Abstract
Cotton (Gossypium hirsutum L.) crop, cultivated between 40°N and 40°S, is currently experiencing 2–11 kJ m–2 d–1 of UV‐B radiation. This is predicted to increase in the near future. An experiment was conducted to study the effect of enhanced UV‐B radiation on vegetative and reproductive morphology and leaf anatomy of cotton in sunlit, controlled environment chambers. From emergence to harvest, cotton plants were exposed to 0, 8 or 16 kJ m–2 d–1 of UV‐B in a square wave approach for 8 h from 0800 to 1600 h. Changes in plant height, internode and branch length, mainstem node number, leaf area, length and area of petals and bracts, and anther number per flower were recorded. Epidermal cell and stomatal density, stomatal index, leaf thickness, and epidermal, palisade and mesophyll tissue thickness were also measured. Initial chlorotic symptoms on leaves turned into necrotic patches on continued exposure to enhanced UV‐B. Exposure to high UV‐B reduced both vegetative and reproductive parameters and resulted in a smaller canopy indicating sensitivity of cotton to UV‐B radiation. Enhanced UV‐B radiation increased epicuticular wax content on adaxial leaf surfaces, and stomatal index on both adaxial and abaxial leaf surfaces. Leaf thickness was reduced following exposure to UV‐B owing to a decrease in thickness of both the palisade and mesophyll tissue, while the epidermal thickness remained unchanged. The vegetative parameters studied were affected only by high levels of UV‐B (16 kJ m–2 d–1), whereas the reproductive parameters were reduced at both ambient (8 kJ m–2 d–1) and high UV‐B levels. The study shows that cotton plants are sensitive to UV‐B at both the whole plant and anatomical level.
Key words: Gossypium hirsutum, cotton, UV‐B, ultraviolet, wax, stomatal index, palisade, mesophyll, epidermal cell, scanning electron microscopy
INTRODUCTION
Projections indicate that solar ultraviolet‐B (UV‐B) radiation will reach peak levels on the Earth’s surface in the next few years. However, it is expected that UV‐B radiation could fall to pre‐ozone depletion levels by 2050 if the Montreal protocol were fully implemented by the member countries (Van der Leun et al., 1998). Current levels of UV‐B radiation (280–315 nm) in the US Cotton Belt vary between 2 kJ m–2 d–1 in spring and 11 kJ m–2 d–1 in summer (USDA‐UVMRP, 2002). UV‐B radiation is readily absorbed by biomolecules such as amino acids, polypeptides and nucleic acids (Sullivan and Teramura, 1989). Experiments carried out under both controlled environments and field conditions have demonstrated that enhanced UV‐B radiation reduces crop productivity (Corlett et al., 1997). Enhanced UV‐B radiation causes a significant reduction in plant growth (Sullivan and Teramura, 1989; Teramura et al., 1991b), photosynthetic capacity (Ziska et al., 1993; Teramura and Sullivan, 1994) and pigment levels (Strid and Porra, 1992; Sullivan and Rozema, 1999). Although the effects on cotton growth and development of other environmental stress factors, such as high temperature (Reddy et al., 2000), water deficit (Bondada et al., 1995) and nutrient stresses are widely reported in literature, knowledge concerning the effects of UV‐B radiation on cotton growth and development is minimal (Corlett et al., 1997).
Upon exposure to UV‐B radiation, crops respond by altering their morphology and mitigate by adopting shielding or repair mechanisms. Reductions in shoot height, stem internode length and leaf size have been observed in a variety of species (Becwar et al., 1982; Teramura, 1983; Latimer and Mitchell, 1987; Sullivan and Teramura, 1988). Morphological changes of rice seedlings following exposure to UV‐B treatments simulating 5 % depletion of stratospheric ozone in the Philippines included reduced plant height, leaf blade length and leaf area (Barnes et al., 1993). Similarly, the height of pea (Pisum sativum L.) plants exposed to UV‐B decreased (Vu et al., 1984). Decreased leaf area of pea plants exposed to high UV‐B (9 kJ m–2 d–1) was attributed to a reduction in cell division rather than a reduction in cell expansion (Mepsted et al., 1996). Hoffman et al. (2001) also reported a decrease in epidermal cell surface area and stomatal number on exposure of white clover (Trifolium repens L.) to enhanced UV‐B radiation of 13·3 kJ m–2 d–1.
Changes in leaf optical properties alter the surface reflectance which can affect the amount of UV‐B radiation reaching underlying tissues (Cen and Bornman, 1993). Only 10 % of incident UV‐B radiation on the leaf surface is reflected (Clark and Lister, 1975), and transmission through the epidermis ranges from 0 to 40 % (Day et al., 1992). Changes in the thickness of the leaf epidermis and wax layer (Steinmuller and Tevini, 1985; Tevini and Steinmuller, 1987) would modify the response to UV‐B radiation. Epidermal leaf hairs (trichomes) modify the microenvironment of the leaf, primarily through extension of the boundary layer and a reduction in water loss (Ehleringer, 1984). They can also reduce the amount of UV‐B radiation that penetrates through the epidermis (Karabourniotis et al., 1992), probably due to their UV‐absorbing pigment compounds (Karabourniotis et al., 1992, 1994). The wax content on the leaf surface is known to increase when cotton plants are exposed to environmental stresses. A 40 % increase in leaf epicuticular wax content was recorded when cotton plants were exposed to water stress (Bondada et al., 1996). Similarly, deposition of epicuticular waxes reflects 30 % of incident radiation at 290 nm off Eucaluptus leaves, and UV‐B radiation is known to alter the quantity and chemical composition of leaf surface wax deposits (Tevini and Steinmuller, 1987; Barnes et al., 1996). However, exposure to UV‐B radiation sometimes reduces the wax content (Gonzalez et al., 1996) and leads to a decrease in leaf reflectance.
This study is the first step in a larger USDA‐UVB monitoring programme to study the effects of enhanced UV‐B radiation on cotton. A study of these changes would provide useful information about the consequences of future elevated UV‐B levels on cotton growth and development. Therefore, the objective of this study was to examine the effects of enhanced UV‐B radiation on whole plant morphology and vegetative and reproductive characters, with specific focus on the changes in leaf surface ultrastructure and anatomy. We hypothesize that predicted increases in UV‐B radiation will alter the surface and anatomy of cotton leaves and increase the epicuticular wax content, which, in turn, will increase leaf surface reflectance of incident UV‐B radiation.
MATERIALS AND METHODS
Plant material and growth conditions
Experiments were conducted in the sunlit, controlled environment plant growth chamber facility known as the Soil–Plant–Atmosphere–Research (SPAR) facility on the North farm (88·8°W, 33·5°N, and 85 m a.s.l.) of Mississippi State University, USA. Seeds of cotton variety NuCOTN‐33B were sown on 1 Aug. 2001 in the sand bins of the SPAR units. Seeds were sown in 11 rows of five seeds per row, and emergence was observed 6 d later. Six alternate rows of seedlings removed at 15 d after emergence (DAE), and two rows at 25 DAE were used for collecting growth data, leaving three rows with 15 plants m–2 until the end of the experiment, i.e. mid‐fruiting stage (66 DAE). Plants were irrigated three times a day with half‐strength Hoagland’s nutrient solution through an automated drip system. Variable‐density shade cloths around the edges of the plants were used to match plant heights simulating the presence of other plants and eliminating the need for border plants.
Each SPAR unit consisted of a steel soil bin (1 m tall × 2 m long × 0·5 m wide), a Plexiglas chamber (2·5 m tall × 2·0 m long × 1·5 m wide) opaque to ambient solar UV radiation, a heating and cooling system, and an environment monitoring and control system (Reddy et al., 2001). Air ducts located on the northern side of each SPAR unit connected heating and cooling devices to each unit. Conditioned air was passed down through the plant canopy with sufficient flux to cause leaf flutter, and returned to the air‐handling unit just above the soil level. Chilled ethylene glycol supplied the cooling, and two electrical resistance heaters in each SPAR unit provided heat. A dedicated computer controlled air temperature, CO2 concentration and soil watering in each SPAR unit. Air temperatures were controlled at 30/22 °C (± 0·5 °C) (day/night) and were monitored and adjusted every 10 s. The daytime temperature was initiated at sunrise and returned to the night‐time temperature 1 h after sunset. The CO2 concentration in each SPAR unit was also monitored every 10 s and integrated over 900‐s intervals throughout the day. SPAR units were maintained at 360 (± 15) µl l–1 CO2. The computer also conducted continuous monitoring of all the important environmental and plant gas exchange variables.
UV‐B irradiation protocol
Beginning at emergence and continuing until harvest, three levels of UV‐B radiation were imposed in a square wave fashion: control (No UV‐B), and a total daily flux of biologically effective UV‐B radiation of 8 (ambient) and 16 kJ m–2 d–1 (high). UV‐B doses simulated 0 %, ambient and 30 % depletion of stratospheric ozone. The UV‐B radiation from eight fluorescent lamps (UV‐313 lamps; Q‐Panel Company, Cleveland, OH, USA) arranged on a rack perpendicular to the canopy was delivered for 8 h from 0800 to 1600 h and driven by 40 W dimming ballasts. The intensity of UV‐B radiation in each of the treatments was adjusted using dimmable ballasts. To filter out UV‐C radiation (<280 nm), the lamps were wrapped in pre‐solarized 0·07 mm cellulose diacetate (CA) film. The CA on the lamps was changed at 3‐d intervals to account for the degradation of the CA properties caused by UV‐B radiation. Unilluminated bulbs were placed in the control chamber above the plant canopy to simulate the shading effect, if any, produced by the lamps in the other UV‐B treatments. The UV‐B radiation delivered at the top of the canopy was checked daily using a radiometer (UVX digital radiometer; UVP Inc., San Gabriel, CA, USA). Rack height and lamp power were adjusted, as needed, to maintain the respective UV‐B radiation levels. Lamps were always at a height of 0·5 m above the canopy.
Growth measurements
Plant height, mainstem node number, and internode and branch lengths (sum of individual branch lengths) were recorded on nine plants in each treatment at 66 DAE. Branch number and lengths were also noted. Lengths of petals, bracts and staminal columns (maximum measurable length) and anther number per flower were measured for five flowers in each treatment. The uppermost fully expanded mainstem leaves from three plants were sampled at flowering (50 DAE) to investigate leaf morphology and anatomy. Areas of petals and bracts of each of five flowers, and the area of the three individual leaves used for wax extraction and epidermal and stomatal cell counts were measured using a leaf area meter (LI‐3100; LI‐COR, Lincoln, NB, USA).
Stomatal measurements
A light microscope was used to count the epidermal cell and stomatal number. Length and width of the stomata in each field were also measured. The nail polish peel method was used to count cell numbers: portions of abaxial and adaxial surface of three fully expanded leaves in each treatment were coated with colourless nail polish, which was allowed to dry for 30 min to give a clear impression of the leaf surface. The peels were then removed using forceps and placed on a slide. Epidermal cell and stomatal counts were made at random in five fields under the light microscope at ×400 magnification. Stomatal length and width were measured using an eyepiece micrometer, which was calibrated using a stage micrometer prior to measurement. Final numbers are presented as epidermal cell density (mm–2), stomatal density (mm–2) and stomatal index, calculated as the number of stomata per number of epidermal cells per unit leaf area.
Leaf anatomical measurements
Surface anatomy and epicuticular wax structure of the cotton leaves were studied using a scanning electron microscope (SEM). Specimens from three leaves per chamber were dissected and fixed in 2·5 % glutaraldehyde in 0·1 m phosphate buffer, pH 7·0 overnight at 4 °C. Specimens were rinsed, post‐fixed in 2 % osmium tetroxide, dehydrated in series to 100 % ethanol and critical‐point dried, before being mounted on aluminium stubs, sputter‐coated with gold palladium and imaged using a LEO Stereoscan 360 SEM (LEO Electron Microscopy Inc., Thornwood, NY, USA) at an accelerating voltage of 15 kV. Images were recorded on Polaroid Type 55 P/N film. To study internal anatomy, leaf tissue was fixed, osmicated and dehydrated in an ethanolic series as for SEM and then infiltrated and embedded in Spurr’s epoxy resin. Samples were sectioned on a Leica Ultracut E Ultramicrotome and semi‐thin 1 µm sections were obtained and stained with toluidine blue. Images of the sections were obtained using a confocal laser scanning microscope (Leica TCSNT, Leica Microsystems AG, Wetzair, Germany).
Epicuticular wax content determination
The uppermost fully expanded leaf of the canopy (fourth or fifth leaf from the top of the mainstem) was used to determine epicuticular wax content. Three leaves from each treatment were used for wax extraction and these were considered replicates in the statistical analysis. Each of the leaves was immersed and agitated for 20 s in a test tube with chloroform. The chloroform was then evaporated using a nitrogen evaporator and the test tubes were weighed. The wax content was calculated by subtracting the initial weight of the test tube from its final weight. Results were expressed on an area basis (µg mm–2).
Statistical analysis
Statistical analysis was carried out using GENSTAT for Windows (Genstat 5 Committee, 1997). One‐way ANOVA was used to determine differences between the treatments for the parameters presented in the study.
RESULTS
Vegetative morphology
Fully expanded leaves in the high UV‐B treatment developed chlorotic patches within 4–5 d after exposure and these later turned into necrotic patches, whereas leaves of plants in the ambient UV‐B treatments took between 8 and 10 d to develop chlorotic patches. Developmental events, duration from emergence to square and square to flower, were not modified by UV‐B radiation.
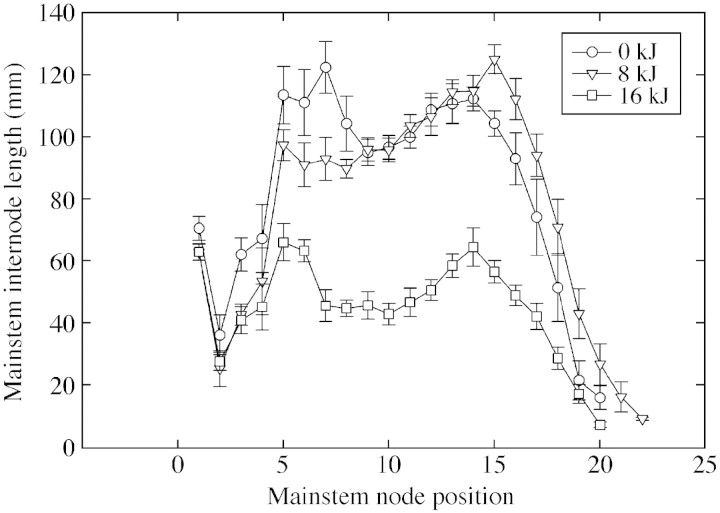
A highly significant UV‐B‐induced reduction in plant height was observed (Table 1), with plants exposed to high levels of UV‐B being 47 % shorter than control plants. Exposure to ambient UV‐B radiation (8 kJ m–2 d–1) reduced plant height compared with that of controls, but the difference was not significant. No significant differences in mainstem node number were observed among the treatments. Internode lengths of plants exposed to control and ambient UV‐B treatments did not differ (Fig. 1), whereas those of plants exposed to high UV‐B radiation were severely shortened. Leaf area of the fully expanded fourth or fifth leaf from the top of the canopy was reduced following exposure to UV‐B radiation (Table 1). The individual leaf area was smaller by 15 and 30 % in plants exposed to ambient and high UV‐B radiation treatments, respectively.
Table 1.
Influence of ultraviolet‐B radiation on cotton vegetative growth parameters
| UV‐B treatment (kJ m–2 d–1) | ||||
| Vegetative character | 0 | 8 | 16 | s.e.d |
| Plant height (m) | 1·52 | 1·45 | 0·80 | 0·032*** |
| Node number (per plant) | 17·70 | 18·20 | 17·70 | 0·29 |
| Branch number (per plant) | 14 | 16 | 14 | 0·6 |
| Total branch length (m per plant) | 3·39 | 4·76 | 1·74 | 0·284*** |
| Fully mature leaf area (cm2) | 158·10 | 133·80 | 110·00 | 6·52** |
Plant height, node number, and branch number and lengths were measured at the final harvest, 66 d after emergence. Leaf area is from the fully expanded fourth or fifth leaf on the mainstem used for stomatal measurements.
*** P ≤ 0·001; ** P ≤ 0·01; * P ≤ 0·05.
Fig. 1. Effects of ultraviolet‐B radiation (0, 8 or 16 kJ m–2 d–1) on internode length of cotton plants recorded at 66 d after emergence. Error bars are ± s.e. of mean of nine plants in each treatment.
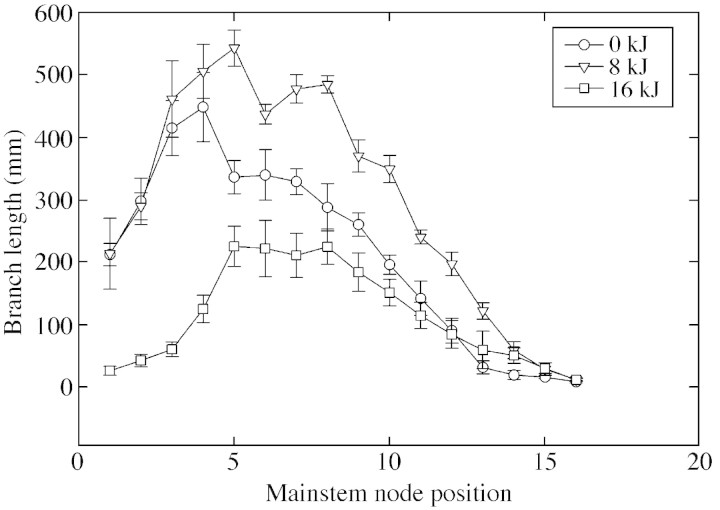
No significant differences in branch number per plant were detected between UV‐B treatments (Table 1). However, total branch length differed between the UV‐B radiation treatments (Fig. 2). The total length of all branches per plant was highest in plants treated with ambient UV‐B—an increase of 40 % over branch length in control plants. Plants in the high UV‐B treatment had the shortest branch length: 49 % lower than that in control plants (Table 1; Fig. 2).
Fig. 2. Effects of ultraviolet‐B radiation (0, 8 or 16 kJ m–2 d–1) on cotton branch lengths at various internode positions on the mainstem as observed at 66 d after emergence. Error bars are ± s.e. of mean of nine plants in each treatment.
Reproductive morphology
Compared with those borne on control plants, flowers borne on plants exposed to both ambient and high levels of UV‐B were smaller (Table 2). The reduction was observed in all floral parts. Compared with values for control plants, petal length and area were reduced following treatment with ambient UV‐B, but exposure to high levels of UV‐B caused a significant (P < 0·05) reduction in both petal length and area compared with values for plants in the control and ambient UV‐B treatments (Table 2). Bract length and area were also significantly reduced by the high UV‐B treatment. Anther number differed significantly between the treatments, being greatest in the control (Table 2). On exposure to UV‐B, flowers on plants treated with ambient and high levels of UV‐B had 15 and 33 % fewer anthers per flower, respectively. There were no significant differences in the staminal column length between the flowers exposed to UV‐B radiation treatments (Table 2).
Table 2.
Effect of ultraviolet‐B radiation on cotton flower characters
| UV‐B treatment (kJ m–2 d–1) | ||||
| Reproductive character | 0 | 8 | 16 | s.e.d |
| Flower length (mm) | 56·8 | 51·5 | 47·3 | 1·57** |
| Petal length (mm) | 50·5 | 46·0 | 41·6 | 2·12* |
| Petal area (mm–2) | 5910 | 5130 | 4130 | 354·6** |
| Bract length (mm) | 45·1 | 42·1 | 35·7 | 3·34 |
| Bract area (mm–2) | 1811 | 1529 | 1036 | 172·7** |
| Anthers (no. per flower) | 100·2 | 85·0 | 67·5 | 4·56*** |
| Staminal column length (mm) | 20·7 | 21·2 | 21·2 | 1·35 |
Petal, bract and staminal column lengths were measured and anther number counted on a fully opened flower at 50 d after emergence. Petal and bract area is cumulative of all petals and bracts, respectively, of a single flower.
*** P ≤ 0·001; ** P ≤ 0·01; * P ≤ 0·05.
Epidermal ultrastructure and epicuticular wax content
There were no statistically significant differences in leaf epidermal cell density on either the adaxial or abaxial surfaces of leaves when plants were exposed to different levels of UV‐B radiation (Table 3). On the other hand, stomatal density on both leaf surfaces differed significantly with UV‐B treatments (Table 3). Treatment with ambient and high levels of UV‐B increased the number of stomata on the adaxial surface by 36 and 65 %, respectively, over values for control plants. The corresponding increases on the abaxial surface of leaves were 22 and 10 %. UV‐B treatments resulted in similar increases in stomatal index on both adaxial and abaxial surfaces of the leaves (Table 3). An increase in the stomatal length on the adaxial surface was recorded in the high UV‐B treatment. However, the width of stomates did not differ between treatments.
Table 3.
Differences in response of cotton leaf epidermal and ultrastructural characters to ultraviolet‐B radiation
| UV‐B treatment (kJ m–2 d–1) | ||||
| Leaf epidermal character | 0 | 8 | 16 | s.e.d |
| Epidermal cell density (no. mm–2) | ||||
| Adaxial | 779 | 848 | 786 | 49·6 |
| Abaxial | 758 | 703 | 772 | 39·3 |
| Stomatal density (no. mm–2) | ||||
| Adaxial | 75 | 102 | 124 | 4·7*** |
| Abaxial | 183 | 224 | 202 | 6·4** |
| Stomatal index | ||||
| Adaxial | 0·10 | 0·12 | 0·16 | 0·011* |
| Abaxial | 0·24 | 0·32 | 0·26 | 0·009* |
| Stomatal length (µm) Adaxial | 11·90 | 12·00 | 12·80 | 0·24* |
| Stomatal width (µm) Adaxial | 7·50 | 6·40 | 7·20 | 0·25* |
Each value is a mean of counts from five fields on each leaf (0·145 mm in radius) from three different leaves. Cell counts are expressed as number of cells per square millimeter of adaxial and abaxial leaf area.
*** P ≤ 0·001; ** P ≤ 0·01; * P ≤ 0·05.
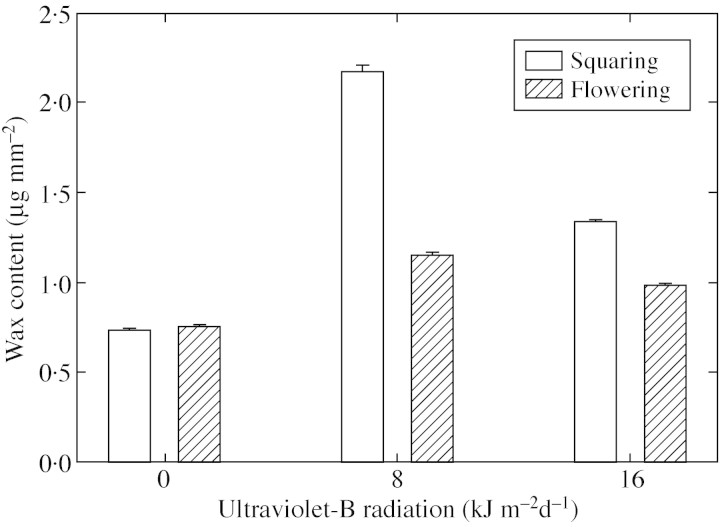
At both squaring and flowering stages, amounts of epicuticular wax deposited on leaf surfaces were significantly larger for plants exposed to UV‐B radiation than for those exposed to control levels (Fig. 3). The epicuticular wax content of leaves was greater in plants exposed to ambient UV‐B radiation than in those exposed to high UV‐B radiation at both squaring and flowering. At squaring, the wax content of plants treated with ambient and high levels of UV‐B increased by 200 and 84 % over values for the controls, respectively. The corresponding increases at flowering were 52 and 29 %, respectively.
Fig. 3. Epicuticular wax content of cotton leaves at squaring and flowering following exposure to ultraviolet‐B radiation. Error bars are ± s.e. of mean of three leaves in each treatment.
Scanning electron microscopy revealed that an increase in UV‐B increased the waxy appearance of the leaf surface (Fig. 4). The epicuticular wax morphology in the form of wax ridges was much denser and interwoven in UV‐B‐treated leaves. This observation corroborates the gravimetric wax content increase on leaves exposed to UV‐B treatments. An increase in the density of wax ridges was observed on the adaxial surface, but not on the abaxial surface.
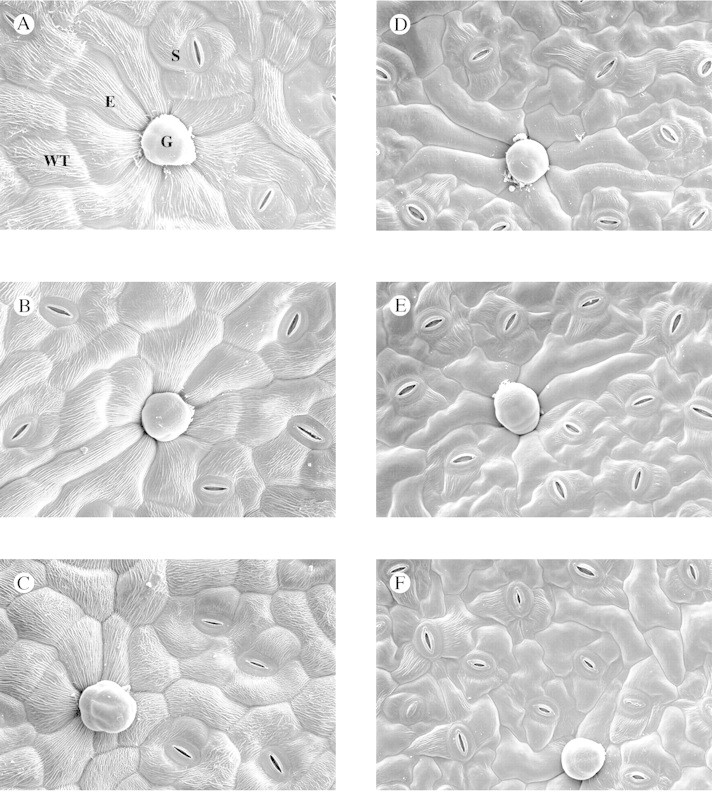
Fig. 4. Epicuticular wax morphology on leaf surfaces of cotton plants exposed to enhanced ultraviolet‐B radiation treatments. The figure shows wax tubes on both adaxial (A–C) and abaxial (D–F) leaf surfaces exposed to 0 (A and D), 8 (B and E) and 16 (C and F) kJ m–2 d–1 of UV‐B radiation. An increase in wax tube (WT) density is observed only on the adaxial surface with increase in ultraviolet‐B intensity. The image also shows epidermal cells (E), stomata (S) and glandular trichomes (G).
Structural observations
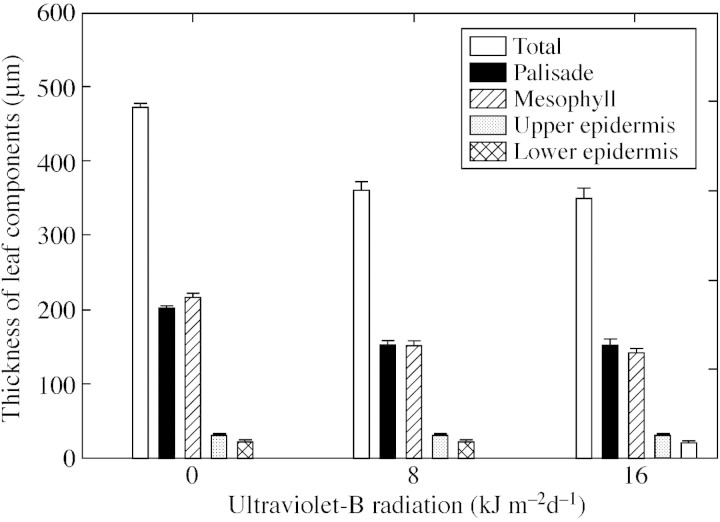
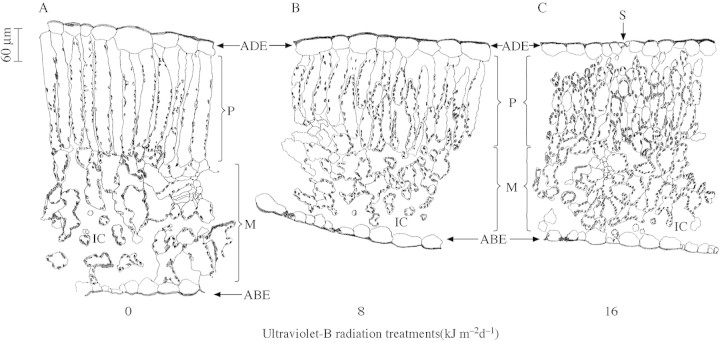
UV‐B treatment changed leaf thickness (Fig. 5). Plants in the control treatment had significantly thicker leaves (472 µm) than those exposed to ambient (361 µm) and high (351 µm) levels of UV‐B. The decrease in leaf thickness was due to a decrease in the thickness of both the palisade and mesophyll layers. The palisade layer was 24 % thinner in plants exposed to both UV‐B treatments, and the mesophyll was thinner by 29 and 34 % in plants treated with ambient and high levels of UV‐B, respectively. There were no changes in epidermal cell thickness on the adaxial or abaxial side.
Fig. 5. Changes in total leaf thickness and thickness of various leaf ultrastucture component layers on exposure of cotton plants to ultraviolet‐B radiation treatments. Error bars indicate ± s.e. of mean of three leaves in each treatment.
Studying leaf sections, the causes of the change in leaf thickness on exposure to UV‐B (Fig. 6) become apparent. In the control treatment, a single layer of tightly packed adaxial palisade mesophyll cells occupied half of the blade thickness, and large spongy mesophyll cells were present on the abaxial side. In leaves exposed to UV‐B radiation, the palisade again occupied half of the leaf thickness, but was multilayered with short cells and intercellular spaces. The size of the mesophyll cells was also reduced in leaves exposed to UV‐B radiation, more severely in the high UV‐B treatment, with increasing number of intercellular air spaces.
Fig. 6. Anatomical features of cotton leaves exposed to 0 (A), 8 (B) and 16 (C) kJ m–2 d–1 of ultraviolet‐B radiation. The diagrams are traced from images obtained using a Leica TCSNT Confocal laser scanning microscope attached to a pictomicrography system. The adaxial (ADE), abaxial epidermis (ABE), palisade layer (P) mesophyll layer (M) stomata (S) and intercellular cavities (IC) can be clearly seen in the diagrams. Bar = 60 µm for length and width in all panels.
DISCUSSION
The main objective of this work was to study changes in whole plant morphology and leaf‐level morphological and anatomical changes of cotton plants as affected by enhanced UV‐B radiation. Studies were conducted as early as 1987 by Jolley et al. and Pushnik et al. to evaluate the influences of ultraviolet light using cool white fluorescent lamps as a source of ultraviolet light. Specifically, studies to evaluate the effects of UV‐B radiation on cotton have not been conducted to date (Corlett et al., 1997). Hence, this is the first report on the changes in morphology and anatomy of cotton on exposure to enhanced UV‐B radiation.
Sensitivity of cotton plants to UV‐B radiation resulted in a reduction of plant height, internode lengths, branch lengths and individual leaf sizes, and the appearance of chlorotic and necrotic patches on leaf surfaces. The appearance of chlorotic and necrotic patches following exposure to UV‐B has been widely reported (Vu et al., 1981; Strid and Porra, 1992; Smith et al., 2000). On continued exposure to high levels of UV‐B radiation, the chlorotic patches turned into necrotic or dead tissue patches.
The overall decrease in size of the plant canopy can be attributed to a decrease in plant height and branch lengths. Researchers studying other crop species have also reported a decrease in height on exposure to enhanced UV‐B radiation. In monocots, rice (Oryza sativa) seedlings showed a decrease in plant height, leaf blade length and total leaf area when exposed to 15·7 kJ m–2 d–1 (Teramura et al., 1991a) or 10·3 kJ m–2 d–1 (Barnes et al., 1993) UV‐B radiation. In dicotyledons, exposure to enhanced UV‐B severely reduced plant height and leaf area. Plant heights of the soybean species Glycine max and G. soja were 18 % shorter following exposure to 13·6 kJ m–2 d–1 UV‐B radiation. Similar results have been observed in other dicot species (Tevini and Teramura, 1989; Searles et al., 1995). The reduction in plant height is mainly the result of a reduction in internodal length rather than a reduction in node number (Table 1). In the current study, the lack of significant differences between the control and the ambient treatments for plant height shows that cotton plants can tolerate current levels of UV‐B radiation (around 8 kJ m–2 d–1), but any increase in these levels, as predicted by climate scientists (UNEP/WMO, 2002), would have the potential to reduce plant height.
In addition to vegetative morphology, the reproductive or floral morphology of cotton was also modified by UV‐B radiation. No data are available on the floral morphology of crop plants exposed to enhanced UV‐B radiation. Flowering increased when UV‐B was excluded by covering plants with mylar sheet that filters UV‐B radiation (Caldwell, 1968). In the present study, flowers produced by plants exposed to UV‐B radiation were smaller than those produced by control plants owing to a reduction in both petal and bract size. The floral morphology of cotton is very sensitive to enhanced UV‐B radiation, and pollination, boll formation and development, and lint yield could all be affected. Cotton flowers exposed to enhanced UV‐B radiation had a reduced number of anthers. We have also observed that anthers are smaller in UV‐B‐treated plants. Reduction in anther number and size reduces the amount of pollen available for fertilization. The decrease in anther number would also hamper commercial hybrid cotton production. Evidence from in vitro experiments shows that pollen germination is inhibited by enhanced UV‐B (Chang and Campbell, 1976; Caldwell, 1979; Flint and Caldwell, 1984). Further studies are needed to evaluate the effects of UV‐B on cotton pollen production, germination and growth.
Along with changes in whole plant (canopy) morphology, UV‐B treatment also resulted in morphological and anatomical changes in cotton. Leaf structure is important as it attenuates most of the UV‐B and visible solar radiation incident on the leaf. Changes in leaf anatomy as a result of enhanced UV‐B radiation would modify light attenuation by the leaf and in turn affect photosynthesis. Exposure of cotton plants to environmental stress, such as water deficit, reduces leaf size without altering the stomatal index (Singh et al., 1990). In this study, the UV‐B‐induced increase in the adaxial stomatal index was the result of an increase in stomatal density without any change in epidermal cell number per unit area. This suggests that UV‐B radiation affects plants in a manner similar to water deficit, by reducing leaf size (Singh et al., 1990), and to temperature stress, by increasing stomatal number (Radin, 1992). That there was no change in the epidermal cell density on the leaf surface strongly suggests that the decrease in leaf area was the result of a reduction in cell division rather than slower cell expansion (Hoffman et al., 2001). Decrease in plant height, internode length and total branch length can also be attributed to reduced cell division, as both node and branch numbers were unaffected by UV‐B radiation.
The epicuticular wax layer, which acts as an interface between the environment and internal structures of the leaf, is an important surface character that responds to environmental stresses (e.g. water stress; Bondada et al., 1995). Enhanced UV‐B radiation treatments are known to alter the quantity and chemical composition of the leaf surface wax (Tevini and Steinmuller, 1987; Barnes et al., 1996). In the present study, exposure to both ambient and high levels of UV‐B radiation increased the wax content. The increase in wax content might be a protective mechanism as epicuticular wax reflects a minimum of 10 % (Caldwell et al., 1983) to a maximum of 30 % (in Eucalyptus; Holmes, 1997) of the incident UV‐B radiation. As early as 1975, Clark and Lister confirmed that epicuticular wax increased reflectance markedly in the ultraviolet and blue regions of the spectrum. A similar increase in wax content was recorded in pea cultivars when exposed to UV‐B radiation (Corlett et al., 1997). The measured increase in wax is supported by the observed increase in density of wax tubes on the adaxial surface of UV‐B‐treated leaves (Fig. 4). Greater wax deposition was seen in the uppermost leaves at squaring than at flowering (Fig. 3). The lower wax content observed at flowering could be attributed to diversion of carbon assimilates for boll development. Among the UV‐B treatments, exposure to high levels of UV‐B resulted in a lower wax content than did exposure to ambient UV‐B. Gordon et al. (1998) showed that the UV‐B sensitivity threshold for epicuticular wax deposition in sugar maple (Acer saccharum) was 6·2 kJ m–2 d–1. Thus, in cotton, the sensitivity threshold of UV‐B radiation, based on wax content, appears to fall between 8 and 16 kJ m–2 d–1, unless increased wax is a response to stress rather than a defence mechanism against enhanced UV‐B.
Changes in internal leaf anatomy were related to changes in leaf morphology. Species differ in their anatomical response to UV‐B radiation (Nagel et al., 1998), and while an increase in leaf thickness following UV‐B exposure is common, a decrease in leaf thickness was observed in this study. Studying Populus trichocarpa, Schumaker et al. (1997) found that ambient UV‐B treatment resulted in thinner leaves compared with those of plants exposed to sub‐ambient UV‐B radiation owing to decreased development of palisade parenchyma tissue. The present anatomical study indicated that the reduction in leaf thickness in response to UV‐B radiation was caused by a reduction in the thickness of both the palisade and spongy mesophyll layers. The palisade layer is typically a single layer of elongated cells occupying half of the leaf thickness (Bondada et al., 1994; Wise et al., 2000). In contrast to a decrease in epidermal cell division, an increase in palisade cell number was observed. In the present study, the palisade in leaves exposed to UV‐B radiation was two to three layers thick, with short cells stacked on top of each other with air spaces. Weston et al. (2000) investigated palisade development in wild‐type and blue‐light‐perception mutants of Arabidopsis thaliana and observed that a second and even a third palisade layer was formed at high light intensity. The increase in cell number would increase the cell wall surface that would block and prevent harmful UV‐B radiation from reaching the abaxial photosynthetically active mesophyll. The increase in palisade cell number would increase the amount of air–cell wall interfaces, an important parameter that affects reflectance (Knipling, 1970). Further studies are needed to verify the effects of altered leaf morphology on reflectance and transmittance of UV‐B radiation.
CONCLUSIONS
Exposure of cotton plants to UV‐B radiation reduced the overall canopy size by decreasing plant height, branch length and leaf area without slowing the developmental events. Leaf anatomical and morphological changes, such as a decrease in thickness, and increases in stomatal index, palisade layers and wax content, are adaptive mechanisms for high UV‐B radiation. Cotton floral morphology was also altered on exposure to UV‐B radiation. A decrease in floral morphological characters and anther number may impact on the fertilization process and fruit set. Future studies should aim to study the effects of UV‐B on cotton boll development, lint yield and quality.
ACKNOWLEDGEMENTS
This work was partially supported by grants from USDA UV‐B Monitoring and Research Program, Colorado State University, Fort Collins, Colorado and the National Aeronautical and Space Administration (NASA)—funded Remote Sensing Technologies Center at Mississippi State University, NASA grant number NCC13‐9901. We thank Wendell Ladner, Sam Turner, David Brand, Kim Gourley and Bill Monroe (Electron Microscope Center, Mississippi State University) for technical support, Drs Joe Sullivan and Dennis Gitz for initial discussions and help during the course of the experiment, and Vara Prasad Pagadala (University of Florida, Gainesville) and John Read (USDA‐ARS, Mississippi State) for reviewing the manuscript. Contribution from the Department of Plant and Soil Sciences, Mississippi State University, Mississippi Agricultural and Forestry Experiment Station paper no. J10129.
Supplementary Material
Received: 4 November 2002; Returned for revision: 6 January 2003; Accepted: 10 February 2003
References
- BarnesPW, Ballare CL, Caldwell MM.1996. Photomorphogenic effects of UV‐B radiation on plants: consequences for light competition. Journal of Plant Physiology 148: 15–20. [Google Scholar]
- BarnesPW, Maggard S, Holman, SR, Vergara BS.1993. Intraspecific variation in sensitivity to UV‐B radiation in rice. Crop Science 33: 1041–1046. [Google Scholar]
- BecwarMR, Moore FD, Burke MJ.1982. Effects of depletion and enhancement of ultraviolet‐B (280–315 nm) radiation on plants grown at 3000 m elevation. Journal of the American Society of Horticultural Science 107: 771–774. [Google Scholar]
- BondadaBR, Oosterhuis DM, Murphy JB, Kim KS.1996. Effect of water stress on the epicuticular wax composition and ultrastructure of cotton (Gossypium hirsutum L.) leaf, bract, and boll. Environmental and Experimental Botany 36: 61–69. [Google Scholar]
- BondadaBR, Oosterhuis DM, Wullschleger SD, Kim KS, Harris WM.1994. Anatomical considerations related to photosynthesis in cotton leaves, bracts, and the capsule wall. Journal of Experimental Botany 36: 111–118. [Google Scholar]
- CaldwellMM.1968. Solar ultraviolet radiation as an ecological factor for alpine plants. Ecological Monographs 38: 243–268. [Google Scholar]
- CaldwellMM.1979. Plant life and ultraviolet radiation: some pers pectives in the history of the earth’s UV climate. Bioscience 29: 520–525. [Google Scholar]
- CaldwellMM, Robberecht R, Flint SD.1983. Internal filters: prospects for UV acclimation in higher plants. Physiologia Plantarum 58: 445–450. [Google Scholar]
- CenYP, Bornman JF.1993. The effect of exposure to enhanced UV‐B radiation on the penetration of monochromatic and polychromatic UV‐B radiation in leaves of Brassica napus Physiologia Plantarum 87: 249–255 [Google Scholar]
- ChangDCN, Campbell WF.1976. Responses of Tradescantia stamen hairs and pollen to UV‐B (ultraviolet) irradiation. Environmental and Experimental Botany 16: 195–199. [Google Scholar]
- ClarkJB, Lister GR.1975. Photosynthetic action spectra of trees. II. The relationship of cuticle structure to the visible and ultraviolet properties of needles from four coniferous species. Plant Physio logy 55: 407–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CorlettJE, Stephen J, Jones HG, Woodfin R, Mepsted R, Paul ND.1997. Assessing the impact of UV‐B radiation on the growth and yield of field crops. In: Lumsden PJ, ed. Plants and UV‐B: responses to environmental change Society for Experimental Biology, Seminar Series 64. Cambridge: Cambridge University Press. [Google Scholar]
- DayTA, Vogelmann TC, Delucia EH.1992. Are some plant life forms more effective than others in screening out ultraviolet‐B radiation? Oecologia 92: 513–519. [DOI] [PubMed] [Google Scholar]
- EhleringerJR.1984. Ecology and ecophysiology of leaf pubescence in North American desert plants. In: Rodriguez E, Healy PL, Mehta I, eds. Biology and chemistry of plant trichomes New York: Plenum Press, 113–132. [Google Scholar]
- FlintSD, Caldwell MM.1984. Partial inhibition of in vitro pollen germination by simulated solar ultraviolet‐B radiation. Ecology 65: 792–795. [Google Scholar]
- Genstat 5 Committee.1997.Genstat 5 Release 3 Reference manual. Oxford: Clarendon Press. [Google Scholar]
- GonzalezR, Paul ND, Percy K, Ambrose M, McLaughlin CK, Barnes JD, Areses M, Welburn AR.1996. Responses to ultraviolet‐B radiation (280–315 nm) of pea (Pisum sativum) lines differing in leaf surface wax. Physiologia Plantarum 98: 852–860. [Google Scholar]
- GordonDC, Perry KE, Riding RT, Paoletti E.1998. Effect of enhanced UV‐B radiation of adaxial leaf surface micromorphology and epicuticular wax biosynthesis of sugar maple. Chemosphere 36: 853–858. [Google Scholar]
- HoffmanRW, Campbell BD, Fountain DW, Jordan BR, Greer DH, Hunt DY, Hunt CL.2001. Multivariate analysis of intraspecific responses to UV‐B radiation in white clover (Trifolium repens L.). Plant, Cell and Environment 24: 917–927. [Google Scholar]
- HolmesMG.1997. Action spectra for UV‐B effects on plants: monochromatic approaches for analysing plant responses. In: Lumsden PJ, ed. Plants and UV‐B: responses to environmental change Society for Experimental Biology, Seminar Series 64. Cambridge: Cambridge University Press. [Google Scholar]
- JolleyDV, Brown JC, Pushnik JC, Miller GW.1987. Influences of ultraviolet (UV) blue light radiation on the growth of cotton. I. Effect of iron nutrition and iron stress response. Journal of Plant Nutrition 10: 333–351. [Google Scholar]
- KarabourniotisG, Kotsabassidis D, Manetas D.1994. Trichome leaf density and its protective potential against ultraviolet‐B radiation damage during leaf development. Canadian Journal of Botany 73: 376–383. [Google Scholar]
- KarabourniotisG, Papadopoulos K, Papamarkou M, Manetas Y.1992. Ultraviolet‐B radiation absorbing capacity of leaf hairs. Physiologia Plantarum 86: 414–418. [Google Scholar]
- KniplingEB.1970. Physical and physiological basis for the reflection of visible and near‐infrared radiation from vegetation. Remote Sensing of Environment 1: 155–159. [Google Scholar]
- LatimerJG, Mitchell GA.1987. UV‐B radiation and photosynthetic irradiance acclimate eggplant for outdoor exposure. HortScience 22: 426–429. [PubMed] [Google Scholar]
- MepstedR, Paul N, Stephen J, Nogues S, Corlett JE, Baker NR, Jones HG, Ayres PG.1996. Effects of enhanced UV‐B radiation on pea (Pisum sativum L.) grown under field conditions. Global Change Biology 2: 325–334. [Google Scholar]
- NagelLM, Bassman JH, Edwards GE, Robberecht R, Franceshi VR.1998. Leaf anatomical changes in Poplus trichocarpa, Quercus rubra, Pseudotsuga menziesii and Pinus ponderosa exposed to enhanced ultraviolet‐B radiation. Physiologia Plantarum 104: 385–396. [Google Scholar]
- PushnikJC, Miller GW, Jolly VD, Brown JC, Davis TD, Barnes AN.1987. Influences of ultraviolet (UV) blue light radiation on the growth of cotton. II. Photosynthesis, leaf anatomy, and iron reduction. Journal of Plant Nutrition 10: 2283–2297. [Google Scholar]
- RadinJW.1992. Reconciling water use efficiencies of cotton in field and laboratory. Crop Science 32: 13–18. [Google Scholar]
- ReddyKR, Hodges HF, Kimball BA.2000. Crop ecosystem responses to global climate change: cotton. In: Reddy KR, Hodges HF, eds. Climate change and global productivity Wallingford: CAB Inter national, 161–187. [Google Scholar]
- ReddyKR, Hodges HF, Read JJ, McKinion JM, Baker JT, Tarpley L, Reddy VR.2001. Soil‐plant‐atmosphere‐research (SPAR) facility: a tool for plant research and modeling. Biotronics 30: 27–50. [Google Scholar]
- SchumakerMA, Bassman JH, Robberecht R, Radamaker GK.1997. Growth, leaf anatomy, and physiology of Populus clones in response to solar ultraviolet‐B radiation. Tree Physiology 17: 617–626. [DOI] [PubMed] [Google Scholar]
- SearlesPS, Caldwell MM, Winter K.1995. The response of five tropical dicotyledon species to solar ultraviolet‐B radiation. American Journal of Botany 82: 445–453. [Google Scholar]
- SinghJ, Bhardwaj SN, Singh M.1990. Leaf size and specific leaf weight in relation to its water potential and relative water content in Upland cotton (Gossypium hirsutum). Indian Journal of Agricultural Sciences 60: 215–216. [Google Scholar]
- SmithJL, Burritt DJ, Bannister P.2000. Shoot dry weight, chlorophyll and UV‐B‐absorbing compounds as indicators of a plant’s sensitivity to UV‐B radiation. Annals of Botany 86: 1057–1063. [Google Scholar]
- SteinmullerD, Tevini M.1985. Action of ultraviolet radiation (UV‐B) upon cuticular waxes in some crop plants. Planta 164: 557–564. [DOI] [PubMed] [Google Scholar]
- StridA, Porra RJ.1992. Alterations in pigment content in leaves of Pisum sativum after exposure to supplementary UV‐B. Plant and Cell Physiology 33: 1015–1023. [Google Scholar]
- SullivanJH, Rozema J.1999. UV‐B effects on terrestrial plant growth and photosynthesis. In: Rozema J, ed. Stratospheric ozone depletion: the effects of enhanced UV‐B radiation on terrestrial ecosystems Leiden: Backhuys Publishers, 39–57. [Google Scholar]
- SullivanJH, Teramura AH.1988. Effects of ultraviolet‐B irradiation on seedling growth in the Pinaceae. American Journal of Botany 75: 225–230. [Google Scholar]
- SullivanJH, Teramura AH.1989. The effects of ultraviolet‐B radiation on loblolly pine. I. Growth, photosynthesis and pigment production in greenhouse‐grown seedlings. Physiologia Plantarum 77: 202–207. [Google Scholar]
- TeramuraAH.1983. Effects of ultraviolet‐B radiation on the growth and yield of crop plants. Physiologia Plantarum 58: 415–427. [Google Scholar]
- TeramuraAH, Sullivan JH.1994. Effects of UV‐B radiation on photosynthesis and growth of terrestrial plants. Photosynthesis Research 39: 463–473. [DOI] [PubMed] [Google Scholar]
- TeramuraAH, Ziska LH, Sztein AE.1991a. Changes in growth and photosynthetic capacity of rice with increased UV‐B radiation. Physiologia Plantarum 83: 373–380. [Google Scholar]
- TeramuraAH, Sullivan JH, Abrol YP, Govindjee, Wattal PW, Ort DR, Gnanam A.1991b. Field studies of UV‐B radiation effects on plants: case histories of soybean and loblolly pine. In: Abrol YP, Govindjee, Wattal PW, Ort DR, Gnanam A, eds. Impact of global climatic changes on photosynthesis and plant productivity Oxford & New Delhi: IBH Publishing Co. Pvt. Ltd, 147–161. [Google Scholar]
- TeviniM, Teramura AH.1989. UV‐B effects on terrestrial plants. Journal of Photochemistry and Photobiology 50: 479–487. [Google Scholar]
- TeviniM, Steinmuller D.1987. Influence of light, UV‐B radiation, and herbicides on wax biosynthesis of cucumber seedlings. Journal of Plant Physiology 131: 111–121. [Google Scholar]
- UNEP/WMO.2002.Executive summary, Scientific assessment of ozone depletion:2002 Prepared by the Scientific Assessment Panel of the Montreal Protocol on Substances that Deplete Ozone Layer. Released by WMO/UNEP on 23 Aug. 2002. http://www.unep.ch/ozone/pdf/ execsumm‐sap2002.pdf [Google Scholar]
- USDA‐UVMRP.2002.United States Department of Agriculture – UV‐B Monitoring and Research Program [Online] http://uvb. nrel.colostate.edu/UVB/uvb_climate_ network.html [Google Scholar]
- Van der LeunJC, Tang X, Tevini M.1998. Environmental effects of ozone depletion: 1998 assessment. Journal of Photochemistry and Photobiology 46: 5–19. [Google Scholar]
- VuCV, Allen LH Jr, Garrard LA.1981. Effects of supplemental UV‐B radiation on growth and leaf photosynthetic reactions of soybean (Glycine max). Physiologia Plantarum 52: 353–362. [Google Scholar]
- VuCV, Allen LH Jr, Garrard LA.1984. Effects of enhanced UV‐B radiation (280–320 nm) on ribulose‐1,5‐bisphosphate carboxylase in pea and soybean. Environmental and Experimental Botany 24: 131–143. [Google Scholar]
- WestonE, Thorogood K, Vinti G, Lopez‐Juez E.2000. Light quantity controls leaf‐cell and chloroplast development in Arabidopsis thaliana wild type and blue‐light‐perception mutants. Planta 211: 807–815. [DOI] [PubMed] [Google Scholar]
- WiseRR, Sassenrath‐Cole GF, Percy RG.2000. A comparison of leaf anatomy in field‐grown Gossypium hirsutum and G. barbadense Annals of Botany 86: 731–738. [Google Scholar]
- ZiskaLH, Teramura AH, Sullivan AH, McCoy A.1993. Influence of ultraviolet‐B (UV‐B) radiation on photosynthetic and growth characteristics in field‐grown cassava (Manihot esculentum Crantz). Plant, Cell and Environment 16: 73–79. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.