Abstract
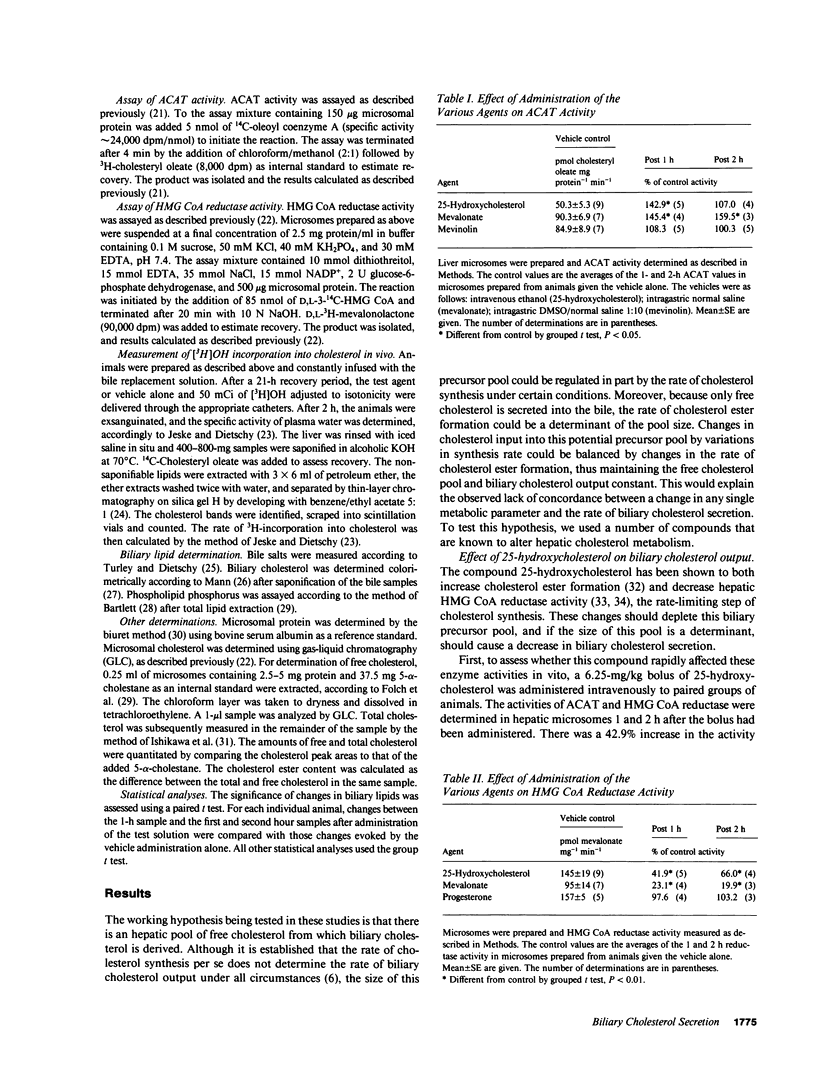
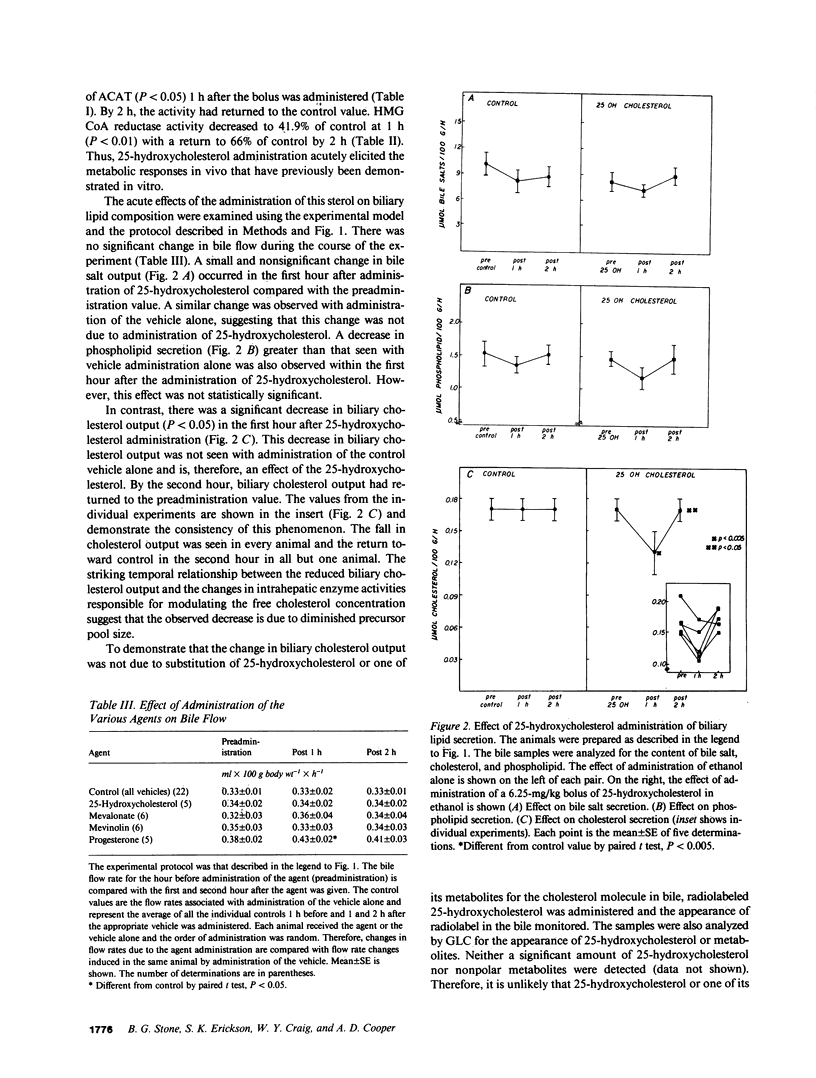
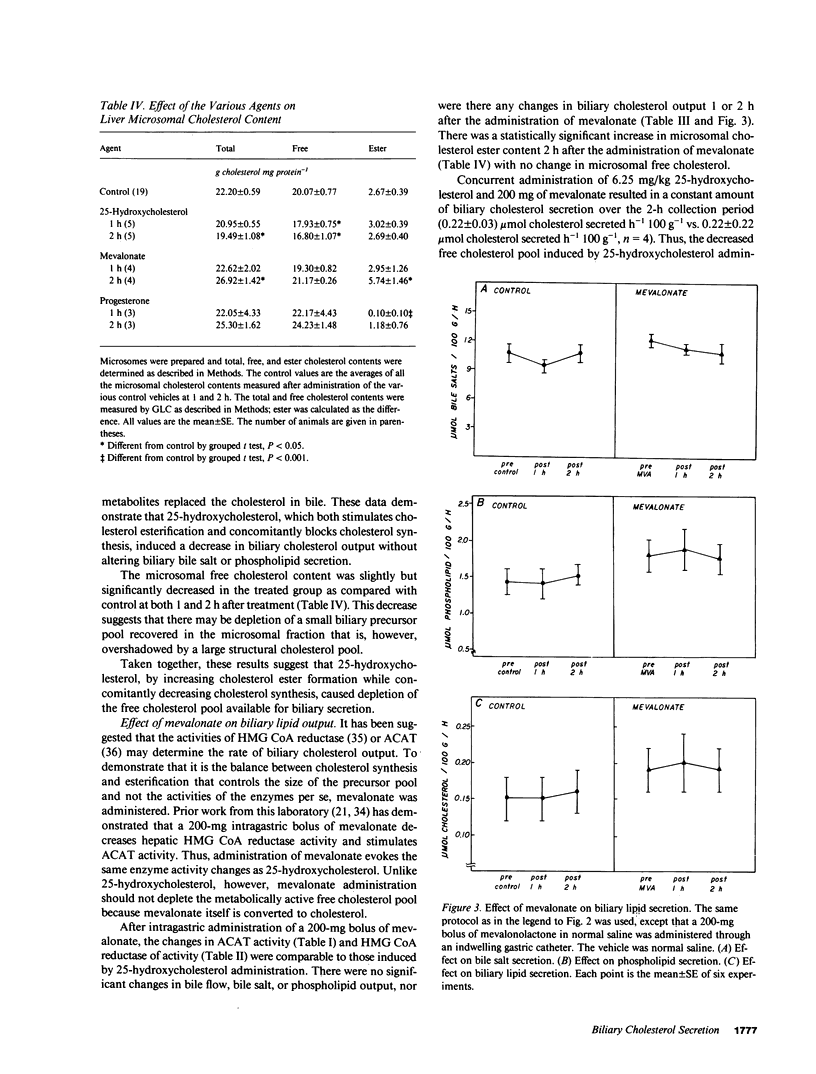
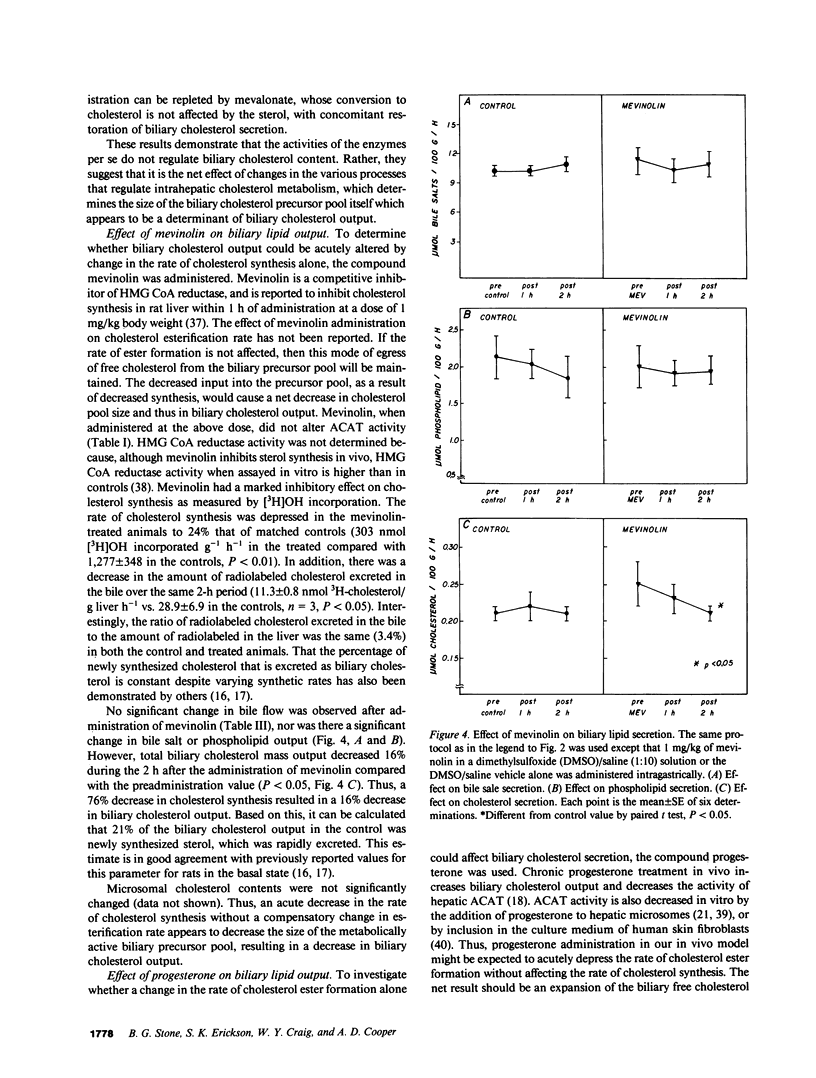
Propensity for cholesterol gallstone formation is determined in part by biliary cholesterol content relative to bile salts and phospholipid. We examined the hypothesis that the rate of biliary cholesterol secretion can be controlled by availability of an hepatic metabolically active free cholesterol pool whose size is determined in part by rates of sterol synthesis, as reflected by activity of the primary rate-limiting enzyme 3-hydroxy-3-methylglutaryl coenzyme A (HMG CoA) reductase and of sterol esterification, as reflected by the activity of the enzyme acyl coenzyme A/cholesterol acyltransferase (ACAT). Rats were prepared with biliary, venous, and duodenal catheters. The enterohepatic circulation of biliary lipids was maintained constant by infusion of a bile salt, lecithin, cholesterol replacement solution. Administration of 25-hydroxycholesterol decreased HMG CoA reductase activity, increased ACAT activity, and decreased biliary cholesterol output 26% by 1 h. By 2 h, ACAT activity and biliary cholesterol secretion were at control levels. Administration of mevinolin, a competitive inhibitor of HMG CoA reductase, had no effect on ACAT activity and decreased biliary cholesterol secretion 16%. Administration of progesterone, an inhibitor of ACAT, had no effect on HMG CoA reductase and increased biliary cholesterol output 32% at 1 h. By 2 h, all parameters were near control levels. None of these agents had any significant effect on biliary bile salt or phospholipid secretion. Thus, acutely altering rates of esterification and/or synthesis can have profound effects on biliary cholesterol secretion independent of the other biliary lipids. These experiments suggest the existence of a metabolically active pool of free cholesterol that serves as a precursor pool for biliary cholesterol secretion. Furthermore, the size of this precursor pool is determined in part both by rates of cholesterol synthesis and esterification and is a key determinant of biliary cholesterol secretion.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABEL L. L., LEVY B. B., BRODIE B. B., KENDALL F. E. A simplified method for the estimation of total cholesterol in serum and demonstration of its specificity. J Biol Chem. 1952 Mar;195(1):357–366. [PubMed] [Google Scholar]
- AVIGAN J., GOODMAN D. S., STEINBERG D. THIN-LAYER CHROMATOGRAPHY OF STEROLS AND STEROIDS. J Lipid Res. 1963 Jan;4:100–101. [PubMed] [Google Scholar]
- Admirand W. H., Small D. M. The physicochemical basis of cholesterol gallstone formation in man. J Clin Invest. 1968 May;47(5):1043–1052. doi: 10.1172/JCI105794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberts A. W., Chen J., Kuron G., Hunt V., Huff J., Hoffman C., Rothrock J., Lopez M., Joshua H., Harris E. Mevinolin: a highly potent competitive inhibitor of hydroxymethylglutaryl-coenzyme A reductase and a cholesterol-lowering agent. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3957–3961. doi: 10.1073/pnas.77.7.3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Balasubramaniam S., Mitropoulos K. A., Myant N. B., Mancini M., Postiglione A. Acyl-coenzyme A--cholesterol acyltransferase activity in human liver. Clin Sci (Lond) 1979 Apr;56(4):373–375. doi: 10.1042/cs0560373. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Dana S. E., Goldstein J. L. Cholesterol ester formation in cultured human fibroblasts. Stimulation by oxygenated sterols. J Biol Chem. 1975 May 25;250(10):4025–4027. [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L., Dietschy J. M. Active and inactive forms of 3-hydroxy-3-methylglutaryl coenzyme A reductase in the liver of the rat. Comparison with the rate of cholesterol synthesis in different physiological states. J Biol Chem. 1979 Jun 25;254(12):5144–5149. [PubMed] [Google Scholar]
- Brown M. S., Ho Y. K., Goldstein J. L. The cholesteryl ester cycle in macrophage foam cells. Continual hydrolysis and re-esterification of cytoplasmic cholesteryl esters. J Biol Chem. 1980 Oct 10;255(19):9344–9352. [PubMed] [Google Scholar]
- Cooper A. D. The regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase in the isolated perfused rat liver. J Clin Invest. 1976 Jun;57(6):1461–1470. doi: 10.1172/JCI108416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper A. D., Yu P. Y. Rates of removal and degradation of chylomicron remnants by isolated perfused rat liver. J Lipid Res. 1978 Jul;19(5):635–643. [PubMed] [Google Scholar]
- Coyne M. J., Bonorris G. G., Goldstein L. I., Schoenfield L. J. Effect of chenodeoxycholic acid and phenobarbital on the rate-limiting enzymes of hepatic cholesterol and bile acid synthesis in patients with gallstones. J Lab Clin Med. 1976 Feb;87(2):281–291. [PubMed] [Google Scholar]
- Del Pozo R., Nervi F., Covarrubias C., Ronco B. Reversal of progesterone-induced biliary cholesterol output by dietary cholesterol and ethynylestradiol. Biochim Biophys Acta. 1983 Sep 20;753(2):164–172. doi: 10.1016/0005-2760(83)90004-8. [DOI] [PubMed] [Google Scholar]
- Einarsson K., Grundy S. M. Effects of feeding cholic acid and chenodeoxycholic acid on cholesterol absorption and hepatic secretion of biliary lipids in man. J Lipid Res. 1980 Jan;21(1):23–34. [PubMed] [Google Scholar]
- Erickson S. K., Cooper A. D. Acyl-coenzyme A:cholesterol acyltransferase in human liver. In vitro detection and some characteristics of the enzyme. Metabolism. 1980 Oct;29(10):991–996. doi: 10.1016/0026-0495(80)90045-1. [DOI] [PubMed] [Google Scholar]
- Erickson S. K., Cooper A. D., Matsui S. M., Gould R. G. 7-Ketocholesterol. Its effects on hepatic cholesterogenesis and its hepatic metabolism in vivo and in vitro. J Biol Chem. 1977 Aug 10;252(15):5186–5193. [PubMed] [Google Scholar]
- Erickson S. K., Matsui S. M., Shrewsbury M. A., Cooper A. D., Gould R. G. Effects of 25-hydroxycholesterol on rat hepatic 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in vivo, in perfused liver, and in hepatocytes. J Biol Chem. 1978 Jun 25;253(12):4159–4164. [PubMed] [Google Scholar]
- Erickson S. K., Shrewsbury M. A., Brooks C., Meyer D. J. Rat liver acyl-coenzyme A:cholesterol acyltransferase: its regulation in vivo and some of its properties in vitro. J Lipid Res. 1980 Sep;21(7):930–941. [PubMed] [Google Scholar]
- Erickson S. K., Shrewsbury M. A., Gould R. G., Cooper A. D. Studies on the mechanisms of the rapid modulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase in intact liver by mevalonolactone and 25-hydroxycholesterol. Biochim Biophys Acta. 1980 Oct 6;620(1):70–79. doi: 10.1016/0005-2760(80)90186-1. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Goldstein J. L., Faust J. R., Dygos J. H., Chorvat R. J., Brown M. S. Inhibition of cholesteryl ester formation in human fibroblasts by an analogue of 7-ketocholesterol and by progesterone. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1877–1881. doi: 10.1073/pnas.75.4.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy S. M., Metzger A. L., Adler R. D. Mechanisms of lithogenic bile formation in American Indian women with cholesterol gallstones. J Clin Invest. 1972 Dec;51(12):3026–3043. doi: 10.1172/JCI107130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISAKSSON B. On the dissolving power of lecithin and bile salts for cholesterol in human bladder bile. Acta Soc Med Ups. 1954 Sep 30;59(5-6):296–306. [PubMed] [Google Scholar]
- Ishikawa T. T., MacGee J., Morrison J. A., Glueck C. J. Quantitative analysis of cholesterol in 5 to 20 microliter of plasma. J Lipid Res. 1974 May;15(3):286–291. [PubMed] [Google Scholar]
- Jeske D. J., Dietschy J. M. Regulation of rates of cholesterol synthesis in vivo in the liver and carcass of the rat measured using [3H]water. J Lipid Res. 1980 Mar;21(3):364–376. [PubMed] [Google Scholar]
- Lichtenstein A. H., Brecher P. Esterification of cholesterol and 25-hydroxycholesterol by rat liver microsomes. Biochim Biophys Acta. 1983 May 16;751(3):340–348. doi: 10.1016/0005-2760(83)90292-8. [DOI] [PubMed] [Google Scholar]
- MANN G. V. A method for measurement of cholesterol in blood serum. Clin Chem. 1961 Jun;7:275–284. [PubMed] [Google Scholar]
- Maton P. N., Ellis H. J., Higgins M. J., Dowling R. H. Hepatic HMGCoA reductase in human cholelithiasis: effects of chenodeoxycholic and ursodeoxycholic acids. Eur J Clin Invest. 1980 Aug;10(4):325–332. doi: 10.1111/j.1365-2362.1980.tb00040.x. [DOI] [PubMed] [Google Scholar]
- Metzger A. L., Adler R., Heymsfield S., Grundy S. M. Diurnal variation in biliary lipid composition. Possible role in cholesterol gallstone formation. N Engl J Med. 1973 Feb 15;288(7):333–336. doi: 10.1056/NEJM197302152880702. [DOI] [PubMed] [Google Scholar]
- Nervi F. O., Del Pozo R., Covarrubias C. F., Ronco B. O. The effect of progesterone on the regulatory mechanisms of biliary cholesterol secretion in the rat. Hepatology. 1983 May-Jun;3(3):360–367. doi: 10.1002/hep.1840030314. [DOI] [PubMed] [Google Scholar]
- Robins S. J., Brunengraber H. Origin of biliary cholesterol and lecithin in the rat: contribution of new synthesis and preformed hepatic stores. J Lipid Res. 1982 May;23(4):604–608. [PubMed] [Google Scholar]
- Rodwell V. W., Nordstrom J. L., Mitschelen J. J. Regulation of HMG-CoA reductase. Adv Lipid Res. 1976;14:1–74. doi: 10.1016/b978-0-12-024914-5.50008-5. [DOI] [PubMed] [Google Scholar]
- Salen G., Nicolau G., Shefer S., Mosbach E. H. Hepatic cholesterol metabolism in patients with gallstones. Gastroenterology. 1975 Sep;69(3):676–684. [PubMed] [Google Scholar]
- Schwartz C. C., Halloran L. G., Vlahcevic Z. R., Gregory D. H., Swell L. Preferential utilization of free cholesterol from high-density lipoproteins for biliary cholesterol secretion in man. Science. 1978 Apr 7;200(4337):62–64. doi: 10.1126/science.204996. [DOI] [PubMed] [Google Scholar]
- Schwartz C. C., Vlahcevic Z. R., Halloran L. G., Gregory D. H., Meek J. B., Swell L. Evidence for the existence of definitive hepatic cholesterol precursor compartments for bile acids and biliary cholesterol in man. Gastroenterology. 1975 Dec;69(6):1379–1382. [PubMed] [Google Scholar]
- Shaffer E. A., Small D. M. Biliary lipid secretion in cholesterol gallstone disease. The effect of cholecystectomy and obesity. J Clin Invest. 1977 May;59(5):828–840. doi: 10.1172/JCI108705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shefer S., Hauser S., Lapar V., Mosbach E. H. Regulatory effects of sterols and bile acids on hepatic 3-hydroxy-3-methylglutaryl CoA reductase and cholesterol 7alpha-hydroxylase in the rat. J Lipid Res. 1973 Sep;14(5):573–580. [PubMed] [Google Scholar]
- Small D. M., Rapo S. Source of abnormal bile in patients with cholesterol gallstones. N Engl J Med. 1970 Jul 9;283(2):53–57. doi: 10.1056/NEJM197007092830201. [DOI] [PubMed] [Google Scholar]
- Tanaka R. D., Edwards P. A., Lan S. F., Knöppel E. M., Fogelman A. M. The effect of cholestyramine and Mevinolin on the diurnal cycle of rat hepatic 3-hydroxy-3-methylglutaryl coenzyme A reductase. J Lipid Res. 1982 Sep;23(7):1026–1031. [PubMed] [Google Scholar]
- Turley S. D., Dietschy J. M. Re-evaluation of the 3 alpha-hydroxysteroid dehydrogenase assay for total bile acids in bile. J Lipid Res. 1978 Sep;19(7):924–928. [PubMed] [Google Scholar]
- Turley S. D., Dietschy J. M. Regulation of biliary cholesterol output in the rat: dissociation from the rate of hepatic cholesterol synthesis, the size of the hepatic cholesteryl ester pool, and the hepatic uptake of chylomicron cholesterol. J Lipid Res. 1979 Nov;20(8):923–934. [PubMed] [Google Scholar]
- Turley S. D., Dietschy J. M. The contribution of newly synthesized cholesterol to biliary cholesterol in the rat. J Biol Chem. 1981 Mar 10;256(5):2438–2446. [PubMed] [Google Scholar]
- Vlahcevic Z. R., Bell C., Jr, Swell L. Significance of the liver in the production of lithogenic bile in man. Gastroenterology. 1970 Jul;59(1):62–69. [PubMed] [Google Scholar]
- Wagner C. I., Trotman B. W., Soloway R. D. Kinetic analysis of biliary lipid excretion in man and dog. J Clin Invest. 1976 Feb;57(2):473–477. doi: 10.1172/JCI108299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zsigmond G., Solymoss B. Increased canalicular bile production induced by pregnenolone-16alpha-carbonitrile, spironolactone and cortisol in rats. Proc Soc Exp Biol Med. 1974 Feb;145(2):631–635. doi: 10.3181/00379727-145-37864. [DOI] [PubMed] [Google Scholar]


