Abstract
Background
Recent molecular studies have revealed high species diversity of Diplostomum in central and northern Europe. However, our knowledge of the distribution of Diplostomum spp. in the southern distributional range in Europe of the snail intermediate hosts (Lymnaea stagnalis and Radix spp.) is rather limited. This study aims to fill this gap in our knowledge using molecular and morphological evidence.
Methods
Nineteen fish species and six fish-eating bird species were sampled opportunistically in three regions (Catalonia, Extremadura and Aragon) in Spain. All isolates of Diplostomum spp. were characterised morphologically and molecularly. Partial sequences of the barcode region of the cox1 mitochondrial gene and complete sequences of the ribosomal ITS1-5.8S-ITS2 gene cluster were used for molecular identification of the isolates.
Results
Integrated morphological and molecular analyses demonstrated the presence of three species among the larval and adult isolates of Diplostomum spp. sampled in Spain: Diplostomum spathaceum (in fish and birds), D. pseudospathaceum (in birds) and Diplostomum sp. (in fish) referred to as Clade Q sensu Georgieva et al. (Int J Parasitol, 43:57–72, 2013). We detected ten cox1 haplotypes among the isolates of D. spathaceum with only one haplotype shared with adult isolates from central and northern Europe. No specific geographic pattern of the distribution of the novel haplotypes was found.
Conclusion
This first molecular exploration of the diversity of Diplostomum spp. in southern Europe indicates much lower species richness compared with the northern regions of Europe.
Keywords: Diplostomum spathaceum, Diplostomum pseudospathaceum, Lens metacercariae, Freshwater fish, Gulls, Spain, Cox1, ITS1-5.8S-ITS2
Background
Diplostomum von Nordmann, 1832 is a relatively large genus of widely distributed digeneans with three-host life-cycles involving lymnaeid snails and fish as intermediate hosts and fish-eating birds (predominantly gulls) as definitive hosts. There are 41 nominal species described within the Palaearctic, mainly from Europe (see [1] for details). However, treatment of the data on the geographic and host ranges of Diplostomum spp. have long been hindered by taxonomic and identification problems concerning all life-cycle stages.
The use of molecular markers has proved to be valuable and more efficient than experimental approaches in elucidating parasite life-cycles by linking larvae with adults, e.g. [1-5]. The mitochondrial cytochrome c oxidase subunit 1 (cox1) barcode region was found to be suitable for this goal as well as for the identification and recognition of cryptic species diversity within Diplostomum [1,6,7].
Recent molecular studies linking cox1 and ITS1-5.8S-ITS2 sequences for larval and adult isolates, which were identified based on parasite morphology, have revealed high species diversity of Diplostomum in central and northern Europe [1,7]. However, our knowledge of the distribution of Diplostomum spp. in the southern distributional range in Europe of the snail intermediate hosts (Lymnaea stagnalis and Radix spp.) is rather limited. Virtually no data exist for infections with Diplostomum spp. in the intermediate and definitive hosts in southern Europe. In Spain, two species have been recorded in populations of the gull definitive hosts. Diplostomum spathaceum was reported in four out of 324 yellow-legged gulls referred to as “Larus cachinnans” [8] and “Larus michahellis” [9] in Galicia and D. pseudospathaceum was recorded in one of 122 “L. cachinnans” from Medes Islands [10,11]. Similarly, there is a lack of data from the intermediate fish hosts; only unidentified metacercariae of Diplostomum sp. were reported in Anguilla anguilla in the Rivers Ulla and Tea in Galicia [12].
In this study, we used the molecular framework and the recently generated genetic datasets for Nearctic and Palaearctic species of the genus [1,6,13] to investigate species diversity of Diplostomum in birds and fishes sampled opportunistically in three regions in the northern and southern Spain. We provide the first molecular evidence associated with descriptions of the hologenophores sensu Pleijel et al. [14] for three species of Diplostomum.
Methods
Sample collection and processing
An opportunistic sampling strategy was adopted for this study, which was focused on examination of a diverse array of hosts rather than large samples of a single host species. Table 1 provides a list of the fish hosts and localities in different regions in Spain. Fish were obtained in collaboration with the regional governments of Extremadura, Aragón and Catalunya. A total of 230 fish belonging to 19 species and 10 families was examined in 2012 for the presence of eye dwelling metacercariae. The samples of Pseudochondrostoma willkommi and Salmo trutta collected in Villafranco del Guadiana and Jerte were obtained from aquiculture centres of the regional government of Extremadura whereas the remaining fish species/samples were collected in rivers. The largest number of individuals and species was collected in the Ebro Delta. The aquaculture system in Villafranco del Guadiana Aquaculture Centre comprises a central octagonal pool (depth 1 m; surface c.100 m2) surrounded by a group of pentagonal pools (depth <1 m; surface c.100 m2) (Figure 1A). The central pool is used for culturing mature breeders of P. willkommi of different ages whereas the peripheral pools are used for fish fry for up to two seasons; the latter are transferred to the central pool after reaching maturity. All pools are covered with nets to decrease predation by fish-eating birds and have an open water circulation system with a steady flow of 20 L/min. Although efforts are made to keep the water quality within the accepted ranges, the degree of eutrophication is high. Pools have been completely dried on various occasions but soon afterwards were repopulated by freshwater snails.
Table 1.
Summary data for the fish species examined/infected with Diplostomum spp.
| Fish species | Fish family | Locality | Date of collection | No. examined (infected) | Total length (range, mm) |
|---|---|---|---|---|---|
| *Carassius auratus (L.) | Cyprinidae | Ebro Deltaa | 18.ii.2012 | 2 | 121 − 248 |
| *Cyprinus carpio L. | Cyprinidae | 13 (1) | 290 − 379 | ||
| *Silurus glanis L. | Siluridae | 2 | 440 − 460 | ||
| *Pseudorasbora parva (Temminck & Schlegel) | Cyprinidae | 15 | 45 − 103 | ||
| *Lepomis gibbosus (L.) | Centrarchidae | 1 | 52 | ||
| Liza ramada (Risso) juv. | Mugilidae | 10 | 90 − 183 | ||
| *Misgurnus anguillicaudatus (Cantor) | Cobitidae | 15 (1) | 50 − 128 | ||
| Anguilla anguilla (L.) | Anguillidae | Ebro Deltaa | 17.v.2012 | 5 | 158 − 255 |
| Atherina boyeri Risso | Atherinidae | 10 | 34 − 44 | ||
| *Cyprinus carpio L. | Cyprinidae | 1 | 192 | ||
| *Gambusia holbrooki Girard | Poeciliidae | 18 | 24 − 50 | ||
| Liza ramada (Risso) juv. | Mugilidae | 1 | 58 | ||
| *Lepomis gibbosus (L.) | Centrarchidae | 14 | 43 − 65 | ||
| *Misgurnus anguillicaudatus (Cantor) | Cobitidae | 16 (2) | 52 − 122 | ||
| Pomatoschistus microps (Krøyer) | Gobiidae | 1 | 32 | ||
| *Pseudorasbora parva (Temminck & Schlegel) | Cyprinidae | 27 | 49 − 79 | ||
| *Silurus glanis L. | Siluridae | 1 (1) | 409 | ||
| Tropidophoxinellus alburnoides (Steindachner) | Cyprinidae | River Albarragenab | 21.ii.2012 | 4 | 57 − 89 |
| Tropidophoxinellus alburnoides (Steindachner) | Cyprinidae | River Luorianillab | 06.vi.2012 | 8 | 55 − 75 |
| Pseudochondrostoma willkommii (Steindachner) | Cyprinidae | Villafranco del Guadianab | 06.iii.2012 | 10 (10) | 235 − 262 |
| Salmo trutta L. | Salmonidae | Jertec | 07.iii.2012 | 3 | 262 − 291 |
| Parachondrostoma miegii (Steindachner) | Cyprinidae | River Piedrad | 24.ix.2012 | 5 | 139 − 177 |
| Oncorhynchus mykiss (Walbaum) | Salmonidae | 2 | 170 − 195 | ||
| Squalius pyrenaicus (Günther) | Cyprinidae | 10 | 84 − 135 | ||
| Salmo trutta L. | Salmonidae | Lake Espejod | 24.ix.2012 | 2 | 490 − 497 |
| Luciobarbus graellsii (Steindachner) | Cyprinidae | 3 | 236 − 405 | ||
| Oncorhynchus mykiss (Walbaum) | Salmonidae | 1 | 441 | ||
| Salmo trutta L. | Salmonidae | River Aragond | 25.ix.2012 | 12 | 70 − 188 |
| Salmo trutta L. | Salmonidae | River Arae | 25.ix.2012 | 12 | 68 − 146 |
| Gobio lozanoi Doadrio & Madeira | Cyprinidae | River Cincae | 25.ix.2012 | 1 | 53 |
| *Gambusia holbrooki Girard | Poeciliidae | 5 | 21 − 29 |
*Invasive species are marked with a star; aTarragona; bBadajoz; cCaceres; dZaragoza; eHuesca.
Figure 1.

Focus of infection with D. spathaceum at the Aquaculture Centre in Villafranco del Guadiana. A, Pool system for culturing Pseudochondrostoma willkommi at the Aquaculture Centre in Villafranco del Guadiana; B, P. willkommii infected with large numbers of lens-dwelling metacercariae of Diplostomum spathaceum; C, Eye of P. willkommii with lens capsule close to rupture due to the large numbers of metacercariae of D. spathaceum.
A total of 31 fish eating birds were obtained from bird recovery centres in Catalunya (Spain) in 2012 in order to obtain adult specimens of Diplostomum (Table 2). Six species of birds of four families were examined: (i) Laridae [Larus ridibundus L., Larus argentatus michahellis Naumann]; (ii) Sternidae [Sterna sandvicensis Latham]; (iii) Ardeidae [Ardea cinerea L. and Ixobrychus minutus (L.)]; (iv) Phalacrocoracidae [Phalacrocorax aristotelis (L.)]. The largest number of birds was obtained from the Ebro Delta.
Table 2.
Summary data for the bird species examined/ infected with Diplostomum spp.
| Bird species | Collection site | No. examined (infected) |
|---|---|---|
| Larus argentatus michahellis Naumann | Ebro Delta (Tarragona) | 6 (2) |
| Larus argentatus michahellis Naumann | Barcelona | 2 |
| Larus argentatus michahellis Naumann | Alella (Barcelona) | 1 |
| Larus argentatus michahellis Naumann | Sabadell (Barcelona) | 1 |
| Larus argentatus michahellis Naumann | Empuria Brava (Girona) | 1 |
| Larus argentatus michahellis Naumann | Figueres (Girona) | 1 |
| Larus argentatus michahellis Naumann | Roses (Girona) | 2 |
| Larus argentatus michahellis Naumann | Tarragona | 1 |
| Larus argentatus michahellis Naumann | Cambrils (Tarragona) | 1 |
| Larus ridibundus L. | Ebro Delta (Tarragona) | 5 (3) |
| Larus ridibundus L. | Cunit (Tarragona) | 1 (1) |
| Sterna sandvicensis (Latham) | Roda de Bará (Tarragona) | 1 |
| Phalacrocorax aristotelis (L.) | Tarragona | 1 |
| Ardea cinerea L. | Ebro Delta (Tarragona) | 5 |
| Ixobrychus minutus (L.) | Ebro Delta (Tarragona) | 2 |
All metacercariae were dissected out from fresh fish, fixed in hot saline solution and preserved in molecular biology grade ethanol whereas all adult worms were collected from birds found dead and frozen until necropsy; these were also preserved in molecular grade ethanol. The morphology of the larval and adult stages of Diplostomum spp. was studied on live and fixed material from series of photomicrographs made for each isolate with a digital camera of an Olympus BX51 microscope prior to sequencing; measurements were taken from the digital images with the aid of Quick Photo Camera 2.3 image analysis software. The structure of the secondary excretory system was reconstructed from serial microphotographs and the number of excretory concretions was counted.
All measurements in the descriptions and tables are in micrometres and are presented as the range followed by the mean in parentheses.
Sequence generation
Total genomic DNA was isolated from single ethanol-fixed adult individuals using the Chelex method (see [15] for details). Partial fragments of the barcode region of the cox1 mitochondrial gene [16] were obtained by polymerase chain reaction (PCR) amplifications using Ready-To-Go PCR beads (GE Healthcare, UK) and the diplostomid-specific PCR primers Plat-diploCOX1F (5′-CGT TTR AAT TAT ACG GAT CC-3′) and Plat-diploCOX1R (5′-AGC ATA GTA ATM GCA GCA GC-3′) designed by Moszczynska et al. [16] (see [1] for details). PCR amplifications of the ITS1-5.8S-ITS2 gene cluster were performed as above using the primers D1 (forward: 5′-AGG AAT TCC TGG TAA GTG CAA G-3′) and D2 (reverse: 5′-CGT TAC TGA GGG AAT CCT GGT-3′) and thermocycling conditions of Galazzo et al. [17].
PCR amplicons were purified using a QIAquick PCR purification kit (Qiagen Ltd, UK) and sequenced directly from both strands using the PCR primers (cox1) and the primers from [18]: BD1 (forward: 5′-GTC GTA ACA AGG TTT CCG TA-3′) and BD2: (reverse: 5′-TAT GCT TAA ATT CAG CGG GT-3′) (ITS1-5.8S-ITS2) with ABI BigDye chemistry (ABI Perkin-Elmer, UK), alcohol-precipitated, and run on an ABI Prism 3130 x 1 automated sequencer. Contiguous sequences were assembled with MEGA v5 [19] and submitted to GenBank (details and accession numbers are shown in Table 3).
Table 3.
Summary data for the isolates of Diplostomum spp. from fishes and birds collected in Spain and used for generation of the cox 1 and ITS1-5.8S-ITS2 sequences
| Species | Life-cycle stage a | Isolate | Haplotype | Host | Locality | GenBank accession numbers | |
|---|---|---|---|---|---|---|---|
| cox 1 | ITS1-5.8S-ITS2 | ||||||
| Diplostomum sp. (Clade Q) | M | CCED | − | Cyprinus carpio | Ebro Delta | KP025770 | KP025788 |
| Diplostomum pseudospathaceum | A | LRED1 | − | Larus ridibundus | Ebro Delta | KP025771 | JX986854b |
| Diplostomum spathaceum | A | LCED1 | 1 | Larus argentatus michahellis | Ebro Delta | KP025772 | – |
| Diplostomum spathaceum | A | LCED2 | 6 | Larus argentatus michahellis | Ebro Delta | KP025773 | – |
| Diplostomum spathaceum | A | LCED3 | 4 | Larus argentatus michahellis | Ebro Delta | KP025774 | – |
| Diplostomum spathaceum | A | LRC | 3 | Larus ridibundus | Cunit | KP025775 | KP025789 |
| Diplostomum spathaceum | A | LRED2 | 10 | Larus ridibundus | Ebro Delta | KP025776 | – |
| Diplostomum spathaceum | A | LRED3 | 8 | Larus ridibundus | Ebro Delta | KP025777 | – |
| Diplostomum spathaceum | M | MAED1 | 4 | Misgurnus anguillicaudatus | Ebro Delta | KP025778 | KP025790 |
| Diplostomum spathaceum | M | MAED2 | 2 | Misgurnus anguillicaudatus | Ebro Delta | KP025779 | KP025791 |
| Diplostomum spathaceum | M | PWVG1 | 5 | Pseudochondrostoma willkommii | Villafranco del Guadiana | KP025780 | – |
| Diplostomum spathaceum | M | PWVG2 | 4 | Pseudochondrostoma willkommii | Villafranco del Guadiana | KP025781 | KP025792 |
| Diplostomum spathaceum | M | PWVG3 | 7 | Pseudochondrostoma willkommii | Villafranco del Guadiana | KP025782 | KP025793 |
| Diplostomum spathaceum | M | PWVG4 | 1 | Pseudochondrostoma willkommii | Villafranco del Guadiana | KP025783 | – |
| Diplostomum spathaceum | M | PWVG5 | 9 | Pseudochondrostoma willkommii | Villafranco del Guadiana | KP025784 | – |
| Diplostomum spathaceum | M | PWVG6 | 3 | Pseudochondrostoma willkommii | Villafranco del Guadiana | KP025785 | – |
| Diplostomum spathaceum | M | PWVG7 | 7 | Pseudochondrostoma willkommii | Villafranco del Guadiana | KP025786 | – |
| Diplostomum spathaceum | M | SGED | 6 | Silurus glanis | Ebro Delta | KP025787 | – |
Alignments and data analysis
The newly-generated and published sequences were aligned together with MUSCLE implemented in MEGA v5; cox1 sequences were aligned with reference to the amino acid translation, using the echinoderm and flatworm mitochondrial code [20]. The cox1 alignment (410 nt; 46 sequences) comprised the 18 newly-generated (Table 3) and 28 published sequences, the latter including 1 − 5 representative sequences per species/lineage identified in previous studies in Europe [1,13]; see Table 4 for details. The ITS1-5.8S-ITS2 alignment (997 nt; 35 sequences) comprised seven new sequences for Spanish isolates sub-sampled within the cox1-derived clades and 29 published sequences, representative for the species/lineages sequenced in Europe [1,13] and Canada [6,17] (for details see Table 4). Sequences for Tylodelphys clavata were used as outgroups.
Table 4.
Summary data for the isolates of Diplostomum spp. retrieved from GenBank
| Trematode species | Isolate | Life-cycle stage a | Host species | Locality | Accession No. ( cox 1) | Accession No. (ITS1-5.8S-ITS2) | Reference |
|---|---|---|---|---|---|---|---|
| ‘Diplostomum baeri’ 1 | STR3 | M | Salmo trutta fario | Germany: River Ruhr (Henne) | JX986862 | JX986837 | Georgieva et al. [1] |
| ‘Diplostomum baeri’ 1 | STL1 | M | Salmo trutta fario | Germany: River Lenne | JX986863 | - | Georgieva et al. [1] |
| ‘Diplostomum baeri’ 1 | STR4 | M | Salmo trutta fario | Germany: River Ruhr (Henne) | JX986864 | Georgieva et al. [1] | |
| ‘Diplostomum baeri’ 1 | STL2 | M | Salmo trutta fario | Germany: River Lenne | JX986865 | - | Georgieva et al. [1] |
| ‘Diplostomum baeri’ 1 | STR7 | M | Salmo trutta fario | Germany: River Ruhr (Henne) | JX986869 | - | Georgieva et al. [1] |
| Diplostomum baeri | PF5D3 | M | Perca fluviatilis | Germany: Lake Constance | JQ639195 | - | Behrmann-Godel [13] |
| Diplostomum baeri | PF15D9 | M | Perca fluviatilis | Germany: Lake Constance | JQ639193 | - | Behrmann-Godel [13] |
| Diplostomum baeri | PF15D4 | M | Perca fluviatilis | Germany: Lake Constance | JQ639187 | - | Behrmann-Godel [13] |
| Diplostomum baeri | PF8D7 | M | Perca fluviatilis | Germany: Lake Constance | JQ639191 | - | Behrmann-Godel [13] |
| Diplostomum baeri | PF6D3 | M | Perca fluviatilis | Germany: Lake Constance | JQ639189 | - | Behrmann-Godel [13] |
| Diplostomum baeri | – | A | Larus delawarensis (exp.)b | Canada | - | AY123042 | Galazzo et al. [17] |
| Diplostomum huronense | – | A | Larus delawarensis (exp.)b | Canada | - | AY123044 | Galazzo et al. [17] |
| Diplostomum huronense | D.LL.IVT.Cc.3 F.1 | M | Catostomus commersoni | Canada | - | GQ292513 | Locke et al. [6] |
| Diplostomum indistinctum | D.RL.D.Cc.1.2 | M | Catostomus commersoni | Canada | GQ292508 | Locke et al. [6] | |
| ‘Diplostomum mergi’ 1 | RAH1 | C | Radix auricularia | Germany: Hengsteysee | JX986873 | JX986838 | Georgieva et al. [1] |
| ‘Diplostomum mergi’ 2 | RAH2 | C | Radix auricularia | Germany: Hengsteysee | JX986874 | - | Georgieva et al. [1] |
| ‘Diplostomum mergi’ 2 | RAH3 | C | Radix auricularia | Germany: Hengsteysee | JX986875 | JX986839 | Georgieva et al. [1] |
| ‘Diplostomum mergi’ 2 | RAH4 | C | Radix auricularia | Germany: Hengsteysee | JX986876 | - | Georgieva et al. [1] |
| ‘Diplostomum mergi’ 3 | GGR2 | M | Gobio gobio | Germany: River Ruhr (Henne) | JX986877 | JX986840 | Georgieva et al. [1] |
| ‘Diplostomum mergi’ 3 | STR10 | M | Salmo trutta fario | Germany: River Ruhr (Henne) | JX986878 | JX986841 | Georgieva et al. [1] |
| ‘Diplostomum mergi’ 3 | STR11 | M | Salmo trutta fario | Germany: River Ruhr (Henne) | JX986879 | - | Georgieva et al. [1] |
| ‘Diplostomum mergi’ 3 | STR12 | M | Salmo trutta fario | Germany: River Ruhr (Henne) | JX986880 | - | Georgieva et al. [1] |
| ‘Diplostomum mergi’ 3 | GGR3 | M | Gobio gobio | Germany: River Ruhr (Henne) | - | JX986842 | Georgieva et al. [1] |
| ‘Diplostomum mergi’ 3 | GGR4 | M | Gobio gobio | Germany: River Ruhr (Henne) | - | JX986843 | Georgieva et al. [1] |
| ‘Diplostomum mergi’ 3 | STR15 | M | Salmo trutta fario | Germany: River Ruhr (Henne) | JX986886 | - | Georgieva et al. [1] |
| Diplostomum mergi | RR45 | M | Rutilus rutilus | Germany: Lake Constance | JQ639178 | - | Behrmann-Godel [13] |
| Diplostomum mergi | RR43 | M | Rutilus rutilus | Germany: Lake Constance | JQ639177 | - | Behrmann-Godel [13] |
| Diplostomum mergi | RA97 | C | Radix auricularia | Germany: Lake Constance | JQ639179 | JQ665458 | Behrmann-Godel [13] |
| Diplostomum paracaudum | CL100 | M | Coregonus lavaretus | Germany: Lake Constance | - | JQ665457 | Behrmann-Godel [13] |
| Diplostomum pseudospathaceum | LCT3 | A | Larus cachinnans | Czech Republic: near Tovačov | JX986896 | JX986849 | Georgieva et al. [1] |
| Diplostomum pseudospathaceum | LSB2 | C | Lymnaea stagnalis | Germany: Baldeneysee | - | JX986850 | Georgieva et al. [1] |
| Diplostomum pseudospathaceum | LSH1 | C | Lymnaea stagnalis | Germany: Harkortsee | - | JX986851 | Georgieva et al. [1] |
| Diplostomum pseudospathaceum | GAH6 | M | Gasterosteus aculeatus | Germany: Hengsteysee | - | JX986852 | Georgieva et al. [1] |
| Diplostomum pseudospathaceum | LAG2 | A | Larus argentatus | Poland: near Gdańsk | JX986904 | JX986853 | Georgieva et al. [1] |
| Diplostomum pseudospathaceum | LCT4 | A | Larus cachinnans | Czech Republic: near Tovačov | JX986905 | JX986854 | Georgieva et al. [1] |
| Diplostomum pseudospathaceum | GC87 | M | Gymnocephalus cernuus | Germany: Lake Constance | - | JQ665456 | Behrmann-Godel [13] |
| Diplostomum spathaceum | LCT1 | A | Larus cachinnans | Czech Republic: near Tovačov | JX986887 | JX986844 | Georgieva et al. [1] |
| Diplostomum spathaceum | RAH6 | C | Radix auricularia | Germany: Hengsteysee | JX986846 | Georgieva et al. [1] | |
| Diplostomum spathaceum | RAH5 | C | Radix auricularia | Germany: Hengsteysee | JX986845 | Georgieva et al. [1] | |
| Diplostomum spathaceum | LAG1 | A | Larus argentatus | Poland: near Gdańsk | JX986892 | JX986847 | Georgieva et al. [1] |
| Diplostomum spathaceum | LCT2 | A | Larus cachinnans | Czech Republic: near Tovačov | JX986895 | JX986848 | Georgieva et al. [1] |
| Diplostomum sp. 1 SAL-2008 | D.IN.SSO.Ld.2 F.6 | A | Larus delawarensis (exp.)b | Canada | - | GQ292519 | Locke et al. [6] |
| Diplostomum sp. 2 SAL-2008 | D.BR.S.B.20.1 | M | Pimephales notatus | Canada | - | GQ292505 | Locke et al. [6] |
| Diplostomum sp. 3 SAL-2008 | D.RL.B08.Ms.1 F.1 | M | Micropterus salmoides | Canada | - | GQ292511 | Locke et al. [6] |
| Diplostomum sp. 4 SAL-2008 | D.IN.SSO.Ld.2 F.10 | A | Larus delawarensis | Canada | - | GQ292520 | Locke et al. [6] |
| Tylodelphys clavata | PFL1 | M | Perca fluviatilis | Germany: River Lippe | JX986909 | - | Georgieva et al. [1] |
| Tylodelphys clavata | CL91 | M | Coregonus lavaretus | Germany: Lake Constance | - | JQ665459 | Behrmann-Godel [13] |
aC, cercaria, M, metacercaria; A, adult; braised in experimental infection.
Distance-based [neighbour-joining (NJ)] and model-based [maximum likelihood (ML) and Bayesian inference (BI)] algorithms were used for tree reconstruction. Prior to analyses the best-fit nucleotide substitution models were selected in jModelTest 2.1.1 [21,22] using the Akaike Information Criterion (AIC). These were the Hasegawa-Kishino-Yano model including estimates of invariant sites and among-site rate heterogeneity (HKY + I + G) for the cox1 dataset and the Hasegawa-Kishino-Yano model including estimates of among-site rate heterogeneity (HKY + G) for the ITS dataset. ML analyses were performed in PhyML 3.0 [23] with a non-parametric bootstrap validation based on 1,000 replicates. BI analyses were carried out in MrBayes 3.2 [24] using Markov Chain Monte Carlo (MCMC) searches on two simultaneous runs of four chains during 107 generations, sampling trees every 103 generations. The first 25% of the sampled trees were discarded as “burn-in” for each data set and the consensus tree topology and the nodal support were estimated from the remaining samples as posterior probability values [25]. Distance matrices (p-distance model, i.e. the percentage of pairwise character differences with pairwise deletion of gaps) were also calculated and explored with MEGA v5.
Results
Diplostomum spp. infections in fish and birds
Of the 230 fish of 19 species studied, only 15 were infected with Diplostomum spp.: one Cyprinus carpio (Cyprinidae), one Silurus glanis (Siluridae), three Misgurnus anguillicaudatus (Cobitidae) and ten Pseudochondrostoma willkommii (Cyprinidae). All infected fishes were collected in the Ebro Delta (Tarragona, Spain) with the exception of P. willkomii originating from the aquaculture centre of Villafranco del Guadiana (Badajoz, Spain) (Table 1). It is worth noting that infections with metacercariae of Diplostomum spp. were detected in some (C. carpio and M. anguillicaudatus) and not in other relatively well-sampled species (Pseudorasbora parva, Gambusia holbrooki and Lepomis gibbosus) in the Ebro Delta but also in one of the three S. glanis sampled in this locality. All infections with Diplostomum spp. in the fish from Ebro Delta were of low intensity (1 to 4 metacercariae).
All P. willkommii (n = 10) examined from the aquaculture centre in Villafranco de Guadiana were infected with 95–139 metacercariae. Due to the high parasite load, infections were detectable by visual examination especially in older mature fish (Figure 1B,C). The overall prevalence of infection is estimated as 60–65% with a trend of increase with fish age: 0–25% in fish during the first year; 25–50% during the second year; 50–75% during the third year; up to 90% during the fourth year.
A total of 31 fish-eating birds belonging to six species was examined (Table 2). Of these, only six gulls were infected with Diplostomum spp.: two Larus argentatus michahellis and three L. ridibundus originating from Ebro Delta (Tarragona) and one L. ridibundus from Cunit (Tarragona). Representative adult specimens of the two Diplostomum spp. identified in the material from gulls based on morphology, i.e. D. spathaceum and D. pseudospathaceum, and all metacercariae recovered from fish were selected for sequencing.
Molecular identification
Partial cox1 sequences were obtained for seven adult isolates collected from two gull hosts (Larus ridibundus and L. cachinnans) and 11 metacercarial isolates collected from the lenses of four fish hosts (Cyprinus carpio, Misgurnus anguillicaudatus, Pseudochondrostoma willkommii and Silurus glanis). Similar to a previous study on Diplostomum spp. in Europe [1], phylogenetic analyses of the cox1 dataset (410 nt) recovered eight species/lineages comprising D. spathaceum, D. pseudospathaceum, D. spathaceum/parviventosum referred to as Clade Q sensu Georgieva et al. [1], ‘D. mergi’ complex (including three putative species) and ‘D. baeri’ complex (representing two sibling species) (Figure 2). The analyses provided robust evidence that most of the isolates are conspecific with D. spathaceum sensu Georgieva et al. [1] (Figure 2). These represented five adult isolates ex L. ridibundus and L. argentatus michahellis from Ebro Delta, one adult isolate ex L. ridibundus from Cunit, seven metacercarial isolates ex P. willkommii from Villafranco del Guadiana, two metacercarial isolates ex M. anguillicaudatus and a single isolate ex S. glanis, the last two fish species both collected from Ebro Delta.
Figure 2.
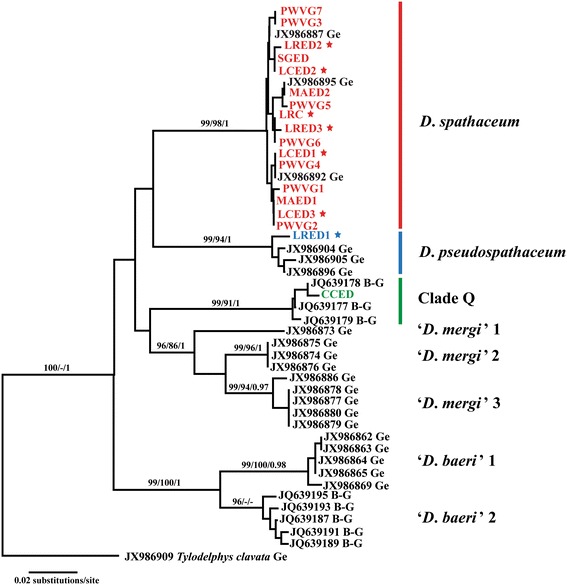
Neighbour-joining (NJ) phylogram reconstructed using the newly-generated and retrieved from GenBank cox 1 sequences for Diplostomum spp. Nodal support from Maximum Likelihood (ML) and Bayesian Inference (BI) analyses indicated as NJ/ML/BI. Outgroup: Tylodelphys clavata. The scale-bar indicates the expected number of substitutions per site. Isolates from Spain are coded as in Table 3; stars indicate adult isolates from gulls.
The intraspecific divergence within the D. spathaceum clade ranged between 0 and 1.5%, i.e. within the known range of intraspecific variation for Diplostomum spp. [1]. The material collected in Spain was represented by a total of 10 haplotypes (Table 3) with only one haplotype shared with adult isolates from central and northern Europe (haplotype 2, isolate ex M. anguillicaudatus and JX986892). There was no specific geographic pattern of the distribution of the novel haplotypes. Thus isolates from Pseudochondrostoma willkommii from the population of Villafranco del Guadiana were represented by six haplotypes with only one shared and there were shared haplotypes among isolates from geographically distant host samples, e.g. among larval isolates from Villafranco del Guadiana and adult isolates from Ebro Delta and Cunit (haplotypes 1, 3 and 4) (see Table 3 for details).
Numerous attempts were made to obtain sequences for isolates of adult D. pseudospathaceum identified based on morphology but only one was successful; this may be due to the fact that the infected birds were collected long after their death. The sequence for the single isolate ex Larus ridibundus clustered within the strongly supported clade (Figure 2) representing sequences for adult isolates of D. pseudospathaceum identified based on morphology [1]. The Spanish isolate was represented by a unique haplotype which differed by 1.2-1.7% from the remaining three haplotypes within the D. pseudospathaceum clade.
Finally, a sequence from a single metacercaria ex Cyprinus carpio from the Ebro Delta clustered together with sequences for one cercarial isolate ex Radix auricularia (RA97) and two metacercarial isolates ex R. rutilus (RR43 and RR45) from Lake Constance, all reported as D. spathaceum [13] but labelled as D. mergi in GenBank (see Clade Q in Figure 2).
A total of seven ITS1-5.8S-ITS2 sequences was generated after a selective sub-sampling of the Spanish isolates within the three cox1 clades of Diplostomum spp. The analysis of the ITS data (997 nt positions) resulted in molecular identification of these isolates concordant with that based on the cox1 gene trees with strong support (Figure 3). The intraspecific divergence within the D. spathaceum clade ranged between 0 and 0.4%. The five representative isolates from the cox1 dataset corresponded to four genotypes (with one genotype shared between an isolate ex M. anguillicaudatus from Ebro Delta and one ex L. ridibundus from Cunit).
Figure 3.
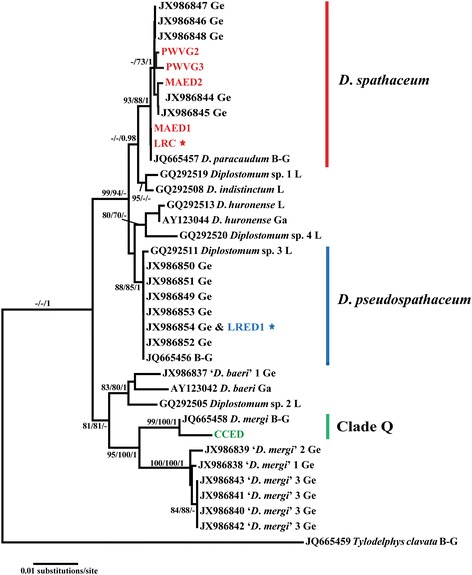
Neighbour-joining (NJ) phylogram reconstructed using the newly-generated and retrieved from GenBank ITS1-5.8S-ITS2 sequences for Diplostomum spp. Nodal support from Maximum Likelihood (ML) and Bayesian Inference (BI) analyses indicated as NJ/ML/BI. Outgroup: Tylodelphys clavata. The scale-bar indicates the expected number of substitutions per site. Isolates from Spain are coded as in Table 3; stars indicate adult isolates from gulls.
The sequence from the single adult isolate identified as D. pseudospathaceum based on morphology and cox1 phylogeny was identical with six sequences of Georgieva et al. [1] based on larval and adult isolates from the Czech Republic and Germany and one sequence of Berhrmann-Godel [13]; all these sequences formed a strongly supported clade representing D. pseudospathaceum (Figure 3) which also included Diplostomum sp. 3 of Locke et al. [6] as in previous studies [1,7].
As in the cox1 solution, the sequence for the metacercarial isolate ex C. carpio clustered together with a sequence labelled in GenBank as “D. mergi” for a cercarial isolate (RA97) ex Radix auricularia from Lake Constance [13] within the Clade Q sensu Georgieva et al. [1]. The divergence between the two sequences was 0.8%.
Descriptions of the molecular voucher material
Diplostomum spathaceum (Rudolphi, 1819) (adult)
Hosts:Larus argentatus michahellis Naumann; L. ridibundus L.
Localities: Ebro Delta, Cunit (Tarragona, Spain).
Site in host: Small intestine.
[Based on five frozen specimens (hologenophores) preserved in ethanol (molecular biology grade)]. Body 1,971 − 2,189 (2,085) long (Figure 4). Forebody oval, dorso-ventrally flattened, 782 − 1,155 long [40 − 43 (42)% of total body length], with maximum width 504 − 726 (592) at level of holdfast organ. Hindbody, elongate-oval, narrower anteriorly, 1,252 − 1,368 (1,285) long, with maximum width 387 − 575 (477) at level of anterior testis.
Figure 4.
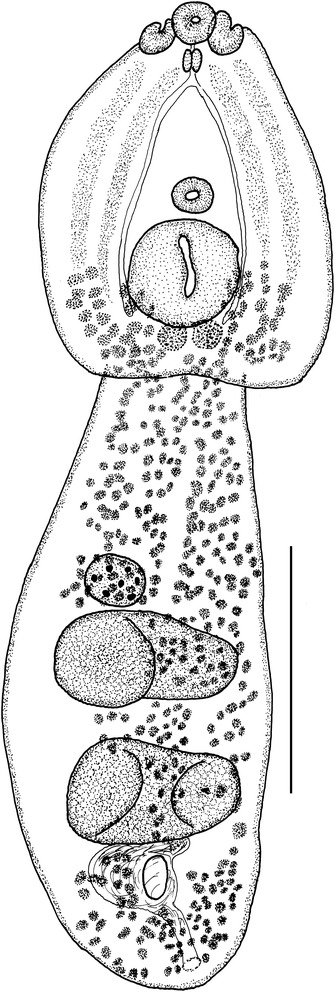
Diplostomum spathaceum . Adult ex Larus argentatus michahellis (hologenophore). Scale-bar: 500 μm.
Oral sucker ventro-subterminal, subspherical, 71 − 93 × 70 − 92 (81 × 78). Pseudosuckers well developed, 109 − 155 × 44 − 62 (139 × 56). Ventral sucker subglobular, 65 − 95 × 80 − 99 (83 × 89), similar in size to oral sucker, located just anterior to mid-forebody. Holdfast organ large, subglobular, 150 − 236 × 202 − 288 (215 × 224), fairly close to or contiguous with ventral sucker. Prepharynx short or absent; pharynx elongate-oval, 55 − 89 × 45 − 59 (73 × 52); oesophagus indistinct; caeca narrow.
Testes 2, large, in posterior half of hindbody; anterior testis transversely elongate, asymmetrical, 171 − 203 × 154 − 224 (183 × 191); posterior testis transversely elongate, symmetrical, horseshoe-shaped, 190 − 317 × 240 − 399 (247 × 334). Seminal vesicle voluminous. Gentital pore dorso-subterminal. Ovary small, dextral, pretesticular, subglobular, 87 × 83, contiguous with anterior testis. Vitellarium follicular, follicles numerous, small, arranged in four lateral bands surrounding holdfast organ in forebody; bands reach to mid-level of holdfast organ, converge close to posterior margin of forebody, posteriorly to holdfast organ; vitelline follicles in hindbody in two wide, not well-delimited lateral bands, converging medially at level of testes, reaching fairly close to posterior extremity of body. Eggs few, 89 − 99 × 61 − 66 (95 × 63).
Diplostomum spathaceum (Rudolphi, 1819) (metacercaria)
Hosts: Pseudochondrostoma willkommii (Steindachner); Misgurnus anguillicaudatus (Cantor); Silurus glanis L.
Localities: Villafranco del Guadiana (P. willkommii) and Ebro Delta (M. anguillicaudatus and S. glanis), Spain.
Site in host: Eye lens.
[Based on 10 metacercariae (hologenophores) fixed in hot saline solution and preserved in ethanol (molecular biology grade)]. Body elongate-oval, flattened, 277 − 453 × 198 − 295 (376 × 248); primordial hindbody 10 − 26 (16) long (Figure 5). Oral sucker elongate-oval, 40 − 57 × 36 − 41 (45 × 39). Ventral sucker transversely oval, 30 − 43 × 33 − 48 (38 × 43). Two contractile lappets (pseudosuckers) present on each side of oral sucker, 44 − 55 (48) long, with maximum width 22 − 30 (26). Prepharynx very short; pharynx elongate-oval, 29 − 43 × 19 − 26 (37 × 23); oesophagus short; caeca long, wide, reach posterior to holdfast organ. Holdfast organ large, elongate-oval, 63 − 89 × 59 − 90 (75 × 80). Reserve excretory system with numerous, relatively large excretory granules (170 − 184 in number), distributed in a median and two lateral fields.
Figure 5.
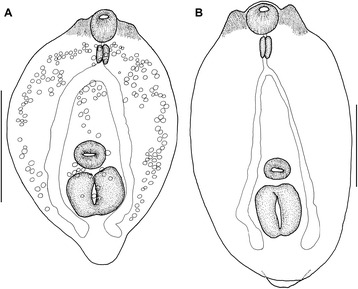
Metacercariae of Diplostomum spathaceum ex Pseudochondrostoma willkommii . A, Live metacercaria (hologenophore); B, Fixed metacercariae (hologenophore). Scale-bars: A, 200 μm; B, 100 μm.
Diplostomum pseudospathaceum Niewiadomska, 1984 (adult)
Host:Larus ridibundus L.
Locality: Ebro Delta (Tarragona, Spain).
Site in host: Small intestine.
[Based on a single frozen specimen (hologenophore) preserved in ethanol (molecular biology grade)]. Body 2,884 long (Figure 6). Forebody elongate-oval, narrow, dorso-ventrally flattened, tapering anteriorly, 1,075 long (37% of total body length), with maximum width at level of ventral sucker, 526. Hindbody, elongate, sub-cylindrical, narrower anterior to ovary, 1,891 long, with maximum width at level of posterior testis, 163.
Figure 6.
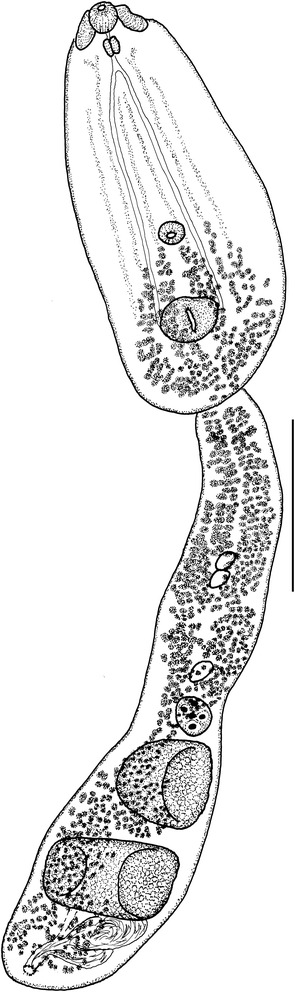
Diplostomum pseudospathaceum . Adult ex Larus ridibundus (hologenophore). Scale-bar: 500 μm.
Oral sucker ventro-subterminal, subspherical, 69 × 73. Pseudosuckers well developed, 128 × 49. Ventral sucker transversely oval, 67 × 85, slightly larger than oral sucker, located just posterior to mid-forebody. Holdfast organ subglobular, 126 × 118, located well posterior to ventral sucker (at a distance >2 ventral sucker diameters). Prepharynx fairly short; pharynx elongate-oval, 53 × 35; oesophagus short; caeca narrow.
Testes 2, large, in posterior half of hindbody; anterior testis transversely elongate, asymmetrical, 132 × 75; posterior testis larger, transversely elongate, symmetrical, horseshoe-shaped, 237 × 315. Seminal vesicle voluminous. Gentital pore dorso-subterminal. Ovary small, submedian, pretesticular, subglobular, 79 × 78, nearly contiguous with anterior testis. Vitellarium follicular, follicles numerous, small, arranged in two median inter-caecal and four lateral extra-caecal bands in forebody, reaching to the posterior margin of ventral sucker anteriorly; bands, converge close to posterior margin of forebody, posteriorly to holdfast organ; vitelline follicles in hindbody in two wide, dense lateral bands, converging medially at level of gonads, reach fairly close to posterior extremity of body. Eggs few, 96 − 110 × 58 − 63.
Diplostomum sp. (metacercaria)
Host:Cyprinus carpio L.
Locality: Ebro Delta (Tarragona, Spain).
Site in host: Eye lens.
[Based on a single metacercaria (hologenophore) fixed and preserved in ethanol (molecular biology grade).] Body elongate-oval, flattened, 229 × 180; primordial hindbody not evident (Figure 7). Oral sucker spherical, 29 × 29. Ventral sucker subspherical, 37 × 42. Two small contractile lappets (pseudosuckers) present on each side of oral sucker, 31 − 32 long, with maximum width 15 − 16. Prepharynx absent; pharynx subspherical, 24 × 23; oesophagus very short; caeca long, narrow, reach posterior to holdfast organ. Holdfast organ large, transversely elongate, 50 × 84. Reserve excretory system with numerous, dispersed, relatively large excretory granules (c. 215 in number).
Figure 7.
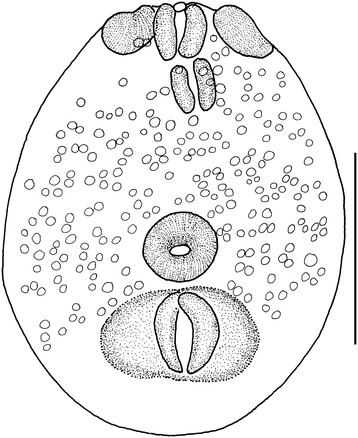
Diplostomum sp. ex Cyprinus carpio . Fixed metacercaria (hologenophore). Scale-bar: 100 μm.
Discussion
This first molecular exploration of the diversity of Diplostomum spp. in southern Europe indicates much lower species richness compared with the northern regions of Europe (3 vs 12 species). Of the six species of fish-eating birds studied in the north of Spain only two gull species were found to host adult Diplostomum spp.; however, sample sizes were rather small. The detection of metacercariae in fish also might have been influenced by the differential sample sizes. However, we found infections in an under-sampled fish host as well in some but not in other hosts with relatively large sample sizes. Notably, metacercariae of Diplostomum spp. were recovered in three out of the seven invasive fish species examined (C. carpio, M. anguillicaudatus and S. glanis; Table 1) thus indicating that these hosts may have a considerable contribution to the transmission of Diplostomum spp. in the Ebro Delta and elsewhere. M. anguillicaudatus and S. glanis are new host records for D. spathaceum.
Another important finding is the high prevalence and abundance of infection with D. spathaceum in P. willkommii, a native vulnerable species [26] with distribution restricted to the southern Iberian Peninsula in Spain and Portugal. The high levels of infections in the aquaculture centre in Villafranco de Guadiana, where mature breeders from natural populations are being added yearly to the cultured population, reveal a further threat upon this fish species in both natural and fish farming conditions. The shallow, open nature of the pools probably contributes significantly to the establishment of a focus of infection with D. spathaceum.
To the best of our knowledge, this study is the first to provide detailed morphometric data and morphological description of the isolates of Diplostomum spp. in association with the molecular data used for identification. The morphology of the adult specimens of D. spathaceum and D. pseudospathaceum used for sequence generation agrees well with the descriptions of D. spathaceum sensu stricto and D. pseudospathaceum of Niewiadomska [27], respectively. The material of D. spathaceum ex Larus spp. from Ebro Delta is characterised by lower values (outside the lower range for the material ex Larus fuscus L. and L. ridibundus from Poland studied by Niewiadomska [27] for the size of the hindbody, holdfast organ, ovary and testes (Table 5). Similarly, the specimen of D. pseudospathaceum ex L. ridibundus from Ebro Delta had smaller holdfast organ, ovary and testes and much narrower hindbody and longer pseudosuckers compared with the specimens from the same host studied in Poland (Table 5). These data indicate much higher geographic variation in the morphometric features in both Diplostomum spp.
Table 5.
Comparative metrical data for adults of Diplostomum spathaceum and D . pseudospathaceum
| Species | Dipostomum spathaceum | Dipostomum pseudospathaceum | ||
|---|---|---|---|---|
| Host | Larus fuscus L., Larus ridibundus L. | Larus argentatus michahellis Naumann; Larus ridibundus L. | Larus ridibundus L. | Larus ridibundus L. |
| Locality | Lake Mamry (Poland) | Ebro Delta (Spain) | Lake Mamry (Poland) | Ebro Delta (Spain) |
| Source | Niewiadomska [ 27 ] | Present study | Niewiadomska [ 27 ] | Present study |
| TL | up to 4,000 | 1,971 − 2,189 | up to 3,600 | 2,884 |
| FBL | 1,110 − 1,480 | 782 − 1,155 | 1,030 − 1,720 | 1,075 |
| FBW | 590 − 850 | 504 − 726 | 400 − 680 | 526 |
| HBL | 1,560 − 2,920 | 1,252 − 1,368 | 960 − 2,190 | 1,891 |
| HBW | 560 − 660 | 387 − 575 | 420 − 720 | 163 |
| OSL | 57 − 95 | 71 − 93 | 67 − 78 | 69 |
| OSW | 74 − 102 | 70 − 92 | 68 − 95 | 73 |
| PSL | 102 − 153 | 109 − 155 | 51 − 115 | 128 |
| PSW | – | 44 − 62 | – | 49 |
| VSL | 78 − 95 | 65 − 95 | 68 − 103 | 67 |
| VSW | 89 − 102 | 80 − 99 | 62 − 119 | 85 |
| HOL | 238 − 374 | 150 − 236 | 153 − 335 | 126 |
| HOW | 259 − 399 | 202 − 288 | 163 − 388 | 118 |
| PHL | 59 − 74 | 55 − 89 | 44 − 74 | 53 |
| PHW | 51 − 74 | 45 − 59 | 47 − 66 | 35 |
| ATL | 185 − 540 | 171 − 203 | 188 − 503 | 132 |
| ATW | 421 − 629 | 154 − 224 | 296 − 629 | 75 |
| PTL | 348 − 592 | 190 − 317 | 255 − 666 | 237 |
| PTW | 466 − 658 | 240 − 399 | 370 − 666 | 315 |
| OVL | 138 − 222 | 87 | 111 − 187 | 79 |
| OVW | 163 − 236 | 83 | 142 − 238 | 78 |
| FO/BL (%) | 31 − 48 | 40 − 43 | 41 − 58 | 37 |
| Egg-length | – | 89 − 99 | – | 96 − 110 |
| Egg-width | – | 61 − 66 | – | 58 − 63 |
Abbreviations: TL total body length, FBL forebody length, FBW forebody width, HBL hindbody length, HBW hindbody width, OSL oral sucker length, OSW oral sucker width, PSL pseudosucker length, PSW pseudosucker width, VSL ventral sucker length, VSW ventral sucker width, HOL holdfast organ length, HOW holdfast organ width, PHL pharynx length, PHW pharynx width, ATL anterior testis length, ATW anterior testis width, PTL posterior testis length, PTW posterior testis width, OVL ovary length, OVW ovary width, FO/BL (%) forebody as a percentage of body length.
The dimensions of the metacercariae from the three fish hosts identified molecularly as D. spathaceum varied within the range provided by Niewiadomska [28] for the metacercariae of this species raised experimentally in C. carpio. However, the mean values for the length of body and the size of suckers were lower in the specimens obtained in Spain (Table 6). The metacercaria of Diplostomum sp. that was found to be conspecific with the isolates of Clade Q sensu Georgieva et al. [1] had distinctly smaller oral sucker and shorter holdfast organ compared with both Spanish and Polish isolates of D. spathaceum (Table 6). Finally, the metacercariae of both Diplostomum spp. examined in Spain had distinctly lower number of excretory granules in the secondary excretory system than the experimentally raised metacercariae ex C. carpio (see [28]; Table 6).
Table 6.
Comparative metrical data for the metacercariae of Diplostomum spathaceum and Diplostomum sp. (Clade Q)
| Species | Dipostomum spathaceum | Diplostomum sp. (Clade Q) | |||
|---|---|---|---|---|---|
| Host | Cyprinus carpio L. | Pseudochondrostoma willkommii (Steindachner); Misgurnus anguillicaudatus (Cantor); Silurus glanis L. | Cyprinus carpio | ||
| Locality | Experimental infection | Villafranco del Guadiana and Ebro Delta (Spain) | |||
| Source | Niewiadomska [ 28 ] | Present study | Present study | ||
| Range | Mean | Range | Mean | n =1 | |
| BL | 340 − 451 | 398 | 277 − 453 | 376 | 229 |
| BW | 170 − 296 | 217 | 198 − 295 | 248 | 180 |
| HL | – | – | 10 − 26 | 16 | 0 |
| OSL | 42 − 54 | 48 | 40 − 57 | 45 | 29 |
| OSW | 42 − 52 | 45 | 36 − 41 | 39 | 29 |
| PSL | – | – | 44 − 55 | 48 | 31 − 32 |
| PSW | – | – | 22 − 30 | 26 | 15 − 16 |
| VSL | 39 − 56 | 46 | 30 − 43 | 38 | 37 |
| VSW | 42 − 59 | 53 | 33 − 48 | 43 | 42 |
| PHL | 25 − 39 | 31 | 29 − 43 | 37 | 24 |
| PHW | 12 − 25 | 20 | 19 − 26 | 23 | 23 |
| HOL | 68 − 93 | 77 | 63 − 89 | 75 | 50 |
| HOW | 62 − 102 | 85 | 59 − 90 | 80 | 84 |
| No. of excretory granules | c. 300 | – | 170 − 184 | 178 | c. 215 |
Abbreviations: BL body length, BW body width, HL primordial hindbody length, OSL oral sucker length, OSW oral sucker width, PSL pseudosucker length, PSW pseudosucker width, VSL ventral sucker length, VSW ventral sucker width, HOL holdfast organ length, HOW holdfast organ width, PHL pharynx length, PHW pharynx width.
Although the molecular and morphological identification of the larval and adult isolates of D. spathaceum and D. pseudospathaceum were straightforward, we failed to identify one isolate recovered in C. carpio. The analysis of both cox1 and ITS1-5.8S-ITS2 sequences placed this isolate within the Clade Q (i.e. questionable), a label used by Georgieva et al. [1] to indicate five identical ITS1 sequences from Europe: two for cercariae ex R. ovata identified as D. spathaceum and one for cercariae ex R. ovata identified as D. parviventosum by Niewiadomska & Laskowski [29] in Poland; one for a metacercaria ex R. rutilus from Finland submitted to GenBank as D. cf. parviventosum/spathaceum by Rellstab et al. [30]; and one for cercariae ex R. auricularia (isolate RA97) from Lake Constance [13]; the latter was designated as D. spathaceum but submitted to GenBank as D. mergi. Using the sequences of Behrmann-Godel [13] for both cox1 and ITS1-5.8S-ITS2, we found that this clade, incorporating our sequence for the metacercaria ex C. carpio, is strongly supported and reconstructed as sister to the species-level lineages of the ‘Diplostomum mergi’ species complex sensu Georgieva et al. [1]. Unfortunately, no identification to the species level can be attempted for the isolates within this clade since all represent larval stages for which, with the exception of the present data, no morphological evidence has been provided. The congruent morphological and molecular identification of the adult isolates of D. spathaceum achieved here, supports the suggestion of Georgieva et al. [1] that isolates in Clade Q may represent D. parviventosum. Further molecular and morphological evidence is required, preferably based on adult isolates, in order to solve the species-level identification of this clade.
Conclusion
This first molecular exploration of the diversity of Diplostomum spp. in southern Europe indicates much lower species richness compared with the northern regions of Europe.
Acknowledgements
This study was partially funded by the Czech Science Foundation (ECIP P505/12/G112). We thank Nati Franch (Parc Natural Delta de l’Ebre), Emilio Valbuena-Ureña (Centre de Recuperació de Fauna Salvatge de Torreferrussa), Imanol Ruiz, Ignacio de Blas and Tania Pérez (University of Zaragoza) for their help with the fish and bird sampling.
Footnotes
Ana Pérez-del-Olmo and Simona Georgieva contributed equally to this article.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
APO conceived and designed the study, obtained the samples, undertook the morphological characterisation and helped draft the MS. HJP obtained samples, discussed the results and took part in the preparation of the MS and figures. SG carried out the sequencing and phylogenetic analyses, took part in the morphological assessment, and prepared the first draft of the MS and figures. AK coordinated the project and helped draft the MS. All authors read and approved the final manuscript.
Contributor Information
Ana Pérez-del-Olmo, Email: ana.perez-olmo@uv.es.
Simona Georgieva, Email: georgieva@paru.cas.cz.
Héctor J Pula, Email: hjpula@gmail.com.
Aneta Kostadinova, Email: aneta.kostadinova@uv.es.
References
- 1.Georgieva S, Soldánová M, Pérez-del-Olmo A, Dangel RD, Sitko J, Sures B, Kostadinova A. Molecular prospecting for European Diplostomum (Digenea: Diplostomidae) reveals cryptic diversity. Int J Parasitol. 2013;43:57–72. doi: 10.1016/j.ijpara.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 2.Jousson O, Bartoli P, Pawlowski J. Cryptic speciation among intestinal parasites (Trematoda: Digenea) infecting sympatric host fishes (Sparidae) J Evol Biol. 2000;13:778–785. doi: 10.1046/j.1420-9101.2000.00221.x. [DOI] [Google Scholar]
- 3.Georgieva S, Kostadinova A, Skirnisson K. The life-cycle of Petasiger islandicus Kostadinova & Skirnisson, 2007 (Digenea: Echinostomatidae) elucidated with the aid of molecular data. Syst Parasitol. 2012;82:177–183. doi: 10.1007/s11230-012-9354-y. [DOI] [PubMed] [Google Scholar]
- 4.Born-Torrijos A, Kostadinova A, Raga JA, Holzer AS. Molecular and morphological identification of larval opecoelids (Digenea: Opecoelidae) parasitising prosobranch snails in a Western Mediterranean lagoon. Parasitol Int. 2012;61:450–460. doi: 10.1016/j.parint.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 5.Alcántar-Escalera FJ, García-Varela M, Vázquez-Domínguez E, Pérez-Ponce de León G. Using DNA barcoding to link cystacanths and adults of the acanthocephalan Polymorphus brevis in central Mexico. Mol Ecol Resour. 2013;13:1116–1124. doi: 10.1111/1755-0998.12090. [DOI] [PubMed] [Google Scholar]
- 6.Locke SA, McLaughlin JD, Dayanandan S, Marcogliese DJ. Diversity, specificity and evidence of hybridization in Diplostomum spp. metacercariae in freshwater fishes is revealed by DNA barcodes and ITS sequences. Int J Parasitol. 2010;40:333–343. doi: 10.1016/j.ijpara.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 7.Blasco-Costa I, Faltýnková A, Georgieva S, Skírnisson K, Scholz T, Kostadinova A. Fish pathogens near the Arctic Circle: molecular, morphological and ecological evidence for unexpected diversity of Diplostomum (Digenea: Diplostomidae) in Iceland. Int J Parasitol. 2014;44:703–715. doi: 10.1016/j.ijpara.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 8.Sanmartín ML, Cordeiro JA, Álvarez MF, Leiro JM. Helminth fauna of the yellow-legged gull Larus cachinnans in Galicia, north-west Spain. J Helm. 2005;79:361–371. doi: 10.1079/JOH2005309. [DOI] [PubMed] [Google Scholar]
- 9.Álvarez MF, Cordeiro JA, Leiro JM, Sanmartín ML. Influence of host age and sex on the helminth fauna of the yellowlegged gull (Larus michahellis) in Galicia (Northwestern Spain) J Parasitol. 2006;92:454–458. doi: 10.1645/GE-3546.1. [DOI] [PubMed] [Google Scholar]
- 10.Ribas J, Miquel J, Torres J. New record of Diplostomum pseudospathaceum (Szidat, 1924) Niewiadomska, 1984 in Spain. Res Rev Parasitol. 1999;59:19–21. [Google Scholar]
- 11.Bosch M, Torres J, Figuerola J. A helminth community in breeding yellow-legged gulls (Larus cachinnans): pattern of association and its effect on host fitness. Can J Zool. 2000;78:777–786. doi: 10.1139/cjz-78-5-777. [DOI] [Google Scholar]
- 12.Aguilar A, Alvarez MF, Leiro JM, Sanmartín ML. Parasite populations of the European eel (Anguilla anguilla L.) in the Rivers Ulla and Tea (Galicia, northwest Spain) Aquaculture. 2005;249:85–94. doi: 10.1016/j.aquaculture.2005.04.052. [DOI] [Google Scholar]
- 13.Behrmann-Godel J. Parasite identification, succession and infection pathways in perch fry (Perca fluviatilis): new insights through a combined morphological and genetic approach. Parasitology. 2013;140:509–520. doi: 10.1017/S0031182012001989. [DOI] [PubMed] [Google Scholar]
- 14.Pleijel F, Jondelius U, Norlinder E, Nygren A, Oxelman B, Schander C, Sundberg P, Thollesson M. Phylogenies without roots? A plea for the use of vouchers in molecular phylogenetic studies. Mol Phylogen Evol. 2008;48:369–371. doi: 10.1016/j.ympev.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 15.Georgieva S, Selbach C, Faltýnková A, Soldánová M, Sures B, Skírnisson K, Kostadinova A. New cryptic species of the ‘revolutum’ group of Echinostoma (Digenea: Echinostomatidae) revealed by molecular and morphological data. Parasites & Vectors. 2013;6:64. doi: 10.1186/1756-3305-6-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moszczynska A, Locke SA, McLaughlin JD, Marcogliese DJ, Crease TJ. Development of primers for the mitochondrial cytochrome c oxidase I gene in digenetic trematodes illustrates the challenge of barcoding parasitic helminths. Mol Ecol Resour. 2009;9:75–82. doi: 10.1111/j.1755-0998.2009.02634.x. [DOI] [PubMed] [Google Scholar]
- 17.Galazzo DE, Dayanandan S, Marcogliese DJ, McLaughlin JD. Molecular systematics of some North American species of Diplostomum (Digenea) based on rDNA-sequence data and comparisons with European congeners. Can J Zool. 2002;80:2207–2217. doi: 10.1139/z02-198. [DOI] [Google Scholar]
- 18.Luton K, Walker D, Blair D. Comparison of ribosomal internal transcribed spacers from two congeneric species of flukes (Platyhelminthes: Trematoda: Digenea) Mol Biochem Parasitol. 1992;56:323–328. doi: 10.1016/0166-6851(92)90181-I. [DOI] [PubMed] [Google Scholar]
- 19.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Telford MJ, Herniou EA, Russell RB, Littlewood DTJ. Changes in mitochondrial genetic codes as phylogenetic characters: Two examples from the flatworms. Proc Natl Acad Sci U S A. 2000;97:11359–11364. doi: 10.1073/pnas.97.21.11359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guindon S, Gascuel O. A simple, fast and accurate method to estimate large phylogenies by maximum-likelihood. Syst Biol. 2003;52:696–704. doi: 10.1080/10635150390235520. [DOI] [PubMed] [Google Scholar]
- 22.Darriba D, Taboada GL, Doallo R, Posada D. jModelTest 2: more models, new heuristics and parallel computing. Nat Methods. 2012;9:772. doi: 10.1038/nmeth.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol. 2010;59:307–321. doi: 10.1093/sysbio/syq010. [DOI] [PubMed] [Google Scholar]
- 24.Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Hohna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol. 2012;61:539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huelsenbeck JP, Ronquist F, Nielsen R, Bollback JP. Bayesian Inference of phylogeny and its impact on evolutionary biology. Science. 2001;294:2310–2314. doi: 10.1126/science.1065889. [DOI] [PubMed] [Google Scholar]
- 26.Anonymous: The IUCN Red List of threatened species.http://www.iucnredlist.org/details/4785/0. Accessed on 10 July 2014.
- 27.Niewiadomska K. Present status of Diplostomum spathaceum (Rudolphi, 1819) and differentiation of Diplostomum pseudospathaceum nom. nov. (Trematoda: Diplostomatidae) Syst Parasitol. 1984;6:81–86. doi: 10.1007/BF02185515. [DOI] [Google Scholar]
- 28.Niewiadomska K. Verification of the life-cycles of Diplostomum spathaceum (Rudolphi, 1819) and D. pseudospathaceum Niewiadomska, 1984 (Trematoda: Diplostomidae) Syst Parasitol. 1986;8:23–31. doi: 10.1007/BF00010306. [DOI] [Google Scholar]
- 29.Niewiadomska K, Laskowski Z. Systematic relationships among six species of Diplostomum Nordmann, 1832 (Digenea) based on morphological and molecular data. Acta Parasitol. 2002;47:20–28. [Google Scholar]
- 30.Rellstab C, Louhi K-R, Karvonen A, Jokela J. Analysis of trematode parasite communities in fish eye lenses by pyrosequencing of naturally pooled DNA. Infect Genet Evol. 2011;11:1276–1286. doi: 10.1016/j.meegid.2011.04.018. [DOI] [PubMed] [Google Scholar]


