Kim JW, Yustein JT, Lee LA, Dang CV. Myc stimulates nuclearly encoded mitochondrial genes and mitochondrial biogenesis. Mol Cell Biol. 2005;25:6225–6234. doi: 10.1128/MCB.25.14.6225-6234.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.van Riggelen J, Yetil A, Felsher DW. MYC as a regulator of ribosome biogenesis and protein synthesis. Nat Rev Cancer. 2010;10:301–309. doi: 10.1038/nrc2819. [DOI] [PubMed] [Google Scholar]
- 221.Zhang Z, Sun H, Dai H, Walsh RM, Imakura M, Schelter J, Burchard J, Dai X, Chang AN, Diaz RL, Marszalek JR, Bartz SR, Carleton M, Cleary MA, Linsley PS, Grandori C. MicroRNA miR-210 modulates cellular response to hypoxia through the MYC antagonist MNT. Cell Cycle. 2009;8:2756–2768. doi: 10.4161/cc.8.17.9387. [DOI] [PubMed] [Google Scholar]
- 222.Iizuka K, Horikawa Y. ChREBP: a glucose-activated transcription factor involved in the development of metabolic syndrome. Endocr J. 2008;55:617–624. doi: 10.1507/endocrj.k07e-110. [DOI] [PubMed] [Google Scholar]
- 223.Davies MN, O’Callaghan BL, Towle HC. Glucose activates ChREBP by increasing its rate of nuclear entry and relieving repression of its transcriptional activity. J Biol Chem. 2008;283:24029–24038. doi: 10.1074/jbc.M801539200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Davies MN, O’Callaghan BL, Towle HC. Activation and repression of glucose-stimulated ChREBP requires the concerted action of multiple domains within the MondoA conserved region. Am J Physiol Endocrinol Metab. 2010;299:E665–E674. doi: 10.1152/ajpendo.00349.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Peterson CW, Stoltzman CA, Sighinolfi MP, Han KS, Ayer DE. Glucose controls nuclear accumulation, promoter binding, and transcriptional activity of the MondoA-Mlx heterodimer. Mol Cell Biol. 2010;30:2887–2895. doi: 10.1128/MCB.01613-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Ma L, Robinson LN, Towle HC. ChREBP*Mlx is the principal mediator of glucose-induced gene expression in the liver. J Biol Chem. 2006;281:28721–28730. doi: 10.1074/jbc.M601576200. [DOI] [PubMed] [Google Scholar]
- 227.Ishii S, Iizuka K, Miller BC, Uyeda K. Carbohydrate response element binding protein directly promotes lipogenic enzyme gene transcription. Proc Natl Acad Sci U S A. 2004;101:15597–15602. doi: 10.1073/pnas.0405238101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Wang Y, Botolin D, Xu J, Christian B, Mitchell E, Jayaprakasam B, Nair MG, Peters JM, Busik JV, Olson LK, Jump DB. Regulation of hepatic fatty acid elongase and desaturase expression in diabetes and obesity. J Lipid Res. 2006;47:2028–2041. doi: 10.1194/jlr.M600177-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Jeong YS, Kim D, Lee YS, Kim HJ, Han JY, Im SS, Chong HK, Kwon JK, Cho YH, Kim WK, Osborne TF, Horton JD, Jun HS, Ahn YH, Ahn SM, Cha JY. Integrated expression profiling and genome-wide analysis of ChREBP targets reveals the dual role for ChREBP in glucose-regulated gene expression. PLoS One. 2011;6:e22544. doi: 10.1371/journal.pone.0022544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Ma L, Tsatsos NG, Towle HC. Direct role of ChREBP. Mlx in regulating hepatic glucose-responsive genes. J Biol Chem. 2005;280:12019–12027. doi: 10.1074/jbc.M413063200. [DOI] [PubMed] [Google Scholar]
- 231.Collier JJ, Doan TT, Daniels MC, Schurr JR, Kolls JK, Scott DK. c-Myc is required for the glucose-mediated induction of metabolic enzyme genes. J Biol Chem. 2003;278:6588–6595. doi: 10.1074/jbc.M208011200. [DOI] [PubMed] [Google Scholar]
- 232.Collier JJ, Zhang P, Pedersen KB, Burke SJ, Haycock JW, Scott DK. c-Myc and ChREBP regulate glucose-mediated expression of the L-type pyruvate kinase gene in INS-1-derived 832/13 cells. Am J Physiol Endocrinol Metab. 2007;293:E48–56. doi: 10.1152/ajpendo.00357.2006. [DOI] [PubMed] [Google Scholar]
- 233.Zhang P, Metukuri MR, Bindom SM, Prochownik EV, O’Doherty RM, Scott DK. c-Myc is required for the CHREBP-dependent activation of glucose-responsive genes. Mol Endocrinol. 2010;24:1274–1286. doi: 10.1210/me.2009-0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Qing G, Li B, Vu A, Skuli N, Walton ZE, Liu X, Mayes PA, Wise DR, Thompson CB, Maris JM, Hogarty MD, Simon MC. ATF4 regulates MYC-mediated neuroblastoma cell death upon glutamine deprivation. Cancer Cell. 2012;22:631–644. doi: 10.1016/j.ccr.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Le A, Cooper CR, Gouw AM, Dinavahi R, Maitra A, Deck LM, Royer RE, Vander Jagt DL, Semenza GL, Dang CV. Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proc Natl Acad Sci U S A. 2010;107:2037–2042. doi: 10.1073/pnas.0914433107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Greenberg RA, O’Hagan RC, Deng H, Xiao Q, Hann SR, Adams RR, Lichtsteiner S, Chin L, Morin GB, DePinho RA. Telomerase reverse transcriptase gene is a direct target of c-Myc but is not functionally equivalent in cellular transformation. Oncogene. 1999;18:1219–1226. doi: 10.1038/sj.onc.1202669. [DOI] [PubMed] [Google Scholar]
- 237.Gunes C, Lichtsteiner S, Vasserot AP, Englert C. Expression of the hTERT gene is regulated at the level of transcriptional initiation and repressed by Mad1. Cancer Res. 2000;60:2116–2121. [PubMed] [Google Scholar]
- 238.Oh S, Song YH, Yim J, Kim TK. Identification of Mad as a repressor of the human telomerase (hTERT) gene. Oncogene. 2000;19:1485–1490. doi: 10.1038/sj.onc.1203439. [DOI] [PubMed] [Google Scholar]
- 239.Xu D, Popov N, Hou M, Wang Q, Bjorkholm M, Gruber A, Menkel AR, Henriksson M. Switch from Myc/Max to Mad1/Max binding and decrease in histone acetylation at the telomerase reverse transcriptase promoter during differentiation of HL60 cells. Proc Natl Acad Sci U S A. 2001;98:3826–3831. doi: 10.1073/pnas.071043198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Lutz W, Leon J, Eilers M. Contributions of Myc to tumorigenesis. Biochim Biophys Acta. 2002;1602:61–71. doi: 10.1016/s0304-419x(02)00036-7. [DOI] [PubMed] [Google Scholar]
- 241.Bouchard C, Thieke K, Maier A, Saffrich R, Hanley-Hyde J, Ansorge W, Reed S, Sicinski P, Bartek J, Eilers M. Direct induction of cyclin D2 by Myc contributes to cell cycle progression and sequestration of p27. EMBO J. 1999;18:5321–5333. doi: 10.1093/emboj/18.19.5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Bouchard C, Dittrich O, Kiermaier A, Dohmann K, Menkel A, Eilers M, Luscher B. Regulation of cyclin D2 gene expression by the Myc/Max/Mad network: Myc-dependent TRRAP recruitment and histone acetylation at the cyclin D2 promoter. Genes Dev. 2001;15:2042–2047. doi: 10.1101/gad.907901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Rottmann S, Menkel AR, Bouchard C, Mertsching J, Loidl P, Kremmer E, Eilers M, Luscher-Firzlaff J, Lilischkis R, Luscher B. Mad1 function in cell proliferation and transcriptional repression is antagonized by cyclin E/CDK2. J Biol Chem. 2005;280:15489–15492. doi: 10.1074/jbc.C400611200. [DOI] [PubMed] [Google Scholar]
- 244.Galaktionov K, Chen X, Beach D. Cdc25 cell-cycle phosphatase as a target of c-myc. Nature. 1996;382:511–517. doi: 10.1038/382511a0. [DOI] [PubMed] [Google Scholar]
- 245.Zhao H, Xu YH. Mad-overexpression down regulates the malignant growth and p53 mediated apoptosis in human hepatocellular carcinoma BEL-7404 cells. Cell Res. 1999;9:51–59. doi: 10.1038/sj.cr.7290005. [DOI] [PubMed] [Google Scholar]
- 246.Lin CJ, Cencic R, Mills JR, Robert F, Pelletier J. c-Myc and eIF4F are components of a feedforward loop that links transcription and translation. Cancer Res. 2008;68:5326–5334. doi: 10.1158/0008-5472.CAN-07-5876. [DOI] [PubMed] [Google Scholar]
- 247.Jones RM, Branda J, Johnston KA, Polymenis M, Gadd M, Rustgi A, Callanan L, Schmidt EV. An essential E box in the promoter of the gene encoding the mRNA cap-binding protein (eukaryotic initiation factor 4E) is a target for activation by c-myc. Mol Cell Biol. 1996;16:4754–4764. doi: 10.1128/mcb.16.9.4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Postic C, Dentin R, Denechaud PD, Girard J. ChREBP, a transcriptional regulator of glucose and lipid metabolism. Annu Rev Nutr. 2007;27:179–192. doi: 10.1146/annurev.nutr.27.061406.093618. [DOI] [PubMed] [Google Scholar]
- 249.Uyeda K, Repa JJ. Carbohydrate response element binding protein, ChREBP, a transcription factor coupling hepatic glucose utilization and lipid synthesis. Cell Metab. 2006;4:107–110. doi: 10.1016/j.cmet.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 250.Shih HM, Liu Z, Towle HC. Two CACGTG motifs with proper spacing dictate the carbohydrate regulation of hepatic gene transcription. J Biol Chem. 1995;270:21991–21997. doi: 10.1074/jbc.270.37.21991. [DOI] [PubMed] [Google Scholar]
- 251.Ma L, Sham YY, Walters KJ, Towle HC. A critical role for the loop region of the basic helix-loop-helix/leucine zipper protein Mlx in DNA binding and glucose-regulated transcription. Nucleic Acids Res. 2007;35:35–44. doi: 10.1093/nar/gkl987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Stoeckman AK, Ma L, Towle HC. Mlx is the functional heteromeric partner of the carbohydrate response element-binding protein in glucose regulation of lipogenic enzyme genes. J Biol Chem. 2004;279:15662–15669. doi: 10.1074/jbc.M311301200. [DOI] [PubMed] [Google Scholar]
- 253.Schuhmacher M, Kohlhuber F, Holzel M, Kaiser C, Burtscher H, Jarsch M, Bornkamm GW, Laux G, Polack A, Weidle UH, Eick D. The transcriptional program of a human B cell line in response to Myc. Nucleic Acids Res. 2001;29:397–406. doi: 10.1093/nar/29.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Littlewood TD, Kreuzaler P, Evan GI. All things to all people. Cell. 2012;151:11–13. doi: 10.1016/j.cell.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 255.Walz S, Eilers M, Eisenman R, Dang CV. Unlocking the mysterious mechanisms of Myc. Nat Med. 2013;19:26–27. doi: 10.1038/nm.3060. [DOI] [PubMed] [Google Scholar]
- 256.McCarthy N. Tumorigenesis: Megaphone MYC. Nat Rev Cancer. 2012;12:733. doi: 10.1038/nrc3384. [DOI] [PubMed] [Google Scholar]
- 257.Sabo’ A, Amati B. Genome Recognition by Myc. Cold Spring Harb Perspect Med. 2013 doi: 10.1101/cshperspect.a014191. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Grignani F, Lombardi L, Inghirami G, Sternas L, Cechova K, Dalla-Favera R. Negative autoregulation of c-myc gene expression is inactivated in transformed cells. EMBO J. 1990;9:3913–3922. doi: 10.1002/j.1460-2075.1990.tb07612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Breit S, Schwab M. Suppression of MYC by high expression of NMYC in human neuroblastoma cells. J Neurosci Res. 1989;24:21–28. doi: 10.1002/jnr.490240105. [DOI] [PubMed] [Google Scholar]
- 260.Rosenbaum H, Webb E, Adams JM, Cory S, Harris AW. N-myc transgene promotes B lymphoid proliferation, elicits lymphomas and reveals cross-regulation with c-myc. EMBO J. 1989;8:749–755. doi: 10.1002/j.1460-2075.1989.tb03435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Kime L, Wright SC. Mad4 is regulated by a transcriptional repressor complex that contains Miz-1 and c-Myc. Biochem J. 2003;370:291–298. doi: 10.1042/BJ20021679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Marcotte R, Chen JM, Huard S, Wang E. c-Myc creates an activation loop by transcriptionally repressing its own functional inhibitor, hMad4, in young fibroblasts, a loop lost in replicatively senescent fibroblasts. J Cell Biochem. 2005;96:1071–1085. doi: 10.1002/jcb.20503. [DOI] [PubMed] [Google Scholar]
- 263.Poloni A, Serrani F, Berardinelli E, Maurizi G, Mariani M, Costantini B, Trappolini S, Mancini S, Olivieri A, Leoni P. Telomere length, c-myc and mad-1 expression could represent prognosis markers of myelodysplastic syndrome. Leuk Res. 2013;37:1538–1544. doi: 10.1016/j.leukres.2013.07.022. [DOI] [PubMed] [Google Scholar]
- 264.Boult JK, Taniere P, Hallissey MT, Campbell MJ, Tselepis C. Oesophageal adenocarcinoma is associated with a deregulation in the MYC/MAX/MAD network. Br J Cancer. 2008;98:1985–1992. doi: 10.1038/sj.bjc.6604398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Crona J, Maharjan R, Delgado Verdugo A, Stalberg P, Granberg D, Hellman P, Bjorklund P. MAX mutations status in Swedish patients with pheochromocytoma and paraganglioma tumours. Fam Cancer. 2013 doi: 10.1007/s10689-013-9666-3. [DOI] [PubMed] [Google Scholar]
- 266.Guo XL, Pan L, Zhang XJ, Suo XH, Niu ZY, Zhang JY, Wang F, Dong ZR, Da W, Ohno R. Expression and mutation analysis of genes that encode the Myc antagonists Mad1, Mxi1 and Rox in acute leukaemia. Leuk Lymphoma. 2007;48:1200–1207. doi: 10.1080/10428190701342018. [DOI] [PubMed] [Google Scholar]
- 267.Li XJ, Wang DY, Zhu Y, Guo RJ, Wang XD, Lubomir K, Mukai K, Sasaki H, Yoshida H, Oka T, Machinami R, Shinmura K, Tanaka M, Sugimura H. Mxi1 mutations in human neurofibrosarcomas. Jpn J Cancer Res. 1999;90:740–746. doi: 10.1111/j.1349-7006.1999.tb00809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Prochownik EV, Eagle Grove L, Deubler D, Zhu XL, Stephenson RA, Rohr LR, Yin X, Brothman AR. Commonly occurring loss and mutation of the MXI1 gene in prostate cancer. Genes Chromosomes Cancer. 1998;22:295–304. [PubMed] [Google Scholar]
- 269.Kuczyk MA, Serth J, Bokemeyer C, Schwede J, Herrmann R, Machtens S, Grunewald V, Hofner K, Jonas U. The MXI1 tumor suppressor gene is not mutated in primary prostate cancer. Oncol Rep. 1998;5:213–216. [PubMed] [Google Scholar]
- 270.Bartsch D, Peiffer SL, Kaleem Z, Wells SA, Jr., Goodfellow PJ. Mxi1 tumor suppressor gene is not mutated in primary pancreatic adenocarcinoma. Cancer Lett. 1996;102:73–76. doi: 10.1016/0304-3835(96)04167-5. [DOI] [PubMed] [Google Scholar]
- 271.Eagle LR, Yin X, Brothman AR, Williams BJ, Atkin NB, Prochownik EV. Mutation of the MXI1 gene in prostate cancer. Nat Genet. 1995;9:249–255. doi: 10.1038/ng0395-249. [DOI] [PubMed] [Google Scholar]
- 272.Sommer A, Waha A, Tonn J, Sorensen N, Hurlin PJ, Eisenman RN, Luscher B, Pietsch T. Analysis of the Max-binding protein MNT in human medulloblastomas. Int J Cancer. 1999;82:810–816. doi: 10.1002/(sici)1097-0215(19990909)82:6<810::aid-ijc7>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 273.Takahashi T, Konishi H, Kozaki K, Osada H, Saji S, Takahashi T, Takahashi T. Molecular analysis of a Myc antagonist, ROX/Mnt, at 17p13.3 in human lung cancers. Jpn J Cancer Res. 1998;89:347–351. doi: 10.1111/j.1349-7006.1998.tb00569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Cvekl A, Jr., Zavadil J, Birshtein BK, Grotzer MA, Cvekl A. Analysis of transcripts from 17p13.3 in medulloblastoma suggests ROX/MNT as a potential tumour suppressor gene. Eur J Cancer. 2004;40:2525–2532. doi: 10.1016/j.ejca.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 275.Vermeer MH, van Doorn R, Dijkman R, Mao X, Whittaker S, van Voorst Vader PC, Gerritsen MJ, Geerts ML, Gellrich S, Soderberg O, Leuchowius KJ, Landegren U, Out-Luiting JJ, Knijnenburg J, Ijszenga M, Szuhai K, Willemze R, Tensen CP. Novel and highly recurrent chromosomal alterations in Sezary syndrome. Cancer Res. 2008;68:2689–2698. doi: 10.1158/0008-5472.CAN-07-6398. [DOI] [PubMed] [Google Scholar]
- 276.Link JM, Hurlin PJ. MYC needs MNT. Cell Cycle. 2013;12:385–386. doi: 10.4161/cc.23551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Kawamata N, Sugimoto KJ, Sakajiri S, Oshimi K, Koeffler HP. Mxi1 isoforms are expressed in hematological cell lines and normal bone marrow. Int J Oncol. 2005;26:1369–1375. [PubMed] [Google Scholar]
- 278.Edelmann J, Holzmann K, Miller F, Winkler D, Buhler A, Zenz T, Bullinger L, Kuhn MW, Gerhardinger A, Bloehdorn J, Radtke I, Su X, Ma J, Pounds S, Hallek M, Lichter P, Korbel J, Busch R, Mertens D, Downing JR, Stilgenbauer S, Dohner H. High-resolution genomic profiling of chronic lymphocytic leukemia reveals new recurrent genomic alterations. Blood. 2012;120:4783–4794. doi: 10.1182/blood-2012-04-423517. [DOI] [PubMed] [Google Scholar]
- 279.Eisen MB, Spellman PT, Brown PO, Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci U S A. 1998;95:14863–14868. doi: 10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Yuneva MO, Fan TW, Allen TD, Higashi RM, Ferraris DV, Tsukamoto T, Mates JM, Alonso FJ, Wang C, Seo Y, Chen X, Bishop JM. The metabolic profile of tumors depends on both the responsible genetic lesion and tissue type. Cell Metab. 2012;15:157–170. doi: 10.1016/j.cmet.2011.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]