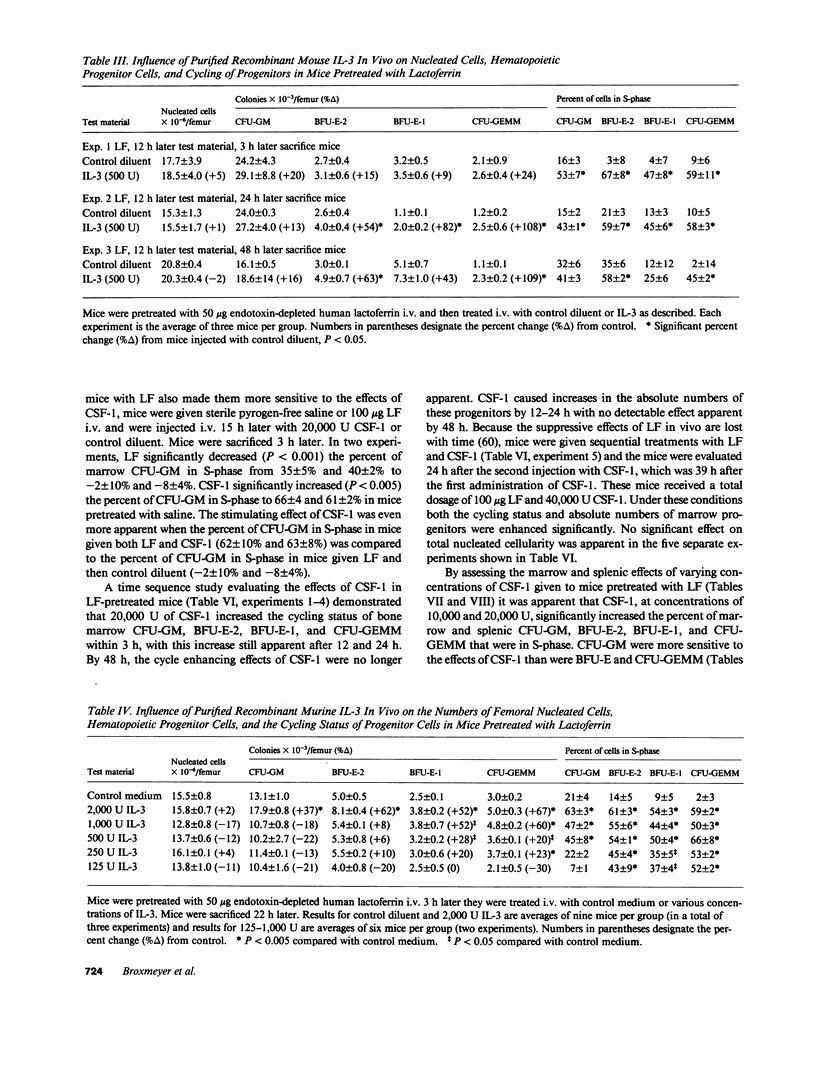
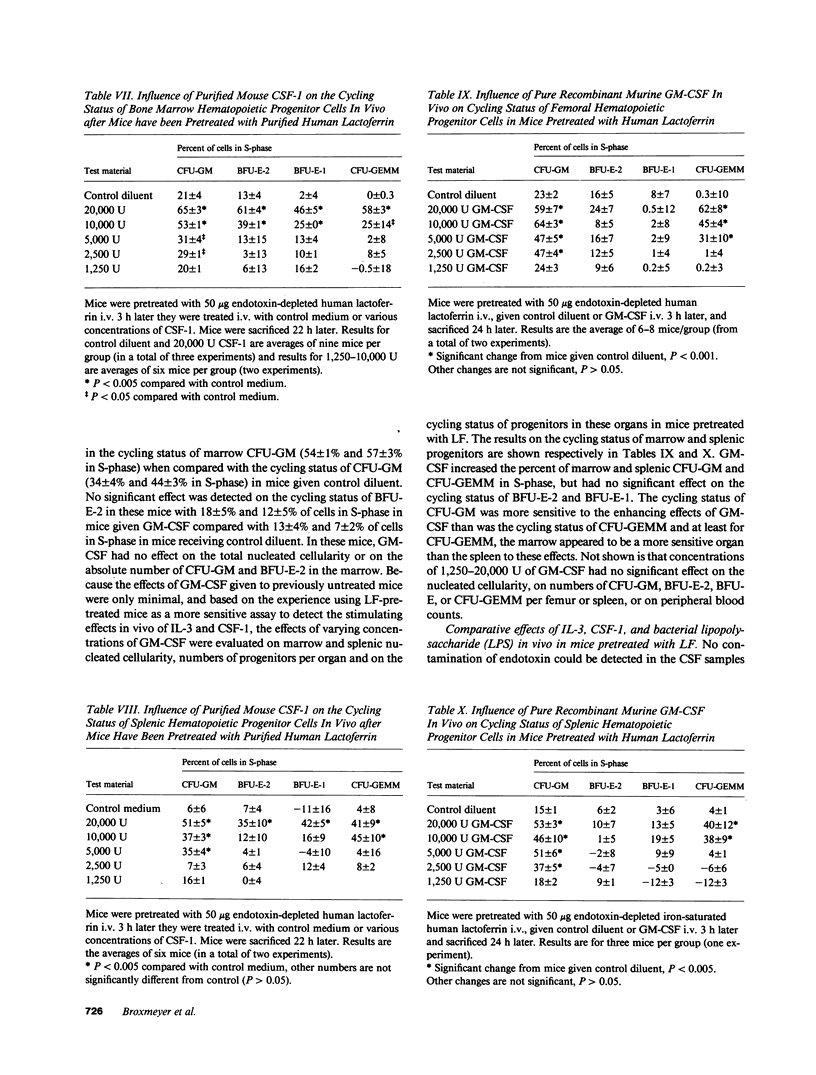
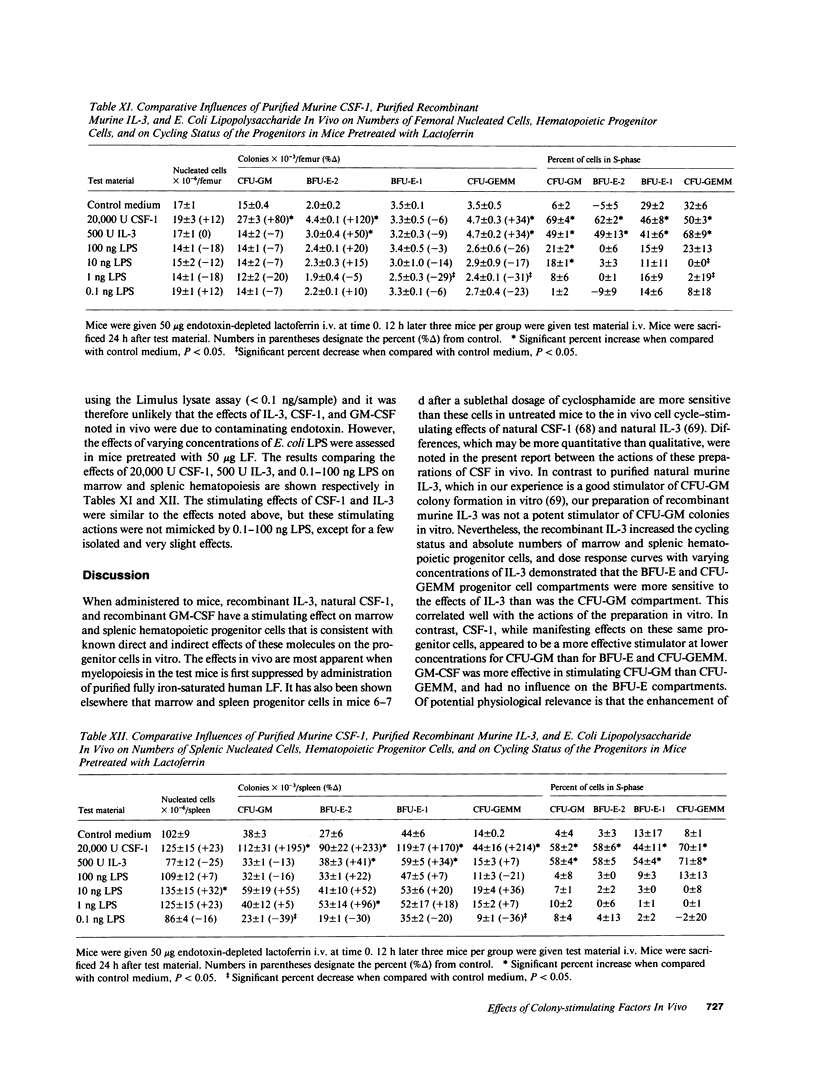
Abstract
Purified murine colony-stimulating factors (CSF) recombinant interleukin 3 (IL-3), natural CSF-1, and recombinant granulocyte-macrophage (GM) CSF were assessed in vivo for their effects on BDF1 mouse bone marrow and spleen granulocyte-macrophage (CFU-GM), erythroid (BFU-E), and multipotential (CFU-GEMM) progenitor cells in untreated mice and in mice pretreated with purified iron-saturated human lactoferrin (LF). The CSF and LF preparations did not contain detectable endotoxin (less than 0.1 ng). Mice pretreated with LF were more sensitive to the effects of CSF. In mice pretreated with LF, 2,000 U IL-3 or 20,000 U CSF-1 significantly enhanced the cycling status and absolute numbers of all progenitors, whereas 20,000 U GM-CSF significantly increased the cycling status of CFU-GM and CFU-GEMM, but had no effect on cycling of BFU-E or on numbers of any of the progenitors. The effects of CSF in mice pretreated with LF were not mimicked by 0.1-100 ng E. coli lipopolysaccharide.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagby G. C., Jr, McCall E., Bergstrom K. A., Burger D. A monokine regulates colony-stimulating activity production by vascular endothelial cells. Blood. 1983 Sep;62(3):663–668. [PubMed] [Google Scholar]
- Bagby G. C., Jr, McCall E., Layman D. L. Regulation of colony-stimulating activity production. Interactions of fibroblasts, mononuclear phagocytes, and lactoferrin. J Clin Invest. 1983 Feb;71(2):340–344. doi: 10.1172/JCI110774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagby G. C., Jr, Rigas V. D., Bennett R. M., Vandenbark A. A., Garewal H. S. Interaction of lactoferrin, monocytes, and T lymphocyte subsets in the regulation of steady-state granulopoiesis in vitro. J Clin Invest. 1981 Jul;68(1):56–63. doi: 10.1172/JCI110254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broxmeyer H. E., Bicknell D. C., Gillis S., Harris E. L., Pelus L. M., Sledge G. W., Jr Lactoferrin: affinity purification from human milk and polymorphonuclear neutrophils using monoclonal antibody (II 2C) to human lactoferrin, development of an immunoradiometric assay using II 2C, and myelopoietic regulation and receptor-binding characteristics. Blood Cells. 1986;11(3):429–446. [PubMed] [Google Scholar]
- Broxmeyer H. E., Bognacki J., Ralph P., Dörner M. H., Lu L., Castro-Malaspina H. Monocyte-macrophage-derived acidic isoferritins: normal feedback regulators of granulocyte-macrophage progenitor cells in vitro. Blood. 1982 Sep;60(3):595–607. [PubMed] [Google Scholar]
- Broxmeyer H. E. Colony assays of hematopoietic progenitor cells and correlations to clinical situations. Crit Rev Oncol Hematol. 1984;1(3):227–257. doi: 10.1016/s1040-8428(84)80013-x. [DOI] [PubMed] [Google Scholar]
- Broxmeyer H. E., DeSousa M., Smithyman A., Ralph P., Hamilton J., Kurland J. I., Bognacki J. Specificity and modulation of the action of lactoferrin, a negative feedback regulator of myelopoiesis. Blood. 1980 Feb;55(2):324–333. [PubMed] [Google Scholar]
- Broxmeyer H. E., Smithyman A., Eger R. R., Meyers P. A., de Sousa M. Identification of lactoferrin as the granulocyte-derived inhibitor of colony-stimulating activity production. J Exp Med. 1978 Oct 1;148(4):1052–1067. doi: 10.1084/jem.148.4.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broxmeyer H., Van Zant G., Zucali J. R., LoBue J., Gordon A. S. Mechanisms of leukocyte production and release. XII. A comparative assay of the leukocytosis-inducing factor (LIF) and the colony-stimulating factor (CSF). Proc Soc Exp Biol Med. 1974 Apr;145(4):1262–1267. doi: 10.3181/00379727-145-37993. [DOI] [PubMed] [Google Scholar]
- Burgess A. W., Camakaris J., Metcalf D. Purification and properties of colony-stimulating factor from mouse lung-conditioned medium. J Biol Chem. 1977 Mar 25;252(6):1998–2003. [PubMed] [Google Scholar]
- Burgess A. W., Metcalf D., Kozka I. J., Simpson R. J., Vairo G., Hamilton J. A., Nice E. C. Purification of two forms of colony-stimulating factor from mouse L-cell-conditioned medium. J Biol Chem. 1985 Dec 15;260(29):16004–16011. [PubMed] [Google Scholar]
- Campbell H. D., Ymer S., Fung M. C., Young I. G. Cloning and nucleotide sequence of the murine interleukin-3 gene. Eur J Biochem. 1985 Jul 15;150(2):297–304. doi: 10.1111/j.1432-1033.1985.tb09020.x. [DOI] [PubMed] [Google Scholar]
- Cantrell M. A., Anderson D., Cerretti D. P., Price V., McKereghan K., Tushinski R. J., Mochizuki D. Y., Larsen A., Grabstein K., Gillis S. Cloning, sequence, and expression of a human granulocyte/macrophage colony-stimulating factor. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6250–6254. doi: 10.1073/pnas.82.18.6250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caramatti C., Pigoli G., Shadduck R. K., Waheed A. The effect of preincubation of bone marrow cells on the binding of colony-stimulating factor. J Lab Clin Med. 1983 Jul;102(1):1–16. [PubMed] [Google Scholar]
- Chen B. D., Kuhn C., 3rd, Lin H. S. Receptor-mediated binding and internalization of colony-stimulating factor (CSF-1) by mouse peritoneal exudate macrophages. J Cell Sci. 1984 Aug;70:147–166. doi: 10.1242/jcs.70.1.147. [DOI] [PubMed] [Google Scholar]
- Clark-Lewis I., Kent S. B., Schrader J. W. Purification to apparent homogeneity of a factor stimulating the growth of multiple lineages of hemopoietic cells. J Biol Chem. 1984 Jun 25;259(12):7488–7494. [PubMed] [Google Scholar]
- Cutler R. L., Metcalf D., Nicola N. A., Johnson G. R. Purification of a multipotential colony-stimulating factor from pokeweed mitogen-stimulated mouse spleen cell conditioned medium. J Biol Chem. 1985 Jun 10;260(11):6579–6587. [PubMed] [Google Scholar]
- Donahue R. E., Wang E. A., Stone D. K., Kamen R., Wong G. G., Sehgal P. K., Nathan D. G., Clark S. C. Stimulation of haematopoiesis in primates by continuous infusion of recombinant human GM-CSF. 1986 Jun 26-Jul 2Nature. 321(6073):872–875. doi: 10.1038/321872a0. [DOI] [PubMed] [Google Scholar]
- Emerson S. G., Sieff C. A., Wang E. A., Wong G. G., Clark S. C., Nathan D. G. Purification of fetal hematopoietic progenitors and demonstration of recombinant multipotential colony-stimulating activity. J Clin Invest. 1985 Sep;76(3):1286–1290. doi: 10.1172/JCI112087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung M. C., Hapel A. J., Ymer S., Cohen D. R., Johnson R. M., Campbell H. D., Young I. G. Molecular cloning of cDNA for murine interleukin-3. Nature. 1984 Jan 19;307(5948):233–237. doi: 10.1038/307233a0. [DOI] [PubMed] [Google Scholar]
- Gabrilove J. L., Welte K., Harris P., Platzer E., Lu L., Levi E., Mertelsmann R., Moore M. A. Pluripoietin alpha: a second human hematopoietic colony-stimulating factor produced by the human bladder carcinoma cell line 5637. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2478–2482. doi: 10.1073/pnas.83.8.2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasson J. C., Kaufman S. E., Weisbart R. H., Tomonaga M., Golde D. W. High-affinity binding of granulocyte-macrophage colony-stimulating factor to normal and leukemic human myeloid cells. Proc Natl Acad Sci U S A. 1986 Feb;83(3):669–673. doi: 10.1073/pnas.83.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentile P., Broxmeyer H. E. Suppression of mouse myelopoiesis by administration of human lactoferrin in vivo and the comparative action of human transferrin. Blood. 1983 May;61(5):982–993. [PubMed] [Google Scholar]
- Gough N. M., Gough J., Metcalf D., Kelso A., Grail D., Nicola N. A., Burgess A. W., Dunn A. R. Molecular cloning of cDNA encoding a murine haematopoietic growth regulator, granulocyte-macrophage colony stimulating factor. 1984 Jun 28-Jul 4Nature. 309(5971):763–767. doi: 10.1038/309763a0. [DOI] [PubMed] [Google Scholar]
- Guilbert L. J., Stanley E. R. Specific interaction of murine colony-stimulating factor with mononuclear phagocytic cells. J Cell Biol. 1980 Apr;85(1):153–159. doi: 10.1083/jcb.85.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilbert L. J., Stanley E. R. The interaction of 125I-colony-stimulating factor-1 with bone marrow-derived macrophages. J Biol Chem. 1986 Mar 25;261(9):4024–4032. [PubMed] [Google Scholar]
- Huebner K., Isobe M., Croce C. M., Golde D. W., Kaufman S. E., Gasson J. C. The human gene encoding GM-CSF is at 5q21-q32, the chromosome region deleted in the 5q- anomaly. Science. 1985 Dec 13;230(4731):1282–1285. doi: 10.1126/science.2999978. [DOI] [PubMed] [Google Scholar]
- Ihle J. N., Keller J., Henderson L., Klein F., Palaszynski E. Procedures for the purification of interleukin 3 to homogeneity. J Immunol. 1982 Dec;129(6):2431–2436. [PubMed] [Google Scholar]
- Ishizaka Y., Motoyoshi K., Hatake K., Saito M., Takaku F., Miura Y. Mode of action of human urinary colony-stimulating factor. Exp Hematol. 1986 Jan;14(1):1–8. [PubMed] [Google Scholar]
- Kajigaya S., Suda T., Suda J., Saito M., Miura Y., Iizuka M., Kobayashi S., Minato N., Sudo T. A recombinant murine granulocyte/macrophage (GM) colony-stimulating factor derived from an inducer T cell line (IH5.5). Functional restriction to GM progenitor cells. J Exp Med. 1986 Oct 1;164(4):1102–1113. doi: 10.1084/jem.164.4.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki E. S., Ladner M. B., Wang A. M., Van Arsdell J., Warren M. K., Coyne M. Y., Schweickart V. L., Lee M. T., Wilson K. J., Boosman A. Molecular cloning of a complementary DNA encoding human macrophage-specific colony-stimulating factor (CSF-1). Science. 1985 Oct 18;230(4723):291–296. doi: 10.1126/science.2996129. [DOI] [PubMed] [Google Scholar]
- Kindler V., Thorens B., de Kossodo S., Allet B., Eliason J. F., Thatcher D., Farber N., Vassalli P. Stimulation of hematopoiesis in vivo by recombinant bacterial murine interleukin 3. Proc Natl Acad Sci U S A. 1986 Feb;83(4):1001–1005. doi: 10.1073/pnas.83.4.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Beau M. M., Westbrook C. A., Diaz M. O., Larson R. A., Rowley J. D., Gasson J. C., Golde D. W., Sherr C. J. Evidence for the involvement of GM-CSF and FMS in the deletion (5q) in myeloid disorders. Science. 1986 Feb 28;231(4741):984–987. doi: 10.1126/science.3484837. [DOI] [PubMed] [Google Scholar]
- Lee F., Yokota T., Otsuka T., Gemmell L., Larson N., Luh J., Arai K., Rennick D. Isolation of cDNA for a human granulocyte-macrophage colony-stimulating factor by functional expression in mammalian cells. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4360–4364. doi: 10.1073/pnas.82.13.4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord B. I., Molineux G., Testa N. G., Kelly M., Spooncer E., Dexter T. M. The kinetic response of haemopoietic precursor cells, in vivo, to highly purified, recombinant interleukin-3. Lymphokine Res. 1986 Spring;5(2):97–104. [PubMed] [Google Scholar]
- Lu L., Broxmeyer H. E. Comparative influences of phytohemagglutinin-stimulated leukocyte conditioned medium, hemin, prostaglandin E, and low oxygen tension on colony formation by erythroid progenitor cells in normal human bone marrow. Exp Hematol. 1985 Nov;13(10):989–993. [PubMed] [Google Scholar]
- Lu L., Broxmeyer H. E. The selective enhancing influence of hemin and products of human erythrocytes on colony formation by human multipotential (CFUGEMM) and erythroid (BFUE) progenitor cells in vitro. Exp Hematol. 1983 Sep;11(8):721–729. [PubMed] [Google Scholar]
- Metcalf D., Begley C. G., Johnson G. R., Nicola N. A., Lopez A. F., Williamson D. J. Effects of purified bacterially synthesized murine multi-CSF (IL-3) on hematopoiesis in normal adult mice. Blood. 1986 Jul;68(1):46–57. [PubMed] [Google Scholar]
- Metcalf D., Burgess A. W. Clonal analysis of progenitor cell commitment of granulocyte or macrophage production. J Cell Physiol. 1982 Jun;111(3):275–283. doi: 10.1002/jcp.1041110308. [DOI] [PubMed] [Google Scholar]
- Metcalf D., Burgess A. W., Johnson G. R., Nicola N. A., Nice E. C., DeLamarter J., Thatcher D. R., Mermod J. J. In vitro actions on hemopoietic cells of recombinant murine GM-CSF purified after production in Escherichia coli: comparison with purified native GM-CSF. J Cell Physiol. 1986 Sep;128(3):421–431. doi: 10.1002/jcp.1041280311. [DOI] [PubMed] [Google Scholar]
- Metcalf D., Johnson G. R., Burgess A. W. Direct stimulation by purified GM-CSF of the proliferation of multipotential and erythroid precursor cells. Blood. 1980 Jan;55(1):138–147. [PubMed] [Google Scholar]
- Metcalf D., Nicola N. A. Synthesis by mouse peritoneal cells of G-CSF, the differentiation inducer for myeloid leukemia cells: stimulation by endotoxin, M-CSF and multi-CSF. Leuk Res. 1985;9(1):35–50. doi: 10.1016/0145-2126(85)90020-7. [DOI] [PubMed] [Google Scholar]
- Metcalf D. The granulocyte-macrophage colony-stimulating factors. Science. 1985 Jul 5;229(4708):16–22. doi: 10.1126/science.2990035. [DOI] [PubMed] [Google Scholar]
- Miyatake S., Yokota T., Lee F., Arai K. Structure of the chromosomal gene for murine interleukin 3. Proc Natl Acad Sci U S A. 1985 Jan;82(2):316–320. doi: 10.1073/pnas.82.2.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R. N., Larsen H. S., Horohov D. W., Rouse B. T. Endogenous regulation of macrophage proliferative expansion by colony-stimulating factor-induced interferon. Science. 1984 Jan 13;223(4632):178–181. doi: 10.1126/science.6606850. [DOI] [PubMed] [Google Scholar]
- Morgan C. J., Stanley E. R. Chemical crosslinking of the mononuclear phagocyte specific growth factor CSF-1 to its receptor at the cell surface. Biochem Biophys Res Commun. 1984 Feb 29;119(1):35–41. doi: 10.1016/0006-291x(84)91614-0. [DOI] [PubMed] [Google Scholar]
- Nagata S., Tsuchiya M., Asano S., Yamamoto O., Hirata Y., Kubota N., Oheda M., Nomura H., Yamazaki T. The chromosomal gene structure and two mRNAs for human granulocyte colony-stimulating factor. EMBO J. 1986 Mar;5(3):575–581. doi: 10.1002/j.1460-2075.1986.tb04249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola N. A., Metcalf D. Binding of 125I-labeled granulocyte colony-stimulating factor to normal murine hemopoietic cells. J Cell Physiol. 1985 Aug;124(2):313–321. doi: 10.1002/jcp.1041240222. [DOI] [PubMed] [Google Scholar]
- Nicola N. A., Metcalf D., Matsumoto M., Johnson G. R. Purification of a factor inducing differentiation in murine myelomonocytic leukemia cells. Identification as granulocyte colony-stimulating factor. J Biol Chem. 1983 Jul 25;258(14):9017–9023. [PubMed] [Google Scholar]
- Palaszynski E. W., Ihle J. N. Evidence for specific receptors for interleukin 3 on lymphokine-dependent cell lines established from long-term bone marrow cultures. J Immunol. 1984 Apr;132(4):1872–1878. [PubMed] [Google Scholar]
- Park L. S., Friend D., Gillis S., Urdal D. L. Characterization of the cell surface receptor for a multi-lineage colony-stimulating factor (CSF-2 alpha). J Biol Chem. 1986 Jan 5;261(1):205–210. [PubMed] [Google Scholar]
- Park L. S., Friend D., Gillis S., Urdal D. L. Characterization of the cell surface receptor for granulocyte-macrophage colony-stimulating factor. J Biol Chem. 1986 Mar 25;261(9):4177–4183. [PubMed] [Google Scholar]
- Pelus L. M., Broxmeyer H. E., Kurland J. I., Moore M. A. Regulation of macrophage and granulocyte proliferation. Specificities of prostaglandin E and lactoferrin. J Exp Med. 1979 Aug 1;150(2):277–292. doi: 10.1084/jem.150.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigoli G., Waheed A., Shadduck R. K. Observations on the binding and interaction of radioiodinated colony-stimulating factor with murine bone marrow cells in vivo. Blood. 1982 Feb;59(2):408–420. [PubMed] [Google Scholar]
- Prestidge R. L., Watson J. D., Urdal D. L., Mochizuki D., Conlon P., Gillis S. Biochemical comparison of murine colony-stimulating factors secreted by a T cell lymphoma and a myelomonocytic leukemia. J Immunol. 1984 Jul;133(1):293–298. [PubMed] [Google Scholar]
- Quesenberry P., Levin J., Zuckerman K., Rencricca N., Sullivan R., Tyler W. Stem cell migration induced by erythropoietin or haemolytic anaemia: the effects of actinomycin and endotoxin contamination of erythropoietin preparations. Br J Haematol. 1979 Feb;41(2):253–269. doi: 10.1111/j.1365-2141.1979.tb05854.x. [DOI] [PubMed] [Google Scholar]
- Shadduck R. K., Pigoli G., Caramatti C., Degliantoni G., Rizzoli V., Porcellini A., Waheed A., Shiffer L. Identification of hemopoietic cells responsive to colony-stimulating factor by autoradiography. Blood. 1983 Dec;62(6):1197–1202. [PubMed] [Google Scholar]
- Shadduck R. K., Waheed A., Porcellini A., Rizzoli V., Levin J. A method for the removal of endotoxin from purified colony-stimulating factor. Proc Soc Exp Biol Med. 1980 May;164(1):40–44. doi: 10.3181/00379727-164-40821. [DOI] [PubMed] [Google Scholar]
- Sherr C. J., Rettenmier C. W., Sacca R., Roussel M. F., Look A. T., Stanley E. R. The c-fms proto-oncogene product is related to the receptor for the mononuclear phagocyte growth factor, CSF-1. Cell. 1985 Jul;41(3):665–676. doi: 10.1016/s0092-8674(85)80047-7. [DOI] [PubMed] [Google Scholar]
- Souza L. M., Boone T. C., Gabrilove J., Lai P. H., Zsebo K. M., Murdock D. C., Chazin V. R., Bruszewski J., Lu H., Chen K. K. Recombinant human granulocyte colony-stimulating factor: effects on normal and leukemic myeloid cells. Science. 1986 Apr 4;232(4746):61–65. doi: 10.1126/science.2420009. [DOI] [PubMed] [Google Scholar]
- Stanley E. R., Heard P. M. Factors regulating macrophage production and growth. Purification and some properties of the colony stimulating factor from medium conditioned by mouse L cells. J Biol Chem. 1977 Jun 25;252(12):4305–4312. [PubMed] [Google Scholar]
- Waheed A., Shadduck R. K. Purification and properties of L cell-derived colony-stimulating factor. J Lab Clin Med. 1979 Jul;94(1):180–193. [PubMed] [Google Scholar]
- Waheed A., Shadduck R. K. Purification of colony-stimulating factor by affinity chromatography. Blood. 1982 Jul;60(1):238–244. [PubMed] [Google Scholar]
- Walker F., Burgess A. W. Specific binding of radioiodinated granulocyte-macrophage colony-stimulating factor to hemopoietic cells. EMBO J. 1985 Apr;4(4):933–939. doi: 10.1002/j.1460-2075.1985.tb03721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker F., Nicola N. A., Metcalf D., Burgess A. W. Hierarchical down-modulation of hemopoietic growth factor receptors. Cell. 1985 Nov;43(1):269–276. doi: 10.1016/0092-8674(85)90032-7. [DOI] [PubMed] [Google Scholar]
- Warren M. K., Ralph P. Macrophage growth factor CSF-1 stimulates human monocyte production of interferon, tumor necrosis factor, and colony stimulating activity. J Immunol. 1986 Oct 1;137(7):2281–2285. [PubMed] [Google Scholar]
- Welte K., Platzer E., Lu L., Gabrilove J. L., Levi E., Mertelsmann R., Moore M. A. Purification and biochemical characterization of human pluripotent hematopoietic colony-stimulating factor. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1526–1530. doi: 10.1073/pnas.82.5.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing E. J., Waheed A., Shadduck R. K., Nagle L. S., Stephenson K. Effect of colony stimulating factor on murine macrophages. Induction of antitumor activity. J Clin Invest. 1982 Feb;69(2):270–276. doi: 10.1172/JCI110449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota T., Lee F., Rennick D., Hall C., Arai N., Mosmann T., Nabel G., Cantor H., Arai K. Isolation and characterization of a mouse cDNA clone that expresses mast-cell growth-factor activity in monkey cells. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1070–1074. doi: 10.1073/pnas.81.4.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]


