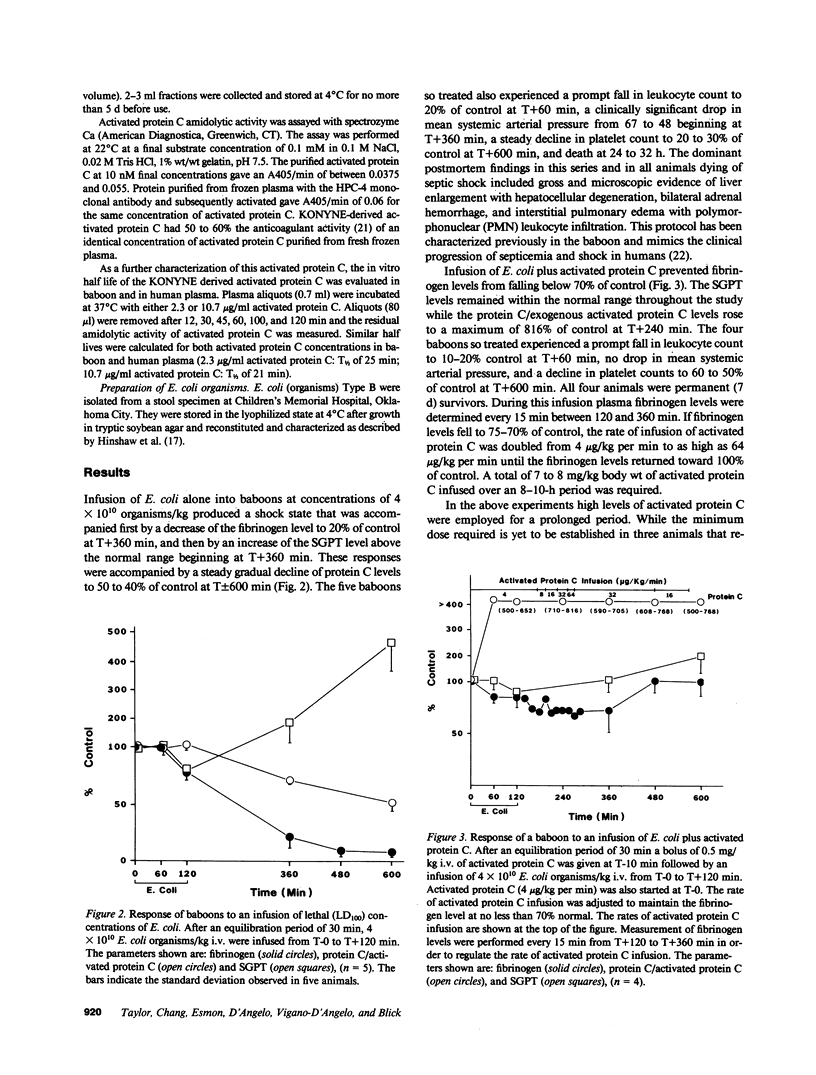
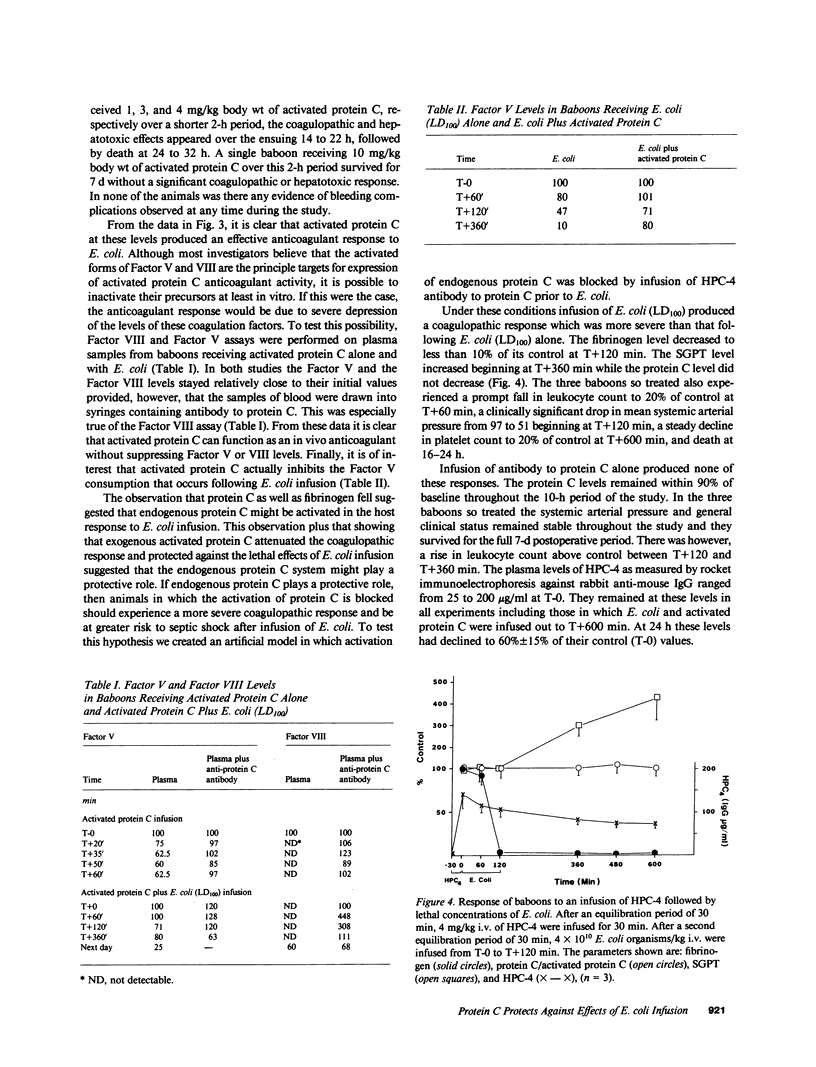
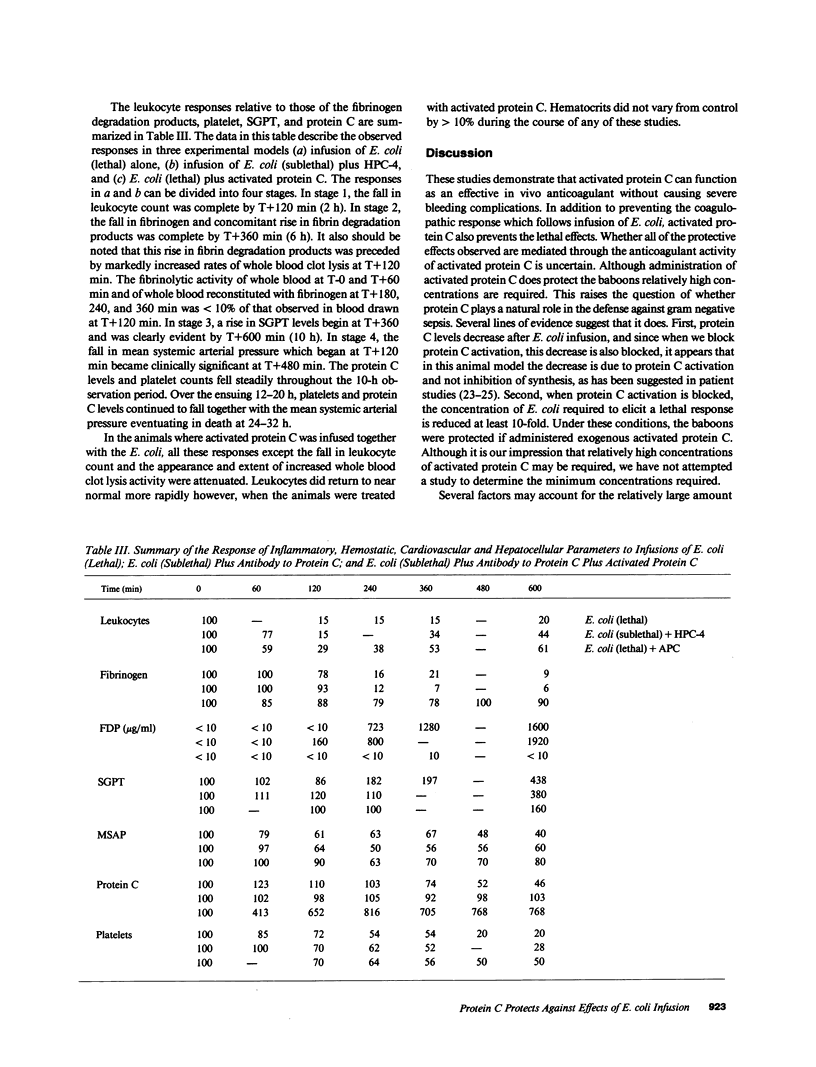
Abstract
Gram-negative septicemia elicits multiple abnormalities of the coagulation system. Although products of coagulation can lead to clot formation, thereby potentiating organ damage, recent work has shown that low concentrations of thrombin can protect animals from the shock state. Because these amounts of thrombin also lead to formation in vivo of the anticoagulant enzyme, activated protein C, we examined the role of protein C in modulation of Escherichia coli shock in baboons. First, we infused activated protein C and lethal concentrations of E. coli organisms, which prevented the coagulopathic, hepatotoxic, and lethal effects of E. coli. Second, using an antibody to protein C we blocked protein C activation in vivo to determine if this influenced the response to lethal and sublethal concentrations of E. coli organisms. Under these conditions the response to lethal concentrations of E. coli organisms was made more severe and the response to sublethal concentrations of E. coli was made lethal. The coagulopathic, hepatotoxic, and lethal responses in this latter case were prevented by infusion of exogenous protein C.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beutler B., Milsark I. W., Cerami A. C. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 1985 Aug 30;229(4716):869–871. doi: 10.1126/science.3895437. [DOI] [PubMed] [Google Scholar]
- Coalson J. J., Benjamin B. A., Archer L. T., Beller B. K., Spaet R. H., Hinshaw L. B. A pathologic study of Escherichia coli shock in the baboon and the response to adrenocorticosteroid treatment. Surg Gynecol Obstet. 1978 Nov;147(5):726–736. [PubMed] [Google Scholar]
- Comp P. C., Esmon C. T. Generation of fibrinolytic activity by infusion of activated protein C into dogs. J Clin Invest. 1981 Nov;68(5):1221–1228. doi: 10.1172/JCI110368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comp P. C., Jacocks R. M., Ferrell G. L., Esmon C. T. Activation of protein C in vivo. J Clin Invest. 1982 Jul;70(1):127–134. doi: 10.1172/JCI110584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmon C. T., Owen W. G. Identification of an endothelial cell cofactor for thrombin-catalyzed activation of protein C. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2249–2252. doi: 10.1073/pnas.78.4.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajdusek C., Carbon S., Ross R., Nawroth P., Stern D. Activation of coagulation releases endothelial cell mitogens. J Cell Biol. 1986 Aug;103(2):419–428. doi: 10.1083/jcb.103.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin J. H., Mosher D. F., Zimmerman T. S., Kleiss A. J. Protein C, an antithrombotic protein, is reduced in hospitalized patients with intravascular coagulation. Blood. 1982 Jul;60(1):261–264. [PubMed] [Google Scholar]
- Harris K. W., Esmon C. T. Protein S is required for bovine platelets to support activated protein C binding and activity. J Biol Chem. 1985 Feb 25;260(4):2007–2010. [PubMed] [Google Scholar]
- Hinshaw L. B., Brackett D. J., Archer L. T., Beller B. K., Wilson M. F. Detection of the 'hyperdynamic state' of sepsis in the baboon during lethal E. coli infusion. J Trauma. 1983 May;23(5):361–365. doi: 10.1097/00005373-198305000-00001. [DOI] [PubMed] [Google Scholar]
- Kisiel W., Ericsson L. H., Davie E. W. Proteolytic activation of protein C from bovine plasma. Biochemistry. 1976 Nov 2;15(22):4893–4900. doi: 10.1021/bi00667a022. [DOI] [PubMed] [Google Scholar]
- Mannucci P. M., Vigano S. Deficiencies of protein C, an inhibitor of blood coagulation. Lancet. 1982 Aug 28;2(8296):463–467. doi: 10.1016/s0140-6736(82)90494-9. [DOI] [PubMed] [Google Scholar]
- Marlar R. A., Kleiss A. J., Griffin J. H. Mechanism of action of human activated protein C, a thrombin-dependent anticoagulant enzyme. Blood. 1982 May;59(5):1067–1072. [PubMed] [Google Scholar]
- Marlar R. A., Kleiss A. J., Griffin J. H. Mechanism of action of human activated protein C, a thrombin-dependent anticoagulant enzyme. Blood. 1982 May;59(5):1067–1072. [PubMed] [Google Scholar]
- Nesheim M. E., Canfield W. M., Kisiel W., Mann K. G. Studies of the capacity of factor Xa to protect factor Va from inactivation by activated protein C. J Biol Chem. 1982 Feb 10;257(3):1443–1447. [PubMed] [Google Scholar]
- Rodeghiero F., Mannucci P. M., Viganò S., Barbui T., Gugliotta L., Cortellaro M., Dini E. Liver dysfunction rather than intravascular coagulation as the main cause of low protein C and antithrombin III in acute leukemia. Blood. 1984 Apr;63(4):965–969. [PubMed] [Google Scholar]
- Rodeghiero F., Mannucci P. M., Viganò S., Barbui T., Gugliotta L., Cortellaro M., Dini E. Liver dysfunction rather than intravascular coagulation as the main cause of low protein C and antithrombin III in acute leukemia. Blood. 1984 Apr;63(4):965–969. [PubMed] [Google Scholar]
- Sacks T., Moldow C. F., Craddock P. R., Bowers T. K., Jacob H. S. Oxygen radicals mediate endothelial cell damage by complement-stimulated granulocytes. An in vitro model of immune vascular damage. J Clin Invest. 1978 May;61(5):1161–1167. doi: 10.1172/JCI109031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern D. M., Nawroth P. P., Harris K., Esmon C. T. Cultured bovine aortic endothelial cells promote activated protein C-protein S-mediated inactivation of factor Va. J Biol Chem. 1986 Jan 15;261(2):713–718. [PubMed] [Google Scholar]
- Taylor F. B., Jr, Chang A., Hinshaw L. B., Esmon C. T., Archer L. T., Beller B. K. A model for thrombin protection against endotoxin. Thromb Res. 1984 Oct 15;36(2):177–185. doi: 10.1016/0049-3848(84)90339-6. [DOI] [PubMed] [Google Scholar]
- Taylor F. B., Jr, Lockhart M. S. Whole blood clot lysis: in vitro modulation by activated protein C. Thromb Res. 1985 Mar 15;37(6):639–649. doi: 10.1016/0049-3848(85)90193-8. [DOI] [PubMed] [Google Scholar]
- Vehar G. A., Davie E. W. Preparation and properties of bovine factor VIII (antihemophilic factor). Biochemistry. 1980 Feb 5;19(3):401–410. doi: 10.1021/bi00544a001. [DOI] [PubMed] [Google Scholar]
- Vigano D'Angelo S., Comp P. C., Esmon C. T., D'Angelo A. Relationship between protein C antigen and anticoagulant activity during oral anticoagulation and in selected disease states. J Clin Invest. 1986 Feb;77(2):416–425. doi: 10.1172/JCI112319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WROBLEWSKI F., LADUE J. S. Serum glutamic pyruvic transaminase in cardiac with hepatic disease. Proc Soc Exp Biol Med. 1956 Apr;91(4):569–571. doi: 10.3181/00379727-91-22330. [DOI] [PubMed] [Google Scholar]
- Walker F. J., Sexton P. W., Esmon C. T. The inhibition of blood coagulation by activated Protein C through the selective inactivation of activated Factor V. Biochim Biophys Acta. 1979 Dec 7;571(2):333–342. doi: 10.1016/0005-2744(79)90103-7. [DOI] [PubMed] [Google Scholar]
- Wetmore R., Gurewich V. The role of fibrin monomer and an in vivo thrombin-induced-anticoagulant in experimental venous thrombosis. Scand J Haematol. 1974;12(3):204–212. doi: 10.1111/j.1600-0609.1974.tb00200.x. [DOI] [PubMed] [Google Scholar]


