Abstract
A novel environment-friendly method to access bioactive oroxin A through a one-pot/two-step process from naturally abundant and inexpensive baicalin is described. The procedure presented here has several advantages including clean, one-pot, synthetic ease, and large-scale feasibility. This work also provides a model strategy for rapid and diverse access to natural molecules sharing the common skeleton of this family.
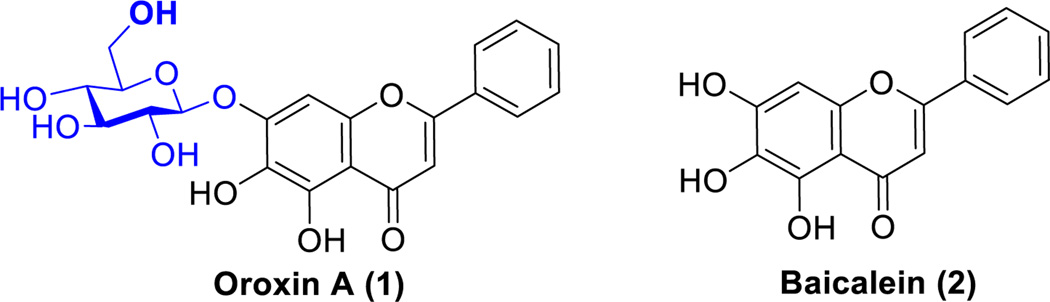
Plant-derived natural products have the potential to treat various human diseases. More than 65% people in the world rely primarily on traditional herbal medicines for the health. It is estimated that above 50% of current clinical drugs are derived from natural products.1–3 Notably, there is increasing interest on the development of low toxic natural antioxidants as promising leads especially those compounds that are widely distributed in the plant kingdom.4 Polyphenolic flavonoids as a large group in dietary plants exhibit a diverse range of pharmacological and biological properties including anticancer, antioxidant, antithrombotic, antiplatelet, and antibacterial effects.5 Till now, more than 5,000 polyphenolic flavonoids have been isolated and characterized, which are classified into over 10 subgroups.6 The multifunctional properties of these promising natural products are due to the presence of multiple oxygenated moieties.7,8 Accumulating evidence has demonstrated that flavonoids exhibit potential health protective effects in vivo, resulting in several lead compounds in preclinical and clinical trials.9,10 Besides the flavonoids having favorable pharmacological effects, flavonoid glucosides as another subgroup of flavonoids also gained much of interest in the research recently. It was found that glycosylated flavonoids possessing similar bioactivity, stability and improved solubility, are more efficacious than their aglycones in pharmaceutical studies.11 However, in contrast to an extensive investigation on flavonoids, flavonoid glycosides have not yet been thoroughly explored, owing to the limited preparative accessibility and the lack of an efficient and convenient synthetic methodology. Among them, oroxin A (1, baicalein-7-O-glucoside, Figure 1) was identified from a traditional herbal medicine of Asian countries, Oroxylum indicum.12,13 Accumulating studies have demonstrated the beneficial biological effects of oroxin A including antioxidant, anticancer, antibacterial and anti-virulence properties.14–17 Despite the promising biological evidence, extensive investigation including in vivo toxicological study and efficacy evaluation of oroxin A is limited because of scarce availability.
Figure 1.
Chemical structures of oroxin A (1) and baicalein (2).
In order to obtain sufficient oroxin A for pharmacological evaluation, several groups have made substantial efforts in recent years. Generally, oroxin A was previously produced either by natural product purification or through biological engineering. For instance, oroxin A can be isolated as one of the major constituents in the seeds of Oroxylum indicum by high-speed counter-current chromatography (HSCCC).18–20 However, the presence of strong polar hydroxyl groups in oroxin A results in a low solubility in organic solvents. Hence, the separation and purification of oroxin A by HSCCC using conventional solvents is very difficult. To overcome this limitation, Liu et al. established a preparative HSCCC by using ionic liquids as the modifier of the two-phase solvent system.21 Despite application of ionic liquids in separation procedure makes it possible to produce oroxin A in a relatively large scale; however, the cost of natural purification limits it further application. To address this issue, Sohng and coworkers developed the biotransformation of baicalein (2, Figure 1) into oroxin A by applying engineered Escherichia coli(E. coli).22 Glucosylation of baicalein in engineered E. coli might be beneficial for the large scale industrial production of oroxin A; however, various uncertain factors including time-consuming, complex of products, low yield and high cost in biological engineering still make it far from practical application.
Chemical synthesis remains to be an ideal option to yield pure desired natural products and plenty of key intermediates for further investigation of structure–activity relationships and potential applications in drug discovery. To this end, we report the chemical synthesis of oxorin A by a facile and efficient synthetic strategy.
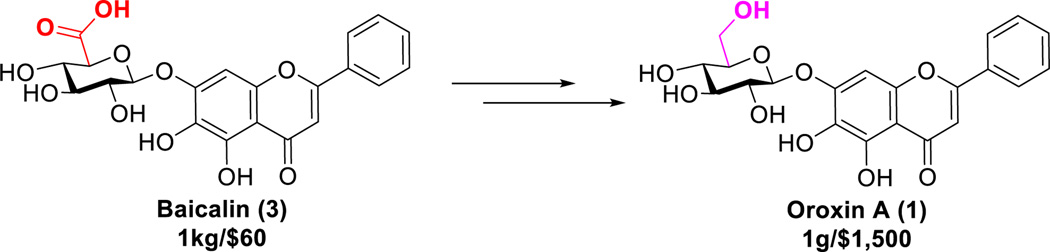
According to the chemical structure of oroxin A, baicalin (3) has the similar structure which contains a glucuronide moiety at 7-O-position of baicalein, while, oroxin A is the glucoside of baicalein (Scheme 1). Notably, baicalin is a cheap natural product (only $60/kg), which is abundantly available as the major ingredient extracted from traditional Chinese medicine Scutellaria baicalensis.23 Our efficient method follows the semisynthetic route using baicalin as the starting material.
Scheme 1.
Synthesis of oroxin A (1) from baicalin (3).
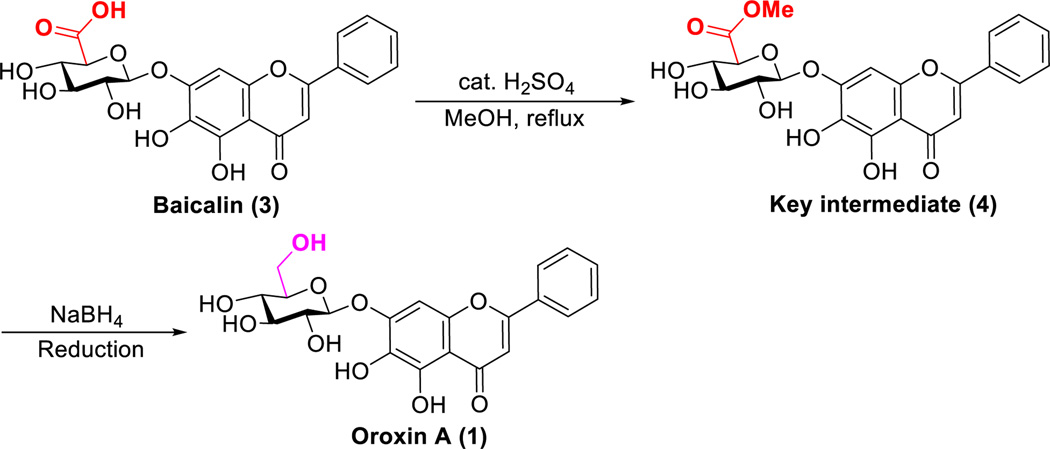
As shown in Scheme 2, key intermediate (4) was prepared in high yield by treating baicalin with catalytic amount of concentrated H2SO4 in the solution of methanol without purification. Sufficient amount of methanol was crucial for the outcome of this reaction due to the low solubility of baicalin. The formation of gel/colloidal material becomes feasible when the concentration of key intermediate is high. In this regard, the concentration of the starting material of baicalin in methanol should not be less than 25 mg/mL. This phenomenon indicates that baicalin and this key intermediate may be a good natural material for cosmetics and food industry.
Scheme 2.
Reagents and conditions: (a) cat. H2SO4, MeOH, reflux; (b) NaBH4, different solvents, 0 °C to 25 °C.
With the key intermediate 4 in hand, we set out to find a reliable condition for the simple conversion of 4 to the desired productoroxin A. Sodium borohydride (NaBH4) is the traditional reagent for the reduction of esters. We utilized this reducing agent in order to get a feasible and scalable process. The key intermediate4 is suspended in methanol and cooled to 0 °C, and then 1.0 equiv of NaBH4 is added portionwise (Table 1, entry 1). By reaction of compound 4 with NaBH4, we observed that the reduction of glucuronic acid methyl ester was capable of affording the desired product after 1 h of reaction time at 0 °C. Despite the yield is only 14%, it was noteworthy that the desired product was smoothly generated and about 85% of the starting material was recovered. Since this key reduction step appeared to be applicable for cost-effective and fast synthesis, the reduction of 4 by using NaBH4 was investigated under different reaction conditions in terms of time and temperature as well as a variety of solvents (Table 1).
Table 1.
Optimization of the NaBH4 Reduction Condition.
| Entry | NaBH4 (equiv) | solvent | T (°C) | Time (h) | Conversion (%)a | Yield (%)b |
|---|---|---|---|---|---|---|
| 1 | 1.0 | MeOH | 0 | 1 | 15% | 14% |
| 2 | 1.0 | MeOH | 0 | 24 | 51% | 19% (decomposed) |
| 3 | 5.0 | MeOH | 0 | 1 | 40% | 38% |
| 4 | 5.0 | MeOH | 0 | 4 | 60% | 49% |
| 5 | 1.0 | MeOH | 25 | 1 | 36% | 34% |
| 6 | 5.0 | MeOH | 25 | 1 | 76% | 69% |
| 7 | 10.0 | MeOH | 25 | 1 | 82% | 75% |
| 8 | 10.0 | EtOH | 25 | 1 | 69% | 64% |
| 9 | 10.0 | THF | 25 | 1 | 2% | trace |
| 10 | 10.0 | MeOH | 25 | 24 | 91% | 15% (decomposed) |
The conversion is based on the amount of isolated starting material.
Isolated yield.
Based upon our preliminary result mentioned above, we extended the reaction time from 1 h to 24 h. However, this reduction reaction was still incomplete and the reductive product was a complex reaction mixture of several components (entry 2). It seems that the desired product might decompose under this condition for a long time. In order to improve the yield and to avoid the undesired reaction, we added 5.0 equiv of NaBH4 and the reaction was stirred at 0 °C for 1 h or 4 h, respectively. In the case of 1 h, no undesired by product was detected on silica gel TLC (entry 3), while the reaction was still incomplete after a reaction time of 4 h and some small amounts of by products can be found in TLC (thin-layer chromatography) (entry 4). We decided to carry out the reduction at 25 °C with 1.0 equiv of NaBH4. As expected, this reaction proceeded faster at elevated temperature (entry 5). Increasing the amount of NaBH4 resulted in significant improving the synthetic effect, especially when we used 10.0 equiv of NaBH4, the isolated yield was up to 75% (entry6 and entry 7). Among different solvents including EtOH and THF, the results showed that MeOH was superior for this reaction (entries 7–9). In this regard, the evaporation of methanol in the first step seems unnecessary. Therefore, a more straightforward procedure was possible. After reaction of baicalin with cat. H2SO4 in MeOH, followed by reduction with NaBH4and subsequent workup procedure by adding appropriate amount of 10% AcOH/H2O, the mixture was concentrated to afford the desired product in an excellent yield.
Conclusions
In summary, a facile and efficient synthetic approach to access oroxin A from inexpensive baicalin has been developed. Optimized NaBH4 reduction as the key step after methyl esterification of baicalin, resulted in a satisfactory yield of oroxinA. Development of this method will greatly facilitate the biological studies of oroxin A that has demonstrated great potentials in food and pharmaceutical applications. The reported synthetic protocol is concise and may be readily extended to the synthesis of other flavonoid glycosides.
Experimental section
Synthesis of baicalin methylate (4)24
To the solution of baicalin (2.0 g, 4.48 mmol) in methanol (80 mL) was added catalytic amount of sulfuric acid (0.01 mL), and the solution was heated at reflux temperature for 3 h. The mixture was cooled at room temperature, and the solvent was concentrated under reduced pressure to give the key intermediate as a yellow solid for direct use in the next step without further purification (2.05 g, 99%).
Synthesis of oroxin A (1)
The key intermediate baicalin methylate (4) (0.916 g, 2.0 mmol) was suspended in methanol (40 mL) and cooled to 0 °C. Then NaBH4 (0.76 g, 20.0 mmol) was added portionwise, so that the temperature did not rise above 4 °C and the hydrogen evolution was under control. After the addition was complete, the mixture was stirred for an additional 1–2 h at 25 °C and then quenched with 20 mL of 10% AcOH/H2O. The solution was evaporated to obtain the crude product as a yellow solid. The residue was suspended in H2O/MeOH (10 mL, 1/1), and oroxin A (1) was precipitated at 0 °C. The suspension was seeded with the product mixture, cooled in the ice bath, filtered, and washed with cold water. The product was dried in vacuo for 12 h at 40 °C to yield 650 mg (75%) of oroxin A (1) as a light yellow solid (mp 221–222°C, in lit25: 222–223 °C). 1H NMR (400 MHz, DMSO-d6): 12.57 (s, 1H), 8.59 (s, 1H), 8.08 (d, 2H, J = 8.0 Hz), 7.57–7.63 (m, 3H), 7.06 (s, 1H), 7.02 (s, 1H), 5.42 (d, 1H, J = 4.0 Hz), 5.16 (d, 1H, J = 4.0 Hz), 5.11 (d, 1H, J = 4.0 Hz), 5.02 (d, 1H, J = 8.0 Hz), 4.68 (t, 1H, J = 4.0 Hz), 3.74–3.78 (m, 1H), 3.48–3.52 (m, 2H), 3.18–3.24 (m, 1H). 13C NMR (100 MHz, DMSO-d6): 183.1, 164.0, 152.1, 149.7, 147.0, 132.5, 131.3, 131.1, 129.6, 126.9, 106.6, 105.2, 101.5, 94.8, 77.8, 76.4, 73.7, 70.2, 61.2. HRMS (ESI) calcd for C21H19O10 431.0984 (M-H)−, found 431.0973.
One-pot large-scale synthesis oforoxin A (1)
To the solution of baicalin (10.0 g, 22.4 mmol) in methanol (400 mL) was added catalytic amount of sulfuric acid (0.05 mL), and the mixture was heated at reflux temperature for 4 h. The mixture was cooled to 0 °C and then NaBH4 (8.51 g,224 mmol) was added portionwise in 1 h. After the addition was complete, the mixture was stirred for an additional 2 h at 25 °C and then quenched with 200 mL of 10% AcOH/H2O. The solution was evaporated to obtain the crude product. The residue was suspended in H2O/MeOH (500 mL/100 mL, v/v 5/1), and the reaction mixture was acidified to pH 4 by dropwise addition of 1N HCl (aq). The desired product was precipitated and filtered. The solid was washed with cold water and dried in vacuo to yield 6.97 g (72%) of oroxin A (1). The structural characterization data are same as those described above.
Supplementary Material
Acknowledgements
This work was supported by the Technology Development Foundation of Fuzhou University (Project Numbers 2013-XQ-8 and 2013-XQ-9), grants P30 DA028821, R21 MH093844 from the National Institutes of Health, R. A. Welch Foundation Chemistry and Biology Collaborative Grant from the Gulf Coast Consortia (GCC), John Sealy Memorial Endowment Fund, Institute for Translational Sciences (ITS), and the Center for Addiction Research (CAR) at UTMB.
Footnotes
The authors declare no competing financial interest.
Electronic Supplementary Information (ESI) available:See DOI: 10.1039/b000000x/
Notes and references
- 1.Koehn FE, Carter GT. Nat. Rev. Drug Discov. 2005;4:206–220. doi: 10.1038/nrd1657. [DOI] [PubMed] [Google Scholar]
- 2.Harvey AL. Drug Discov. Today. 2008;13:894–901. doi: 10.1016/j.drudis.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Li JW, Vederas JC. Science. 2009;325:161–165. doi: 10.1126/science.1168243. [DOI] [PubMed] [Google Scholar]
- 4.Cragg GM, Grothaus PG, Newman DJ. Chem. Rev. 2009;109:3012–3043. doi: 10.1021/cr900019j. [DOI] [PubMed] [Google Scholar]
- 5.Srinivas NR. Curr. Clin. Pharmacol. 2009;4:67–70. doi: 10.2174/157488409787236065. [DOI] [PubMed] [Google Scholar]
- 6.Ross JA, Kasum CM. Annu. Rev. Nutr. 2002;22:19–34. doi: 10.1146/annurev.nutr.22.111401.144957. [DOI] [PubMed] [Google Scholar]
- 7.Havsteen B. Biochem. Pharmacol. 1983;32:1141–1148. doi: 10.1016/0006-2952(83)90262-9. [DOI] [PubMed] [Google Scholar]
- 8.Chen AY, Chen YC. Food Chem. 2013;138:2099–2107. doi: 10.1016/j.foodchem.2012.11.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Romano B, Pagano E, Montanaro V, Fortunato AL, Milic N, Borrelli F. Phytother. Res. 2013;27:1588–1596. doi: 10.1002/ptr.5023. [DOI] [PubMed] [Google Scholar]
- 10.Chen H, Mrazek AA, Wang X, Ding C, Ding Y, Porro LJ, Liu H, Chao C, Hellmich MR, Zhou J. Bioorg. Med. Chem. 2014;22:3393–3404. doi: 10.1016/j.bmc.2014.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Y, Hui H, Yang H, Zhao K, Qin Y, Gu C, Wang X, Lu N, Guo Q. Blood. 2013;121:3682–3691. doi: 10.1182/blood-2012-11-466219. [DOI] [PubMed] [Google Scholar]
- 12.Cheng CL, Chao TY. Yao Xue Xue Bao. 1964;11:762–767. [PubMed] [Google Scholar]
- 13.Harminder V. Singh, Chaudhary AK. Indian J. Pharm. Sci. 2011;73:483–490. doi: 10.4103/0250-474X.98981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sannigrahi S, Mazumder UK, Mondal A, Pal D, Mishra SL, Roy S. Nat. Prod. Commun. 2010;5:1239–1242. [PubMed] [Google Scholar]
- 15.Qiu J, Wang D, Zhang Y, Dong J, Wang J, Niu X. PLoS One. 2013;8:e80197. doi: 10.1371/journal.pone.0080197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li DQ, Zhao J, Li SP, Zhang QW. Anal. Bioanal. Chem. 2014;406:1975–1984. doi: 10.1007/s00216-013-7612-8. [DOI] [PubMed] [Google Scholar]
- 17.Yan R, Cao Y, Yang B. Molecules. 2014;19:4409–4417. doi: 10.3390/molecules19044409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen LJ, Games DE, Jones J. J. Chromatogr. A. 2003;988:95–105. doi: 10.1016/s0021-9673(02)01954-4. [DOI] [PubMed] [Google Scholar]
- 19.Chen LJ, Song H, Lan XQ, Games DE, Sutherland IA. J. Chromatogr. A. 2005;1063:241–245. doi: 10.1016/j.chroma.2004.11.072. [DOI] [PubMed] [Google Scholar]
- 20.Yuan Y, Luo H, Chen L. Se Pu. 2008;26:489–493. doi: 10.1016/s1872-2059(08)60024-3. [DOI] [PubMed] [Google Scholar]
- 21.Liu R, Xu L, Li A, Sun A. J. Sep. Sci. 2010;33:1058–1063. doi: 10.1002/jssc.200900612. [DOI] [PubMed] [Google Scholar]
- 22.Thuan NH, Park JW, Sohng JK. Process Biochem. 2013;48:1744–1748. [Google Scholar]
- Chen H, Gao Y, Wu J, Chen Y, Chen B, Hu J, Zhou J. Cancer Lett. 2014 doi: 10.1016/j.canlet.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang S, Ma J, Bao Y, Yang P, Zou L, Li K, Sun X. Bioorg. Med. Chem. 2008;16:7128–7133. doi: 10.1016/j.bmc.2008.06.055. [DOI] [PubMed] [Google Scholar]
- 25.Mezey-Vandor G, Farkas L, Kanzel I, Nogradi M. Chem. Ber. 1980;113:1945–1949. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.