Abstract
Obesity is a major and growing health care concern. Large epidemiologic studies that evaluated the relationship between obesity and mortality, observed that a higher body-mass index (BMI) is associated with increased rate of death from several causes, among them cardiovascular disease; which is particularly true for those with morbid obesity. Being overweight was also associated with decreased survival in several studies. Unfortunately, obese subjects are often exposed to public disapproval because of their fatness which significantly affects their psychosocial behavior. All obese patients (BMI ≥ 30 kg/m2) should receive counseling on diet, lifestyle, exercise and goals for weight management. Individuals with BMI ≥ 40 kg/m2 and those with BMI > 35 kg/m2 with obesity-related comorbidities; who failed diet, exercise, and drug therapy, should be considered for bariatric surgery. In current review article, we will shed light on important medical principles that each surgeon/gastroenterologist needs to know about bariatric surgical procedure, with special concern to the early post operative period. Additionally, we will explain the common complications that usually follow bariatric surgery and elucidate medical guidelines in their management. For the first 24 h after the bariatric surgery, the postoperative priorities include pain management, leakage, nausea and vomiting, intravenous fluid management, pulmonary hygiene, and ambulation. Patients maintain a low calorie liquid diet for the first few postoperative days that is gradually changed to soft solid food diet within two or three weeks following the bariatric surgery. Later, patients should be monitored for postoperative complications. Hypertension, diabetes, dumping syndrome, gastrointestinal and psychosomatic disorders are among the most important medical conditions discussed in this review.
Keywords: Obesity, Bariatric surgery, Postoperative care, Body-mass index, El banna
Core tip: Obesity is a growing health concern worldwide that impacts the life of individuals both physically and psychologically. There are several well-established health hazards associated with obesity. Additionally, obese subjects are often exposed to public disapproval because of their fatness which significantly affects their psychosocial behavior. Bariatric surgery is one of the definite solutions for obesity. In this review, we will briefly discuss the general guidelines that should be considered before bariatric surgery. Also, we discuss the protocols of patients’ postoperative care and the management of medical disorders that must be considered after bariatric surgery.
INTRODUCTION
Obesity is a chronic disease that impairs health-related quality of life in adolescents and children. In 2010, overweight and obesity were estimated to cause 3.4 million deaths, 3.9% of years of life loss, and 3.8% of disability-adjusted life-years worldwide. Obesity is increasing in prevalence, currently, the proportion of adults with a body-mass index (BMI) of 25 kg/m2 or greater is 36.9% in men and 38.0% in women worldwide[1]. Attempts to explain the large increase in obesity in the past 30 years focused on several potential contributors including increase in caloric intake, changes in the composition of diet, decrease in the levels of physical activity and changes in the gut microbiome. More than 50% of the obese individuals in the world are located in ten countries (listed in order of number of obese individuals): United States, China, India, Russia, Brazil, Mexico, Egypt, Germany, Pakistan and Indonesia. Although age-standardized rates were lower in developing than in developed countries overall, 62% of the world’s obese individuals live in developing countries. Recently, United States accounted for 13% of obese people worldwide, the prevalence of obesity was 31.7% and 33.9% among adult men and women, respectively. In Canada 21.9% of men and 20.5% of women are obese. Reported prevalence rates of obesity include: 27.5% of men and 29.8% of women in Australia, 24.5% of men and 25.4% of women in the United Kingdom, in Germany 21.9% of men and 22.5% of women, in Mexico 20.6% of men and 32.7% of women, in South Africa 13.5% of men and 42% of women, in Egypt 26.4% of men and 48.4% of women, in Saudi Arabia 30% of men and 44.4% of women and in Kuwait 43.4% of men and 58.6% of women (Table 1, Figure 1)[2]. There are several well-established health hazards associated with obesity, e.g., nonalcoholic steatohepatitis (NASH), type 2 diabetes, heart disease, chronic kidney disease, gastroesophageal reflux disease, gastrointestinal motility disorders, sexual disorders, cerebrovascular stroke, certain cancers, osteoarthritis, depression and others[3-10]. The risk of development of such complications rises with the increase of adiposity, while weight loss can reduce the risk. Bariatric surgery could be the definitive clue in many situations[11-15]. Bariatric surgery is one of the fastest growing operative procedures performed worldwide, with an estimated > 340000 operations performed in 2011. While the absolute growth rate of bariatric surgery in Asia was 44.9% between 2005 and 2009, the numbers of procedures performed in the United States plateaued at approximately 200000 operations per year[16,17]. Starting in 2006, the Center for Medicare and Medicaid Services, United States, restricted the coverage of bariatric surgery to hospitals designated as “Centers of Excellence” by two major professional organizations[18]. Medical management and follow up of patients who have undergone bariatric surgery is a challenge due to post operative complications.
Table 1.
Prevalence of obesity in different countries worldwide
| Country | Male | Female |
| United States | 31.70% | 33.90% |
| Canada | 21.90% | 20.50% |
| United Kingdom | 24.50% | 25.40% |
| Australia | 27.50% | 29.80% |
| Germany | 21.90% | 22.50% |
| Mexico | 20.60% | 32.70% |
| South Africa | 13.50% | 42% |
| Egypt | 26.40% | 48.40% |
| Saudi Arabia | 30% | 44.40% |
| Kuwait | 43.40% | 58.60% |
Figure 1.
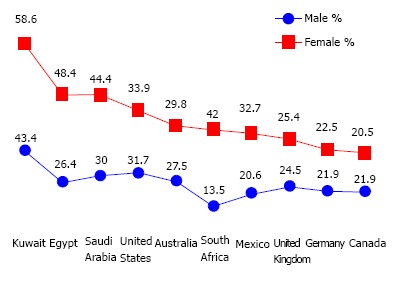
Male to female prevalence in different countries worldwide.
GENERAL GUIDELINES FOR SURGEONS/GASTROENTEROLOGISTS
A well skilled physician or a surgeon has to consider the followings: (1) as the prevalence of obesity increases so does the prevalence of the comorbidities associated with obesity. Losing weight means overcoming illness at the present, complications in future and alleviating the economic burden in the present and future; (2) Overweight; BMI between 25 and 30, technically refers to excessive body weight, whereas “obesity” BMI ≥ 30 kg/m2 refers excessive body fat, “Severe obesity”, BMI ≥ 35 kg/m2, or “morbid obesity” refers to individuals with obesity-related comorbidities. Furthermore, severe obesity and morbid obesity groups who failed dietary and medical regimens are candidates for bariatric surgery; (3) Children obesity; refers to children with BMI > 95th percentile for their age and sex and “overweight” refers to children with BMI between the 85th and 95th percentile for their age and sex; (4) Patients undergoing a bariatric operation should have a nutritional assessment for deficiencies in macro and micronutrients, also with no contraindication for such a major operation; (5) Most of bariatric procedures are performed in women (> 80%) and approximately half of these (> 40% of all bariatric procedures) are performed in reproductive aged women, accordingly, pregnancy planning and contraception options should be discussed in details with women who will undergo bariatric procedures. Fertility improves soon after bariatric surgery, particularly in middle-aged women, who were anovulatory. Additionally, oral contraceptives may be less effective in women who have undergone malabsorptive bariatric procedure. Therefore, it is better to delay pregnancy for 6-12 mo following bariatric surgery. Risk of preeclampsia, gestational diabetes, and macrosomia significantly decrease post bariatric surgery, but the risk of intrauterine growth restriction/small infants for their gestational age may increase. Body contouring surgery is in high demand following bariatric surgery; (6) All bariatric operations are accompanied with restrictive and/or malabsorption maneuvers; less food intake and malabsorption concepts; (7) The most common types of bariatric surgeries performed worldwide are Sleeve gastrectomy (SG): This procedure involves the longitudinal excision of the stomach and thus shaping the remaining part of the stomach into a tube or a “sleeve” like structure. SG removes almost 85% of the stomach (Figure 2); Roux-en-Y gastric bypass (RYGB): It reduces the size of the stomach to the size of a small pouch that is directly surgically attached to the lower part of the small intestine. In this procedure, most of the stomach and the duodenum are surgically stapled and therefore, bypassed (Figure 3); The laparoscopic adjustable gastric band (AGB): This is one of the least invasive procedures, where the surgeon inserts an adjustable band around a portion of the stomach and therefore, patients feel fuller after eating smaller food portions (Figure 4). Bariatric surgical procedures, particularly RYGB, plus medical therapy, are effective interventions for treating type 2 diabetes. Improvement in metabolic control is often evident within days to weeks following RYGB; and (8) Complications reported following bariatric surgery vary based upon the procedure performed. Cholilithiasis, renal stone formation and incisional hernia could be the delayed phase complications; on the other hand, bleeding, leaking, infection and pulmonary embolism could be the early phase complications following the bariatric procedure. The overall 30-d mortality for bariatric surgical procedures worldwide is less than 1%.
Figure 2.
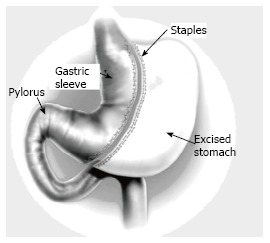
Schematic presentation of sleeve gastrectomy.
Figure 3.
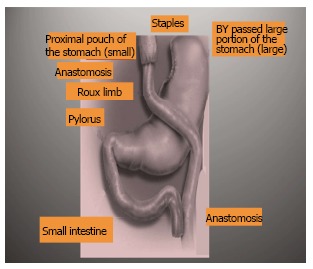
Schematic presentation of Roux-in Y Gastrectomy.
Figure 4.
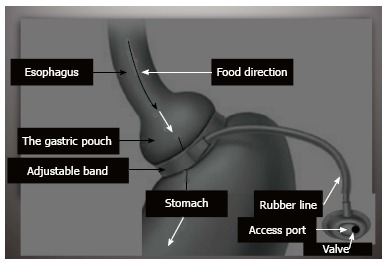
Schematic presentation of adjustable gastric band.
POST OPERATIVE CARE AND FOLLOW UP
Early post operative period; (1-3) d post bariatric surgery
Patients undergoing a bariatric operation are admitted to the post-anesthesia care unit (PACU) immediately at the conclusion of the operation. Usually, on postoperative day (POD) one, we begin oral therapy in tablet or crushed-tablet and liquid form if there is a naso-gastric tube after the gastrografin leak test. A basic metabolic profile (e.g., complete blood count, electrolytes, renal function, liver function, prothrombin time and partial thromboplastin time) should be obtained every 12 h for the successive two PODs, then every 24 h for another 3 d. Oxygen is administered by nasal cannula and weaned thereafter. The likelihood that, early specific complication, will arise for a given patient is determined by the nature of the procedure, the anesthetic techniques used, and the patient’s preoperative diseases. Respiratory problems are common complication in the early postoperative period following bariatric surgery. Patients with significant comorbidities, particularly neuromuscular, pulmonary, or cardiac problems are at a higher risk for respiratory compromise, but any patient can develop hypoxemia following bariatric surgery. For prophylaxis against Deep Venous Thrombosis (DVT) following bariatric surgeries, ultrasound evaluation is recommended for all patients, D-dimer test should be applied for suspected patients with DVT, especially after long operative time, repeat ultrasound or venography may be required for those with suspected calf vein DVT and a negative initial ultrasound investigation[19,20].
Late post operative monitoring
After the PACU period, most patients are transferred to the inpatient surgical postoperative unit. For the next 24-72 h, the postoperative priorities include ruling out an anastomotic leak following laparoscopic RYGB or laparoscopic SG. If no leak is observed, patients are allowed to start a clear liquid diet and soft drinks. The postoperative care team cares for the following: control of pain, care of the wound, continuous monitoring of blood pressure, intravenous fluid management, pulmonary hygiene, and ambulation. Post-bariatric nausea and vomiting is directly correlated with the length of the surgery; it also increases in females, non-smokers, and those patients with prior history of vomiting or motion sickness. Prophylaxis with pharmacologic treatment before the development of post operative nausea and vomiting significantly reduces its incidence after surgery[21-23].
After hospital discharge
Diet: Usually patients are discharged 4-6 d after surgery. Most patients are typically discharged from the hospital on a full liquid diet, patients should be taught to keep monitoring their hydration and urine output. Approximately two-three weeks after surgery, the diet is gradually changed to soft, solid foods. The average caloric intake ranges from (400) to (800) kcal/d for the first month, and thus the daily glycemic load is greatly reduced. We encourage patients to consume a diet consisting of salads, fruits, vegetables and soft protein daily.
To control the epigastric pain and vomiting, patients should be taught to eat slowly, to stop eating as soon as they reach satiety and not to consume food and beverages at the same time. For most patients suffering chronic vomiting, prokinetic therapy and proton-pump inhibitors (PPIs) should be considered. Patients, who underwent SG, LAGB or RYGB, benefit from a well-planned dietary advancement. Patients should understand that the surgery has changed their body but not the environment, they have to choose healthy foods, do not skip meals and to visit the dietitian regularly in the first 12 mo after surgery. However, if food intolerance develops, patients may choose a more vegetarian-based diet. Nevertheless, fresh fruits and vegetables are usually tolerated without a problem. The daily protein intake should be between 1.0 to 1.5 g/kg ideal body weight per day[24]. The biliopancreatic diversion/duodenal switch (BPD/DS) is a malabsorptive procedure for both macro- and micronutrients. Hence, we encourage higher protein intake of 1.5 g to 2.0 g of protein/kg ideal body weight per day, making the average protein requirement per day approximately 90 g/d[25,26]. Alcohol is better prevented in the first 6-12 mo after surgery[27].
Monitoring: Patients should generally have their weight and blood pressure measured weekly until the rapid weight loss phase diminishes, usually within 4-6 mo, then again at 8, 10 and 12 mo, and annually thereafter. Patients with diabetes are encouraged to check their blood glucose daily. Glycemic control typically improves rapidly following bariatric surgery. Patients maintained on antihypertensive or diabetic medications at discharge should be monitored closely for hypotension and hypoglycemia, respectively, and medications should be adjusted accordingly. We recommend that the following laboratory tests be performed at three, six, nine months and annually thereafter: (1) Complete Blood Count; (2) Electrolytes; (3) Glucose and Glucose Tolerance test; (4) Complete iron studies; (5) Vitamin B12; (6) Aminotransferases, alkaline phosphatase, bilirubin, GGT; (7) Total protein and Albumin; (8) Complete lipid profile; (9) 25-hydroxyvitamin D, parathyroid hormone; (10)Thiamine; (11) Folate; (12) Zinc; and (13) Copper.
Complications following the surgical treatment of severe obesity vary based upon the procedure performed. Secondary hyperparathyroidism, Hypocalcemia, Gastric remnant distension, Stomal stenosis/Obstruction, Marginal ulcerations, Cholilithiasis, Ventral incisional hernia, Internal hernia, Hiatus Hernia, Short bowel syndrome, Renal failure, Gastric prolapse, infection, Esophagitis, Reflux, Vomiting, Hepatic abnormalities and dumping syndrome are common late-phase complications after bariatric surgery. However, the clinician should aware of complications specific for every bariatric procedure[28,29]. Before therapy, the clinician should understand that the impact of various bariatric surgeries on drug absorption and metabolism are scarce. On the other hand, RYGB and other malabsorptive procedures that significantly exclude the proximal part of the small intestine, decrease the surface area where most drug absorption occurs and may result in a reduction in systemic bioavailability[30-32].
COMMON MEDICAL CONDITIONS FOLLOWING BARIATRIC SURGERY
Hypertension
Hypertension is not always related to obesity, and dietary interventions do not assure the normalization of blood pressure. However weight loss, whether by an intensive lifestyle medical modification program or by a bariatric operation, improves obesity-linked hypertension. Patients should be monitored weekly until the blood pressure has stabilized, and patients may need to resume antihypertensive medications, but often at adjusted doses[33].
Diabetes
Patients with diabetes should have frequent monitoring of blood glucose in the early postoperative period and should be managed with sliding scale insulin. Many diabetic patients have a decreased need for insulin and oral hypoglycemic agents after bariatric surgery. Oral sulfonylureas and meglitinides should be discontinued postoperatively as these medications can lead to hypoglycemia after bariatric surgery. Metformin is the safest oral drug in the postoperative period, since it is not associated with dramatic fluctuations in blood glucose. RYGB is associated with durable remission of type 2 diabetes in many, but not all, severely obese diabetic adults. However those who underwent LAGB generally exhibit a slower improvement in glucose metabolism and diabetes as they lose weight in a gradual fashion[34,35].
Reflux
Medications for gastroesophageal reflux disease (GERD) may be discontinued after RYGB and Laparoscopic AGB, however, SG has been associated with an increased incidence of GERD in some procedures. Recurrent GERD symptoms after RYGB, particularly when accompanied by weight regain, should raise the possibility of a gastrogastric fistula between the gastric pouch and remnant, and should be investigated by an upper GI contrast study or CT scan and referred to the bariatric surgeon. Upper endoscopy is the best investigation to exclude other esophagogastroduodenal disorders. GERD may be associated with esophageal complications including esophagitis, peptic stricture, Barrett’s metaplasia, esophageal cancer and other pulmonary complications. Failure of the PPI treatment to resolve GERD-related symptoms has become one of the most common complications of GERD after bariatric surgery. Most patients who fail PPI treatment have Non Erosive Reflux Disease and without pathological reflux on pH testing. In patients with persistent heartburn despite of medical therapy, it is reasonable to recommend avoidance of specific lifestyle activities that have been identified by patients or physicians to trigger GERD-related symptoms[36-38].
Nausea and vomiting
Nausea and vomiting can often be helped by antiemetic or prokinetic drugs, however, some patients have chronic functional nausea and/or vomiting that does not fit the pattern of cyclic vomiting syndrome or other gastrointestinal disorders, hence particular attention should be directed to potential psychosocial factors post bariatric surgery. Therefore, low dose antidepressant medications and psychotherapy should be addressed. On demand CT scan and Gastroscopy could be the gold standard investigations in chronic situations[39,40].
Marginal ulceration
Due to increased risk of ulcer formation from nonsteroidal anti-inflammatory drugs (NSAIDs), these medications should be discontinued postoperatively, especially after RYGB. NSAID use is associated with an increased risk of bleeding. If analgesic or anti-inflammatory treatment is needed, the use of acetaminophen is preferred in a dose of 1-2 g/daily[41-45]. Other factors associated with increased risk of ulcer formation are smoking, alcohol, spicy food, gastrogastric fistulas, ischemia at the site of surgical anastomosis, poor tissue perfusion due to tension, presence of foreign material, such as staples and/or Helicobacter pylori infection. Diagnosis is established by upper endoscopy. According to our strategy, all patients should undergo diagnostic upper endoscopy to exclude congenital or GI diseases prior to bariatric procedures. Medical management is usually successful and surgical intervention is rarely needed[46-48].
DUMPING SYNDROME
Dumping syndrome or rapid gastric emptying is a group of symptoms that most likely occur following bariatric bypass. It occurs when the undigested contents of the stomach move too rapidly into the small intestine. Many patients who underwent bariatric bypass experienced postprandial hypoglycemia. However, the dumping syndrome usually occurs early (within one hour) after eating and is not associated with hypoglycemia. It is presumed to be caused by contraction of the plasma volume due to fluid shifts into the gastrointestinal tract. Dumping syndrome may result in tachycardia, abdominal pain, diaphoresis, nausea, vomiting, diarrhea, and sometimes, hypoglycemia. The late dumping syndrome is a result of the hyperglycemia and the subsequent insulin response leading to hypoglycemia that occurs around 2-3 h after a meal. Dumping syndrome is a common problem that occurs in patients who have undergone RYGB and when high levels of simple carbohydrates are ingested. Accordingly, patients who have experienced postgastric bypass bariatric surgery should avoid foods that are high in simple sugar content and replace them with a diet consisting of high fiber and protein rich food. Eating vegetables and salad is encouraged; beverages and alcohol consumption are better avoided[49].
PSYCHOSOMATIC DISORDERS/DEPRESSION
Many patients usually experience enhanced self esteem and improved situational depression following weight loss. Depression often requires continued treatment, specially that, many patients with severe obesity often use food for emotional reasons. Therefore, when those patients experience a small gastric pouch postoperatively they may grieve the loss of food. Many studies documented the relationship between eating disorder and anxiety disorder, depression or schizophrenia[50,51]. Displaced emotions can result in somatization with symptoms of depression and psychosomatic disorders. It is important that clinicians recognize the psychological aspect of food loss after bariatric surgery, and reassure patients that the symptoms are related to the small gastric pouch size. Antidepressants often help to decrease the anxiety related to grieving associated with food loss, although the use of antidepressants needs to be approached with an empathetic style. Behavioral and emotive therapies are reported to be very helpful[52,53].
OUTCOME
Bariatric surgery remains the only effective sustained weight loss option for morbidly obese patients. The American Society for Metabolic and Bariatric Surgery estimated that in 2008 alone, about 220000 patients in the United States underwent a weight loss operation. The optimal choice for type of bariatric procedure, i.e., RYGB, SG, AGB or the selected surgical approach, i.e., open versus laparoscopic depends upon each individualized goals, i.e., weight loss, glycemic control, surgical skills, center experience, patient preferences, personalized risk assessment and other medical facilities. Laparoscopic sleeve gastrectomy is the most common bariatric procedure. However weight re-gain after long-term follow-up was reported[54-58]. Prospective studies and reviews report a general tendency for patients with metabolic disorders to improve or normalize after bariatric surgery. However weight loss is highly variable following each procedure. Recent studies have evaluated the potential impact of obesity on outcomes in organ-transplant recipients, for example bariatric surgery may be an important bridge to transplantation for morbidly obese patients with severe heart failure[59-63].
RECENT ADVANCES IN BARIATRIC SURGERY
A modified intestinal bypass bariatric procedure (Elbanna operation), reported a novel surgical technique designed to maintain good digestion, better satiety, and selective absorption with less medical and surgical complications (Figure 5). This procedure preserves the proximal duodenum and the terminal ileum and thus preserving the anatomical biliary drainage and enterohepatic circulation[64,65].
Figure 5.
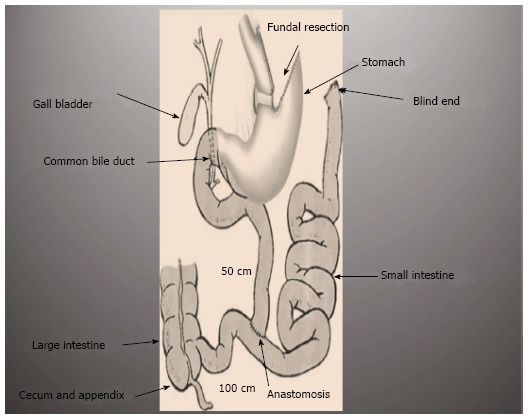
Novel ElBanna surgical procedure.
Recently, a novel bariatric technique dedicated; Modified Elbanna technique in childhood bariatric, showed promising success in pediatric surgeries (non published data).
CONCLUSION
The rising prevalence of overweight and obesity in several countries has been described as a global pandemic. Obesity can be considered like the driving force towards the pre-mature deaths. It increases the like hood for the development of diabetes, hypertension and NASH. The American Heart Association identified obesity as an independent risk factor for the development of coronary heart disease. In order to minimize post-surgical cardiovascular risk, surgical weight loss may become a more frequently utilized option to address obesity. Currently, bariatric surgery passes through a plateau phase, hence medical management and follow up of patients who have undergone bariatric surgery is a challenge.
FUTURE RECOMMENDATIONS
Children obesity has become one of the most important public health problems in many industrial countries. In the United States alone, 5% of children have severe obesity. It is imperative that health care providers should identify overweight and obese children so as to start early counseling and therapy. To establish a therapeutic relationship and enhance effectiveness, the communication and interventions should be supported by the entire family, society, school, public media and primary health care. Bariatric surgery could be considered in complicated cases that failed all other options.
Footnotes
P- Reviewer: Amiya E, Firstenberg MS, Narciso-Schiavon JL S- Editor: Tian YL L- Editor: A E- Editor: Lu YJ
References
- 1.Schauer PR, Kashyap SR, Wolski K, Brethauer SA, Kirwan JP, Pothier CE, Thomas S, Abood B, Nissen SE, Bhatt DL. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366:1567–1576. doi: 10.1056/NEJMoa1200225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, Mullany EC, Biryukov S, Abbafati C, Abera SF, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hogan MC, Foreman KJ, Naghavi M, Ahn SY, Wang M, Makela SM, Lopez AD, Lozano R, Murray CJ. Maternal mortality for 181 countries, 1980-2008: a systematic analysis of progress towards Millennium Development Goal 5. Lancet. 2010;375:1609–1623. doi: 10.1016/S0140-6736(10)60518-1. [DOI] [PubMed] [Google Scholar]
- 4.Rajaratnam JK, Marcus JR, Flaxman AD, Wang H, Levin-Rector A, Dwyer L, Costa M, Lopez AD, Murray CJ. Neonatal, postneonatal, childhood, and under-5 mortality for 187 countries, 1970-2010: a systematic analysis of progress towards Millennium Development Goal 4. Lancet. 2010;375:1988–2008. doi: 10.1016/S0140-6736(10)60703-9. [DOI] [PubMed] [Google Scholar]
- 5.Bleich S, Cutler D, Murray C, Adams A. Why is the developed world obese? Annu Rev Public Health. 2008;29:273–295. doi: 10.1146/annurev.publhealth.29.020907.090954. [DOI] [PubMed] [Google Scholar]
- 6.Food and Agriculture Organization Corporate Statistical Database. Food balance sheets. Available from: http://faostat3.fao.org/faostat-gateway/go/to/home/E.
- 7.UN Department of Economic and Social Affairs, Population Division. World population prospects: the 2010 revision. Volume 1: Comprehensive tables. New York: United Nations; 2011. [Google Scholar]
- 8.Astrup A, Brand-Miller J. Diet composition and obesity. Lancet. 2012;379:1100; author reply 1100–1101. doi: 10.1016/S0140-6736(12)60456-5. [DOI] [PubMed] [Google Scholar]
- 9.Drewnowski A, Popkin BM. The nutrition transition: new trends in the global diet. Nutr Rev. 1997;55:31–43. doi: 10.1111/j.1753-4887.1997.tb01593.x. [DOI] [PubMed] [Google Scholar]
- 10.Briefel RR, Johnson CL. Secular trends in dietary intake in the United States. Annu Rev Nutr. 2004;24:401–431. doi: 10.1146/annurev.nutr.23.011702.073349. [DOI] [PubMed] [Google Scholar]
- 11.Swinburn B, Sacks G, Ravussin E. Increased food energy supply is more than sufficient to explain the US epidemic of obesity. Am J Clin Nutr. 2009;90:1453–1456. doi: 10.3945/ajcn.2009.28595. [DOI] [PubMed] [Google Scholar]
- 12.Popkin BM. The nutrition transition and obesity in the developing world. J Nutr. 2001;131:871S–873S. doi: 10.1093/jn/131.3.871S. [DOI] [PubMed] [Google Scholar]
- 13.Ng M, Freeman MK, Fleming TD, Robinson M, Dwyer-Lindgren L, Thomson B, Wollum A, Sanman E, Wulf S, Lopez AD, et al. Smoking prevalence and cigarette consumption in 187 countries, 1980-2012. JAMA. 2014;311:183–192. doi: 10.1001/jama.2013.284692. [DOI] [PubMed] [Google Scholar]
- 14.Ben-Menachem T. Risk factors for cholangiocarcinoma. Eur J Gastroenterol Hepatol. 2007;19:615–617. doi: 10.1097/MEG.0b013e328224b935. [DOI] [PubMed] [Google Scholar]
- 15.Younossi ZM, Stepanova M, Negro F, Hallaji S, Younossi Y, Lam B, Srishord M. Nonalcoholic fatty liver disease in lean individuals in the United States. Medicine (Baltimore) 2012;91:319–327. doi: 10.1097/MD.0b013e3182779d49. [DOI] [PubMed] [Google Scholar]
- 16.American Society for Metabolic and Bariatric Surgery. Fact Sheet: Metabolic and Bariatric Surgery. Available from: http://www.asbs.org/ Newsite07/media/asbs_presskit.htm.
- 17.Nguyen NT, Masoomi H, Magno CP, Nguyen XM, Laugenour K, Lane J. Trends in use of bariatric surgery, 2003-2008. J Am Coll Surg. 2011;213:261–266. doi: 10.1016/j.jamcollsurg.2011.04.030. [DOI] [PubMed] [Google Scholar]
- 18.Dimick JB, Nicholas LH, Ryan AM, Thumma JR, Birkmeyer JD. Bariatric surgery complications before vs after implementation of a national policy restricting coverage to centers of excellence. JAMA. 2013;309:792–799. doi: 10.1001/jama.2013.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen KN. Managing complications I: leaks, strictures, emptying, reflux, chylothorax. J Thorac Dis. 2014;6 Suppl 3:S355–S363. doi: 10.3978/j.issn.2072-1439.2014.03.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mechanick JI, Youdim A, Jones DB, Garvey WT, Hurley DL, McMahon MM, Heinberg LJ, Kushner R, Adams TD, Shikora S, et al. Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient--2013 update: cosponsored by American Association of Clinical Endocrinologists, The Obesity Society, and American Society for Metabolic & amp; Bariatric Surgery. Obesity (Silver Spring) 2013;21 Suppl 1:S1–27. doi: 10.1002/oby.20461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tucker ON, Szomstein S, Rosenthal RJ. Nutritional consequences of weight-loss surgery. Med Clin North Am. 2007;91:499–514, xii. doi: 10.1016/j.mcna.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 22.Sjöström L, Peltonen M, Jacobson P, Sjöström CD, Karason K, Wedel H, Ahlin S, Anveden Å, Bengtsson C, Bergmark G, et al. Bariatric surgery and long-term cardiovascular events. JAMA. 2012;307:56–65. doi: 10.1001/jama.2011.1914. [DOI] [PubMed] [Google Scholar]
- 23.Bouldin MJ, Ross LA, Sumrall CD, Loustalot FV, Low AK, Land KK. The effect of obesity surgery on obesity comorbidity. Am J Med Sci. 2006;331:183–193. doi: 10.1097/00000441-200604000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Schweiger C, Weiss R, Keidar A. Effect of different bariatric operations on food tolerance and quality of eating. Obes Surg. 2010;20:1393–1399. doi: 10.1007/s11695-010-0233-9. [DOI] [PubMed] [Google Scholar]
- 25.Ortega J, Ortega-Evangelio G, Cassinello N, Sebastia V. What are obese patients able to eat after Roux-en-Y gastric bypass? Obes Facts. 2012;5:339–348. doi: 10.1159/000339769. [DOI] [PubMed] [Google Scholar]
- 26.Nelson WK, Fatima J, Houghton SG, Thompson GB, Kendrick ML, Mai JL, Kennel KA, Sarr MG. The malabsorptive very, very long limb Roux-en-Y gastric bypass for super obesity: results in 257 patients. Surgery. 2006;140:517–522, discussion 522-523. doi: 10.1016/j.surg.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 27.Shen Z, Li Y, Yu C, Shen Y, Xu L, Xu C, Xu G. A cohort study of the effect of alcohol consumption and obesity on serum liver enzyme levels. Eur J Gastroenterol Hepatol. 2010;22:820–825. doi: 10.1097/MEG.0b013e3283328b86. [DOI] [PubMed] [Google Scholar]
- 28.Koenig SM. Pulmonary complications of obesity. Am J Med Sci. 2001;321:249–279. doi: 10.1097/00000441-200104000-00006. [DOI] [PubMed] [Google Scholar]
- 29.Holes-Lewis KA, Malcolm R, O’Neil PM. Pharmacotherapy of obesity: clinical treatments and considerations. Am J Med Sci. 2013;345:284–288. doi: 10.1097/MAJ.0b013e31828abcfd. [DOI] [PubMed] [Google Scholar]
- 30.Sakcak I, Avsar FM, Cosgun E, Yildiz BD. Management of concurrent cholelithiasis in gastric banding for morbid obesity. Eur J Gastroenterol Hepatol. 2011;23:766–769. doi: 10.1097/MEG.0b013e3283488adb. [DOI] [PubMed] [Google Scholar]
- 31.Herrara MF, Lozano-Salazar RR, González-Barranco J, Rull JA. Diseases and problems secondary to massive obesity. Eur J Gastroenterol Hepatol. 1999;11:63–67. doi: 10.1097/00042737-199902000-00002. [DOI] [PubMed] [Google Scholar]
- 32.Lassailly G, Caiazzo R, Hollebecque A, Buob D, Leteurtre E, Arnalsteen L, Louvet A, Pigeyre M, Raverdy V, Verkindt H, et al. Validation of noninvasive biomarkers (FibroTest, SteatoTest, and NashTest) for prediction of liver injury in patients with morbid obesity. Eur J Gastroenterol Hepatol. 2011;23:499–506. doi: 10.1097/MEG.0b013e3283464111. [DOI] [PubMed] [Google Scholar]
- 33.Hofsø D, Nordstrand N, Johnson LK, Karlsen TI, Hager H, Jenssen T, Bollerslev J, Godang K, Sandbu R, Røislien J, et al. Obesity-related cardiovascular risk factors after weight loss: a clinical trial comparing gastric bypass surgery and intensive lifestyle intervention. Eur J Endocrinol. 2010;163:735–745. doi: 10.1530/EJE-10-0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Service GJ, Thompson GB, Service FJ, Andrews JC, Collazo-Clavell ML, Lloyd RV. Hyperinsulinemic hypoglycemia with nesidioblastosis after gastric-bypass surgery. N Engl J Med. 2005;353:249–254. doi: 10.1056/NEJMoa043690. [DOI] [PubMed] [Google Scholar]
- 35.Arterburn DE, Bogart A, Sherwood NE, Sidney S, Coleman KJ, Haneuse S, O’Connor PJ, Theis MK, Campos GM, McCulloch D, et al. A multisite study of long-term remission and relapse of type 2 diabetes mellitus following gastric bypass. Obes Surg. 2013;23:93–102. doi: 10.1007/s11695-012-0802-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fass R, Shapiro M, Dekel R, Sewell J. Systematic review: proton-pump inhibitor failure in gastro-oesophageal reflux disease--where next? Aliment Pharmacol Ther. 2005;22:79–94. doi: 10.1111/j.1365-2036.2005.02531.x. [DOI] [PubMed] [Google Scholar]
- 37.Löfdahl HE, Lane A, Lu Y, Lagergren P, Harvey RF, Blazeby JM, Lagergren J. Increased population prevalence of reflux and obesity in the United Kingdom compared with Sweden: a potential explanation for the difference in incidence of esophageal adenocarcinoma. Eur J Gastroenterol Hepatol. 2011;23:128–132. doi: 10.1097/MEG.0b013e3283424e25. [DOI] [PubMed] [Google Scholar]
- 38.Fornari F, Madalosso CA, Farré R, Gurski RR, Thiesen V, Callegari-Jacques SM. The role of gastro-oesophageal pressure gradient and sliding hiatal hernia on pathological gastro-oesophageal reflux in severely obese patients. Eur J Gastroenterol Hepatol. 2010;22:404–411. doi: 10.1097/MEG.0b013e328332f7b8. [DOI] [PubMed] [Google Scholar]
- 39.Aasheim ET. Wernicke encephalopathy after bariatric surgery: a systematic review. Ann Surg. 2008;248:714–720. doi: 10.1097/SLA.0b013e3181884308. [DOI] [PubMed] [Google Scholar]
- 40.Salgado W, Modotti C, Nonino CB, Ceneviva R. Anemia and iron deficiency before and after bariatric surgery. Surg Obes Relat Dis. 2014;10:49–54. doi: 10.1016/j.soard.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 41.Klockhoff H, Näslund I, Jones AW. Faster absorption of ethanol and higher peak concentration in women after gastric bypass surgery. Br J Clin Pharmacol. 2002;54:587–591. doi: 10.1046/j.1365-2125.2002.01698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maluenda F, Csendes A, De Aretxabala X, Poniachik J, Salvo K, Delgado I, Rodriguez P. Alcohol absorption modification after a laparoscopic sleeve gastrectomy due to obesity. Obes Surg. 2010;20:744–748. doi: 10.1007/s11695-010-0136-9. [DOI] [PubMed] [Google Scholar]
- 43.Woodard GA, Downey J, Hernandez-Boussard T, Morton JM. Impaired alcohol metabolism after gastric bypass surgery: a case-crossover trial. J Am Coll Surg. 2011;212:209–214. doi: 10.1016/j.jamcollsurg.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 44.King WC, Chen JY, Mitchell JE, Kalarchian MA, Steffen KJ, Engel SG, Courcoulas AP, Pories WJ, Yanovski SZ. Prevalence of alcohol use disorders before and after bariatric surgery. JAMA. 2012;307:2516–2525. doi: 10.1001/jama.2012.6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sasse KC, Ganser J, Kozar M, Watson RW, McGinley L, Lim D, Weede M, Smith CJ, Bovee V. Seven cases of gastric perforation in Roux-en-Y gastric bypass patients: what lessons can we learn? Obes Surg. 2008;18:530–534. doi: 10.1007/s11695-007-9335-4. [DOI] [PubMed] [Google Scholar]
- 46.Capella JF, Capella RF. Gastro-gastric fistulas and marginal ulcers in gastric bypass procedures for weight reduction. Obes Surg. 1999;9:22–27; discussion 28. doi: 10.1381/096089299765553674. [DOI] [PubMed] [Google Scholar]
- 47.Abd Elrazek AE, Mahfouz HM, Metwally AM, El-Shamy AM. Mortality prediction of nonalcoholic patients presenting with upper gastrointestinal bleeding using data mining. Eur J Gastroenterol Hepatol. 2014;26:187–191. doi: 10.1097/MEG.0b013e328365c3b0. [DOI] [PubMed] [Google Scholar]
- 48.Abd Elrazek AE, Yoko N, Hiroki M, Afify M, Asar M, Ismael B, Salah M. Endoscopic management of Dieulafoy’s lesion using Isoamyl-2-cyanoacrylate. World J Gastrointest Endosc. 2013;5:417–419. doi: 10.4253/wjge.v5.i8.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ukleja A. Dumping syndrome: pathophysiology and treatment. Nutr Clin Pract. 2005;20:517–525. doi: 10.1177/0115426505020005517. [DOI] [PubMed] [Google Scholar]
- 50.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA. 2012;307:491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 51.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 52.García-García ML, Martín-Lorenzo JG, Campillo-Soto A, Torralba-Martínez JA, Lirón-Ruiz R, Miguel-Perelló J, Mengual-Ballester M, Aguayo-Albasini JL. [Complications and level of satisfaction after dermolipectomy and abdominoplasty post-bariatric surgery] Cir Esp. 2014;92:254–260. doi: 10.1016/j.ciresp.2013.04.024. [DOI] [PubMed] [Google Scholar]
- 53.Wyatt SB, Winters KP, Dubbert PM. Overweight and obesity: prevalence, consequences, and causes of a growing public health problem. Am J Med Sci. 2006;331:166–174. doi: 10.1097/00000441-200604000-00002. [DOI] [PubMed] [Google Scholar]
- 54.Lamers F, van Oppen P, Comijs HC, Smit JH, Spinhoven P, van Balkom AJ, Nolen WA, Zitman FG, Beekman AT, Penninx BW. Comorbidity patterns of anxiety and depressive disorders in a large cohort study: the Netherlands Study of Depression and Anxiety (NESDA) J Clin Psychiatry. 2011;72:341–348. doi: 10.4088/JCP.10m06176blu. [DOI] [PubMed] [Google Scholar]
- 55.de Graaf R, Bijl RV, Smit F, Vollebergh WA, Spijker J. Risk factors for 12-month comorbidity of mood, anxiety, and substance use disorders: findings from the Netherlands Mental Health Survey and Incidence Study. Am J Psychiatry. 2002;159:620–629. doi: 10.1176/appi.ajp.159.4.620. [DOI] [PubMed] [Google Scholar]
- 56.Cesana G, Uccelli M, Ciccarese F, Carrieri D, Castello G, Olmi S. Laparoscopic re-sleeve gastrectomy as a treatment of weight regain after sleeve gastrectomy. World J Gastrointest Surg. 2014;6:101–106. doi: 10.4240/wjgs.v6.i6.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee WJ, Ser KH, Chong K, Lee YC, Chen SC, Tsou JJ, Chen JC, Chen CM. Laparoscopic sleeve gastrectomy for diabetes treatment in nonmorbidly obese patients: efficacy and change of insulin secretion. Surgery. 2010;147:664–669. doi: 10.1016/j.surg.2009.10.059. [DOI] [PubMed] [Google Scholar]
- 58.Mechanick JI, Youdim A, Jones DB, Timothy Garvey W, Hurley DL, Molly McMahon M, Heinberg LJ, Kushner R, Adams TD, Shikora S, et al. Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient--2013 update: cosponsored by American Association of Clinical Endocrinologists, the Obesity Society, and American Society for Metabolic & amp; Bariatric Surgery. Surg Obes Relat Dis. 2013;9:159–191. doi: 10.1016/j.soard.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 59.Adams PL. Long-term patient survival: strategies to improve overall health. Am J Kidney Dis. 2006;47:S65–S85. doi: 10.1053/j.ajkd.2005.12.043. [DOI] [PubMed] [Google Scholar]
- 60.Gore JL, Pham PT, Danovitch GM, Wilkinson AH, Rosenthal JT, Lipshutz GS, Singer JS. Obesity and outcome following renal transplantation. Am J Transplant. 2006;6:357–363. doi: 10.1111/j.1600-6143.2005.01198.x. [DOI] [PubMed] [Google Scholar]
- 61.Meier-Kriesche HU, Arndorfer JA, Kaplan B. The impact of body mass index on renal transplant outcomes: a significant independent risk factor for graft failure and patient death. Transplantation. 2002;73:70–74. doi: 10.1097/00007890-200201150-00013. [DOI] [PubMed] [Google Scholar]
- 62.Wikiel KJ, McCloskey CA, Ramanathan RC. Bariatric surgery: a safe and effective conduit to cardiac transplantation. Surg Obes Relat Dis. 2014;10:479–484. doi: 10.1016/j.soard.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 63.DiCecco SR, Francisco-Ziller N. Obesity and organ transplantation: successes, failures, and opportunities. Nutr Clin Pract. 2014;29:171–191. doi: 10.1177/0884533613518585. [DOI] [PubMed] [Google Scholar]
- 64.Elbanna A, Tawella N, Neff K, Abd Elfattah A, Bakr I. Abstracts from the 18th World Congress of the International Federation for the Surgery of Obesity & Metabolic Disorders (IFSO), Istanbul, Turkey 28-31 August 2013. Obes Surg. 2013;23:1017–1243. [Google Scholar]
- 65.Elbanna A, Taweela NH, Gaber MB, Tag El-Din MM, Labib MF, Emam MA, Khalil OO, Abdel Meguid MM, Abd Elrazek MAA. Medical Management of Patients with Modified Intestinal Bypass: A New Promising Procedure for Morbid Obesity. GJMR. 2014;14:8–19. [Google Scholar]


