Abstract
• Background and Aims Pistia stratiotes produces large amounts of calcium (Ca) oxalate crystals in specialized cells called crystal idioblasts. The potential involvement of Ca2+ channels in Ca oxalate crystal formation by crystal idioblasts was investigated.
• Methods Anatomical, ultrastructural and physiological analyses were used on plants, fresh or fixed tissues, or protoplasts. Ca2+ uptake by protoplasts was measured with 45Ca2+, and the effect of Ca2+ channel blockers studied in intact plants. Labelled Ca2+ channel blockers and a channel protein antibody were used to determine if Ca2+ channels were associated with crystal idioblasts.
• Key Results 45Ca2+ uptake was more than two orders of magnitude greater for crystal idioblast protoplasts than mesophyll protoplasts, and idioblast number increased when medium Ca was increased. Plants grown on media containing 1–50 µm of the Ca2+ channel blockers, isradipine, nifedipine or fluspirilene, showed almost complete inhibition of crystal formation. When fresh tissue sections were treated with the fluorescent dihydropyridine‐type Ca2+ channel blocker, DM‐Bodipy‐DHP, crystal idioblasts were intensely labelled compared with surrounding mesophyll, and the label appeared to be associated with the plasma membrane and the endoplasmic reticulum, which is shown to be abundant in idioblasts. An antibody to a mammalian Ca2+ channel α1 subunit recognized a single band in a microsomal protein fraction but not soluble protein fraction on western blots, and it selectively and heavily labelled developing crystal idioblasts in tissue sections.
• Conclusions The results demonstrate that Ca oxalate crystal idioblasts are enriched, relative to mesophyll cells, in dihydropyridine‐type Ca2+ channels and that the activity of these channels is important to transport and accumulation of Ca2+ required for crystal formation.
Key words: Calcium, calcium channel, calcium oxalate, crystals, dihydropyridine, Pistia stratiotes
INTRODUCTION
Calcium (Ca) in its ionic form, Ca2+, performs critical functions in metabolism and as a signalling agent in cells (Sanders et al., 2002; White and Broadley, 2003). However, to be effectively used as a signalling molecule, cytoplasmic Ca2+ concentrations (resting) must be <1 µm. Since Ca is generally very abundant in the environment and the driving force for entry of Ca2+ into the plant cell is large (up to 10 000‐fold more Ca2+ in the apoplast than the cytoplasm), Ca accumulates within tissues of plants that do not strictly limit uptake at the root (Kirkby and Pilbeam, 1984). Cells have evolved a number of mechanisms for tightly regulating Ca2+ fluxes and Ca pools. At one level, H+/Ca2+ antiporters and Ca2+‐ATPases can actively transport Ca2+ from the cytosol into the vacuole, endoplasmic reticulum (ER), mitochondria, plastids and cell walls (Bush, 1993; Harper, 2001; Pittman and Hirschi, 2003) where Ca2+ can be sequestered. However, such mechanisms are of limited capacity. When these general homeostatic mechanisms are overcome, an alternative mechanism to sequester excess Ca is necessary or tissue damage will result. Calcium oxalate formation represents a system of large‐scale Ca sequestration that is common to many plant species (reviewed by Arnott and Pautard, 1970; Franceschi and Horner, 1980; Horner and Wagner, 1995; Webb, 1999; Nakata, 2003); however, little is known about how this system operates at the biophysical level.
Calcium oxalate is produced in plants as a physiologically and osmotically inactive product, and is often present in large amounts, commonly representing 10 % or more of total plant Ca (Arnott and Pautard, 1970; Franceschi and Horner, 1980; Franceschi and Loewus, 1995; Webb, 1999). While the crystals can be found in cell walls (cf. Pennisi et al., 2001; Hudgins et al., 2003), most often they are formed in the vacuoles of remarkably specialized cells called crystal idioblasts (Foster, 1956). Developing idioblasts represent specialized high‐capacity sinks which have been shown to be induced by Ca in a number of species and can sequester large amounts of Ca (Frank, 1972; Zindler‐Frank, 1975; Franceschi and Horner, 1979; Borchert, 1985, 1986; Franceschi, 1989; DeSilva et al., 1996; Pennisi and McConnell, 2001; Zindler‐Frank et al., 2001; Volk et al., 2002; Mazen et al., 2003). There is considerable information available about the structure of crystal idioblasts and their crystals; however, little is known about the biochemical features of these unusual cells.
Crystal growth and idioblast growth are highly coordinated to prevent damage to the vacuolar membrane (Kostman and Franceschi, 2000) and recent work has demonstrated that they are capable of synthesizing oxalic acid used for crystal formation from l‐ascorbic acid (Horner et al., 2000; Keates et al., 2000; Kostman et al., 2001; Kostman and Koscher, 2003). The regulation of the other substrate for crystal formation, Ca, is not well characterized in crystal idioblasts, although a number of cellular features, such as abundant ER and unusual vacuolar membranes, are likely to be related to accumulation and precipitation of Ca. With respect to crystal precipitation, vacuolar proteins (Bouropoulos et al., 2001) including a recently identified unique Ca‐binding matrix protein (Li et al., 2003; Mazen et al., 2003), along with other uncharacterized matrix materials (Arnott, 1982; Webb and Arnott, 1983; Webb et al., 1995; Volk et al., 2002) are associated with crystal formation. They are thought to play a role in nucleation and regulation of crystal precipitation rates through mechanisms that remain to be determined.
Developing crystal idioblasts are commonly reported to have an abundance of ER relative to surrounding parenchyma cells. Studies have shown that Pistia stratiotes idioblasts are enriched in the Ca‐binding protein, calreticulin (Quitadamo et al., 2000; Nakata et al., 2003), which resides in specialized extended ER subdomains (Kostman et al., 2003; Nakata et al., 2003). It is hypothesized that the ER and calreticulin are involved in regulating cytoplasmic free Ca2+ during rapid accumulation of Ca for crystal production (Nakata et al., 2003). The abundance of ER in the crystal idioblast cytoplasm provides a large surface area necessary for rapid uptake of Ca2+ into the lumen by Ca2+ ATPases as Ca enters the cell. In addition, ER Ca2+ channels might be important in the transfer of Ca between pools in the idioblast during the formation of Ca oxalate crystals, consistent with evidence that Ca2+ can be released from plant endomembranes through voltage‐dependent Ca2+ channels (Klusener and Weiler, 1999). Ca2+ channels would also provide an energetically efficient mechanism for Ca2+ uptake from the apoplast by crystal idioblasts since a large inwardly directed electrochemical potential gradient for Ca2+ exists. In vitro studies on Ca oxalate crystal formation in Lemna minor have provided some evidence that channels may be necessary to accommodate mass uptake of Ca2+ and the relatively rapid rate of crystal formation that is possible in the idioblasts (Franceschi, 1989). Further studies are required to confirm this. In general, Ca oxalate crystal idioblasts may be enriched in Ca2+ channels and could provide a good model system for the study of Ca2+ channels in plants, an area of investigation where there is much interest but little information on the actual protein components.
There are extensive studies showing that Ca2+ channels operate in various capacities in plant systems. While some putative genes for plant Ca2+ channels have been identified, they remain to be functionally characterized (White et al., 2002). However, electrophysiological studies have identified voltage‐dependent Ca2+ channels in the plasma membrane, tonoplast, ER, chloroplast, and nuclear membranes of plant cells (White, 2000) and patch clamp techniques have revealed that plants have Ca2+ channels similar to the L‐type channels characterized in animal systems (Gelli and Blumwald, 1993; Thuleau et al., 1994; White, 2000). Animal Ca2+‐specific L‐type channels have five subunits, of which the α1 pore‐forming subunit is the largest. A putative voltage‐gated two‐pore Ca2+ channel cloned from Arabidopsis (AtTPC1) has an overall structure similar to a portion of the α1 subunit of voltage‐activated Ca2+ channels from animals (Furuichi et al., 2001). This α1 subunit has two or more high‐affinity binding sites for 1,4‐dihydropyridine‐(DHP) and/or phenylalkylamine‐(PAA) type inhibitors, which are often referred to as Ca2+ channel blockers. Binding of verapamil blocks PAA‐type binding sites while nifedipine, nitrendipine and isradipine (PN 200‐110) bind to DHP‐type sites. These inhibitors have proven to be important tools for study of Ca2+ channels and their functions in animals and have also been used in plant studies.
In plants, Ca2+ channel inhibitors have been found to block various Ca‐dependent processes; however, many plant Ca2+‐dependent processes are not affected by channel blockers. In addition, plant Ca2+ channels are not necessarily inhibited by both PAA‐ and DHP‐type inhibitors. For example, treatment of Catharanthus roseus and Glycine max suspension cell cultures with nifedipine inhibited Ca2+ uptake and callose formation but verapamil only gave inhibitory effects at a high concentration (Waldmann et al., 1988). Application of Ca2+ channel blockers can also inhibit physiological processes such as cold acclimation and frost‐induced injuries (Piotrowska, 1998; Orvar et al., 2000), and polar growth of pollen tubes (Reiss and Herth, 1985). Fluorescent‐tagged and radioactive‐labelled channel inhibitors (Knaus et al., 1992a, b) have provided additional tools for probing for the biochemical identity or cellular location of Ca2+ channels in plant systems (Shaw and Quatrano, 1996; Vallee et al., 1997; Bhatla et al., 2002). Ca2+ channel antibodies would provide a particularly powerful tool for examining the presence and position of Ca2+ channels in plant cells, but there are currently no reports of reliable antibodies to identify plant Ca2+ channels. However, antibodies made against animal Ca2+ channel proteins are commercially available, and a monoclonal antibody that recognizes conserved amino acids of the α1 subunit in L‐type animal Ca2+ channels was found to recognize a protein of the correct molecular weight from plant membranes (Volk and Franceschi, 2000).
The purpose of this study was to test the hypothesis that Ca2+ channels are important for Ca oxalate formation in developing Ca oxalate crystal idioblasts. Using Ca2+ channel blockers and an antibody to a mammalian Ca2+ channel protein, it is demonstrated here that Ca2+ channels play a critical role in Ca oxalate crystal formation in P. stratiotes plants. Pistia, a free‐floating aquatic plant, was used because it produces an abundance of Ca oxalate crystals and rapidly assimilates reagents provided in liquid growth media (Keates et al., 2000; Kostman and Franceschi, 2000). It is reported that functional Ca2+ channels are required for crystal formation and they provide further insight into the mechanisms that are operating to allow these specialized cells to perform their function of high capacity Ca sequestration.
MATERIALS AND METHODS
Plant material
Pistia stratiotes L. is a free‐floating aquatic angiosperm that can reproduce rapidly by vegetative offshoots from stolons. Plants were grown in tap water in large tubs in a glasshouse with a 16‐h photoperiod and fertilized bi‐monthly (Peters General Purpose Fertilizer 20 : 20 : 20 NPK; Scotts Brand, Marysville, OH, USA). Plants were propagated clonally and all material used was of the same genetic background.
Light and electron microscopy
Developing leaves were removed and cut into small pieces (2 mm2) in a fixative solution containing 2 % (v/v) paraformaldehyde and 2·5 % (v/v) glutaraldehyde buffered with 50 mm PIPES (pH 7·2). After 12 h at 4 °C the specimens were washed with buffer and post‐fixed in 1 % (w/v) osmium tetroxide in 50 mm sodium cacodylate buffer (pH 7·2) for 2 h at room temperature. The specimens were washed with buffer, dehydrated stepwise with acetone and infiltrated with Spurr epoxy resin (medium hardness). Thick sections were cut with glass knives and stained with aqueous 0·1 % (w/v) Toluidine blue. Thin sections were cut on a diamond knife, placed on nickel grids and post‐stained sequentially with aqueous uranyl acetate and lead citrate prior to examination and photography on a JEOL 1200 EX transmission electron microscope.
For paraffin embedding, Pistia shoot tips containing developing leaves were fixed for 12 h in 2·5 % (v/v) glutaraldehyde and 4 % (v/v) paraformaldehyde in 50 mm PIPES buffer, dehydrated in ethanol, infiltrated with xylene and embedded in 56 °C melting point paraffin. Blocks were sectioned on a rotary microtome. Sections were dried onto gelatin‐coated slides, deparaffinized in xylene, rehydrated in ethanol, and stained with safranin O and fast green. The sections were dehydrated, infiltrated with xylene, then made permanent using Permount mounting medium (Fisher Scientific, Pittsburgh, PA, USA). Sections were examined with bright field illumination or with partially crossed polarizing filters, which makes the crystals appear as bright areas due to their birefringence.
Application of 45Ca2+ and channel blockers to protoplasts
Pistia protoplasts were isolated as described in Franceschi et al. (1993). Developing leaves removed from five or six plants were abraded to remove trichomes, cut into small squares and immediately placed into digestion medium containing cellulase, macerase and pectolyase, buffered with MES and osmotically adjusted with mannitol as described in Franceschi et al. (1993). Developing leaves were used to ensure the idioblasts that were isolated were still producing Ca oxalate. Mesophyll protoplasts were easy to purify. Raphide idioblast protoplasts were very difficult to obtain since the idioblasts represent a minor component of the total cell complement in the plant and they rupture very easily during purification. Thus, samples were small, though pure, as shown in Fig. 1B and C. Three replicate samples of Pistia raphide idioblast protoplasts and mesophyll protoplasts taken from the final pure protoplast pools were incubated with 4 µCi (1·48 × 105 Bq) 45Ca2+ in the isolation buffer minus the enzymes (iso‐osmotic conditions) for 15 min, rinsed with isolation buffer and total counts per minute were determined using a scintillation counter. A small subsample was removed from each vial before addition of the label to count the number of protoplasts per mL using a haemocytometer. Uptake of 45Ca2+ was calculated as counts per minute per 106 protoplasts per hour. A t‐test was performed using JMP 4.0 (SAS Institute, 2000) for analysis of means separation.
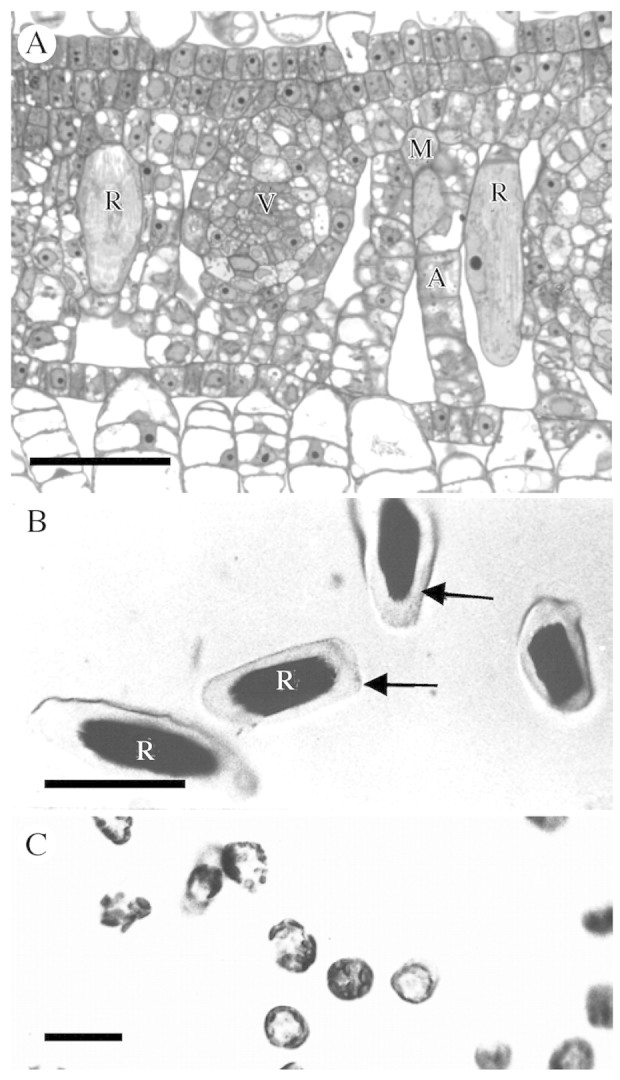
Fig. 1. Raphide idioblasts and protoplasts of Pistia stratiotes. (A) Cross‐section of young leaf containing two developing raphide idioblasts, which are much larger than surrounding cells. (B) Isolated raphide idioblast protoplasts (arrows). (C) Mesophyll protoplasts. Scale bars: A = 40 µm; B = 50 µm; C = 20 µm. A, Aerenchyma; M, mesophyll; R, raphide crystal idioblast; V, vein.
Calcium channel blocker treatment in vivo
Groups of five or six equally sized Pistia plants from glasshouse cultures were placed into separate containers of water supplemented with Peter’s Professional fertilizer (20 : 20 : 20 NPK). Ca in the medium was brought to 2·5 mm by addition of CaCl2. Leaves that were mature or expanding were marked so that leaves that developed during treatment could be identified. The Ca2+ channel blockers, nifedipine (Sigma Chemical Co., St Louis, MO, USA), PN200‐110 [isradipine; gift from Novartis Pharmaceuticals Corporation (formerly Sandoz Pharmaceutical Corp.) East Hanover, NJ, USA] and fluspirilene (Research Biochemicals Inc., Natick, MA, USA) were dissolved in dimethylsulfoxide (DMSO) to give stock solutions of 1 mm blocker. Channel blockers were added to the growth medium to give final concentrations of 50 and 100 µm, except for fluspirilene, which was used at 1·0, 50 and 100 µm. DMSO had a 0·5 % (v/v) final concentration. Control plants were grown with 0·5 % (v/v) DMSO and a separate higher Ca treatment was run at 5 mm CaCl2.
Plants were grown for 7 d and the medium with channel blockers was replaced every 2 d. Pistia grows very rapidly and new leaves were formed within the 7‐d period for all treatments. Leaves that developed during the exposure period were harvested, placed in 70 % ethanol until pigments were removed, treated with 5 % (w/v) NaOH for 4 h, dehydrated with ethanol and infiltrated with xylene prior to infiltration with Permount mounting media. Permanent slides were made and the slides were examined and photographed with a Leitz Orthoplan compound light microscope using crossed polarizing filters.
Fluorescent Ca channel blocker localization
Fresh, developing Pistia leaves (1 cm long) were sectioned by hand and incubated in a 0·5 µm solution of DM‐Bodipy dihydropyridine (DM‐Bodipy‐DHP; Molecular Probes, Eugene, OR, USA) in sucrose buffer (0·4 m sucrose, 5 mm HEPES, 2 mm CaCl2, pH 7). Control sections were pre‐treated with 400 µm nifedipine (in sucrose buffer) as a competitive inhibitor for 4 h prior to incubation with DM‐Bodipy‐DHP. Images were collected using a Bio‐Rad MRC 1024 confocal microscope (Bio‐Rad Laboratories, Hercules, CA, USA).
Vital staining for ER distribution
Crude protoplast preparations were incubated with 5 µg mL–1 DiOC6 (Molecular Probes), a fluorescent compound that preferentially partitions into endoplasmic reticulum. At very low concentrations, DiOC6 labels mitochondria but at higher concentrations, as used here, it appears to preferentially label ER (Sabnis et al., 1997). Treatment was for 15 min followed by a brief wash with protoplast isolation buffer. The protoplasts, which are very sensitive and easily break due to the crystals contained in their vacuoles, were placed onto a well slide and were viewed with epifluorescence on a Leitz Aristoplan microscope.
Western immunoblotting
Soluble and membrane (microsomal fraction) proteins were extracted from Pistia shoot tips as described by Volk and Franceschi (2000). Total protein concentrations were determined using a Pierce Protein Assay kit (Pierce Biotechnology, Rockford, IL, USA) and bovine serum albumin as a standard. Proteins were separated on 10 % SDS–polyacrylamide gels and electroblotted onto pre‐wetted polyvinylidine difluoride membranes (Bio‐Rad Laboratories). Air‐dried blots were wetted in methanol and blocked with western blocking reagent (Roche Molecular Biochemicals, Indianapolis, IN, USA) for 1 h prior to incubation with MAB427 [mouse anti‐dihydropyridine binding complex (α1 subunit); Chemicon International, Inc, Temecula, CA, USA] monoclonal antibody (1 : 500 dilution in 0·5 % blocking solution) overnight at room temperature. Blots were washed with TBST (50 mm Tris, 150 mm NaCl, 0·1 % v/v Tween 20, pH 7·5) and incubated for 1 h with anti‐mouse‐alkaline phosphatase‐tagged antibody (diluted in TBST, 1 : 500 dilution; Sigma Chemical Co., St Louis, MO, USA). Alkaline phosphatase colour reactions were performed according to the manufacturer’s instructions (Roche Molecular Biochemicals, Mannheim, Germany). Images were captured using a Bio‐Rad Gel Doc 1000 (Bio‐Rad Laboratories).
Immunocytochemistry
Paraffin blocks prepared as described above were sectioned on a rotary microtome. Sections were dried onto poly‐l‐lysine‐coated slides, deparaffinized in xylene, rehydrated in ethanol, and then blocked in TBST + BSA (10 mm Tris, 250 mm NaCl, 1 % w/v bovine serum albumin, 0·1 % Tween, pH 7·2) for 1 h. Mounted sections were treated with a 1 : 20 dilution of MAB427 antibody in TBST for 4 h, followed by 1 h in a 1 : 250 dilution of peroxidase labelled goat‐anti‐mouse IgG (Sigma Chemical Co.). After thorough washing with TBST, sections were treated for peroxidase colorimetric detection using a Vector development kit (SK4100, Vector Laboratories, Burlingame, CA, USA). Colour development was stopped by rinsing with distilled water and the sections were subsequently viewed and photographed using a Leitz Orthoplan microscope.
RESULTS
Crystal idioblasts have specialized structure and are enriched in ER
Pistiastratiotes produces two types of crystal idioblasts, raphide and druse. Raphide idioblasts form primarily in the aerenchyma of leaves and other organs as large, elongated cells with a bundle of needle‐shaped crystals in their vacuole (Fig. 1A). Druse idioblasts are smaller and more compact and formed primarily in the compact mesophyll at the adaxial surface of the leave as well as in association with vascular bundles. They contain a stellate crystal conglomerate in their vacuole (see Volk et al., 2002). The raphide idioblasts are much larger than druse or mesophyll cells and protoplasts for these two cell types can be separated based on size and density (Fig. 1B and C) though the yield is extremely low. Most of the observations presented here centre on raphide idioblasts since they are easier to visualize due to their large size.
The cytoplasm of raphide and druse idioblasts is rich in ER and other membrane structures compared with mesophyll cells, as seen in TEM images (Fig. 2A), and the crystals form in association with membranes in the vacuole (Fig. 2B). In some regions of the raphide crystal idioblast the ER takes on an enlarged or extended appearance (Fig. 2B) that has recently been shown to be related to calreticulin and Ca accumulation in these idioblasts (Kostman et al., 2003; Nakata et al., 2003). These extended regions are not connected to the vacuole in any of the numerous micrographs examined, but can occasionally be seen connecting with rough ER. Thicker sections of paraffin embedded tissue show a distinct and extensive reticulate network that is believed to be related to the distribution of the ER in the raphide crystal idioblasts (Fig. 2C–E), although other organelles may also be associated with the network. Plastids remain relatively small and do not develop into chloroplasts, so the denser parts of the network as seen in Fig. 2E are not due to chloroplasts. The network generally appears as a dense reticulum, often with thicker regions that may correlate to the enlarged ER subdomains previously described (Kostman et al., 2003; Nakata et al., 2003).
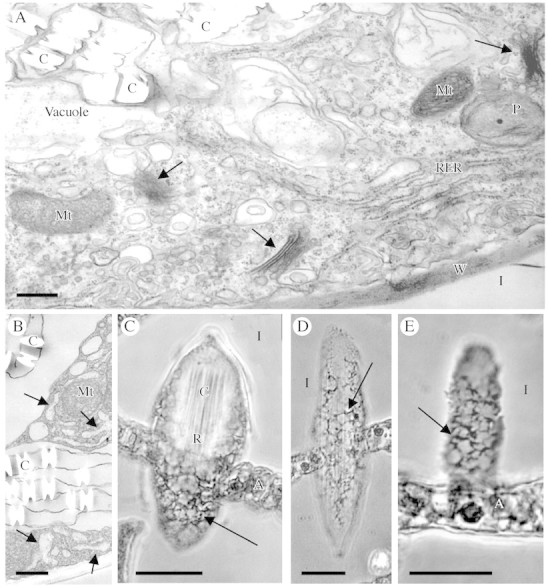
Fig. 2. Endoplasmic reticulum and cytoplasmic features of raphide crystal idioblasts. (A and B) Transmission electron micrographs of part of a developing raphide idioblast cytoplasm and vacuole. (A) Smooth and rough ER, Golgi (arrows) and other membrane structures are abundant in the cytoplasm. (B) Part of a developing idioblast showing regions of enlarged ER (arrows) and the membranes associated with the edges of the crystals in the vacuole. (C–E) Phase contrast images of paraffin sections through raphide idioblasts. (C) Tangential section through an idioblast. The crystal bundle is visible at the top of the cell and the reticulate peripheral cytoplasm (arrow) visible at the bottom. (D) Longitudinal section along the peripheral cytoplasm of an entire idioblast. A reticulate network can be seen (arrows). (E) Enlarged section along the peripheral cytoplasm of an idioblast, showing dense reticulate pattern. Scale bars: A and B = 1 µm; C–E = 20 µm. A, Aerenchyma; C, crystal; I, intercellular space; Mt, mitochondrion; P, plastid; RER, rough endoplasmic reticulum, W, wall.
Crystal idioblasts and Ca accumulation
Ca oxalate crystal idioblasts can be seen in cleared Pistia leaves as bright spots under crossed polarizing filters, and were quite abundant under our control growth conditions containing 2·5 mm Ca (Fig. 3A). When the medium was supplemented with an additional 2·5 mm Ca, the number of crystal idioblasts increased by about 25 % (number per unit surface area of leaf), demonstrating the crystal idioblasts are produced in response to Ca availability, though there does appear to be an upper limit to the number of crystal idioblast that can be formed (Fig. 3B; see also Volk et al., 2002; Mazen et al., 2003). It was hypothesized that crystal idioblasts are designed to accommodate high Ca2+ fluxes relative to mesophyll cells, which is not easily tested in situ. To get an estimate of the relative capacity for Ca transport by the crystal idioblasts, small samples of highly purified raphide idioblast protoplasts and mesophyll protoplasts were produced (Fig. 1B and C). Measurement of 45Ca2+ uptake demonstrates that raphide crystal idioblast protoplasts had a higher rate of uptake than mesophyll protoplasts (Table 1). The Ca2+ uptake was more than two orders of magnitude greater on a protoplast basis in idioblast protoplasts compared with mesophyll protoplasts, indicating significantly enhanced transport capacity of idioblasts. The idioblast protoplasts are highly variable in size, as can be seen in Fig. 1B. An accurate surface area could not be calculated, but even given the larger size of the idioblast protoplasts, the rate of uptake was considerably greater than mesophyll protoplasts.
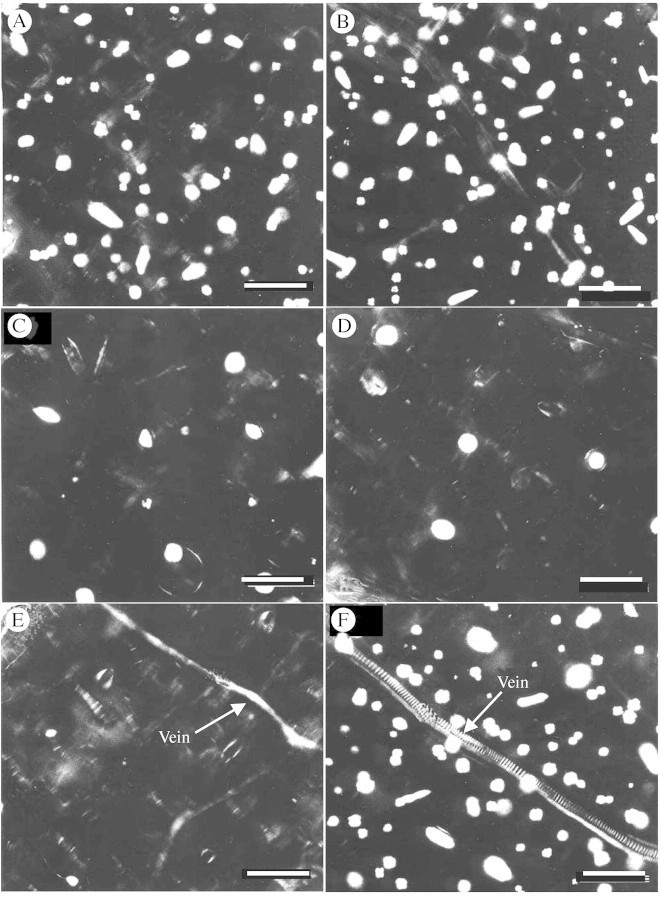
Fig. 3. Effect of Ca2+ channel blockers on Ca oxalate formation. Images are of representative clearings from whole leaves that developed on plants during growth on media containing blocker or control agents. Crystal idioblasts appear as bright spots in these images viewed between crossed polarizing filters. (A) Leaf from plant on normal growth medium with 2·5 mm Ca. (B) Leaf from plant on medium supplemented with 5 mm Ca. There is about a 25 % increase in crystal idioblasts, demonstrating that crystal amount is related to Ca availability. (C) Leaf from plant treated with 1 µm fluspirilene in 0·5 % DMSO. Crystal formation is greatly inhibited. (D) Leaf from plant treated with 50 µm isradipine. Crystal formation is almost completely inhibited. (E) Leaf from plant treated with 50 µm nifedipine. Crystal formation is completely inhibited. (F) Leaf from plant on 0·5 % DMSO. There is no inhibition of crystal formation. Scale bars = 400 µm.
Table 1.
45Ca2+ uptake by Pistia crystal idioblast and mesophyll protoplasts
| Counts per minute for 106 protoplasts h–1 (± s.d.) | |
| Idioblast protoplasts | 4·4 ± 2·2 × 106 |
| Mesophyll protoplasts | 3·0 ± 1·4 × 104 |
The results are significantly different at P = 0·05, using a t‐test for separation of means.
Ca2+ channels are involved in calcium oxalate crystal formation
Ca2+ channels can be involved in large Ca2+ fluxes such as indicated for crystal idioblasts. To determine if functional Ca2+ channels are necessary for Ca oxalate crystal formation, Pistia plants were grown in media containing the DHP‐type inhibitors, nifedipine and isradipine (PN200‐110), the diphenylbutylpiperidine‐type inhibitor, fluspirilene, or the solvent used for the channel blocker stock solutions, DMSO. The leaves (from multiple plants) that formed during growth on these reagents were cleared and observed between crossed polarizers, which allows for all the crystal idioblasts in the sample to be seen. When placed on media containing 1 µm fluspirilene, 50 µm isradipine or 50 µm nifedipine, growth of the plants was not visually affected during the 7‐d treatment, and new leaves were formed during the period. However, media with fluspiriline at 50 and 100 µm was toxic to the plants. Both raphide and druse crystal formation were greatly inhibited upon exposure to either 1 µm fluspirilene (Fig. 3C), 50 µm isradipine (Fig. 3D) or 50 µm nifedipine (Fig. 3E) during the 7‐d growth period. This inhibition was consistent throughout individual leaves and repeatable between plants. Since inhibition was almost complete, as seen in the representative examples in Fig. 3, no attempt was not made to quantify the few idioblasts formed. The solvent used for the Ca2+ channel blockers, DMSO, did not affect crystal idioblast formation (Fig. 3F). The phenylalkylamine Ca2+ channel blockers verapamil and methoxyverapamil were also tried at 50–100 µm concentrations but had inconsistent results even at the higher concentration (not shown). Some plants showed some inhibition while others showed no significant effect on crystal formation.
An attempt was made to determine if binding of channel blockers to protoplasts could be measured. However, it was not possible to isolate enough idioblast protoplasts to measure differences statistically for channel blocker binding, as 3H‐channel binding was low and large amounts of idioblasts were needed to get measurements of radioactivity. Preliminary attempts showed that the 3H‐radiolabelled DHP‐type channel blocker, isradipine, gave low but specific labelling (compared with labelling in the presence of 1000‐fold excess unlabelled compound) of the idioblast protoplasts but no apparent specific labelling of mesophyll protoplasts under the isradipine concentrations and conditions used. 3H‐Fluspirilene labelled both protoplast types, but gave 35‐fold greater binding in idioblasts compared with mesophyll samples. It is not known if the labelled blockers were associated with surface or internal membranes.
DHP‐type Ca2+ channels are localized to crystal idioblasts
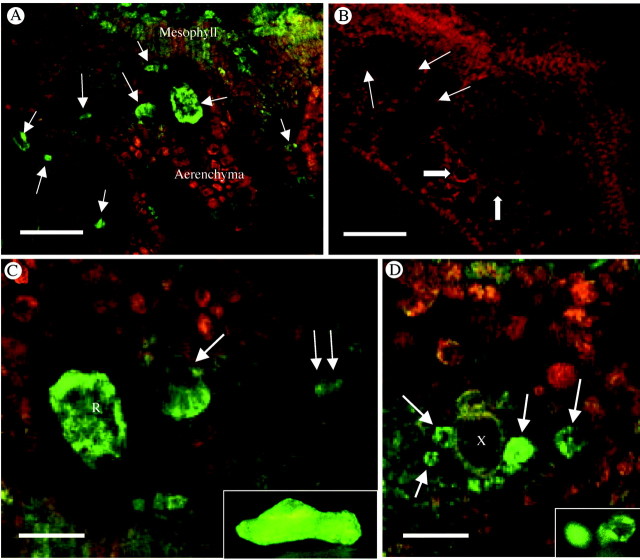
As dihydropyridine Ca2+ channel blockers appeared to be very effective at inhibiting crystal formation a commercially available fluorescent‐tagged dihydropyridine blocker was used as a probe on tissue sections. The DM‐Bodipy‐DHP channel blocker gave very bright fluorescence of both raphide and druse idioblasts compared with surrounding mesophyll cells (Fig. 4A, C and D). Some labelling of mesophyll cells was also seen, particularly for very young developing cells, but it was always much less than in crystal idioblasts. Pretreatment of sections with 400 µm nifedipine as a competitive inhibitor completely inhibited DM‐Bodipy‐DHP binding to crystal idioblasts (Fig. 4B). The DM‐Bodipy‐DHP appeared to penetrate the cells and give heavy labelling within the cytoplasm of both raphide and druse crystal idioblasts (Fig. 4C and D). The pattern of labelling was reticulate to patchy and was similar to what was previously seen using the endoplasmic reticulum probe, DiOC6 (insets in Fig. 4C and D; see Franceschi et al., 1993). In views focused along the surface of the cells, DM‐Bodipy‐DHP fluorescence appeared to be associated with the plasma membrane (Fig. 4C); but given the intense labelling of the internal membranes, it was often hard to distinguish.
Fig. 4. Binding of green fluorescent‐tagged Ca2+ channel blocker, DM‐Bodipy‐DHP, to fresh sections of developing Pistia leaf. (A) Low magnification image. The brightest green fluorescence is associated with Ca oxalate crystal idioblasts at various stages of development (arrows). Some fluorescence is also seen in the young developing mesophyll cells. Red fluorescence is due to chlorophyll. (B) Control. Binding of DM‐Bodipy‐DHP is completely inhibited by a 4‐h pretreatment with the competitive Ca2+ channel blocker, nifedipine. Raphide crystal idioblasts (arrows) and druse idioblasts (block arrows) are pointed out for reference. (C) Enlargement from A showing labelled crystal idioblasts. The raphide idioblast to the left (R) is focused through the cortical cytoplasm indicating Ca2+ channels are abundant in cytoplasmic organelles. At the large single arrow, the same focal plane is near the surface of the raphide idioblast indicating potential association of the fluorescent probe with the plasma membrane as well. At the double arrow is a cell that is just beginning initiation of differentiation to become a raphide idioblast. Inset is a living raphide crystal idioblast stained with DiOC6 to show ER abundance. (D) Image of the xylem region of a vein where druse crystal idioblasts are most common. At least four druse idioblasts at various developmental stages are seen and are fluorescing brightly (arrows). Inset is of living druse idioblasts stained with DiOC6. R, Raphide crystal idioblast; X, xylem. Scale bars: A and B = 50 µm; C and D = 21 µm.
A DHP‐type Ca2+ channel‐like protein is enriched in crystal idioblasts
Previous studies showed that a monoclonal antibody to the α1 subunit of a DHP Ca2+ channel recognized a membrane‐associated protein in plants (Volk and Franceschi, 2000). On western blots of total membrane and total soluble proteins extracted from Pistia leaves, this antibody, MAB427, recognizes a single membrane‐bound protein band (Fig. 5). This band, approx. 175–180 kDa, is close to the size range of the α1 subunit Ca2+ channel protein in animal systems (187–210 kDa), which is the subunit to which the antibody was raised. To determine if this protein was associated with all cells or primarily with crystal idioblasts, immunocytochemical localization was performed using paraffin‐embedded samples of young Pistia leaves. Figure 6A and B shows paraffin sections of developing leaves that were stained for general anatomy to show crystal distribution patterns. Comparison of crystal distribution in general‐stained paradermal sections and cross‐sections with the pattern of immunolabelling shown in Fig. 6C and E demonstrates that strong labelling for Ca2+ channel‐like protein is associated with developing crystal idioblasts. The MAB427 antibody gave strong labelling of both raphide (Fig. 6F) and druse (Fig. 6H) crystal idioblasts and low labelling of surrounding mesophyll cells. A preimmune serum for this antibody was not available but preimmune sera for three other antibodies used on Pistia leaves did not give rise to non‐specific labelling of idioblasts under the same immunostaining protocol (Volk et al., 2002; Li et al., 2003; Nakata et al., 2003). Treatment of sections with only the secondary antibody also did not give rise to labelling (Fig. 6D), demonstrating that the label seen with the primary antibody was not due to endogenous peroxidase activity or nonspecific binding of the secondary antibody. At higher magnification of young raphide crystal idioblasts in cross‐section, the signal was seen to be associated with the periphery of the idioblasts (Fig. 6F), which matched the distribution of cytoplasm as seen by general staining of these young idioblasts (Fig. 6G). A similar labelling pattern was seen with young druse crystal idioblasts (Fig. 6H). Label was clearly associated with the cytoplasm/periphery and not the vacuole of crystal idioblasts. Immunolabelling of some sections that are tangential along the surface of the idioblast (Fig. 6E and F) indicated that the plasma membrane may also be labelled, but the resolution is not high enough in these preparations to definitively distinguish the plasma membrane from the adjacent cytoplasm.
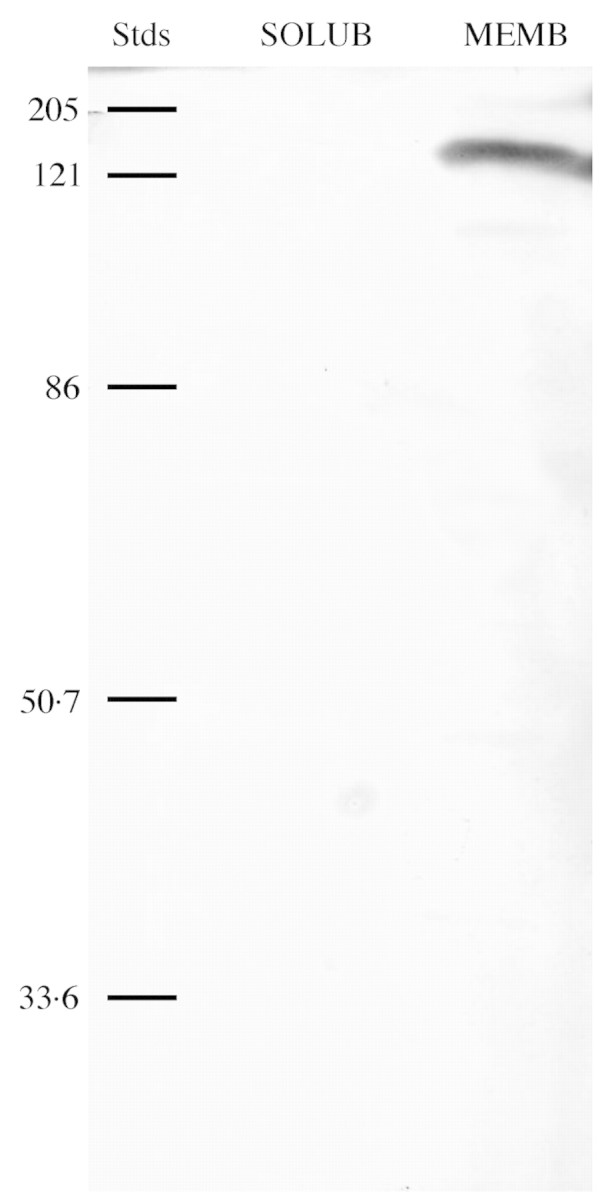
Fig. 5. Western immunoblot of soluble protein (SOLUB) and microsomal membrane protein (MEMB) from Pistia extracts (100 µg protein loaded for each). The blot was probed with an antibody (MAB427) to an α1 subunit of a DHP‐sensitive Ca2+ channel. For reference, relative molecular weight standards (Stds) are given in kDa. A single band of about 175 kDa was detected in the membrane but not soluble fraction.
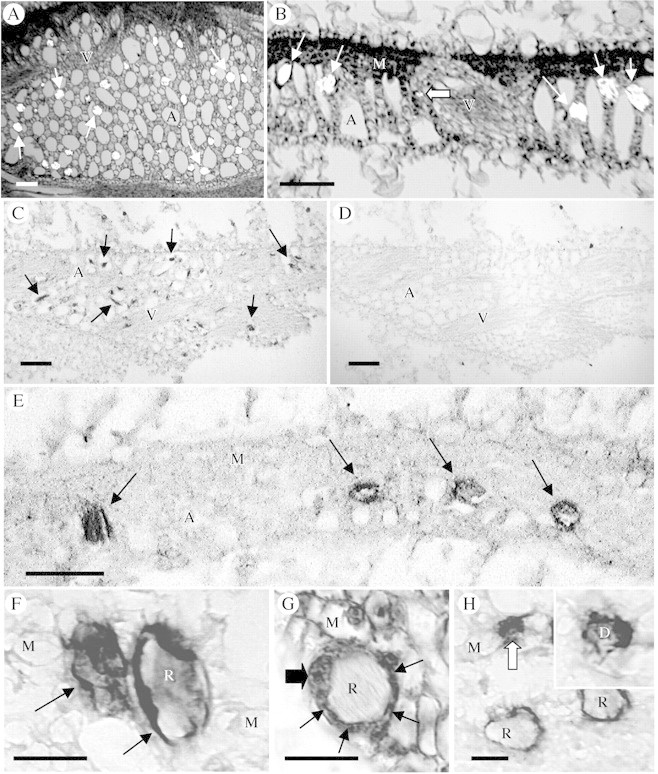
Fig. 6. Immunological localization of putative Ca2+ channels (α1 subunit of a DHP‐sensitive Ca channel) in paraffin sections of developing Pistia leaf tissue. Immunolabelled sections were not counterstained. (A, B and G) Sections of developing leaves stained for general histology for reference. (A) Paradermal section through aerenchyma, viewed with partially crossed polarizing filters. Crystal idioblasts (bright spots, arrows) are distributed throughout the aerenchyma. (B) Cross‐section showing developing raphide idioblasts (arrows) and a druse idioblast (block arrow). (C) Tangential section mostly through aerenchyma of a developing leaf that has been immunolabelled. The many developing idioblasts (arrows) are strongly stained, appearing as dark spots relative to the surrounding cell types. (D) Serial section to that in C but treated only with the secondary antibody. There is no endogenous peroxidase activity or nonspecific binding of the secondary antibody in the paraffin‐embedded sections. The idioblasts are not apparent. (E) Cross‐section through a developing leaf immunostained for α1 subunit of a DHP‐sensitive Ca2+ channel. Four developing idioblasts can be seen (arrows) and are heavily stained. The idioblast on the left is sectioned along the surface and appears completely dark, while the three idioblasts to the right show staining primarily along the periphery and not in the vacuole. (F) Two raphide idibolasts, one sectioned along the surface and one as a cross‐section. The label can be seen associated with the preipheral region. (G) General staining of a developing raphide idioblast for comparison to immunolabel, showing dense ring of cytoplasm (arrows) surrounding the crystal bundle. The enlarged nucleus (big arrow) can also be seen. (H) Section showing two raphide idioblasts and a druse idioblast (block arrow). The idioblasts label strongly with the antibody, but mesophyll cells show no significant label. Inset: Druse idioblast where the star‐shaped druse crystal can be seen surrounded by dense staining material from the cortical region of the cell. Scale bars: A–E = 100 µm, F–G = 20 µm. A, aerenchyma; D, druse crystal; M, mesophyll; R, raphide crystal bundle; V, vein.
The MAB427 antibody was also used on LR White‐embedded resin sections for TEM (as described in Volk et al., 2000) but did not give labelling in the idioblasts. It is likely that the epitope specifically recognized by MAB427 became cross‐linked during fixation or resin polymerization, or the resin interfered with the antibody–epitope interaction, such that labelling could not be achieved in the idioblasts. Previous studies indicated that antigenicity of idioblast membrane epitopes is difficult to preserve, even for abundant proteins like Ca2+ ATPase (not shown).
DISCUSSION
Calcium oxalate crystal formation is a complex process requiring specialized cellular features and the capability to transport large amounts of Ca from the outside of the cell to the vacuole, where crystals are formed. This study demonstrates that Ca2+ uptake by developing crystal idioblasts is much greater than in normal mesophyll cells, and that Ca2+ channels are involved in Ca accumulation in this cell type. The combined results of growth experiments, labelling studies and immunological assays suggest that DHP‐ binding proteins and functional Ca2+ channels are relatively abundant in Ca oxalate crystal idioblasts and are required for the process of crystal formation.
The whole plant studies showed that Ca2+ channel blockers inhibited crystal formation without arresting plant growth. This further suggests that functional Ca2+ channels are involved in Ca2+ transport processes required for Ca oxalate crystal formation. However, given the 7‐d period of growth in the presence of the channel blockers, indirect effects both related and unrelated to inhibition of Ca channel activity cannot be excluded. DHP‐type Ca2+ channel blockers were particularly effective at inhibiting formation of Ca oxalate crystals. While the level of Ca2+ channel blocker applied to the growth medium was 1–50 µm, the level reaching the developing leaves was probably much less than that, further indicating the potency of these blockers and the importance of Ca2+ channels to crystal idioblast formation. A caveat is that there is evidence that Ca2+ channel blockers can affect the activity of Ca2+/H+‐antiporters (Chanson, 1994; Pfeiffer, 1995). Thus, part of the inhibition of crystal formation observed could have been due to an effect on Ca2+/H+‐antiporters if they are operating in the idioblast tonoplast.
The results with a fluorescently tagged DHP Ca2+ channel blocker corroborate the whole plant studies and indicate that DHP type Ca2+ channels are much more numerous in the crystal idioblasts in comparison to surrounding mesophyll cells. The location of the channels could not be precisely determined since the blockers, including the fluorescent compound, are membrane permeable. However, labelling patterns indicate DHP Ca2+ channels are present on the plasma membrane but are particularly high in internal membrane systems in the crystal idioblasts. Plasma membrane labelling in this study was consistent with our hypothesis that Ca2+ channels are likely responsible for transport of Ca2+ into the idioblast, taking advantage of the large electrochemical potential gradient that is present across the plasma membrane (Franceschi, 1989). The cytoplasmic labelling was very specific in its pattern and did not appear to be simple accumulation of DM‐Bodipy‐DHP in the cytoplasm and vacuole. In fact, the vacuole did not accumulate label at all. Previous studies with plant systems have also shown that DM‐Bodipy‐DHP is capable of labelling plasma membrane and entering the cell to give rise to labelling of cytoplasmic organelles in very specific patterns that seem to be related to Ca2+ fluxes (Shaw and Quatrano, 1996; Vallee et al., 1997; Braun and Richter, 1999). Thus, it is believed that the intense cytoplasmic labelling in idioblasts is an indication of Ca2+ channel presence and association with organelles, and not a simple artefact of nonspecific accumulation of fluorescent probe. The pattern of internal labelling by DM‐Bodipy‐DHP correlates to some degree with the extensive network of ER that is present in this specialized cell type (Kostman et al., 2003; Nakata et al., 2003). An indication of this network can be seen in paraffin sections of raphide crystal idioblasts, as shown here (Fig. 6). The correlation of DM‐Bodipy‐DHP staining pattern and the intensity of the ER marker DiOC6 further suggests that at least part of the staining seen for this Ca2+ channel probe is due to association with the idioblast ER.
The Ca2+ channel blocker results are supported by our immunolabelling studies using an antibody to a rabbit T‐tubule Ca2+ channel α1 subunit protein (Morton and Froehner, 1987). Western blot analysis shows that the antibody specifically recognizes a protein of about the same molecular weight as the mammalian Ca2+ channel subunit, and the antibody only recognizes protein associated with the membrane fraction, which further supports the possibility that it is recognizing a Ca2+ channel or related protein in plants. A voltage‐gated arabidopsis Ca channel (AtTPC1) has an overall structure similar to part of the α1 subunit of animal voltage‐activated Ca2+ channels (Furuichi et al., 2001) and it is proposed that the antibody is recognizing conserved domains of a plant Ca2+ channel in Pistia. While sequence comparison gives only 26 % identity, comparison of the 22 amino acids of the active site of the Pore sequence II from AtTPC1 (White et al., 2002) with that of Mus musculus T‐tubule α1 subunit protein sequence (accession no. NM145853) shows that 11 of the 22 amino acids were identical. The animal channel and plant channel α1 subunits may be sufficiently similar for an antibody raised in animals to recognize plant epitopes. In addition, the protein recognized may not necessarily be a homologue of AtTPC1 but may be a different Ca channel, as putative Ca channel subunits have been identified in multiple plant species including arabidopsis, cotton, corn, sorghum, Lotus and soybean (White et al., 2002). With respect to molecular weight, it has been suggested that rat kidney TPC1 may form a dimer to function as an ion channel (Ishibashi et al., 2000), and Furuichi et al. (2001) also indicate that the AtTPC1 overall structure is similar to half of the general structure of α subunits of voltage‐activated Ca2+ channels. It is possible that the antibody used recognized a dimer form of the Ca2+ channel in Pistia, thus giving a molecular weight of about twice the AtTPC1 monomer.
In tissue sections, labelling was very high in developing crystal idioblasts compared with surrounding cell types, and the label was associated with the cortical region of the cell and not the vacuole. The intensity of labelling indicates that the antibody was associated with both the cytoplasm and probably the plasma membrane as well; however, resolution in the paraffin sections prevented us from distinguishing plasma membrane labelling. Attempts to label resin‐ embedded sections were not successful, probably because the epitopes were sensitive to the processing procedure. This is not an uncommon problem when attempting to localize membrane proteins which often requires antibodies to cytoplasmic or apoplasmic domains for immunological activity. The results show that idioblast cytoplasmic compartments are enriched in putative Ca2+ channels consistent with the fluorescent labelling experiments. Since idioblasts can fill with crystals in a matter of hours (Franceschi, 1989), the rate of Ca2+ uptake must be rapid relative to mesophyll cells and mechanisms must exist to carefully regulate free Ca2+ in the cytoplasm. This is likely accomplished by coordination of plasma membrane transport and the activity of Ca2+ ATPases and channels on the ER, an organelle that is particularly abundant in idioblast cells (Arnott and Pautard, 1970; Franceschi and Horner, 1980; Webb, 1999) as shown here for Pistia. It is proposed that the pattern of cytoplasmic DM‐Bodipy‐DHP labelling seen in the crystal idioblasts is likely associated with ER. There is an abundance of information showing that Ca2+ release channels are present in the rough ER and smooth ER of animal cells, and Ca2+ selective channels have also been shown to be present in the ER of plant cells (Klusener and Weiler, 1999). Supporting our interpretation is the observation that the pattern of DM‐Bodipy‐DHP fluorescence is essentially the same as the fluorescent pattern seen in crystal idioblasts that were exposed to DiOC6 for visualization of ER (see also Franceschi et al., 1993). In addition, the crystal idioblast ER is known to be enriched in Ca2+ buffering proteins, such as calreticulin, relative to surrounding cells (Franceschi et al., 1993; Nakata et al., 2003), which is consistent with our hypothesized role for the ER in regulating cytoplasmic Ca2+ activity during idioblast development. Ca2+ channels in the Pistia ER are likely to be involved in regulated release of Ca2+ for subsequent transfer to the vacuole.
Overall, the results of whole plant treatments, fluorescent‐tagged blocker and immunolocalization indicate that a DHP‐sensitive L‐type Ca2+ channel is a major component of Ca2+ transport in the idioblast, but does not exclude other pharmacological types. In animal systems, L‐type Ca2+ channels can be inhibited by the binding of either DHP‐ or PAA‐type inhibitors at specific binding sites. Another compound, fluspiriline can also block L‐channels, as well as N‐ and T‐type Ca2+ channels. While the putative Ca2+ channels described in Pistia are sensitive to 1,4 dihydropyridines and fluspiriline, other Ca2+ channels described in plant systems are not sensitive to dihydropyridines (Andrejauskas et al., 1985; Graziana et al., 1988; Thuleau et al., 1994; Pineros and Tester, 1995; Pineros and Tester, 1997). However, there are various examples of DHP‐sensitive channels in plants. For example, DHP antagonists significantly inhibit bud initial formation in the moss Funaria (Conrad and Hepler, 1988), and treatment with nifedipine causes reduced callose deposition and decreased polarized movement in the vegetative cell of germinating Narcissus pseudonarcissus pollen (Heslop‐Harrison and Heslop‐Harrison, 1992). In addition, callose synthesis was inhibited in Glycine max and Catharanthus roseus suspension‐cultured cells treated with 50 µm nifedipine (Waldmann et al., 1988). The antagonist fluspiriline (50 µm) is also effective in other plant systems. It blocks the ABA‐induced expression of RAB18 in arabidopsis suspension cells (Ghelis et al., 2000). These studies demonstrate that the application of Ca2+ channel antagonists have a physiological effect on various Ca‐dependent processes in plants. Our studies show that treatment with DHP can also inhibit the cell‐specific function of Ca oxalate crystal formation, presumably by interfering with Ca2+ accumulation and transfer.
While Ca2+ influx to vacuoles of the crystal idioblasts is likely to be mediated by Ca2+‐ATPases and/or Ca2+/H+‐antiporters (Pittman and Hirschi, 2003), Ca starvation induces dissolution of crystals in Pistia (Volk et al., 2002), and tonoplast channels might be involved in transfer of Ca to the cytosol under those conditions. Ca2+ channels sensitive to various classes of blockers have been demonstrated in tonoplasts of various cell systems (Ping et al., 1992; Gelli and Blumwald, 1993; Allen and Sanders, 1994). In our study, because the internal organelles labelled so intensely, it was difficult to tell if the idioblast tonoplast had any significant fluorescence associated with it.
In summary, evidence is provided that indicates Ca2+ channels are abundant in Ca oxalate crystal idioblasts and are a necessary component of the physiological process of Ca accumulation required for formation of Ca oxalate crystals. These channels have binding sites for DHP‐type inhibitors and appear to be the L‐type of Ca2+ channel. The channels are likely present in the plasma membrane and the crystal idioblast ER, where they may play a role in cytoplasmic Ca2+ transfer and buffering between Ca2+ pools en route to the vacuole. The results provide further information on the physiological mechanisms required to support Ca oxalate crystal formation in plants, specifically with respect to Ca accumulation in this cell type that is specialized for high capacity Ca sequestration.
ACKNOWLEDGEMENTS
This study was supported by NSF grants MCB9632027 and MCB9904562 to V.R.F. Microscopy was done by the authors at the WSU Electron Microscopy Center. The contents of this publication do not necessarily reflect the views or policies of the US Department of Agriculture, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
Supplementary Material
Received: 20 December 2003; Returned for revision: 22 January 2004; Accepted: 12 February 2004; Published electronically: 15 April 2004
References
- AllenGJ, Sanders D.1994. Two voltage‐gated, calcium release channels coreside in the vacuolar membrane of broad bean guard cells. Plant Cell 6: 685–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AndrejauskasE, Hertel R, Marme D.1985. Specific binding of the calcium antagonist [3H]verapamil to membrane fractions from plants. Journal of Biological Chemistry 260: 5411–5414. [PubMed] [Google Scholar]
- ArnottHJ.1982. Three systems of biomineralization in plants with comments on the associated organic matrix. In: Nancollas GH, ed. Biological mineralization and demineralization. Berlin: Springer‐Verlag, 199–218. [Google Scholar]
- ArnottHJ, Pautard FGE.1970. Calcification in plants. In: Schraer H, ed. Biological calcification. Cellular and molecular aspects. New York: Appleton‐Century‐Crofts, 375–446. [Google Scholar]
- BhatlaSC, Kiessling J, Reski R.2002. Observation of polarity induction by cytochemical localization of phenylalkylamine‐binding sites in regenerating protoplasts of the moss Physcomitrella patens Protoplasma 219: 99–105. [DOI] [PubMed] [Google Scholar]
- BorchertR.1985. Calcium‐induced patterns of calcium‐oxalate crystals in isolated leaflets of Gleditsia triacanthos L. and Albizia julibrissin Durazz. Planta 165: 301–310. [DOI] [PubMed] [Google Scholar]
- BorchertR.1986. Calcium acetate induces calcium uptake and formation of calcium‐oxalate crystals in isolated leaflets of Gleditsia triacanthos L. Planta 168: 571–578. [DOI] [PubMed] [Google Scholar]
- BouropoulosN, Weiner S, Addadi L.2001. Calcium oxalate crystals in tomato and tobacco plants: morphology and in vitro interactions of crystal‐associated macromolecules. Chemistry: a European Journal 7: 1881–1888. [DOI] [PubMed] [Google Scholar]
- BraunM, Richter P.1999. Relocalization of the calcium gradient and a dihydropyridine receptor is involved in upward bending by bulging of Chara protonemata, but not in downward bending by bowing of Chara rhizoids. Planta 209: 414–423. [DOI] [PubMed] [Google Scholar]
- BushDS.1993. Regulation of cytosolic calcium in plants. Plant Physiology 103: 7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ChansonA.1994. Characterization of the tonoplast Ca2+/H+ antiport system from maize roots. Plant Physiology and Biochemistry 32: 341–346. [Google Scholar]
- ConradPA, Hepler PK.1988. The effect of 1,4‐dihydropyridines on the initiation and development of gametophore buds in the moss Funaria Plant Physiology 86: 684–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSilvaDLR, Hetherington AM, Mansfield TA.1996. Where does all the calcium go? Evidence of an important regulatory role for trichomes in two calcicoles. Plant Cell and Environment 19: 880–886. [Google Scholar]
- FosterAS.1956. Plant idioblasts: remarkable examples of cell specialization. Protoplasma 46: 184–193. [Google Scholar]
- FranceschiVR.1989. Calcium oxalate formation is a rapid and reversible process in Lemna minor L. Protoplasma 148: 130–137. [Google Scholar]
- FranceschiVR, Horner HT Jr.1979. Use of Psychotria puncata callus in study of calcium oxalate crystal idioblast formation. Zeitschrift fur Pflanzenphysiologie 67: 61–75. [Google Scholar]
- FranceschiVR, Loewus FA.1995. Oxalate biosynthesis and function in plants and fungi. In: Khan SR, ed. Calcium oxalate in biological systems CRC Press, Boca Raton, FL, 113–130. [Google Scholar]
- FranceschiVR, Horner HT.1980. Calcium oxalate crystals in plants. Botanical Review 46: 361–427. [Google Scholar]
- FranceschiVR, Li XX, Zhang DZ, Okita TW.1993. Calsequestrin‐like calcium‐binding protein is expressed in calcium‐accumulating cells of Pistia stratiotes Proceedings of the National Academy of Sciences of the USA 90: 6986–6990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FrankE.1972. The formation of crystal idioblasts in Canavalia ensiformis D.C. at different levels of calcium supply. Zeitschrift fur Pflanzenphysiologie 67: 350–358. [Google Scholar]
- FuruichiT,Cunningham KW, Muto S.2001. A putative two pore channel AtTPC1 mediates Ca2+ flux in Arabidopsis leaf cells. Plant and Cell Physiology 42: 900–905. [DOI] [PubMed] [Google Scholar]
- GelliA, Blumwald E.1993. Calcium retrieval from vacuolar pools. Plant Physiology 102: 1139–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GhelisT, Dellis O, Jeannette E, Bardat F, Miginiac E, Sotta B.2000. Abscisic acid plasmalemma perception triggers a calcium influx essential for RAB18 gene expression in Arabidopsis thaliana suspension cells. FEBS Letters 483: 67–70. [DOI] [PubMed] [Google Scholar]
- GrazianaA, Fosset M, Ranjeva R, Hetherington AM, Lazdunski M.1988. Ca2+ channel inhibitors that bind to plant cell membranes block Ca2+ entry into protoplasts. Biochemistry 27: 764–768. [Google Scholar]
- HarperJF.2001. Dissecting calcium oscillators in plant cells. Trends in Plant Science 6: 395–397. [DOI] [PubMed] [Google Scholar]
- Heslop‐HarrisonJ, Heslop‐Harrison Y.1992. Germination of monocolpate angiosperm pollen: effects of inhibitory factors and the Ca2+‐channel blocker, nifedipine. Annals of Botany 69: 395–403. [Google Scholar]
- HornerHT, Wagner BL 1995. Calcium oxalate formation in higher plants. In: Khan SR, ed. Calcium oxalate in biological systems CRC Press, Boca Raton, FL, 53–72. [Google Scholar]
- HornerHT, Kausch AP, Wagner BL.2000. Ascorbic acid: a precursor of oxalate in crystal idioblasts of Yucca torreyi in liquid root culture. International Journal of Plant Science 161: 861–868. [Google Scholar]
- HudginsJW, Krekling T, Franceschi VR.2003. Distribution of calcium oxalate crystals in the secondary phloem of conifers: a constitutive defense mechanism? New Phytologist 159: 677–690. [DOI] [PubMed] [Google Scholar]
- IshibashiK, Suzuki M, Imai M.2000. Molecular cloning of a novel form (two‐repeat) protein related to voltage‐gated sodium and calcium channels. Biochemical and Biophysical Research Communications 270: 370–376. [DOI] [PubMed] [Google Scholar]
- KeatesSE, Tarlyn NM Loewus FA Franceschi VR.2000. L‐ascorbic acid and L‐galactose are sources for oxalic acid and calcium oxalate in Pistia stratiotes Phytochemistry 53: 433–440. [DOI] [PubMed] [Google Scholar]
- KirkbyEA, Pilbeam DJ.1984. Calcium as a plant nutrient. Plant Cell and Environment 7: 397–405. [Google Scholar]
- KlusenerB, Weiler EW.1999. A calcium‐selective channel from root‐tip endomembranes of garden cress. Plant Physiology 119: 1399–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KnausH, Moshammer T, Friedrich K, Kang HC, Haugland RP, Glossmann H.1992a.In vivo labelling of L‐type Ca2+ channels by fluorescent dihydropyridines: evidence for a functional, extracellular heparin‐binding site. Proceedings of the National Academy of Sciences of the USA 89: 3586–3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KnausH, Moshammer T, Kang HC, Haugland RP, Glossmann H.1992b. A unique fluorescent phenylalkylamine probe for L‐type Ca2+ channels. Journal of Biological Chemistry 267: 2179–2189. [PubMed] [Google Scholar]
- KostmanTA, Franceschi VR.2000. Cell and calcium oxalate crystal growth is coordinated to achieve high capacity calcium regulation in plants. Protoplasma 214: 166–179. [Google Scholar]
- KostmanTA, Koscher JR.2003. L‐galactono‐γ‐lactone dehydrogenase is present in calcium oxalate crystal idioblasts of two plant species. Plant Physiology and Biochemistry 41: 201–206. [Google Scholar]
- KostmanTA, Franceschi VR, Nakata PA.2003. Specialized ER‐subcompartments may play a role in calcium regulation in crystal idioblasts of Pistia stratiotes Plant Science 165: 205–212. [Google Scholar]
- KostmanTA, Tarlyn NM, Loewus FA, Franceschi VR.2001. Biosynthesis of L‐ascorbic acid and conversion of carbons 1 and 2 of L‐ascorbic acid to oxalic acid occurs within individual calcium oxalate crystal idioblasts. Plant Physiology 215: 634–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LiX, Zhang D, Lynch‐Holm VM, Okita TW, Franceschi VR.2003. Isolation of a crystal matrix protein associated with calcium oxalate precipitation in vacuoles of specialized cells. Plant Physiology 133: 549–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MazenAMA, Zhang D, Franceschi VR.2003. Calcium oxalate formation in Lemna minor L.: physiological and ultrastructural aspects of high capacity calcium sequestration. New Phytologist 161: 435–448. [DOI] [PubMed] [Google Scholar]
- MortonME, Froehner SC.1987. Monoclonal antibody identifies a 200‐kDa subunit of the dihydropyridine‐sensitive calcium channel. Journal of Biological Chemistry 262: 11904–11907. [PubMed] [Google Scholar]
- NakataPA.2003. Advances in our understanding of calcium oxalate crystal formation and function in plants. Plant Science 164: 901–909. [Google Scholar]
- NakataPA, Kostman TA, Franceschi VR.2003. Calreticulin is an important component of the high capacity calcium sequestration mechanism in specialized plant cells. Plant Physiology and Biochemistry 41: 425–430. [Google Scholar]
- OrvarBL, Sangwan V, Omann F, Dhindsa RS.2000. Early steps in cold sensing by plant cells: the role of actin cytoskeleton and membrane fluidity. Plant Journal 23: 785–794. [DOI] [PubMed] [Google Scholar]
- PennisiSV, McConnell DB.2001. Inducible calcium sinks and preferential calcium allocation in leaf primordia of Dracaena sanderiana Hort. Sander ex M.T. Mast (Dracaenaceae). Hortscience 36: 1187–1191. [Google Scholar]
- PennisiSV, McConnell DB, Gower LB, Kane ME, Lucansky T.2001. Periplasmic cuticular calcium oxalate crystal deposition in Dracaena sanderiana New Phytologist 149: 209–218. [DOI] [PubMed] [Google Scholar]
- PfeifferW.1995. Effects of w‐7, w‐5, verapamil and diltiazem on vacuolar proton transport – comparison of vacuolar H+‐ATPase and H+‐PPase from roots of Zea mays Physiologia Plantarum 94: 284–290. [Google Scholar]
- PinerosM, Tester M.1995. Characterization of a voltage‐dependent Ca2+‐selective channel from wheat roots. Planta 195: 478–488. [Google Scholar]
- PinerosM, Tester M.1997. Characterization of the high‐affinity verapamil binding site in a plant plasma membrane Ca2+‐selective channel. Journal of Membrane Biology 157: 139–145. [DOI] [PubMed] [Google Scholar]
- PingZ, Yabe I, Muto S.1992. Identification of K+, Cl–, and Ca2+ channels in the vacuolar membrane of tobacco cell suspension cultures. Protoplasma 171: 7–18. [Google Scholar]
- PiotrowskaG.1998. The role of calcium ions in freezing injuries of winter oilseed rape leaves. Effects of calcium channel blockers and mimesis by calcium ionophore. Acta Physiologiae Plantarum 20: 257–261. [Google Scholar]
- PittmanJK, Hirschi KD.2003. Don’t shoot the (second) messenger: endomembrane transporters and binding proteins modulate cytosolic Ca2+ levels. Current Opinions in Plant Biology 6: 257–262. [DOI] [PubMed] [Google Scholar]
- QuitadamoIJ, Kostman TA, Schelling ME, Franceschi VR.2000. Magnetic bead purification as a rapid and efficient method for enhanced antibody specificity for plant sample immunoblotting and immunolocalization. Plant Science 153: 7–14. [Google Scholar]
- ReissH‐D, Herth W.1985. Nifedipine‐sensitive calcium channels are involved in polar growth of lily pollen tubes. Journal of Cell Science 76: 247–254. [DOI] [PubMed] [Google Scholar]
- SabnisRW, Deligeorgiev TG, Jachak MN, Dalvi TS.1997. DiOC6(3): a useful dye for staining the endoplasmic reticulum. Biotechnic and Histochemistry 72: 253–8. [DOI] [PubMed] [Google Scholar]
- SandersD, Pelloux J, Brownlee C, Harper JF.2002. Calcium at the crossroads of signaling. Plant Cell 14: S401–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAS Institute.2000.JMP User’s Guide, Version 4. SAS Institute Inc., Cary, NC, USA. [Google Scholar]
- ShawSL, Quatrano RS.1996. Polar localization of a dihydropyridine receptor on living Fucus zygotes. Journal of Cell Science 109: 335–342. [DOI] [PubMed] [Google Scholar]
- ThuleauP, Ward JM, Ranjeva R, Schroeder JI.1994. Voltage‐dependent calcium‐permeable channels in the plasma membrane of a higher plant cell. EMBO Journal 13: 2970–2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ValleeN, Briere C, Petitprez M, Barthou H, Souvre A, Alibert G.1997. Studies on the ion channel antagonist‐binding sites in sunflower protoplasts. FEBS Letters 411: 115–118. [DOI] [PubMed] [Google Scholar]
- VolkGM, Franceschi VR.2000. Localization of a calcium channel‐like protein in the sieve element plasma membrane. Australian Journal of Plant Physiology 27: 779–786. [Google Scholar]
- VolkGM, Lynch‐Holm V, Kostman TA, Franceschi VR.2002. The role of druse and raphide calcium oxalate crystals in tissue calcium regulation in Pistia stratiotes leaves. Plant Biology 4: 34–45. [Google Scholar]
- WaldmannT, Jeblick W, Kauss H.1988. Induced net Ca2+ uptake and callose biosynthesis in suspension‐cultured plant cells. Planta 173: 88–95. [DOI] [PubMed] [Google Scholar]
- WebbMA.1999. Cell‐mediated crystallization of calcium oxalate in plants. Plant Cell 11: 751–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WebbMA, Arnott HJ.1983. Inside plant crystals: a study of the noncrystalline core in druses of Vitis vinifera endosperm. Scanning Electron Microscopy 1983: 1759–1770. [Google Scholar]
- WebbMA, Cavaletto JM, Carpita NC, Lopez LE, Arnott HJ.1995. The intravacuolar organic matrix associated with calcium oxalate crystals in leaves of Vitis Plant Journal 7: 633–648. [Google Scholar]
- WhitePJ.2000. Calcium channels in higher plants. Biochemica Biophysica Acta 1465: 171–189. [DOI] [PubMed] [Google Scholar]
- WhitePJ, Broadley MR.2003. Calcium in plants. Annals of Botany 92: 487–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WhitePJ, Bowen HC, Demidchik V, Nichols C, Davies JA.2002. Genes for calcium‐permeable channels in the plasma membrane of plant root cells Biochimica et Biophysica Acta 1564: 299–309. [DOI] [PubMed] [Google Scholar]
- Zindler‐FrankE.1975. On the formation of the pattern of crystal idioblasts in Canavalia ensiformis D. C. VII. Calcium and oxalate content of the leaves in dependence of calcium nutrition. Zeitschrift fur Pflanzenphysiologie 77: 80–85. [Google Scholar]
- Zindler‐FrankE, Honow R, Hesse A.2001. Calcium and oxalate content of the leaves of Phaseolus vulgaris at different calcium supply in relation to calcium oxalate crystal formation. Journal of Plant Physiology 158: 139–144. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.