Abstract
Objective:
To establish different stages of silicosis rat model complicated with tuberculosis infection, and compare the pathological characteristics and analyze the impact of silicosis on tuberculosis infection.
Methods:
SD rats were subjected to intratracheal administration of silica with non-exposure method at the 1st, 30th, or 60th day. At the 50th day, the rats were injected with the suspension of H37Rv (a virulent standard strain of Mycobacterium tuberculosis) via tail-vein. After 40 days post-infection, rats were sacrificed, the lung tissues were isolated, and paraffin was embedded and sectioned. The sections were treated using HE staining for structure observation, acid fast stain of Ziehl–Neelsen for bacterial detection, and Warthin–Starry silver staining for displaying the distribution of dust particles. The bacterial load was quantified by colony counting.
Results:
Primary to tertiary silicosis could be discovered at the 30th, 60th, and 90th day of post-infection. The rats could be infected by injection of M. tuberculosis via tail vein, with tuberculosis load and the degree of lung tissue lesions positively correlated with silicosis.
Conclusion:
The rat model of silicotuberculosis was established successfully, which facilitated understanding the ‘cross-talk’ of silicosis and tuberculosis during the process they drive each other.
Keywords: Silicotuberculosis, Models, Pulmonary fibrosis, Pathology, tuberculosis
Introduction
Patients with silicosis are at increased risk of being infected with Mycobacterium tuberculosis (MTB), with the infection rate of about 20% in patients of primary silicosis, 30% in that of secondary silicosis, and up to 70% in patients of tertiary silicosis. China had 14 495 new cases of silicosis in 2009, among them 748 died. The rate of complication with tuberculosis, silicotuberculosis, is up to 9.7%. So, silicotuberculosis remains a serious disease in China. Compared with single TB infection, silicotuberculosis develops faster and the lesions are more extensive. Moreover, being difficult to diagnosis, with low curative effect and poor prognosis in clinical treatment, silicotuberculosis has received considerable attention.1,2 However, the pathogenesis of the disease still remains unclear, and there is no specific treatment. It is of significance to establish animal models for pathogenesis research and drug screening.3 In the present study, rat model of silicotuberculosis was established by intratracheal administration of silica and injection of H37Rv via tail-vein4 and the pathological characteristic of MTB and silicosis at different stages was analyzed.
Materials and Methods
Materials
The standard virulent strain of M. tuberculosis H37Rv was a gift from the 8th People's Hospital of Guangzhou City.5,6 Female SD mice aged 8–10 weeks, weighed 200–250 g were purchased from the Experimental Animal Center of Anhui Medical University. Silica dust was offered by the National Institute of Occupational Health and Poison Control, Chinese Center for Disease Control and Prevention, 98% of them are less than 5 μm in diameter. Cycloheximide, ampicillin, and OADC broth were purchased from Invitrogen Company (USA). Middlebrook 7H11 agar was purchased from Difco company (USA).
Methods
Injection of silica and MTB
A total of 48 female BALB/c mice were randomly divided into a–d groups randomly. The rats were subjected to injection of H37Rv suspension alone or in combination with intratracheal administration of silica according to the protocol shown in Fig. 1A. The details of injection of silica are as follows. Fresh SiO2 dust grounded for 2 hours was diluted with normal saline into a concentration of 40 mg/ml. After high pressure sterilization, penicillin was added at a final concentration of 8000 U/ml. The syringe needle was replaced by a gavage needle after the suspension was extracted. Rats were anesthetized with ether and the glottis was exposed by pressing the root of the tongue with an otoscope. The gavage needle was placed outside the glottis and inserted in as soon as the glottis opened. The gavage was withdrawn to ensure that it was in the main bronchus. One milliliter of silica suspension was given and the chest was rubbed gently to make it well-distributed.7 After being inoculated in Lownstein-Jenson (L–J) medium for 3 weeks, MTB standard strain H37Rv was collected and homogenized in PBS containing 0.05% Tween 80 to get a final concentration of 20 mg/ml. The bacteria were cultured and the CFU was counted. Each rat was injected 107 CFU of MTB.
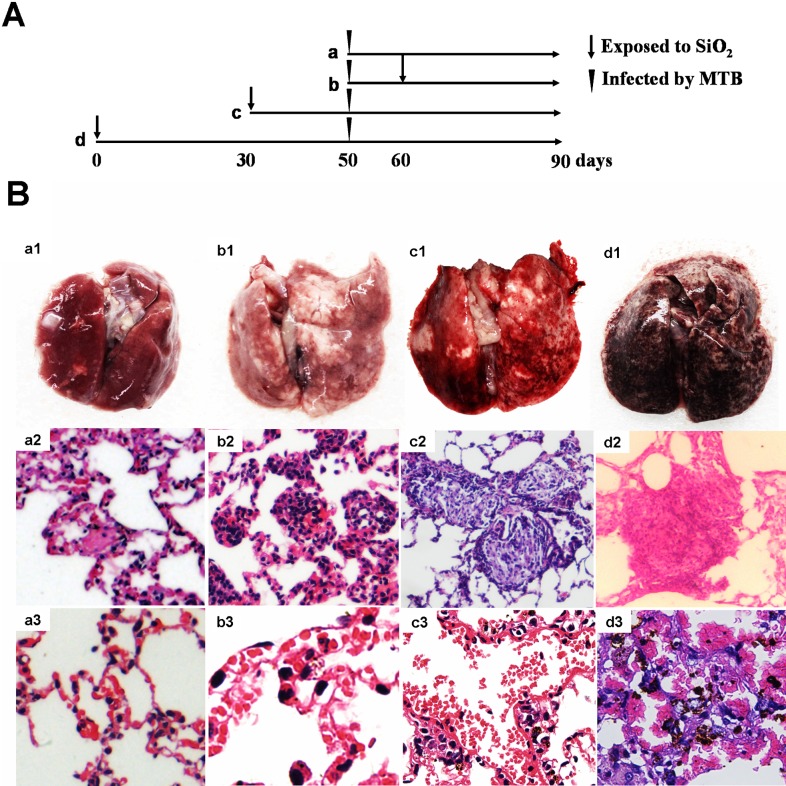
Figure 1.
(A) The protocol of rats subjected to intratracheal administration of silica and injection of H37Rv. (B) Pathological analysis of the lungs by HE staining. a1, b1, c1, and d1 are the morphology of lungs of groups a, b, c, and d, respectively; a2, the Langerhans' cell scattered in the tissue of group a, without no silicotic lesions (×400); b2, primary cellular silicotic nodule of group b (×400); c2, secondary silicotic nodule of group c (×400); d2, tertiary silicotic nodule of group d (×100); a3, pulmonary interstitial tissue of group a (×400); b3, the expanded pulmonary capillary of group b (×800); c3, the congested pulmonary capillary (×400); d3, red blood cells deposited in the alveolar cavities and hemosiderin (×400).
Pathological analysis of the lungs and determination of the MTB load
The rats were sacrificed and a small piece of lung tissue was fixed in 10% neutral formalin. After dehydrated and embedded in paraffin, the histological sections were prepared. Then they were stained with Mayer’s hematoxylin and eosin for tissue examination and with Ziehl–Neelsen method for detection of distribution of MTB. Some lung tissue was homogenized and equal volume of 4% sulfuric acid was added. After standing for 15 minutes, the supernatant was diluted at the ratio of 1:10, 1:100, and 1:1000. Then they were inoculated in Middlebrook 7H11 agar medium (Difco), consisting of 10% OADC broth, 10 mg/ml ampicillin, and 50 mg/ml cycloheximide. The plates were incubated at 37°C for 17 days and then the CFU were counted.8
Warthin–Starry silver staining of the silica
The histological sections were stained with 1% silver nitrate in a 43°C water bath for 30 minutes. After washing, the enzyme reaction was developed with color liquid, consisted of 1.5 ml of 2% silver nitrate, 2 ml of 0.15% hydroquinone, and 3.75 ml of 5% gelatin solution (1% citric acid solution, pH 4). When the sections showed brown yellow they were terminated by 54°C of preheated water. Finally, they were dehydrated and mounted as usually.9
Statistical analysis
Data were presented as mean ± standard error (SEM). They were analyzed using Statistical Package for Social Sciences software (SPSS Inc., Chicago, IL, USA). The heterogeneity of variance was analyzed using one-way analysis of variance (ANOVA). Values of p < 0.05 were considered significant.
Results
Macroscopic observation of the lungs
Pale yellow firm lesions with variable size and a little bit swelling were visible in the lungs of each group. In groups c and d, the lungs congested, swelled, and enlarged slightly. They appeared bright red or dark red, with tip and miliary grayish white spots scattered on the surface. The touch was hard and there was a gravel sense when cut. Gray white nodules or nodular fusion could be observed in the section and those near the hilum were more obvious. Besides, there were tough and meticulous cords and patchy changes in those of group d (Fig. 1B a1–d1). Two rats in group d were dead at 72nd and 80th day. The lung tissue of them inflated with high tension and the alveolar expanded and could not recover.
Microscopic observation of the lungs
In the lung tissue of group a, tubercle epithelioid cells and macrophage accumulated and formed tubercle with leukocyte infiltration around. Caseous necrosis was observed occasionally and Langerhans' cell scattered in the tissue. All these pathological changes characterized a classical crystalline silica-induced lung injury.10 And in those of group b, tubercle and caseous necrosis increased and cell nodules were formed by macrophages, in which the collagen fibers proliferated. This was the so-called primary silicotic nodule. Pulmonary interstitial edema and capillary expansion was also observed. While in group c, there was a lot of serous exudation besides tubercle and caseous necrosis. The silicon nodules were mainly fibrous nodule and began to coalesce (secondary silicon nodules). Pulmonary septal rupture could be observed sometimes and capillary and arteriolar congestion was serious. In those of group d, exudations were characterized as the main pathological changes. The small vascular wall in the middle of the huge fibrous nodule (tertiary silicon nodules) thickened and the proliferated endothelial cells were arranged in concentric circle-like conformation. A large number of red blood cells deposited in the alveolar cavities were destroyed and hemosiderin formed (Fig. 1B a2–d3).11 The pulmonary tissue of the two dead rats was sparse, with pulmonary septa narrowed and broken widely. Pulmonary bulla was formed by the fusion of the emphysema cavity, which could be deduced as suffering from serious obstructive emphysema.
Observation of acid fast staining MTB and colony counting
Mycobacterium tuberculosis in the pulmonary tissue sections could be observed at high magnification after acid fast staining and hematoxylin staining. In group a, red slender bacilli were mainly distributed in Langerhans' cells and tubercles, and appeared to be dissociative. In groups b and c, the number of bacteria increased and the parallel or cross pattern TB appeared in exudation of tuberculous lesion. And in group d, a large number of TB distributed outside the cells and even clustered (Fig. 2A). Compared with group a, the pulmonary tuberculosis load in groups b–d increased significantly by colony counting, which was positive correlated with silicosis progression (Fig. 2B).
Figure 2.
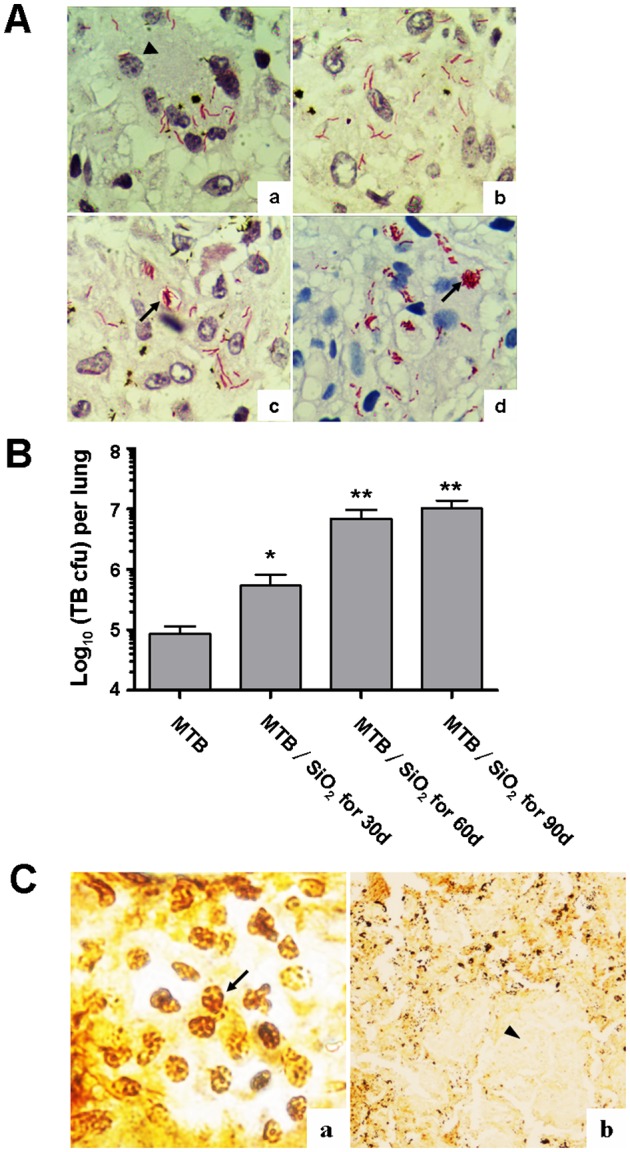
Distribution of Mycobacterium tuberculosis (MTB) and silica dust in pulmonary tissue. (A) The distribution of MTB in pulmonary tissue of rats of groups a–d (acid fast staining, ×1000); black arrow head represents Langerhans' cells. The arrows show TB distributed outside of the cells. (B) Statistical analysis of pulmonary tuberculosis load in each group by colony counting, which increased significantly in groups c and d (*p < 0.05, **p < 0.01). (C) Silica dust in the pulmonary tissue by Warthin–Starry silver staining. (a) The distribution of silica dust in pulmonary interstitial macrophages, the arrow refers to black silver staining granules (×1000); (b) silicon nodules and tubercle are distinguished by silver staining. Arrow head shows the tubercle without silver particles (×100).
Observation of silica dust in the pulmonary tissue
Variable numbers of black silver staining granules, described as silica dust, were observed in pulmonary interstitial macrophages of different groups (Fig. 2C). Silicon nodules and tubercle, formed by fibrosis surrounding quantity of silver particles or no silver particles, were either alone or intermixed occasionally (Fig. 2C).
Discussion
It is an effective method for modeling silicosis by intratracheal administration of silica with the non-exposure method. Primary, secondary, and tertiary silicon nodules could be observed in the organization of rats at the 30th, 60th, and 90th day post-infection, respectively, which was consistent with previous studies.4 In addition, many different pathological changes were observed due to silicosis rats infected with TB simultaneously.
The invasion of silica dust to pulmonary macrophage impaired the immune system and resulted in the increase of the susceptibility to pathogens. In our studies, the defense against MTB was getting poor with the development of the silicosis progress. By HE staining and acid fast staining, our results showed that only hyperplastic lesions appeared in lung tissue of the rats infected with TB alone, with MTB surrounded by Langerhans' cell and tuberculous nodules showing the protective immune response and the strong anti-tuberculosis immunity suggested by the immune reactivity and delayed hypersensitivity.12 While in silicosis rats, not only proliferation but also exudation was observed, which was more severe in tertiary silicosis rats infected with MTB simultaneously. The tuberculous lesions also increased and plenty of MTB appeared in the alveolar, indicating that the immunity of anti-tuberculosis decreased. The tuberculosis loading in rats was consistent with the progress of silicosis. Interestingly, an in vitro study by Shkurupy et al.13,14 demonstrated that preliminary loading of macrophages with silicium dioxide reduced their antibacterial activity, implying that the cytotoxicity of silica dust to macrophage was the main reason for the damage to the anti-tuberculosis function. However, little was known about the detail injury mechanism and immune regulation.
Because the early stage silicon nodule consisted of macrophages with silicium dioxide was much similar with early stage tubercle nodule, Warthin–Starry silver staining was used to make a distinction between them. Our study demonstrated that silicon existed in the lungs’ macrophages of all groups of rats subjected to silica dust. Nevertheless, the amount of silicon deposition was nearly equal. It is generally believed that silicate formed by silica hydrated could act on the lysosomal membrane and result in rupture of lysosome and disintegration of macrophage. And the silica released in turn damages macrophages repeatedly.1 Consistent with previous studies, our study indicated that silicon could not be cleared out and continued to exist in the macrophages. We also observed that the silicon nodules and tubercle could exist independently or merged into larger nodules, which implied that these two kinds of pathogenic factors could promote mutually and then aggravate disease progression, including speeding up the development of emphysema and pulmonary bullae.
In our experiment, different stages of silicosis rats complicated with tuberculosis showed different degrees of pulmonary congestion. There was only mild telangiectasia in primary silicosis rats. While in secondary silicosis rats, small blood vessels of lung tissue expanded, muscularized, and proliferated significantly and bright red plaque was observed in general pathology. And in tertiary silicosis rats, not only dark red and obvious increased lung tissue, with foamy red bloody fluid in the cut, but also erythrocyte sedimentation in the alveolar and wide deposits of hemosiderin were observed. These changes may be related with the enhancement of fibrosis, leading to the reduction of pulmonary capillary bed, pulmonary artery obliterans, and hypoxia, which resulted in pulmonary arteriolar spasm. As a result, increased resistance of pulmonary circulation, enlarged the right ventricle, obstructed body circulation, and left heart failure happened, which caused pulmonary venous reflux obstacle and pulmonary vascular blood sedimentation.
In conclusion, we successfully established different stages of a silicosis rat model complicated with tuberculosis infection and analyzed the pathological characteristics. Our studies revealed that both pathological characteristic and bacteriological indexes in different stages of silicosis rats with tuberculosis were similar to that of corresponding clinical patients,15 which confirmed the reasonableness of it for study. In the future, such a model will be used to explore the specific pathogenesis of silicosis tuberculosis and provide more theoretical support for the treatment.
Disclaimer Statements
Contributors HD WJ ZR CZ conceived and designed the experiments. HD WJ YY YX WW XY performed the experiments. HD WJ MM ZR analyzed the data. CZ XY contributed reagents/materials/analysis tools. HD WJ ZR CZ wrote the paper.
Funding This work was supported by National Natural Science Foundation of China (Nos. 81202294, 81172778, 81302524, and 61170172), and Anhui Provincial Natural Science Foundation of China (Nos. 1208085QH162, KJ2013A105, and 2013SQRL029ZD). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflicts of interest No conflict of interest exits in the submission of this manuscript, and that is approved by all authors for publication. The authors declare that this work is original research that has not been published previously, and not under consideration for publication elsewhere, in whole or in part. All the authors listed have approved the manuscript.
Ethics approval This study was prospectively performed and approved by the Institutional Ethics Committees of Anhui University of Science and Technology and conducted in accordance with the ethical guidelines of the Declaration of Helsinki.
Reference
- 1.Li YL. Pathology. Beijing: People's Publishing House; 2011. pp. 153–5. p. [Google Scholar]
- 2.Barboza CE, Winter DH, Seiscento M, Santos Ude P, Terra Filho M. Tuberculosis and silicosis: epidemiology, diagnosis and chemoprophylaxis. J Bras Pneumol. 2008;34(11):959–66. doi: 10.1590/s1806-37132008001100012. [DOI] [PubMed] [Google Scholar]
- 3.Tiwari RR, Sharma YK. Respiratory health of female stone grinders with free silica dust exposure in Gujarat, India. Int J Occup Environ Health. 2008;14(4):280–2. doi: 10.1179/oeh.2008.14.4.280. [DOI] [PubMed] [Google Scholar]
- 4.Pu XM, Wen H, Dou H, Xu ZX, Liu PC, Li SJ, et al. Pathological observation on animal model of silicosis. Chin J Ind Hyg Occup Dis. 2011;29(10):761–5. [PubMed] [Google Scholar]
- 5.Chen LP, Zhang RB, Hu D, Wu J, Zhang JF, Wu HX, et al. Immune regulation of T-bet adjuvant on Ag85B DNA vaccine against Mycobacterium tuberculosis. Chin J Cell Mol Immunol. 2012;28(7):680–3. [PubMed] [Google Scholar]
- 6.Zhang L, Yang XK, Hu D, Wu J, Wang W, Du J, et al. The construction of Ag85A and ESAT6 co-expression eukaryotic plasmid. Guangdong Med J. 2013;34(20):3102–4. [Google Scholar]
- 7.Zhai RX, Yao L, Yao X, Yan LC, Hao YL, Guan WJ, et al. The change of pulmonary surfactant protein of rat following silica exposure. Chin J Ind Hyg Occup Dis. 2012;30(9):667–71. [PubMed] [Google Scholar]
- 8.Hu D, Wu J, Zhang R, Chen L. T-bet acts as a powerful adjuvant in Ag85B DNAbased vaccination against tuberculosis. Mol Med Report. 2012;6(1):139–44. doi: 10.3892/mmr.2012.883. [DOI] [PubMed] [Google Scholar]
- 9.Huang J, Dai L, Lei S, Liao DY, Wang XQ, Luo TY, et al. Application of Warthin–Starry stain, immunohistochemistry and transmission electron microscopy in diagnosis of cat scratch disease. Zhonghua Bing Li Xue Za Zhi. 2010;39(4):225–9. [PubMed] [Google Scholar]
- 10.Ishihara Y, Yasuhara T, Ishiyama S, Kawashima H, Miyasaka M, Miyazaki T. The role of leukocytes during acute phase inflammation in crystalline silica-induced lung injury. Exp Lung Res. 2001;27(7):589–603. doi: 10.1080/019021401753181845. [DOI] [PubMed] [Google Scholar]
- 11.Karpov MA, Skurupiy VA, Nadeev AP. Analysis of fibrotic depositions in granulomas in chronic silicotuberculosis in mice. Bull Exp Biol Med. 2010;149(5):659–62. doi: 10.1007/s10517-010-1018-9. [DOI] [PubMed] [Google Scholar]
- 12.Chiang CY, Van Weezenbeek C, Mori T, Enarson DA. Challenges to the global control of tuberculosis. Respirology. 2013;18(4):596–640. doi: 10.1111/resp.12067. [DOI] [PubMed] [Google Scholar]
- 13.Arkhipov SA, Shkurupy VA, Bugrimova YS. Effect of preliminary load of macrophages with silicium dioxide on phagocytosis of BCG strain mycobacteria by macrophages and antimicrobial activity. Bull Exp Biol Med. 2010;149(4):534–46. doi: 10.1007/s10517-010-0986-0. [DOI] [PubMed] [Google Scholar]
- 14.Shkurupy VA, Nadeev AP, Karpov MA, Bugrimova YS. Experimental cytomorphological studies of the reaction of mononuclear phagocyte system in granulomatosis of mixed (silicotic and tuberculous) etiology. Bull Exp Biol Med. 2010;149(4):462–5. doi: 10.1007/s10517-010-0971-7. [DOI] [PubMed] [Google Scholar]
- 15.Milovanović A, Nowak D, Milovanović A, Hering KG, Kline JN, Kovalevskiy E, et al. Silicotuberculosis and silicosis as occupational diseases: report of two cases. Srp Arh Celok Lek. 2011;139(7):536–9. [PubMed] [Google Scholar]