Abstract
Type I collagen hydrogels have been used successfully as three-dimensional substrates for cell culture and have shown promise as scaffolds for engineered tissues and tumors. A critical step in the development of collagen hydrogels as viable tissue mimics is quantitative characterization of hydrogel properties and their correlation with fabrication parameters, which enables hydrogels to be tuned to match specific tissues or fulfill engineering requirements. A significant body of work has been devoted to characterization of collagen I hydrogels; however, due to the breadth of materials and techniques used for characterization, published data are often disjoint and hence their utility to the community is reduced. This review aims to determine the parameter space covered by existing data and identify key gaps in the literature so that future characterization and use of collagen I hydrogels for research can be most efficiently conducted. This review is divided into three sections: (1) relevant fabrication parameters are introduced and several of the most popular methods of controlling and regulating them are described, (2) hydrogel properties most relevant for tissue engineering are presented and discussed along with their characterization techniques, (3) the state of collagen I hydrogel characterization is recapitulated and future directions are proposed. Ultimately, this review can serve as a resource for selection of fabrication parameters and material characterization methodologies in order to increase the usefulness of future collagen-hydrogel-based characterization studies and tissue engineering experiments.
Introduction
Three-dimensional (3D) scaffold-based culture models represent a rapidly growing niche in tissue engineering because they more closely mimic in vivo conditions than traditional cellular monolayers.1–4 The class of materials known as hydrogels, which range from synthetic molecules such as poly(ethylene glycol) to native proteins, such as collagen and fibrin, has been demonstrated to be well-suited for use as 3D scaffolds.5–9 Collagen-based hydrogels are gaining widespread popularity as scaffolds for tissue engineering due to the abundance of collagen in natural extracellular matrix (ECM). Collagen comprises 25% (by dry weight) of total protein in vivo10,11; of the various types of collagen, type I is by far the most prevalent form and is popular for tissue engineering due to its ease of extraction and adaptability for multiple applications.
Although collagen I is a viable scaffold for a wide range of applications,12–15 comparison between studies is difficult due to significant variation in hydrogel fabrication protocols used by different research groups. It is well known that scaffold material properties play an important role in cellular behavior.10,12 One of the main drawbacks to using collagen hydrogels as scaffolds for tissue engineering is that these properties are highly variable and dependent on a large number of fabrication parameters, such as collagen source or gelation pH, resulting in a vast design space.10,16–20 The need for quantitative characterization of collagen hydrogels is recognized within the scientific community as a prerequisite for quantitative studies, tissue optimization, and comparative research.14,17,18
Recent publications have aimed to characterize collagen material properties; however, the majority of these studies have investigated relationships between isolated fabrication parameters and material properties without considering the influence of the full design space on material properties. Further, just as there is wide variation in fabrication protocols implemented by each group, there is little commonality between fabrication parameters employed. Since fabrication parameters and material properties are coupled in collagen hydrogels, the data available in the literature form a set of scattered measurements that cannot be extrapolated to form a complete multidimensional data set. Because of this, the results of such studies are difficult to interpret and only qualitatively useful to other research groups.
This review aims to provide a guide to the collagen characterization data available in the literature to facilitate identification of the most relevant studies for design of future experiments. In this review, only fabrication parameters previously identified as having a significant effect on one or more hydrogel material properties are discussed in detail. These parameters include collagen source, solubilization method, polymerization pH, polymerization temperature, ionic strength, and collagen concentration.11,14,21,22 The hydrogel material properties covered in this review, spanning polymerization, mechanics, structure, and transport, have previously been demonstrated to regulate cellular response.9,10,22–24
For information on molecular characterization of collagen, the reader is directed to articles by Abraham et al. and Miller and Rhodes.14,25 Enhanced crosslinking techniques and composite hydrogels, most often used to enhance the strength of low-concentration collagen gels, are beyond the scope of this review as it focuses on basic hydrogel fabrication and properties. However, some works that involve enhanced crosslinking are cited here in the context of their fabrication techniques and characterization methodologies. For further information and examples of enhanced crosslinking of collagen hydrogels, the reader is directed to reviews10,11 as well as several experimental studies.21,26–30 For the reader interested in dynamic remodeling of ECM, excellent reviews by Lu et al. (general perspective on ECM degradation and remodeling)31 and Baaijens et al. (collagen-specific remodeling)32 are recommended.
Collagen Hydrogel Fabrication Parameters
Most collagen hydrogels are prepared using type I collagen, which comprises 90% of the protein in human connective tissues14,20 and is easily extracted from animal tissue with minimal contamination by other collagens or proteins. Type I collagen is a triple-helical protein formed of 67-nm periodic polypeptide chains with a total molecular weight near 300 kDa.14,33 Collagen fibrils self-assemble at neutral pH into bundled fibers typically 12–120-nm diameter that crosslink to produce a matrix structure11,34–36 that ultimately forms a hydrogel in the presence of a water-based solvent.
Collagen source and solubilization
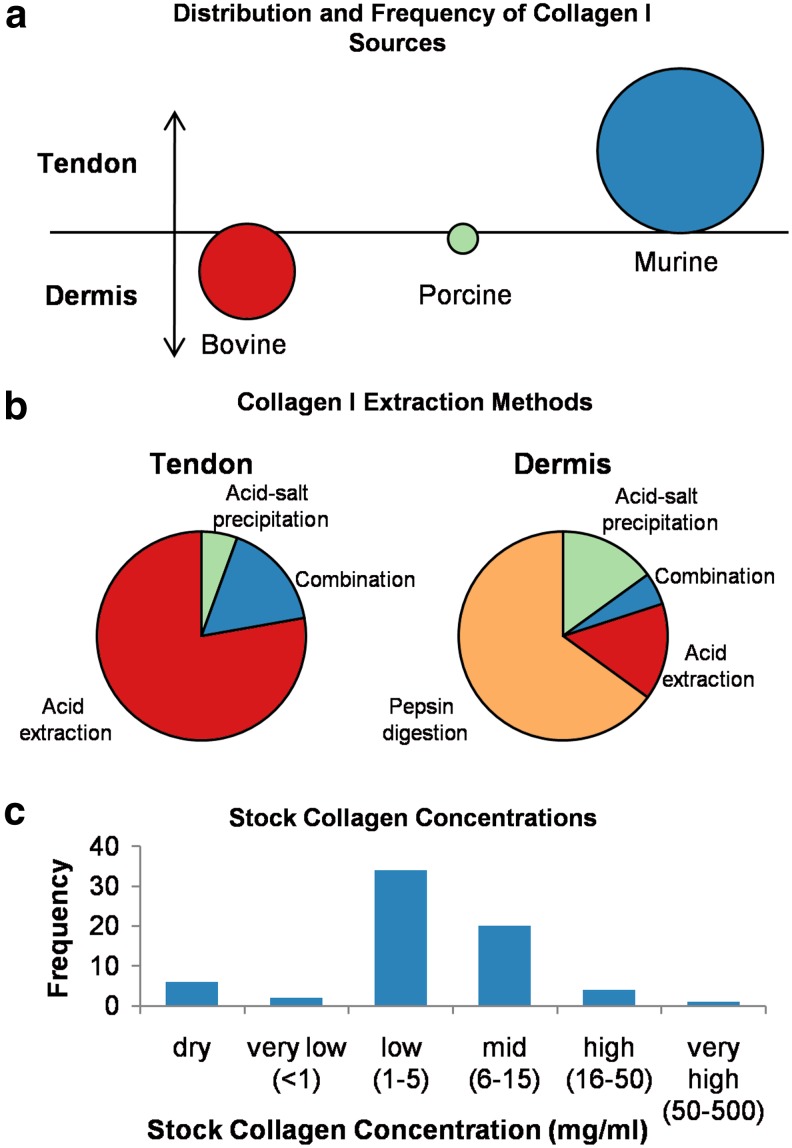
Several groups have demonstrated that the collagen source (e.g., rat tail tendon, porcine skin, and bovine skin) influences hydrogel properties.12,20,37 Relationships between collagen source and polymerization kinetics, hydrogel mechanics, and fiber structure are discussed in more detail in “Polymerization,” “Mechanics,” and “Structure” sections. Further, the method by which collagen is extracted from tissue has been shown to alter the molecular structure of the collagen fibrils as well as the kinetics of assembly.12,38 Acid solubilization is most commonly used for minimally crosslinked collagens, such as rat tail tendon, while a combination of neutral salt solution with proteolytic extraction (pepsin digestion) is often used in order to fully denature highly crosslinked collagens from bovine or porcine skin.11,20,39 Many combinations of collagen sources, solubilization techniques, and solvents are in use for preparation of collagen stock solutions. Further, collagen stock solutions, even those produced commercially, have been found to have significant batch-to-batch variation.12,14 A review of some of the collagen stock solutions referenced in the literature reveals that there is little consistency (Fig. 1).
FIG. 1.
Overview of collagen I sources and solubilization methods. (a) Source animal compared with source tissue; (b) comparison of extraction methods by source tissue; and (c) collagen content of stock solutions. Source data can be found in Supplementary Table S1. Color images available online at www.liebertpub.com/teb
Figure 1 shows the distribution of collagen source, solubilization, and stock solution concentrations obtained from a survey of over 50 research articles—listed in Supplementary Table S1 (Supplementary Data are available online at www.liebertpub.com/teb). While it was not possible to include all collagen-hydrogel-based publications in this review, this sample size is large enough to provide information representative of the field. In Figure 1(a), each bubble corresponds to a source species with the bubble diameter corresponding to relative frequency of experiments using that source. Vertical bubble placement indicates to what extent each tissue type (tendon or dermis) is used from each source species. Figure 1(a) shows that rat tendons represent the primary source of collagen I for hydrogel research, although the contribution of bovine dermal tissue is comparable. Porcine tendon and dermis are infrequently used.
Figure 1(b) provides a breakdown of extraction methods used for each tissue type (regardless of species). Figure 1(b) indicates that collagen denaturation methods in use are relatively consistent with the degree of crosslinking for each source tissue; minimally crosslinked collagens sourced from tendons are denatured using acid alone, while dermal collagens, with their high crosslink density, require pepsin digestion or other complex denaturation procedures.
Finally, Figure 1(c) is a histogram of stock solution concentrations reported in the literature. Whether extracted in-house or obtained commercially, stock concentrations are most commonly in the range 1–5 mg/mL that is lower than the collagen content of many native tissues. As noted in the following sections, this limitation has implications for the utility of collagen hydrogels as tissue mimics.
Collagen concentration
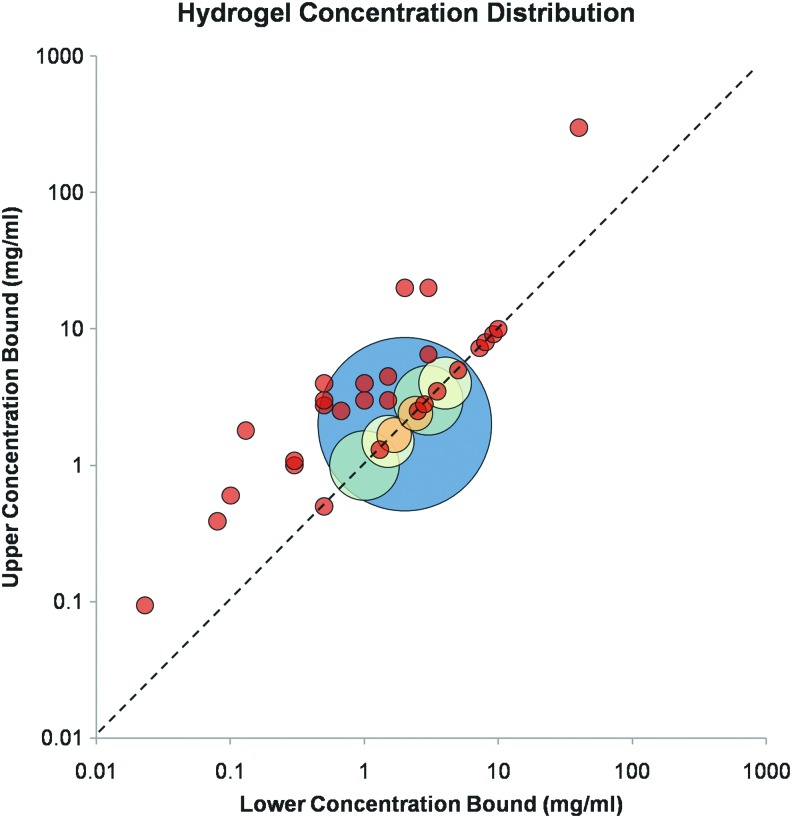
Collagen concentration in vitro and in vivo influences tissue mechanical properties, thereby regulating cellular behavior.40–42 It is suggested to play a role in tumor malignancy, as cancerous tissue contains 9–45 mg/mL (0.9–4.5% wt.) collagen in the interstitium while normal tissue contains significantly less.19 Most research that characterizes collagen hydrogels examines limited and disjoint ranges of collagen concentration, as demonstrated by Figure 2, which presents the distribution of collagen hydrogel concentrations found in the literature (Supplementary Table S2). The majority of these studies were performed within the last decade. In this plot, bubble location corresponds to the range of concentrations examined by each source. If only a single collagen concentration was used for a study, then the bubble falls on the dashed line. Studies that investigate broader concentration ranges are represented by bubbles centered further from the dashed line. Bubble diameter is not linked to concentration range, but rather it indicates number of publications with identical conditions. Bubble color indicates bubbles of the same size. Each red bubble represents a single study. The large blue bubble, located at 2 mg/mL, represents the majority of publications.
FIG. 2.
Collagen concentration ranges used in the tissue engineering literature. Source data can be found in Supplementary Table S2. 1 mg/mL=0.1% wt. Color images available online at www.liebertpub.com/teb
Although most tissue mimics have been fabricated using hydrogels with low collagen content (<4 mg/mL), as shown in Figure 2, they have limited value for mimicking 3D tissues due to their nonphysiological strength and microstructure, which result in an inability to support microfabrication as bulk gels.43,44 Their use in characterization experiments is likely because most commercially available formulations are provided at low concentrations [Fig. 1(c) and Supplementary Table S1]. Conversely, gels with extremely high collagen content (>20 mg/mL) form a fiber structure too dense to permit cell migration and viability.44 Finally, Figure 2 reveals that, as most previous works use single-concentration hydrogels (represented by the bubbles centered along the dashed line), the majority of the literature provides no insight into the effect of varying collagen concentration on gel characteristics and cell response. Notable exceptions include Ramanujan et al. and Erikson et al., who examined the influence of collagen concentration over practical ranges (0–45 and 2–20 mg/mL, respectively) on both diffusivity and fiber structure and found a decrease in diffusion rate and decrease in fiber length and organization with increased collagen concentration.19,27 Cross et al. performed an in-depth study of the relationship between collagen concentration and hydrogel properties, including mechanics and fiber structure, as well as cellular response.44 For further information on correlations of collagen concentration (as well as other fabrication parameters) with hydrogel properties, the reader is directed to the subsequent sections.
Control of collagen concentration in hydrogels is most easily and accurately achieved using lyophilized collagen, as the reconstitution step permits accurate weight measurement of dry collagen and volume measurement of solvent to obtain the stock solution concentration. The Sirius red colorimetric assay45 is commonly used to measure the quantity of soluble collagen in stock solutions.46
Polymerization temperature
Along with collagen content, temperature of polymerization significantly affects hydrogel properties. Because reaction kinetics are temperature dependent, self-assembly of collagen molecules occurs more rapidly at higher temperature and results in fibers with a lower number of bundled fibrils, resulting in a less-ordered structure26,47 and consequently altered mechanical, structural, and transport properties as discussed in later sections. While most previous studies have used hydrogels polymerized at 37°C to facilitate cell seeding and viability, polymerization at room temperature (20–26°C) or below is not uncommon but generally inhibits inclusion of cells within the hydrogel. Chrobak et al. found inconsistent hydrogel formation at polymerization temperatures below 19°C43; however, as other groups have successfully polymerized collagen hydrogels at temperatures down to 4°C, this is likely unique to their experimental protocol. Yang et al. and Raub et al. varied polymerization temperature between 4°C and 37°C to control fiber structure without necessitating variation in concentration and noted that polymerization at low temperatures produced the most desirable pore size for cellular proliferation.26,47 Similarly, Chrobak et al. found that gels formed at room temperature can be used to create microchannels with better stability and less degradation over time than those formed at 37°C.43 Table 1 summarizes polymerization temperatures found in the literature.
Table 1.
Polymerization Temperature of Collagen Hydrogels Used for Tissue Engineering
| Polymerization temperature (°C) | Reference(s) |
|---|---|
| Single temperature | |
| 4 (refrigeration) | 30 |
| 20–26 (room temperature) | 15, 28, 34, 41, 48–50 |
| 37 | 12, 13, 16, 19, 21, 27, 36, 40, 44, 51–78 |
| Range of temperatures | |
| 4, 21, 37 | 35 |
| 4, 14, 24, 37 | 26 |
| 12–37 | 79 |
| 23, 37 | 43 |
| Temperature gradient during polymerization | |
| 4→37 | 80 |
| (4, 26, 35)→4→(4, 26, 35) | 38 |
| (22, 27, 32)→37 | 47 |
Polymerization temperature is difficult to control accurately, as temperature-dependent fiber self-assembly initiates as soon as the solution is neutralized. Most groups work with solutions on ice or in an ice chamber to slow polymerization until the hydrogel is well mixed, at which time it is moved to a chamber at the appropriate polymerization temperature. Therefore, the exact temperature of the hydrogel during the initial stages of polymerization is heterogeneous and regulated by convection and conduction. These effects cannot be strictly controlled in practice; however, if fabrication procedures are consistent, mixing is performed rapidly, and the polymerizing solution is moved immediately from ice to a temperature-controlled chamber, then the properties of the final hydrogel are typically reproducible.
Polymerization pH
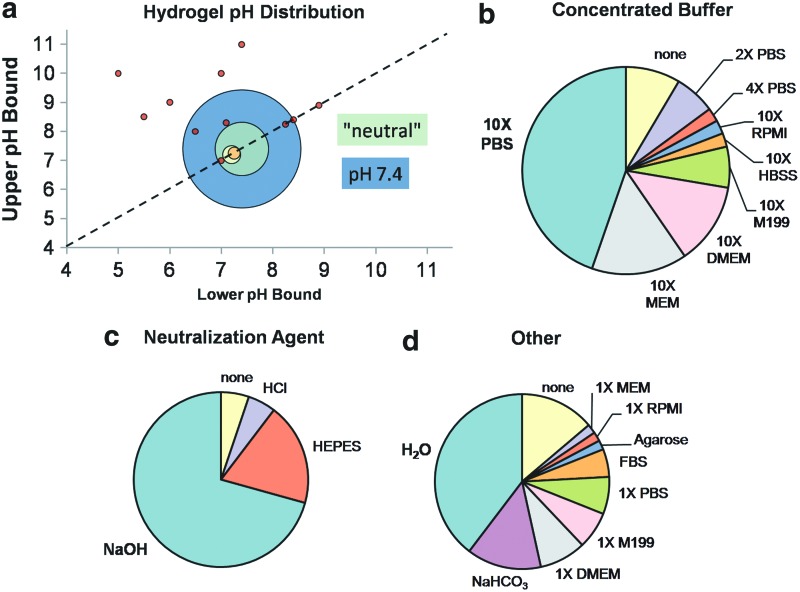
pH during fabrication strongly influences both structural and mechanical properties of the collagen hydrogel.34,47,50 Yamamura et al. created hydrogels with pH ranging from 5 to 10 and found a strong positive correlation between pH and compression modulus,13 and Raub et al. found similar trends with hydrogels polymerized at pH in the range 5.5–8.5.34 Sung et al. and Gobeaux et al. studied the influence of pH on fiber size and organization across pH ranges of 7.1–8.3 and 6–12, respectively, as discussed in detail in “Structure” section.50,80 Although gels polymerized at extreme pH may provide favorable properties for tissue mimics, for practical purposes pH of cellularized hydrogels is restricted to 7.4–8.4, as cell viability suffers outside this range.80,81 Achilli and Mantovani bypassed this restriction by first polymerizing hydrogels at pH=10 and subsequently rinsing with buffer to return the pH to 7.4 before seeding cells on the surface of the gel.35 Although adequate cell viability was achieved with this method, it is not realistic for experiments in which cells must be seeded throughout the gel.21 Figure 3 provides an overview of collagen hydrogel compositions in the literature (Supplementary Table S3). Similarly to Figure 2, Figure 3(a) is a bubble plot indicating pH of collagen hydrogels, where bubble position shows pH values and range (if any), bubble size represents frequency in the literature, and bubble color indicates bubbles of the same size. Many publications did not report pH values for their hydrogels, but instead stated that the solution was at “neutral” or “physiological” pH. For graphical purposes, these studies were grouped together and plotted in Figure 3 at pH=7.4 (green), but kept separate from those studies in which pH was specifically noted to be 7.4 (blue) so that the distinction could be visualized. Figure 3(b–d) shows the breakdown of buffers, neutralization agents, and other components found in collagen hydrogels, respectively. The most common component in each class is highlighted in bold in the legend.
FIG. 3.
Collagen hydrogel configurations used in the tissue engineering literature. (a) pH ranges. (b–d) Distribution of noncollagen hydrogel components. Components in bold occur most frequently and match the commercial BD/Vitrogen hydrogel preparation protocols. Source data can be found in Supplementary Table S3. Color images available online at www.liebertpub.com/teb
Since early hydrogel studies have been primarily concerned with comparison of cell response in two-dimensional versus 3D culture, hydrogels have typically been polymerized at a physiological pH at or near 7.4 (Fig. 3). As a result, published protocols and manufacturer guidelines use “recipes” for proportions of reagents required to obtain hydrogels at pH 7.4. Because pH is a fabrication parameter that can be exploited, at least within the range of cell viability (7.4–8.4), for design of tunable hydrogels, a single recipe is no longer adequate and calibration experiments must be performed.
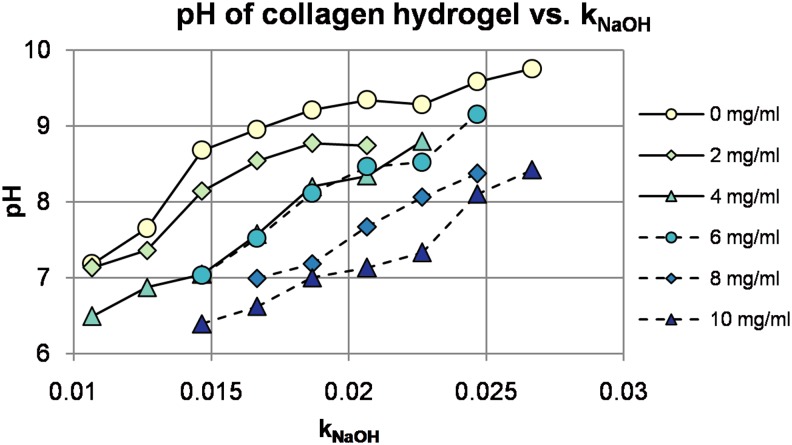
pH of collagen hydrogels is not only dependent on the ratio of neutralization agent to acid-solubilized collagen, but also dependent on buffer type and preparation, dilution ratio of collagen in hydrogel, and even absolute collagen concentration. Figure 3 indicates that one reagent stands out in each category: 10× phosphate-buffered saline (PBS) as the concentrated buffer, sodium hydroxide (NaOH) for neutralization, and distilled water for dilution. However, this unique combination of parameters was used in less than one-fourth of the studies surveyed for Figure 3 (Supplementary Table S3). Because of the variation in reagents comprising hydrogels used by different research groups (Fig. 3), pH should be verified for each individual configuration. A sample pH calibration for the reagents used by our group, with varying collagen concentration, is provided in Figure 4. Details of the experimental methodology used to obtain the data for Figure 4 are provided in Supplementary Data.
FIG. 4.
Dependence of hydrogel pH on NaOH fraction and absolute collagen concentration. Color images available online at www.liebertpub.com/teb
Collagen hydrogels are often fabricated using culture medium containing phenol red, which provides a useful visual indicator of pH. pH paper is also valuable for estimation of hydrogel pH, especially those fabricated using phenol-red-free media or PBS, although it lacks sufficient accuracy for studies that require precise pH regulation. Electronic pH probes provide the most accurate measurements; however, they require immersion of the electrode tip in solution and, therefore, large sample volumes that may be impractical for some applications.
Ionic strength
Finally, ionic strength has been demonstrated to affect hydrogel polymerization and subsequently structural and mechanical properties as described in later sections.47 Gobeaux et al. varied ionic strength from 24 to 1300 mM for very-high-concentration gels (>75 mg/mL) polymerized at pH of 7.4, and measured resulting changes in hydrogel optical and structural properties.50 Achilli and Mantovani varied ionic strength over a much smaller range, from 64.2 to 174 mM, but still they found a significant effect on polymerization and mechanical properties.35
Although few publications directly report ionic strength of collagen hydrogels, most recipes utilize 10× buffers (media or PBS) diluted to 1× strength in the final hydrogel in order to obtain hydrogel ionic strength similar to that of standard buffer solutions (Supplementary Table S3). Reported values using such recipes fall within the range 130–300 mM.12,21,34,47,54,60,70,76,79 Finer control of ionic strength has been achieved by adjusting the concentration of phosphate in the buffer or adding sodium chloride (NaCl) to the hydrogel.35,50,79 Ionic strength is seldom measured directly for collagen hydrogels, but rather it is calculated from known or estimated concentrations of all ionic compounds present in solution.79
Collagen Hydrogel Characteristics
The following sections describe polymerization, mechanics, structure, and transport of collagen hydrogels. Each section provides a brief overview of the importance of the parameter for tissue engineering as well as information about current measurement techniques. Finally, correlations of each property with fabrication parameters and other properties are described.
Polymerization
As noted in previous sections, the kinetics of collagen fiber network assembly—a multistep process, including fibril formation, fiber nucleation and development, and crosslinking—is dependent on almost all fabrication parameters12,26,38,47,50,79; further, the addition of external crosslinking agents also affects fibrillogenesis.21,64 Polymerization kinetics provide insight into the molecular processes linking controllable fabrication parameters with the final state of the hydrogel. For example, Kreger et al. examined how collagen source and extraction method affect material properties.12 Under identical polymerization conditions, they determined that acid-solubilized collagens polymerize more rapidly than pepsin-digested collagens and noted that these results are consistent with previous research that indicates that telopeptides, which are damaged or destroyed by pepsin digestion, play a key role in fiber nucleation.12
The importance of characterization of polymerization becomes clear when one considers that the ideal polymerization temperature or pH to achieve target structural or mechanical properties may not align with optimum conditions for viability in cell-seeded gels. In particular, lag time before polymerization begins and duration of polymerization are critical parameters for the design of mixing and incubation protocols to maximize viability.
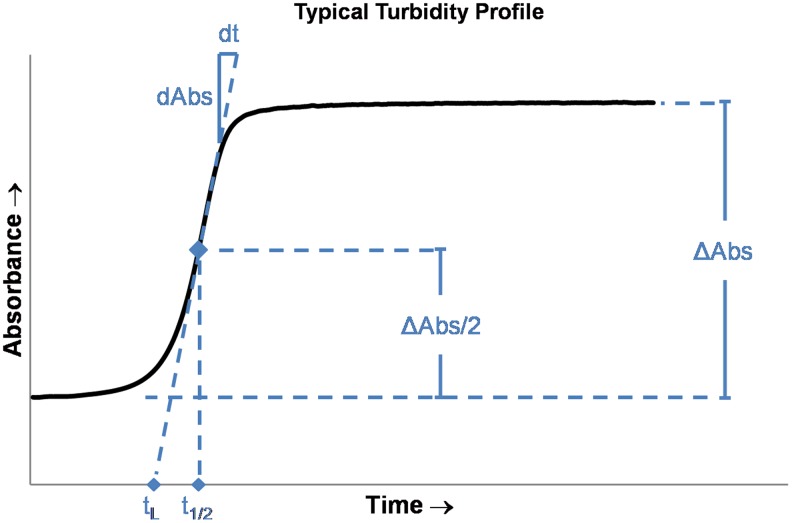
Fibrillogenesis is frequently measured through spectrophotometric measurements of hydrogel turbidity, which have been demonstrated to correlate with degree of polymerization.12,38 Most previous works have measured turbidity at 31321,38,79 or 405 nm12,64 using standard spectrophotometric equipment with temperature-controlled sample chambers. Total change in absorbance (ΔAbs) as well as three temporal parameters—lag time (tL), polymerization rate (dAbs/dt), and half-time (t1/2)—can be easily quantified from typical turbidity data (Fig. 5). Duration of polymerization (twice the half-time) is strongly dependent on temperature but it is typically on the order of 10 min to several hours12,35 for most applications.
FIG. 5.
Diagram of a typical spectrophotometric measurement for quantification of polymerization kinetics. The polymerization half-time t1/2 is defined as the time at which half of the total change in absorbance ΔAbs/2 is attained. The rate of change of absorbance at the half-time is defined as the polymerization rate dAbs/dt, while the lag time is defined as the zero-absorbance intercept of the line with slope dAbs/dt and intercepting (t1/2, ΔAbs/2). Color images available online at www.liebertpub.com/teb
Mechanics
The influence of ECM stiffness on cell biology—including morphology, cytoskeletal stiffness, adhesion, migration, and proliferation—has been well-established; however, these effects have not yet been quantified for many cell types.9,18,40,82–85 The relationship between tissue elasticity and cell signaling and response has significant implications for many types of disease, especially cancer. In fact, increased ECM stiffness, along with elevated collagen concentration, is a hallmark of many tumors.58,86,87 ECM stiffness has also been linked with endothelial integrity and consequently regulation of angiogenesis.13,77,88,89 The link between tissue stiffness and cell response has two major implications for tissue engineering using collagen hydrogels. For in vitro hydrogels meant to mimic specific tissues, hydrogel mechanical properties must be matched to the corresponding in vivo tissue properties in order to obtain physiologic behavior of cells in the hydrogel. Alternatively, hydrogel mechanical properties must be varied in a controlled fashion in order to answer outstanding questions about cell behavior in normal and disease states.
While the term “stiffness” is used in the preceding paragraph in a general sense to describe the mechanical resistance to deformation of a material (tissue or hydrogel), it cannot be used for meaningful discussions of material properties because it is strictly an extrinsic, structure-dependent property. Mechanical properties must first be defined in terms of specific types of deformation: tension, compression, or shear. All of these deformation modes are commonly used in practice for characterization of biological tissues and care must be taken to avoid attempts to compare measurements obtained in different deformation modes. The elastic moduli of biological tissues have been reported to range from 102 Pa (brain) to 108 Pa (tendon).90 However, due to the different deformation modalities used to obtain these and similar in vivo data (elastic modulus can refer to either tensile or compression modulus), comparisons can seldom be drawn between such measurements.17,84,90,91 Further, because soft biological tissues as well as collagen hydrogels are nonlinear viscoelastic materials, mechanical properties are both time and strain dependent. If only a single value is reported for an experimental study, then an implicit approximation of linear elasticity (whether acknowledged or not) is made, and special attention must be paid to the experimental conditions under which the measurement was obtained.
Characterization of collagen hydrogel mechanical properties must be performed so that resulting measurements can be matched with in vivo data; this implies matching both deformation modality and temporal/strain characteristics. Although full mechanical characterization of viscoelastic parameters under all three deformation modalities is possible using dynamic mechanical analysis in tension, compression, and torsion, this is not practical for many researchers and further corresponding data for in vivo tissue are seldom available. As an alternative, if the physiologically relevant type of deformation, strain range, and timescale can be identified, then more limited characterization may be sufficient for adequate ECM matching. For example, tendon and muscle often undergo rapid tension and relatively high strains, and are further anisotropic. In contrast, physiological deformation of brain or breast tissue is more commonly quasi-steady-state compression. Fortunately, most tissues undergo relatively small strains under physiological conditions and exhibit linear behavior within this regime, simplifying analysis.91
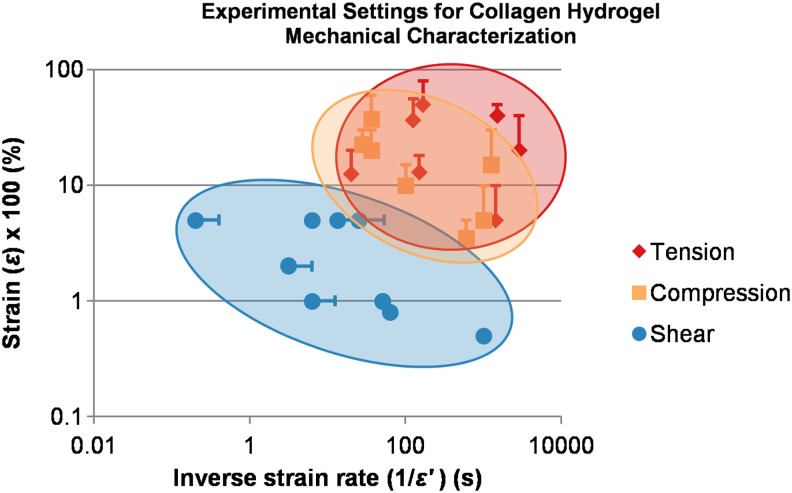
A significant amount of literature exists for mechanical characterization of collagen hydrogels covering a wide range of measurement methodologies, time, and strain scales. Figure 6 provides a comparison of experimental settings used to estimate tensile, compression, and shear moduli. The inverse of the strain rate is plotted on the abscissa as a characteristic time, while the strain (expressed in %) is plotted on the ordinate as a characteristic length. As an example, Yamamura et al. performed confined compression of collagen hydrogels deforming at 0.01 (1%) strain/s from 5% to 15% strain; this study is plotted at (100 s, 10%) with a bar extending to (100 s, 15%) to indicate the strain range used to estimate compression modulus.13 From the figure, it is clear that tensile and compression tests are generally performed with long time scales and at relatively high strains in order to best estimate relaxation moduli, while oscillatory shear measurements are performed at much shorter time scales and smaller strains in order to obtain additional information about the viscous component of deformation. Chandran and Barocas provide an excellent review of experimental methodologies for each deformation mode, discussing underlying physics and highlighting advantages and limitations of each.65
FIG. 6.
Modes and physical scales of collagen hydrogel mechanical characterization. Data points corresponding to studies that investigate a range of scales are centered at the mean value and plotted with a bar extending to the upper bound of the range investigated. Because of the log-log scale, lower bounds are not plotted. Source data and application notes can be found in Supplementary Table S4. Color images available online at www.liebertpub.com/teb
In all deformation modes, it is critical to maintain sample integrity by humidification or immersion in a buffer solution during measurements; further, high-resolution load cells must often be used in order to resolve the small forces associated with compliant hydrogels. Tensile tests in particular require simultaneous imaging of the specimen gauge region in order to quantify the cross-sectional area and obtain an accurate measurement of true strain. In tension, failure stress and strain are often of interest in addition to the elastic modulus. Compression testing can be performed using either confined or unconfined samples. In both cases, accurate positioning of the platen at the sample surface to avoid sample preloading can be difficult. Surface tension at the interface can further complicate matters; this effect has previously been minimized through the use of a porous platen (especially for confined compression)44,65 or hydrophobic platen.92 Oscillatory shear measurements generally require initial frequency and shear sweeps to determine the boundaries of linear response; most of the literature has concluded that ∼1 Hz and 5% strain produce linear response in collagen hydrogels. Although atomic force microscopy (AFM) is extensively used for localized tissue and cell mechanical measurements,7,84 it is poorly suited for measurement of bulk properties due to the heterogeneity of collagen hydrogels.93
The effect on mechanical properties of each of the fabrication parameters described in this review has been quantified. Both collagen source and solubilization affect hydrogel mechanics.12 All studies that investigate the effect of collagen concentration on hydrogel elastic and shear moduli demonstrate positive correlation.12,41,44,60,76,77 Similarly, increasing pH increases all metrics of collagen hydrogel modulus.13,34,35,60 The relationship between ionic strength and compression modulus is less straightforward, and appears to be interdependent with pH and temperature.35 Crosslinking agents, by design, increase mechanical properties of hydrogels.15,21,26,28,30,41,85
In general, there is poor agreement between quantitative mechanical measurements in the literature, not only due to differences in deformation mode and timescale but also due to variation in hydrogel fabrication parameters. This becomes especially apparent when conflicting correlations are found; for instance, Achilli and Mantovani determined that increasing polymerization temperature from 4°C to 37°C decreases mechanical properties, with negligible correlation at pH=7 but strong correlation at pH=10.35 These results contradict the findings of Raub et al. several years earlier26 where mechanical properties increased by two orders of magnitude over the same temperature range, but at a pH of 7.4. Differences between these studies, such as collagen concentration (Raub et al.26: 2 mg/mL; Achilli and Mantovani35: 4 mg/mL) and deformation mode (Achilli and Mantovani35 characterized tensile and compression moduli while Raub et al.26 investigated only shear modulus), may explain the discrepancy; however, further characterization is clearly necessary to fully understand the relationship between hydrogel mechanical properties and polymerization temperature. Further, more detailed reporting of hydrogel fabrication protocols in order to highlight similarities and differences would assist the reader in accurately interpreting mechanical characterization data.
Structure
It is well known that the fiber structure of ECM can regulate cellular morphology, proliferation, migration, and gene expression,9,13,40,47,58,74 and is therefore an important parameter for hydrogel characterization. Fiber structure in collagen gels is complex and often defined quantitatively in terms of parameters, such as mean fiber diameter D, fiber density (or volume fraction), pore size P, degree of crosslinking (or number of crosslinks per fiber), and orientation Ø (Fig. 7). These parameters can be used to help elucidate the cellular characteristics described previously; for example, fiber orientation describes anisotropy of the material and can therefore be a predictor of directionality of cell migration.
FIG. 7.
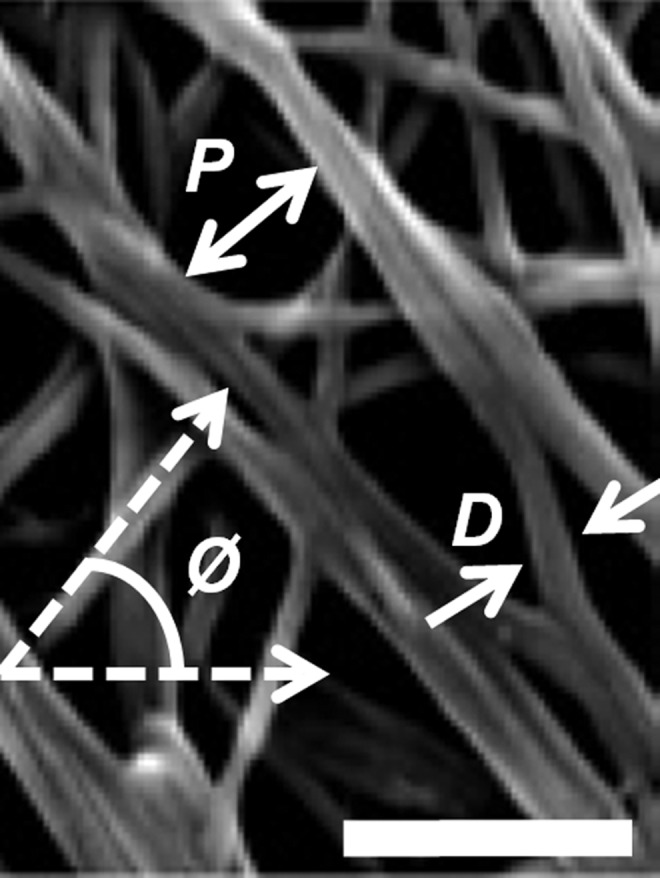
Representative scanning electron microscopy (SEM) image of collagen hydrogel33 annotated to indicate commonly quantified fiber structure parameters P (pore size), D (fiber diameter), and Ø (orientation). Scale bar=500 nm. Figure reproduced with permission of the publisher.
Several methodologies for measurement of hydrogel fiber structure are available. AFM has similar limitations for fiber structure imaging as for mechanical characterization; measurements are too localized for accurate estimation of bulk network properties. Historically, collagen hydrogel fibers have been imaged using scanning electron microscopy (SEM)54,70,75 and transmission electron microscopy38,79 for qualitative/semiquantitative analysis of network and fibril structures, respectively. Although these methods provide high-resolution images and preserve relative information about fiber dimensions,26 they require significant sample manipulation, drying, and chemical processing. These can lead to shrinkage and collapse of the fiber structure, which prevent quantitative analysis of 3D fiber structure and SEM images.14,20,26,34,80 While more advanced electron microscopy techniques, such as cryo-SEM and environmental SEM, have been developed, which do not require sample dehydration, these still necessitate high vacuum or sample fixation that can alter the structure of the hydrogel.
Although electron microscopy remains in use for high-resolution imaging of hydrogel topology where quantitative results are not necessary, its limitations have led to the development of optical imaging techniques for fiber structure measurement of fully hydrated, unmodified hydrogels. These include two closely related techniques, two-photon fluorescence (TPF) and second-harmonic generation (SHG), as well as confocal reflectance microscopy (CRM) and confocal fluorescence microscopy, all of which have become popular modalities for recording fiber structure as they can be used to obtain images of gels in the hydrated state.20,26 Figure 8 provides a qualitative comparison of sample images acquired using each of these modalities.
FIG. 8.
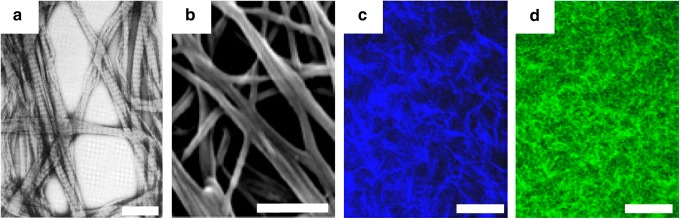
Representative images of collagen hydrogels using various modalities. (a) Transmission electron microscopy37 (scale bar=500 nm), (b) SEM33 (scale bar=500 nm), (c) second-harmonic generation33 (scale bar=50 μm), and (d) confocal reflectance microscopy33 (scale bar=50 μm). Figures reproduced with permission of the publishers. Color images available online at www.liebertpub.com/teb
TPF and SHG are both based on two-photon laser excitation and therefore have the advantages associated with near-infrared illumination: that is, high penetration depth and low phototoxicity. Further, the use of fluorescence or second-harmonic generation provides good signal-to-noise ratio due to suppression of background light. However, they require high laser power and have difficulty penetrating dense tissue.20 CRM, although somewhat noisier than TPF or SHG due to nonspecificity of reflected light, is more commonly used, most likely because of availability of necessary equipment. CRM is an established technique in which images are formed by the light reflected from collagen fibers. Because reflected light does not undergo a spectral shift, CRM requires replacement of the microscope's fluorescent excitation filter with a beamsplitter (typically 80/20) and removal of the emission filters. This permits the reflected light to reach the microscope detector, which is usually a photomultiplier tube for laser-scanning confocal microscopes. CRM has been successfully performed across a range of wavelengths (405–543 nm) and magnifications (40× to 63× using water or oil-immersion objectives).
For all three optical modalities, the distance of the focal plane into the hydrogel is a critical parameter. It has been demonstrated that collagen fibers form a significantly different network at the surface of the coverslip than within the gel; therefore, images acquired too close to the coverslip produce misleading information and are likely a source of inconsistencies in fiber structure data in the literature.40 Therefore, the distance of the focal plane from the sample surface should be clearly reported with all fiber structure data. Further, the overall hydrogel thickness has been shown to influence hydrogel network formation for gels at or below 1.5 mg/mL collagen concentration40; although hydrogels for 3D tissue engineering generally require higher concentrations at which this effect is negligible, this dimension should nonetheless be clearly stated in experimental methodologies.
Regardless of imaging modality, quantitative analysis of fiber network images is necessary for extraction of structural parameters. Although many researchers have investigated collagen hydrogel network structure, no standard automated image analysis technique has yet emerged and therefore wide variation exists in published structural characteristics. Approaches in recent publications utilize algorithms exploiting a wide range of image segmentation and thresholding techniques,12,21,26,27,34,41,44,47,49,64,76 grid-based estimation,27,40,49 and even Fourier transforms and autocorrelation.34,40,68 Further, manual estimation of dimensions of user-identified fibers and pores is not uncommon.12,13,21,34,37,54,62,75 Finally, existing algorithms do not appear to be robust enough to accurately analyze images with a high density of fibers, a parameter primarily regulated by collagen concentration. For example, although Kreger et al. investigated polymerization kinetics and mechanical properties of 0.5–4 mg/mL hydrogels, fiber structure data are notably absent for samples above 3 mg/mL.12 With one exception,44 which presents fiber structure images inconsistent with other publications, no studies have been found that present fiber structure analysis of collagen hydrogels >4 mg/mL collagen concentration. This is a significant limitation, as collagen hydrogels below this concentration, while initially widely studied, have not been successfully matched to in vivo tissues and in particular lack sufficient structural integrity to support microfabrication and perfusion.19,43,44,53
All collagen fabrication parameters discussed in this review have been demonstrated to influence one or more metrics of fiber structure. Increasing collagen concentration has been correlated with increased fiber density and reduced pore size but has no effect on fiber diameter.12,19,41,50,60,64,70,71,75 However, increasing pH or temperature promotes electrostatic interactions and fiber nucleation, accelerating polymerization and produce fibers with reduced diameter and networks with small pore size.26,34,35,47,60,80 Similarly, decreasing ionic strength of the hydrogel produces decreased fiber diameter and pore size.35,50
Although correlation between collagen source and fiber structure is difficult due to differences in collagen extraction and hydrogel preparation, it has been shown that the monomer/oligomer ratio can be used to control the degree of crosslinking within the collagen network.76 In a review by Wolf et al., it is shown that pepsin-digested collagens, most often those produced from bovine tendon or porcine skin (Fig. 1 and Supplementary Table S1), produce fiber networks with long fibers and large pores compared with nonpepsin-treated collagens such as that extracted from rat tail tendon (Fig. 1 and Supplementary Table S1).20 They conclude that this is due to the reduction in fiber nucleation sites associated with telopeptide removal.20 Carey et al. suggested that the culture medium used in hydrogel fabrication (PBS, MEM, DMEM, or M199) influences hydrogel fiber structure but that this relationship is negligible relative to the effect of other fabrication parameters.40 Sung et al. indicated a difference in fiber shape depending on the neutralization medium used to fabricate the hydrogel (HEPES or NaOH).80
Fiber structure has often been measured in conjunction with other material properties, such as polymerization kinetics,12,21,38,50,64,79 mechanical properties,12,13,16,21,26,30,34,35,41,44,47,54,55,60,72,76 or diffusivity.19,27,49,72 The goal of such experiments is usually development of predictive models that eliminate the need for time-consuming, expensive measurement of all relevant properties. However, because of the interdependence of hydrogel properties on fabrication parameters, such correlations have value only under limited conditions.
Transport
Oxygen, nutrients, and other soluble bioactive molecules in avascular tissue are primarily transported by diffusion.27,94 Quantification of diffusion processes is a key step to understanding transport limitations within tumors19,87 as well as designing effective drug-delivery strategies.27 Because diffusion requirements are application dependent, thorough characterization of the transport properties of collagen hydrogels is necessary before hydrogels can be designed to match desired properties.10
The diffusive capacity of a hydrogel, characterized by the diffusion coefficient D, depends on both the gel fiber structure and the size and shape of the diffusing species.10 The effective diffusion coefficient of a molecule in a collagen hydrogel can be estimated from the diffusion coefficient of the molecule in the interstitial fluid if the fiber structure (permeability and fiber shape characteristics) is known8,19; however, a far more reliable estimate can be obtained by direct measurement using the method of fluorescence recovery after photobleaching (FRAP).19,94–96 FRAP is a well-established experimental technique in which a fluorescent probe is homogenously distributed in the diffusion medium, locally bleached, and imaged in a time series to produce an intensity decay profile from which the diffusion coefficient can be measured based on Fick's second law. Using a confocal microscopy, high-resolution data can be obtained and no calibration is necessary. For further details on the method and analysis, see Seiffert and Oppermann.95
The fluorescent probe used in FRAP measurements in collagen hydrogels is often an easily labeled model protein such as dextran19,27,49,94 because it is available in a wide range of molecular weights and thus can be matched to a molecule of interest. Bioactive molecules in ECM range from small soluble cytokines and growth factors, with molecular weight on the order of 100–101 kDa,10,94,97,98 to large insoluble macromolecules, such as glycosaminoglycans (GAGs) and proteoglycans, with molecular weight on the order of 102–103 kDa.94 Examples of other molecular probes that have been used for FRAP diffusivity measurements include bovine serum albumin and lactalbumin.19 It should be noted that care must be taken when performing FRAP using a molecule different than the molecule of interest as molecular weight matching may not be sufficient to mimic all ECM interactions.94
For FRAP measurements, the thickness of the hydrogel is typically limited to several 100 μm to limit out-of-plane diffusion and background noise. It should be noted that, due to the possible dependence of fiber structure on gel thickness,40 this may introduce error when extrapolating FRAP diffusion measurements to thicker hydrogels. FRAP samples are usually formed between coverslips, on special multiwell slides, or in similar molds.19,27,49 The fluorescent probe can be introduced during hydrogel fabrication49 or by soaking the polymerized gel in probe-containing medium for 12–72 h.19,27,72,94 FRAP in collagen hydrogels has most often been performed using laser-scanning confocal microscopy. While wide variation exists in the literature in choice of imaging parameters, such as resolution, magnification, and bleach region size and shape, most measurements require use of all available lasers at full power to obtain sufficient bleaching.
Ramanujan et al. successfully used FRAP to measure the diffusion coefficient of dextrans with a wide range of molecular weights (4–2000 kDa) in collagen hydrogels and correlated decreased diffusivity with an increase in collagen concentration.19 Erikson et al. performed similar experiments with 2000-kDa dextran in collagen supplemented with decorin or hyaluronan,27 and Gillette et al. measured the diffusion coefficient of 3–500-kDa dextrans in collagen/alginate hydrogels.49 Stylianopoulos et al. predicted using numerical models that the ionic strength of the hydrogel solution can affect the diffusion of charged particles.78 To our knowledge, the effects of collagen source and solubilization, hydrogel pH, and hydrogel polymerization temperature on diffusion rates in collagen hydrogels have not yet been experimentally investigated.
Concluding Remarks
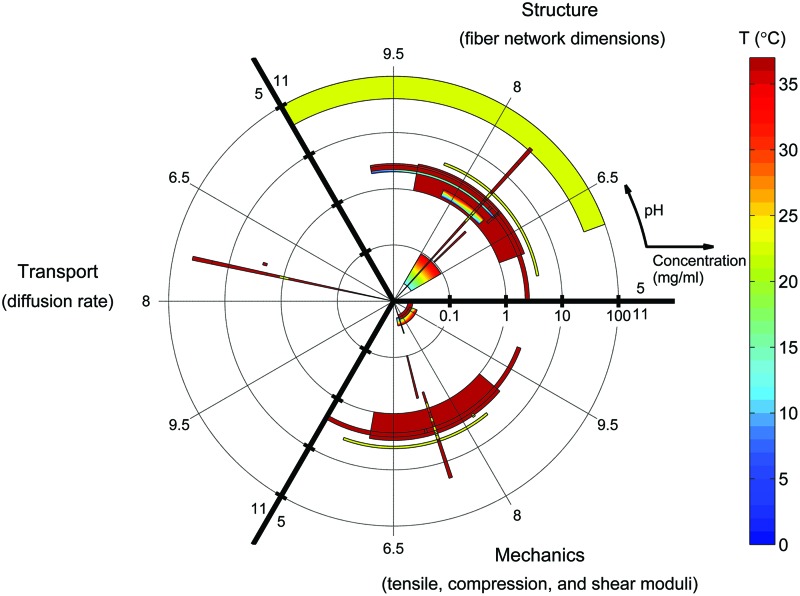
Significant progress has been made in recent years toward characterization of polymerization, mechanical, structural, and transport properties of collagen hydrogels. Figure 9 is a log-polar graphical representation of current knowledge of collagen hydrogel properties in a 3D fabrication parameter space. Each material property investigated—structure, transport, or mechanics—is plotted in one-third of the figure. The fabrication parameters collagen concentration and polymerization pH are plotted on the radial and angular axes, respectively, while polymerization temperature is identified by color. Each object on the plot represents the parameter space investigated in a single publication, with object size indicating concentration and pH ranges and a color gradient used to indicate the maximum and minimum color included in the investigation. In this figure, no distinction is made between studies that investigate hydrogels polymerized at multiple discrete temperatures and studies that investigate the effect of temperature gradients during polymerization.
FIG. 9.
State of collagen hydrogel characterization. Each object represents the fabrication parameter ranges covered by a single experimental study from the literature. Log(collagen concentration) and polymerization pH are plotted as radius and angle, respectively. Polymerization temperature is plotted as a color contour. Studies outside the ranges (0–100) mg/mL, (5–11) pH units, and (0–37) °C are not shown. Diffusing-molecule hydrodynamic radius and deformation mode for mechanical characterization are not represented in this figure. Color images available online at www.liebertpub.com/teb
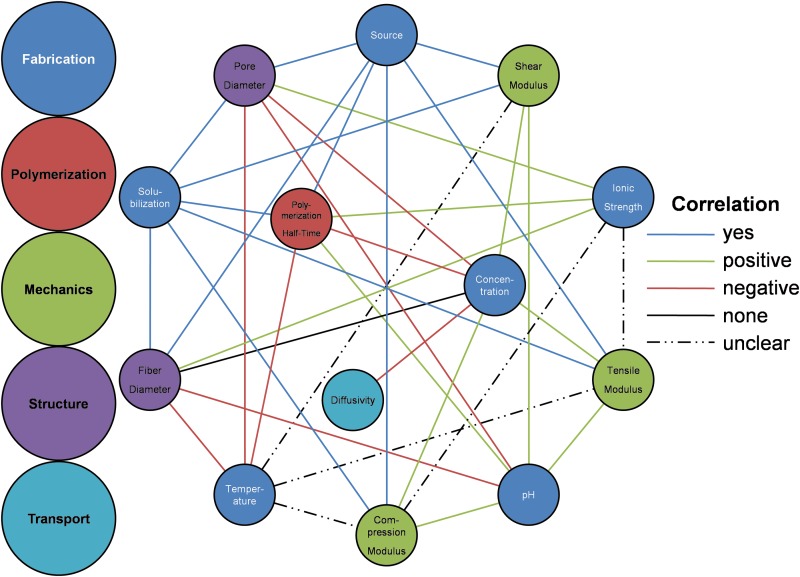
Figure 9 quantifies previously characterized hydrogel configurations; however, only three fabrication parameters could be shown and material properties are categorically presented. Figure 10 provides an overview of known relationships between all fabrication parameters and all material properties described in this review. Each parameter-property correlation is indicated by the color of the line. Green and red links indicate positive and negative correlations, respectively. Blue links are used to indicate relationships that have been investigated but they involve non-numerical fabrication parameters (such as collagen source). The black link signifies that studies have conclusively determined that no significant correlation exists between collagen concentration and fiber diameter. Dashed lines indicate fabrication-property relationships that are not well defined; in some cases this means that conflicting data can be found in the literature, while in other cases a single study has determined that this relationship is complex and dependent on other fabrication parameters. As an example, contradictory correlations have been measured between polymerization temperature and all mechanical properties. Finally, missing lines signify relationships for which no experimental studies were found.
FIG. 10.
Correlations between collagen hydrogel fabrication parameters and material properties. Color images available online at www.liebertpub.com/teb
Figures 9 and 10 highlight the state of collagen characterization as well as areas requiring further investigation. For example, very little data are available in the literature for diffusion of bioactive molecules in collagen hydrogels. The relationships between diffusivity and many fabrication parameters, including polymerization temperature, polymerization pH, and collagen source and solubilization (not shown in Fig. 9), remain unknown. Figures 9 and 10 are meant to be used as references for generation of missing characterization data as well as for design of experiments within the previously studied fabrication space in order to avoid necessitating new characterization.
Not only is the fabrication parameter space of collagen hydrogels broad and not adequately characterized, but also the full potential of many aspects of hydrogel characterization is yet to be realized. The scientific community has not yet reached consensus on a deformation modality for mechanical characterization, even within specialized applications, such as microvascular tissue engineering or tumor engineering. Because individual studies are often restricted to measurements using a single deformation modality, it remains unclear how different modalities correlate with one another. With respect to fiber structure characterization, accurate methods for quantification of structural parameters in hydrogels above 3 mg/mL are generally not available. This is a significant limitation, as the current trend is toward the use of higher concentration hydrogels due to improved replication of the properties of in vivo tissue. Finally, a physics-based model that could predict material properties as a function of fabrication parameters would be of great benefit to the entire community working with collagen hydrogels.
In this review, we note that the literature appears to contain contradictions with respect to material properties of collagen hydrogels, which we trace back to differences in fabrication protocols and characterization techniques. To minimize such ambiguities, it is necessary that researchers report fabrication and measurement protocols in increased detail, including seemingly inconsequential information. For example, all fabrication parameters should be reported: collagen source, solubilization, concentration in the hydrogel, polymerization temperature, polymerization pH, and ionic strength. Fiber structure and mechanical strength are often critical parameters for engineered tissues, and we recommend that they be characterized for collagen hydrogels for most applications. If fiber structure is quantified, then details about the algorithms used should be included or provided in accessible references. Based on the literature, SHG and CRM are the recommended modalities for accurate fiber structure quantification. Mechanical characterization should be performed using carefully designed strain rates and ranges. Not only must these parameters be clearly reported, but also the criteria for their selection should be explained. In general, low strain rates and small deformations will provide the best approximation to linear elasticity as well as the most relevant information for mimicking in vivo tissues. If possible, full viscoelastic characterization should be performed. Such transparency will improve interpretability of the data on collagen hydrogel characterization, and facilitate future research efforts in this promising field.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. Raffaella De Vita for illuminating discussions of hydrogel mechanics and viscoelastic characterization. Funding for this study was provided by the National Science Foundation Early CAREER Award CBET 0955072, a National Institute of Health Grant IR21CA158454-01A1, a Clare Boothe Luce Graduate Fellowship, a Virginia Space Grant Consortium Graduate STEM Research Fellowship, the Virginia Tech AEThER Lab, and the Virginia Tech Tissue Engineering, Nanotechnology, and Cancer Research Lab. We gratefully acknowledge support provided by the MBEDS (Multiscale Bio-Engineered Devices and Systems) Center of the Virginia Tech Institute of Critical Technologies and Applied Sciences (ICTAS).
Disclosure Statement
No competing financial interests exist.
References
- 1.Kumar V., Brewster L., Caves J., and Chaikof E.Tissue engineering of blood vessels: functional requirements, progress, and future challenges. Cardiovasc Eng Technol 2,137, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ingram M., Techy G.B., Ward B.R., Imam S.A., Atkinson R., Ho H., et al. Tissue engineered tumor models. Biotech Histochem 85,213, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Sung J.H., and Shuler M.L.Microtechnology for mimicking in vivo tissue environment. Ann Biomed Eng 40,1289, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Buchanan C., and Rylander M.N.Microfluidic culture models to study the hydrodynamics of tumor progression and therapeutic response. Biotechnol Bioeng 110,2063, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Tibbitt M.W., and Anseth K.S.Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol Bioeng 103,655, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fischbach C., Chen R., Matsumoto T., Schmelzle T., Brugge J.S., Polverini P.J., et al Engineering tumors with 3D scaffolds. Nat Methods 4,855, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Kloxin A.M., Kloxin C.J., Bowman C.N., and Anseth K.S.Mechanical properties of cellularly responsive hydrogels and their experimental determination. Adv Mater 22,3484, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoffman A.S.Hydrogels for biomedical applications. Adv Drug Deliv Rev 54,3, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Aurand E.R., Lampe K.J., and Bjugstad K.B.Defining and designing polymers and hydrogels for neural tissue engineering. Neurosci Res 72,199, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drury J.L., and Mooney D.J.Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials 24,4337, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Parenteau-Bareil R., Gauvin R., and Berthod F.Collagen-based biomaterials for tissue engineering applications. Materials 3,1863, 2010 [Google Scholar]
- 12.Kreger S.T., Bell B.J., Bailey J., Stites E., Kuske J., Waisner B., et al. Polymerization and matrix physical properties as important design considerations for soluble collagen formulations. Biopolymers 93,690, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamamura N., Sudo R., Ikeda M., and Tanishita K.Effects of the mechanical properties of collagen gel on the in vitro formation of microvessel networks by endothelial cells. Tissue Eng 13,1443, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Abraham L.C., Zuena E., Perez-Ramirez B., and Kaplan D.L.Guide to collagen characterization for biomaterial studies. J Biomed Mater Res B Appl Biomater 87B,264, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Charulatha V., and Rajaram A.Influence of different crosslinking treatments on the physical properties of collagen membranes. Biomaterials 24,759, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Ulrich T.A., Jain A., Tanner K., MacKay J.L., and Kumar S.Probing cellular mechanobiology in three-dimensional culture with collagen-agarose matrices. Biomaterials 31,1875, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Gribova V., Crouzier T., and Picart C.A material's point of view on recent developments of polymeric biomaterials: control of mechanical and biochemical properties. J Mater Chem 21,14354, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levy-Mishali M., Zoldan J., and Levenberg S.Effect of scaffold stiffness on myoblast differentiation. Tissue Eng Part A 15,935, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Ramanujan S., Pluen A., McKee T.D., Brown E.B., Boucher Y., and Jain R.K.Diffusion and convection in collagen gels: implications for transport in the tumor interstitium. Biophys J 83,1650, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wolf K., Alexander S., Schacht V., Coussens L.M., von Andrian U.H., van Rheenen J., et al. Collagen-based cell migration models in vitro and in vivo. Sem Cell Dev Biol 20,931, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stuart K., and Panitch A.Influence of chondroitin sulfate on collagen gel structure and mechanical properties at physiologically relevant levels. Biopolymers 89,841, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Gronau G., Krishnaji S.T., Kinahan M.E., Giesa T., Wong J.Y., Kaplan D.L., et al. A review of combined experimental and computational procedures for assessing biopolymer structure-process-property relationships. Biomaterials 33,8240, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu P., Weaver V.M., and Werb Z.The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol 196,395, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Billiet T., Vandenhaute M., Schelfhout J., Van Vlierberghe S., and Dubruel P.A review of trends and limitations in hydrogel-rapid prototyping for tissue engineering. Biomaterials 33,6020, 2012 [DOI] [PubMed] [Google Scholar]
- 25.Miller E.J., and Rhodes R.K.Preparation and characterization of the different types of collagen. Methods Enzymol 82Pt A,33, 1982 [DOI] [PubMed] [Google Scholar]
- 26.Raub C.B., Suresh V., Krasieva T., Lyubovitsky J., Mih J.D., Putnam A.J., et al. Noninvasive assessment of collagen gel microstructure and mechanics using multiphoton microscopy. Biophys J 92,2212, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Erikson A., Andersen H.N., Naess S.N., Sikorski P., and Davies Cde L.Physical and chemical modifications of collagen gels: impact on diffusion. Biopolymers 89,135, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Saddiq Z.A., Barbenel J.C., and Grant M.H.The mechanical strength of collagen gels containing glycosaminoglycans and populated with fibroblasts. J Biomed Mater Res A 89,697, 2009 [DOI] [PubMed] [Google Scholar]
- 29.Wolf K., Muller R., Borgmann S., Brocker E.B., and Friedl P.Amoeboid shape change and contact guidance: T-lymphocyte crawling through fibrillar collagen is independent of matrix remodeling by MMPs and other proteases. Blood 102,3262, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Sheu M.T., Huang J.C., Yeh G.C., and Ho H.O.Characterization of collagen gel solutions and collagen matrices for cell culture. Biomaterials 22,1713, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Lu P., Takai K., Weaver V.M., and Werb Z.Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol 3,a005058, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baaijens F., Bouten C., and Driessen N.Modeling collagen remodeling. J Biomech 43,166, 2010 [DOI] [PubMed] [Google Scholar]
- 33.Kadler K.E., Baldock C., Bella J., and Boot-Handford R.P.Collagens at a glance. J Cell Sci 120,1955, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Raub C.B., Unruh J., Suresh V., Krasieva T., Lindmo T., Gratton E., et al. Image correlation spectroscopy of multiphoton images correlates with collagen mechanical properties. Biophys J 94,2361, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Achilli M., and Mantovani D.Tailoring mechanical properties of collagen-based scaffolds for vascular tissue engineering: the effects of pH, temperature and ionic strength on gelation. Polymers 2,664, 2010 [Google Scholar]
- 36.Gentleman E., Lay A.N., Dickerson D.A., Nauman E.A., Livesay G.A., and Dee K.C.Mechanical characterization of collagen fibers and scaffolds for tissue engineering. Biomaterials 24,3805, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Parenteau-Bareil R., Gauvin R., Cliche S., Gariepy C., Germain L., and Berthod F.Comparative study of bovine, porcine and avian collagens for the production of a tissue engineered dermis. Acta Biomater 7,3757, 2011 [DOI] [PubMed] [Google Scholar]
- 38.Gelman R.A., Poppke D.C., and Piez K.A.Collagen fibril formation in vitro. The role of the nonhelical terminal regions. J Biol Chem 254,11741, 1979 [PubMed] [Google Scholar]
- 39.Walters B.D., and Stegemann J.P.Strategies for directing the structure and function of three-dimensional collagen biomaterials across length scales. Acta Biomater 10,1488, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carey S.P., Kraning-Rush C.M., Williams R.M., and Reinhart-King C.A.Biophysical control of invasive tumor cell behavior by extracellular matrix microarchitecture. Biomaterials 33,4157, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miron-Mendoza M., Seemann J., and Grinnell F.The differential regulation of cell motile activity through matrix stiffness and porosity in three dimensional collagen matrices. Biomaterials 31,6425, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zaman M.H., Trapani L.M., Sieminski A.L., Mackellar D., Gong H., Kamm R.D., et al. Migration of tumor cells in 3D matrices is governed by matrix stiffness along with cell-matrix adhesion and proteolysis. Proc Natl Acad Sci U S A 103,10889, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chrobak K.M., Potter D.R., and Tien J.Formation of perfused, functional microvascular tubes in vitro. Microvasc Res 71,185, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Cross V.L., Zheng Y., Won Choi N., Verbridge S.S., Sutermaster B.A., Bonassar L.J., et al. Dense type I collagen matrices that support cellular remodeling and microfabrication for studies of tumor angiogenesis and vasculogenesis in vitro. Biomaterials 31,8596, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marotta M., and Martino G.Sensitive spectrophotometric method for the quantitative estimation of collagen. Anal Biochem 150,86, 1985 [DOI] [PubMed] [Google Scholar]
- 46.Rodriguez-Rodriguez P., Arribas S.M., de Pablo A.L., Gonzalez M.C., Abderrahim F., and Condezo-Hoyos L.A simple dot-blot-Sirius red-based assay for collagen quantification. Anal Bioanal Chem 405,6863, 2013 [DOI] [PubMed] [Google Scholar]
- 47.Yang Y.L., Motte S., and Kaufman L.J.Pore size variable type I collagen gels and their interaction with glioma cells. Biomaterials 31,5678, 2010 [DOI] [PubMed] [Google Scholar]
- 48.Leung A.D., Wong K.H., and Tien J.Plasma expanders stabilize human microvessels in microfluidic scaffolds. J Biomed Mater Res A 100,1815, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gillette B.M., Jensen J.A., Wang M., Tchao J., and Sia S.K.Dynamic hydrogels: switching of 3D microenvironments using two-component naturally derived extracellular matrices. Adv Mater 22,686, 2010 [DOI] [PubMed] [Google Scholar]
- 50.Gobeaux F., Mosser G., Anglo A., Panine P., Davidson P., Giraud-Guille M.M., et al. Fibrillogenesis in dense collagen solutions: a physicochemical study. J Mol Biol 376,1509, 2008 [DOI] [PubMed] [Google Scholar]
- 51.Chung S., Sudo R., Mack P.J., Wan C.R., Vickerman V., and Kamm R.D.Cell migration into scaffolds under co-culture conditions in a microfluidic platform. Lab Chip 9,269, 2009 [DOI] [PubMed] [Google Scholar]
- 52.Buchanan C.F., Voigt E.E., Szot C.S., Freeman J.W., Vlachos P.P., and Rylander M.N.Three-dimensional microfluidic collagen hydrogels for investigating flow-mediated tumor-endothelial signaling and vascular organization. Tissue Eng Part C Methods 20,11, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Knapp D.M., Barocas V.H., Moon A.G., Yoo K., Petzold L.R., and Tranquillo R.T.Rheology of reconstituted type I collagen gel in confined compression. J Rheol 41,971, 1997 [Google Scholar]
- 54.Kuntz R.M., and Saltzman W.M.Neutrophil motility in extracellular matrix gels: mesh size and adhesion affect speed of migration. Biophys J 72,1472, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lake S.P., and Barocas V.H.Mechanical and structural contribution of non-fibrillar matrix in uniaxial tension: a collagen-agarose co-gel model. Ann Biomed Eng 39,1891, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Krishnan L., Weiss J.A., Wessman M.D., and Hoying J.B.Design and application of a test system for viscoelastic characterization of collagen gels. Tissue Eng 10,241, 2004 [DOI] [PubMed] [Google Scholar]
- 57.Polacheck W.J., Charest J.L., and Kamm R.D.Interstitial flow influences direction of tumor cell migration through competing mechanisms. Proc Natl Acad Sci U S A 108,11115, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Provenzano P.P., Inman D.R., Eliceiri K.W., Knittel J.G., Yan L., Rueden C.T., et al. Collagen density promotes mammary tumor initiation and progression. BMC Med 6,11, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Raeber G.P., Lutolf M.P., and Hubbell J.A.Molecularly engineered PEG hydrogels: a novel model system for proteolytically mediated cell migration. Biophys J 89,1374, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Roeder B.A., Kokini K., and Voytik-Harbin S.L.Fibril microstructure affects strain transmission within collagen extracellular matrices. J Biomech Eng 131,031004, 2009 [DOI] [PubMed] [Google Scholar]
- 61.Song J.W., and Munn L.L.Fluid forces control endothelial sprouting. Proc Natl Acad Sci U S A 108,15342, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sundararaghavan H.G., Monteiro G.A., Firestein B.L., and Shreiber D.I.Neurite growth in 3D collagen gels with gradients of mechanical properties. Biotechnol Bioeng 102,632, 2009 [DOI] [PubMed] [Google Scholar]
- 63.Shreiber D.I., Enever P.A., and Tranquillo R.T.Effects of pdgf-bb on rat dermal fibroblast behavior in mechanically stressed and unstressed collagen and fibrin gels. Exp Cell Res 266,155, 2001 [DOI] [PubMed] [Google Scholar]
- 64.Brightman A.O., Rajwa B.P., Sturgis J.E., McCallister M.E., Robinson J.P., and Voytik-Harbin S.L.Time-lapse confocal reflection microscopy of collagen fibrillogenesis and extracellular matrix assembly in vitro. Biopolymers 54,222, 2000 [DOI] [PubMed] [Google Scholar]
- 65.Chandran P.L., and Barocas V.H.Microstructural mechanics of collagen gels in confined compression: poroelasticity, viscoelasticity, and collapse. J Biomech Eng 126,152, 2004 [DOI] [PubMed] [Google Scholar]
- 66.Whittington C.F., Yoder M.C., and Voytik-Harbin S.L.Collagen-polymer guidance of vessel network formation and stabilization by endothelial colony forming cells in vitro. Macromol Biosci 13,1135, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Haessler U., Teo J.C., Foretay D., Renaud P., and Swartz M.A.Migration dynamics of breast cancer cells in a tunable 3D interstitial flow chamber. Integr Biol (Camb) 4,401, 2012 [DOI] [PubMed] [Google Scholar]
- 68.Ng C.P., Hinz B., and Swartz M.A.Interstitial fluid flow induces myofibroblast differentiation and collagen alignment in vitro. J Cell Sci 118,4731, 2005 [DOI] [PubMed] [Google Scholar]
- 69.Ng C.P., and Swartz M.A.Fibroblast alignment under interstitial fluid flow using a novel 3-D tissue culture model. Am J Physiol Heart Circ Physiol 284,H1771, 2003 [DOI] [PubMed] [Google Scholar]
- 70.Saltzman W.M., Parkhurst M.R., Parsons-Wingerter P., and Zhu W.H.Three-dimensional cell cultures mimic tissues. Ann N Y Acad Sci 665,259, 1992 [DOI] [PubMed] [Google Scholar]
- 71.Sun S., Wise J., and Cho M.Human fibroblast migration in three-dimensional collagen gel in response to noninvasive electrical stimulus. I. Characterization of induced three-dimensional cell movement. Tissue Eng 10,1548, 2004 [DOI] [PubMed] [Google Scholar]
- 72.Guarnieri D., Battista S., Borzacchiello A., Mayol L., De Rosa E., Keene D.R., et al. Effects of fibronectin and laminin on structural, mechanical and transport properties of 3D collageneous network. J Mater Sci Mater Med 18,245, 2007 [DOI] [PubMed] [Google Scholar]
- 73.Jeon J.S., Zervantonakis I.K., Chung S., Kamm R.D., and Charest J.L.In vitro model of tumor cell extravasation. PLoS One 8,e56910, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gunzer M., Friedl P., Niggemann B., Brocker E.B., Kampgen E., and Zanker K.S.Migration of dendritic cells within 3-D collagen lattices is dependent on tissue origin, state of maturation, and matrix structure and is maintained by proinflammatory cytokines. J Leukoc Biol 67,622, 2000 [DOI] [PubMed] [Google Scholar]
- 75.Friedl P., Maaser K., Klein C.E., Niggemann B., Krohne G., and Zanker K.S.Migration of highly aggressive MV3 melanoma cells in 3-dimensional collagen lattices results in local matrix reorganization and shedding of alpha2 and beta1 integrins and CD44. Cancer Res 57,2061, 1997 [PubMed] [Google Scholar]
- 76.Bailey J.L., Critser P.J., Whittington C., Kuske J.L., Yoder M.C., and Voytik-Harbin S.L.Collagen oligomers modulate physical and biological properties of three-dimensional self-assembled matrices. Biopolymers 95,77, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sieminski A.L., Hebbel R.P., and Gooch K.J.The relative magnitudes of endothelial force generation and matrix stiffness modulate capillary morphogenesis in vitro. Exp Cell Res 297,574, 2004 [DOI] [PubMed] [Google Scholar]
- 78.Stylianopoulos T., Poh M.Z., Insin N., Bawendi M.G., Fukumura D., Munn L.L., et al. Diffusion of particles in the extracellular matrix: the effect of repulsive electrostatic interactions. Biophys J 99,1342, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Williams B.R., Gelman R.A., Poppke D.C., and Piez K.A.Collagen fibril formation. Optimal in vitro conditions and preliminary kinetic results. J Biol Chem 253,6578, 1978 [PubMed] [Google Scholar]
- 80.Sung K.E., Su G., Pehlke C., Trier S.M., Eliceiri K.W., Keely P.J., et al. Control of 3-dimensional collagen matrix polymerization for reproducible human mammary fibroblast cell culture in microfluidic devices. Biomaterials 30,4833, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Naciri M., Kuystermans D., and Al-Rubeai M.Monitoring pH and dissolved oxygen in mammalian cell culture using optical sensors. Cytotechnology 57,245, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lo C.M., Wang H.B., Dembo M., and Wang Y.L.Cell movement is guided by the rigidity of the substrate. Biophys J 79,144, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Paszek M.J., Zahir N., Johnson K.R., Lakins J.N., Rozenberg G.I., Gefen A., et al. Tensional homeostasis and the malignant phenotype. Cancer Cell 8,241, 2005 [DOI] [PubMed] [Google Scholar]
- 84.Brandl F., Sommer F., and Goepferich A.Rational design of hydrogels for tissue engineering: impact of physical factors on cell behavior. Biomaterials 28,134, 2007 [DOI] [PubMed] [Google Scholar]
- 85.Haugh M.G., Murphy C.M., McKiernan R.C., Altenbuchner C., and O'Brien F.J.Crosslinking and mechanical properties significantly influence cell attachment, proliferation, and migration within collagen glycosaminoglycan scaffolds. Tissue Eng Part A 17,1201, 2011 [DOI] [PubMed] [Google Scholar]
- 86.Koumoutsakos P., Pivkin I., and Milde F.The fluid mechanics of cancer and its therapy. Annu Rev Fluid Mech 45,325, 2013 [Google Scholar]
- 87.Polacheck W.J., Zervantonakis I.K., and Kamm R.D.Tumor cell migration in complex microenvironments. Cell Mol Life Sci 70,1335, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Saunders R.L., and Hammer D.A.Assembly of human umbilical vein endothelial cells on compliant hydrogels. Cell Mol Bioeng 3,60, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Califano J.P., and Reinhart-King C.A.A balance of substrate mechanics and matrix chemistry regulates endothelial cell network assembly. Cell Mol Bioeng 1,122, 2008 [Google Scholar]
- 90.Levental I., Georges P.C., and Janmey P.A.Soft biological materials and their impact on cell function. Soft Matter 3,299, 2007 [DOI] [PubMed] [Google Scholar]
- 91.Wells R.G.The role of matrix stiffness in regulating cell behavior. Hepatology 47,1394, 2008 [DOI] [PubMed] [Google Scholar]
- 92.Evans D.W., Vavalle N.A., DeVita R., Rajagopalan P., and Sparks J.L.Nano-indentation device for investigating the mechanics of compliant materials. Exp Mech 53,217, 2013 [Google Scholar]
- 93.Thiele J., Ma Y., Bruekers S.M., Ma S., and Huck W.T.25th Anniversary article: designer hydrogels for cell cultures: a materials selection guide. Adv Mater 26,125, 2014 [DOI] [PubMed] [Google Scholar]
- 94.Leddy H.A., Haider M.A., and Guilak F.Diffusional anisotropy in collagenous tissues: fluorescence imaging of continuous point photobleaching. Biophys J 91,311, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Seiffert S., and Oppermann W.Systematic evaluation of FRAP experiments performed in a confocal laser scanning microscope. J Microsc 220,20, 2005 [DOI] [PubMed] [Google Scholar]
- 96.Berk D.A., Yuan F., Leunig M., and Jain R.K.Fluorescence photobleaching with spatial Fourier analysis: measurement of diffusion in light-scattering media. Biophys J 65,2428, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Taylor J.M., Cohen S., and Mitchell W.M.Epidermal growth factor: high and low molecular weight forms. Proc Natl Acad Sci U S A 67,164, 1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mcinnes I.Cytokines. In: Firestein G.S., Kelley W.N., eds., Kelley's textbook of rheumatology. 8th ed., Philadelphia, PA: Saunders/Elsevier, 2009 [Google Scholar]
- 99.Huang Y.C., Wang T.W., Sun J.S., and Lin F.H.Epidermal morphogenesis in an in-vitro model using a fibroblasts-embedded collagen scaffold. J Biomed Sci 12,855, 2005 [DOI] [PubMed] [Google Scholar]
- 100.Chandrakasan G., Torchia D.A., and Piez K.A.Preparation of intact monomeric collagen from rat tail tendon and skin and the structure of the nonhelical ends in solution. J Biol Chem 251,6062, 1976 [PubMed] [Google Scholar]
- 101.Zervantonakis I.K., Hughes-Alford S.K., Charest J.L., Condeelis J.S., Gertler F.B., and Kamm R.D.Three-dimensional microfluidic model for tumor cell intravasation and endothelial barrier function. Proc Natl Acad Sci U S A 109,13515, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.