Abstract
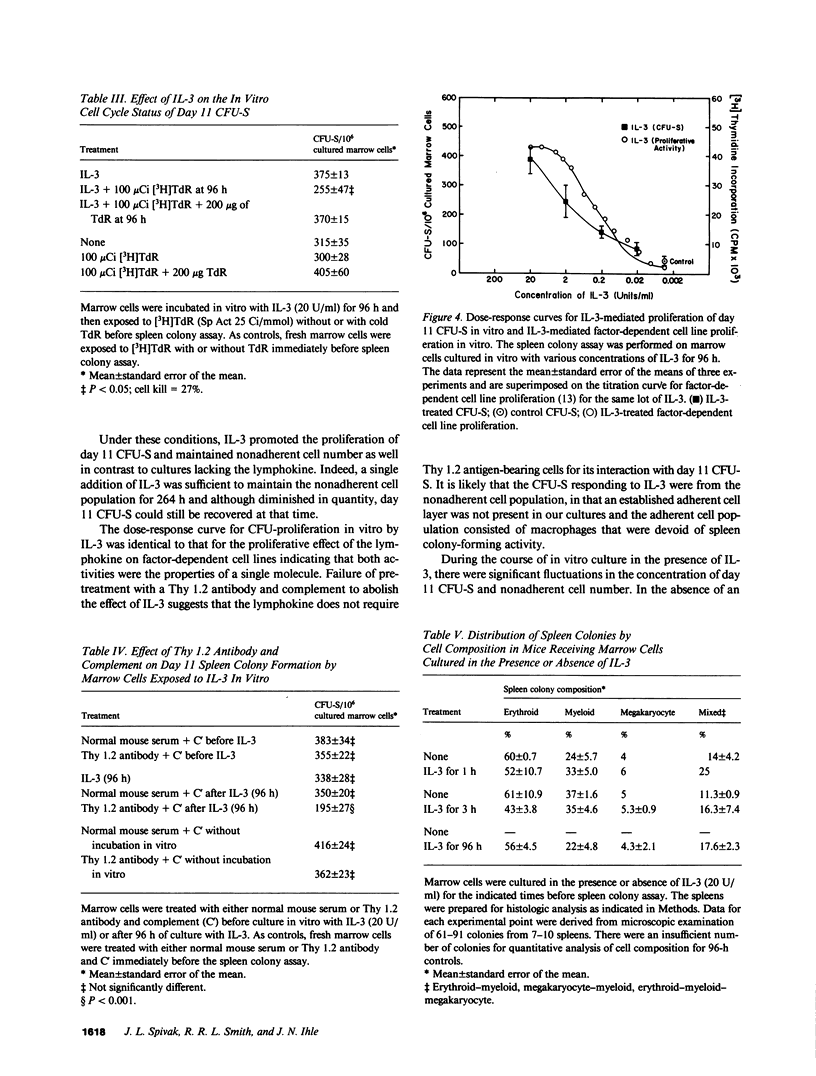
Medium conditioned by activated T lymphocytes stimulates the in vitro proliferation of pluripotent hematopoietic stem cells (spleen colony-forming units [CFU-S]) but the factors involved have not been identified. Because the lymphokine interleukin 3 (IL-3) enhances in vitro colony formation by committed hematopoietic progenitor cells, we examined the effect of IL-3 on the in vitro proliferation of CFU-S using an 11-d spleen colony assay. When mouse marrow cells were placed in liquid culture, CFU-S content declined progressively and by 96 h only 13% of the CFU-S remained. By contrast, after 96 h in the presence of 20 U/ml of IL-3, the number of CFU-S were the same as that in the initial inoculum. Although the number of CFU-S eventually declined, they could still be recovered after 264 h of culture. In the absence of IL-3, the number of CFU-S synthesizing DNA was negligible; in its presence, greater than 20% of the CFU-S were in cycle. IL-3 stimulated CFU-S proliferation at a concentration of 0.2 U/ml. The dose-response curve was similar to that observed for other biologic effects of the lymphokine, and as little as 1 h of exposure to IL-3 enhanced the survival of CFU-S in vitro. Treatment of marrow cells with anti-Thy 1.2 antibody and complement before exposure to IL-3 did not inhibit spleen colony formation, but treatment of the cells with anti-Thy 1.2 antibody and complement after exposure to IL-3 reduced CFU-S recovery after 96 h of culture by 45%. The cell composition of day 11 spleen colonies formed by IL-3-treated marrow cells was similar to that of colonies formed by untreated marrow cells. Finally, day 11 CFU-S persisting in the marrow of mice treated with 5-fluorouracil required IL-3 for proliferation in vitro. Taken together, these data indicate that IL-3 promotes the proliferation of CFU-S in vitro, increases the number of CFU-S synthesizing DNA, but does not alter their commitment program, and the target cell population includes CFU-S with self-renewal and marrow-repopulating ability.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BECKER A. J., MCCULLOCH E. A., SIMINOVITCH L., TILL J. E. THE EFFECT OF DIFFERING DEMANDS FOR BLOOD CELL PRODUCTION ON DNA SYNTHESIS BY HEMOPOIETIC COLONY-FORMING CELLS OF MICE. Blood. 1965 Sep;26:296–308. [PubMed] [Google Scholar]
- Basch R. S., Berman J. W. Thy-1 determinants are present on many murine hematopoietic cells other than T cells. Eur J Immunol. 1982 May;12(5):359–364. doi: 10.1002/eji.1830120502. [DOI] [PubMed] [Google Scholar]
- Boswell H. S., Wade P. M., Jr, Quesenberry P. J. Thy-1 antigen expression by murine high-proliferative capacity hematopoietic progenitor cells. I. Relation between sensitivity to depletion by Thy-1 antibody and stem cell generation potential. J Immunol. 1984 Dec;133(6):2940–2949. [PubMed] [Google Scholar]
- Burgess A. W., Metcalf D., Russell S. H., Nicola N. A. Granulocyte/macrophage-, megakaryocyte-, eosinophil- and erythroid-colony-stimulating factors produced by mouse spleen cells. Biochem J. 1980 Feb 1;185(2):301–314. doi: 10.1042/bj1850301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byron J. W. Manipulation of the cell cycle of the hemopoietic stem cell. Exp Hematol. 1975 Jan;3(1):44–53. [PubMed] [Google Scholar]
- Cerny J. Stimulation of bone marrow haemopoietic stem cells by a factor from activated T cells. Nature. 1974 May 3;249(452):63–66. doi: 10.1038/249063a0. [DOI] [PubMed] [Google Scholar]
- Chervenick P. A., Boggs D. R. Patterns of proliferation and differentiation of hematopoietic stem cells after compartment depletion. Blood. 1971 May;37(5):568–580. [PubMed] [Google Scholar]
- Dexter T. M., Garland J., Scott D., Scolnick E., Metcalf D. Growth of factor-dependent hemopoietic precursor cell lines. J Exp Med. 1980 Oct 1;152(4):1036–1047. doi: 10.1084/jem.152.4.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dexter T. M., Wright E. G., Krizsa F., Lajtha L. G. Regulation of haemopoietic stem cell proliferation in long term bone marrow cultures. Biomedicine. 1977 Dec;27(9-10):344–349. [PubMed] [Google Scholar]
- Garland J. M., Crompton S. A preliminary report: preparations containing Interleukin-3 (IL-3) promote proliferation of multipotential stem cells (CFUs) in the mouse. Exp Hematol. 1983 Sep;11(8):757–761. [PubMed] [Google Scholar]
- Greenberger J. S., Eckner R. J., Sakakeeny M., Marks P., Reid D., Nabel G., Hapel A., Ihle J. N., Humphries K. C. Interleukin 3-dependent hematopoietic progenitor cell lines. Fed Proc. 1983 Jul;42(10):2762–2771. [PubMed] [Google Scholar]
- Gregory C. J., Eaves A. C. Three stages of erythropoietic progenitor cell differentiation distinguished by a number of physical and biologic properties. Blood. 1978 Mar;51(3):527–537. [PubMed] [Google Scholar]
- Harris R. A., Hogarth P. M., Wadeson L. J., Collins P., McKenzie I. F., Penington D. G. An antigenic difference between cells forming early and late haematopoietic spleen colonies (CFU-S). Nature. 1984 Feb 16;307(5952):638–641. doi: 10.1038/307638a0. [DOI] [PubMed] [Google Scholar]
- Hodgson G. S., Bradley T. R. Properties of haematopoietic stem cells surviving 5-fluorouracil treatment: evidence for a pre-CFU-S cell? Nature. 1979 Oct 4;281(5730):381–382. doi: 10.1038/281381a0. [DOI] [PubMed] [Google Scholar]
- Humphries R. K., Jacky P. B., Dill F. J., Eaves A. C., Eaves C. J. CFU-S in individual erythroid colonies derived in vitro from adult mouse marrow. Nature. 1979 Jun 21;279(5715):718–720. doi: 10.1038/279718a0. [DOI] [PubMed] [Google Scholar]
- Ihle J. N., Keller J., Greenberger J. S., Henderson L., Yetter R. A., Morse H. C., 3rd Phenotypic characteristics of cell lines requiring interleukin 3 for growth. J Immunol. 1982 Oct;129(4):1377–1383. [PubMed] [Google Scholar]
- Ihle J. N., Keller J., Henderson L., Klein F., Palaszynski E. Procedures for the purification of interleukin 3 to homogeneity. J Immunol. 1982 Dec;129(6):2431–2436. [PubMed] [Google Scholar]
- Ihle J. N., Keller J., Oroszlan S., Henderson L. E., Copeland T. D., Fitch F., Prystowsky M. B., Goldwasser E., Schrader J. W., Palaszynski E. Biologic properties of homogeneous interleukin 3. I. Demonstration of WEHI-3 growth factor activity, mast cell growth factor activity, p cell-stimulating factor activity, colony-stimulating factor activity, and histamine-producing cell-stimulating factor activity. J Immunol. 1983 Jul;131(1):282–287. [PubMed] [Google Scholar]
- Ihle J. N., Pepersack L., Rebar L. Regulation of T cell differentiation: in vitro induction of 20 alpha-hydroxysteroid dehydrogenase in splenic lymphocytes from athymic mice by a unique lymphokine. J Immunol. 1981 Jun;126(6):2184–2189. [PubMed] [Google Scholar]
- Iscove N. N., Roitsch C. A., Williams N., Guilbert L. J. Molecules stimulating early red cell, granulocyte, macrophage, and megakaryocyte precursors in culture: similarity in size, hydrophobicity, and charge. J Cell Physiol Suppl. 1982;1:65–78. doi: 10.1002/jcp.1041130412. [DOI] [PubMed] [Google Scholar]
- King-Smith E. A., Morley A. Computer simulation of granulopoiesis: normal and impaired granulopoiesis. Blood. 1970 Aug;36(2):254–262. [PubMed] [Google Scholar]
- Lord B. I., Mori K. J., Wright E. G. A stimulator of stem cell proliferation in regenerating bone marrow. Biomedicine. 1977 Jul;27(6):223–226. [PubMed] [Google Scholar]
- Lord B. I., Mori K. J., Wright E. G., Lajtha L. G. Inhibitor of stem cell proliferation in normal bone marrow. Br J Haematol. 1976 Nov;34(3):441–445. doi: 10.1111/j.1365-2141.1976.tb03590.x. [DOI] [PubMed] [Google Scholar]
- Magli M. C., Iscove N. N., Odartchenko N. Transient nature of early haematopoietic spleen colonies. Nature. 1982 Feb 11;295(5849):527–529. doi: 10.1038/295527a0. [DOI] [PubMed] [Google Scholar]
- Metcalf D., Johnson G. R., Mandel T. E. Colony formation in agar by multipotential hemopoietic cells. J Cell Physiol. 1979 Feb;98(2):401–420. doi: 10.1002/jcp.1040980216. [DOI] [PubMed] [Google Scholar]
- Metcalf D., Johnson G. R. Production by spleen and lymph node cells of conditioned medium with erythroid and other hemopoietic colony-stimulating activity. J Cell Physiol. 1978 Jul;96(1):31–42. doi: 10.1002/jcp.1040960105. [DOI] [PubMed] [Google Scholar]
- Miller B. A., Lipton J. M., Linch D. C., Burakoff S. J., Nathan D. G. Thy-1 is a differentiation antigen that characterizes immature murine erythroid and myeloid hematopoietic progenitors. J Cell Physiol. 1985 Apr;123(1):25–32. doi: 10.1002/jcp.1041230105. [DOI] [PubMed] [Google Scholar]
- Nakahata T., Gross A. J., Ogawa M. A stochastic model of self-renewal and commitment to differentiation of the primitive hemopoietic stem cells in culture. J Cell Physiol. 1982 Dec;113(3):455–458. doi: 10.1002/jcp.1041130314. [DOI] [PubMed] [Google Scholar]
- Nakahata T., Ogawa M. Identification in culture of a class of hemopoietic colony-forming units with extensive capability to self-renew and generate multipotential hemopoietic colonies. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3843–3847. doi: 10.1073/pnas.79.12.3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prystowsky M. B., Otten G., Naujokas M. F., Vardiman J., Ihle J. N., Goldwasser E., Fitch F. W. Multiple hemopoietic lineages are found after stimulation of mouse bone marrow precursor cells with interleukin 3. Am J Pathol. 1984 Nov;117(2):171–179. [PMC free article] [PubMed] [Google Scholar]
- Quesenberry P. J., Ihle J. N., McGrath E. The effect of interleukin 3 and GM-CSA-2 on megakaryocyte and myeloid clonal colony formation. Blood. 1985 Jan;65(1):214–217. [PubMed] [Google Scholar]
- Rennick D. M., Lee F. D., Yokota T., Arai K. I., Cantor H., Nabel G. J. A cloned MCGF cDNA encodes a multilineage hematopoietic growth factor: multiple activities of interleukin 3. J Immunol. 1985 Feb;134(2):910–914. [PubMed] [Google Scholar]
- Schrader J. W., Battye F., Scollay R. Expression of Thy-1 antigen is not limited to T cells in cultures of mouse hemopoietic cells. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4161–4165. doi: 10.1073/pnas.79.13.4161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader J. W., Clark-Lewis I. A T cell-derived factor stimulating multipotential hemopoietic stem cells: molecular weight and distinction from T cell growth factor and T cell-derived granulocyte-macrophage colony-stimulating factor. J Immunol. 1982 Jul;129(1):30–35. [PubMed] [Google Scholar]
- Silini G., Pons S., Pozzi L. V. Quantitative histology of spleen colonies in irradiated mice. Br J Haematol. 1968 May;14(5):489–500. doi: 10.1111/j.1365-2141.1968.tb07000.x. [DOI] [PubMed] [Google Scholar]
- Stanley E. R., Jubinsky P. T. Factors affecting the growth and differentiation of haemopoietic cells in culture. Clin Haematol. 1984 Jun;13(2):329–348. [PubMed] [Google Scholar]
- Suda T., Suda J., Ogawa M. Disparate differentiation in mouse hemopoietic colonies derived from paired progenitors. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2520–2524. doi: 10.1073/pnas.81.8.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suda T., Suda J., Ogawa M. Proliferative kinetics and differentiation of murine blast cell colonies in culture: evidence for variable G0 periods and constant doubling rates of early pluripotent hemopoietic progenitors. J Cell Physiol. 1983 Dec;117(3):308–318. doi: 10.1002/jcp.1041170305. [DOI] [PubMed] [Google Scholar]
- TILL J. E., McCULLOCH E. A. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res. 1961 Feb;14:213–222. [PubMed] [Google Scholar]
- Toksöz D., Dexter T. M., Lord B. I., Wright E. G., Lajtha L. G. The regulation of hemopoiesis in long-term bone marrow cultures. II. Stimulation and inhibition of stem cell proliferation. Blood. 1980 Jun;55(6):931–936. [PubMed] [Google Scholar]
- Williams D. A., Lemischka I. R., Nathan D. G., Mulligan R. C. Introduction of new genetic material into pluripotent haematopoietic stem cells of the mouse. Nature. 1984 Aug 9;310(5977):476–480. doi: 10.1038/310476a0. [DOI] [PubMed] [Google Scholar]