Abstract
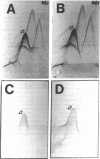
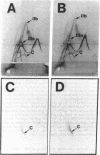
In this study we have used two new monoclonal antibodies, designated LJP5 and LJP9, as well as a previously described one, AP2, all specific for the platelet membrane glycoprotein (GP)IIb/IIIa complex. None of them reacted with dissociated GPIIb or GPIIIa. The monovalent Fab fragment of both LJP5 and LJP9 bound to unstimulated platelets in a saturable manner, but binding was markedly decreased after platelets had been incubated at 37 degrees C in the absence of added extracellular calcium. The binding of LJP9 was not affected by AP2, but was blocked by excess LJP5. On the contrary, the binding of LJP5 was blocked in the presence of both AP2 and LJP9. Thus, these antibodies bound to distinct epitopes of GPIIb/IIIa. At saturation, the binding to unstimulated platelets was between 2.41 and 10.9 X 10(4) molecules/platelet for LJP5 and between 3.47 and 9.1 X 10(4) molecules/platelet for LJP9 (range of 11 and 10 experiments, respectively). Binding increased up to 50% after thrombin stimulation. The estimated association constant, Ka, was 2.7 X 10(7) M-1 for LJP5 and 3.85 X 10(7) M-1 for LJP9. Both LJP5 and LJP9 partially inhibited the association of 45Ca2+ with the surface of unstimulated platelets. Moreover, both antibodies blocked the binding of von Willebrand factor (vWF) to stimulated platelets, whereas only LJP9, but not LJP5, blocked fibrinogen binding. LJP9 was also a potent inhibitor of platelet aggregation, whereas LJP5 was without effect in this regard. The results of the present study demonstrate that independent modulation of vWF and fibrinogen binding to stimulated platelets can be attained with monoclonal antibodies directed against distinct epitopes of GPIIb/IIIa.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett J. S., Hoxie J. A., Leitman S. F., Vilaire G., Cines D. B. Inhibition of fibrinogen binding to stimulated human platelets by a monoclonal antibody. Proc Natl Acad Sci U S A. 1983 May;80(9):2417–2421. doi: 10.1073/pnas.80.9.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J. S., Vilaire G., Cines D. B. Identification of the fibrinogen receptor on human platelets by photoaffinity labeling. J Biol Chem. 1982 Jul 25;257(14):8049–8054. [PubMed] [Google Scholar]
- Brass L. F., Shattil S. J. Changes in surface-bound and exchangeable calcium during platelet activation. J Biol Chem. 1982 Dec 10;257(23):14000–14005. [PubMed] [Google Scholar]
- Brass L. F., Shattil S. J., Kunicki T. J., Bennett J. S. Effect of calcium on the stability of the platelet membrane glycoprotein IIb-IIIa complex. J Biol Chem. 1985 Jul 5;260(13):7875–7881. [PubMed] [Google Scholar]
- Bruck C., Portetelle D., Glineur C., Bollen A. One-step purification of mouse monoclonal antibodies from ascitic fluid by DEAE Affi-Gel blue chromatography. J Immunol Methods. 1982 Sep 30;53(3):313–319. doi: 10.1016/0022-1759(82)90178-8. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Clamp J. R., Hughes K. W., McPherson J. C. Glycoproteins from human spleen cell surfaces. Immunochemistry. 1971 Sep;8(9):874–879. doi: 10.1016/0019-2791(71)90455-1. [DOI] [PubMed] [Google Scholar]
- Coller B. S. A new murine monoclonal antibody reports an activation-dependent change in the conformation and/or microenvironment of the platelet glycoprotein IIb/IIIa complex. J Clin Invest. 1985 Jul;76(1):101–108. doi: 10.1172/JCI111931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coller B. S., Peerschke E. I., Scudder L. E., Sullivan C. A. A murine monoclonal antibody that completely blocks the binding of fibrinogen to platelets produces a thrombasthenic-like state in normal platelets and binds to glycoproteins IIb and/or IIIa. J Clin Invest. 1983 Jul;72(1):325–338. doi: 10.1172/JCI110973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coller H. A., Coller B. S. Statistical analysis of repetitive subcloning by the limiting dilution technique with a view toward ensuring hybridoma monoclonality. Hybridoma. 1983;2(1):91–96. doi: 10.1089/hyb.1983.2.91. [DOI] [PubMed] [Google Scholar]
- De Marco L., Shapiro S. S. Properties of human asialo-factor VIII. A ristocetin-independent platelet-aggregating agent. J Clin Invest. 1981 Aug;68(2):321–328. doi: 10.1172/JCI110259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Minno G., Thiagarajan P., Perussia B., Martinez J., Shapiro S., Trinchieri G., Murphy S. Exposure of platelet fibrinogen-binding sites by collagen, arachidonic acid, and ADP: inhibition by a monoclonal antibody to the glycoprotein IIb-IIIa complex. Blood. 1983 Jan;61(1):140–148. [PubMed] [Google Scholar]
- Ey P. L., Prowse S. J., Jenkin C. R. Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-sepharose. Immunochemistry. 1978 Jul;15(7):429–436. doi: 10.1016/0161-5890(78)90070-6. [DOI] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Fujimoto T., Hawiger J. Adenosine diphosphate induces binding of von Willebrand factor to human platelets. Nature. 1982 May 13;297(5862):154–156. doi: 10.1038/297154a0. [DOI] [PubMed] [Google Scholar]
- Fujimoto T., Ohara S., Hawiger J. Thrombin-induced exposure and prostacyclin inhibition of the receptor for factor VIII/von Willebrand factor on human platelets. J Clin Invest. 1982 Jun;69(6):1212–1222. doi: 10.1172/JCI110560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gralnick H. R., Williams S. B., Coller B. S. Fibrinogen competes with von Willebrand factor for binding to the glycoprotein IIb/IIIa complex when platelets are stimulated with thrombin. Blood. 1984 Oct;64(4):797–800. [PubMed] [Google Scholar]
- Hangen I., Bjerrum O. J., Gogstad G., Korsmo R., Solum N. O. Involvement of divalent cations in the complex between the platelet glycoproteins IIb and IIIa. Biochim Biophys Acta. 1982 Feb 4;701(1):1–6. doi: 10.1016/0167-4838(82)90303-x. [DOI] [PubMed] [Google Scholar]
- Howard L., Shulman S., Sadanandan S., Karpatkin S. Crossed immunoelectrophoresis of human platelet membranes. The major antigen consists of a complex of glycoproteins, GPIIb and GPIIIa, held together by Ca2+ and missing in Glanzmann's thrombasthenia. J Biol Chem. 1982 Jul 25;257(14):8331–8336. [PubMed] [Google Scholar]
- Jennings L. K., Phillips D. R. Purification of glycoproteins IIb and III from human platelet plasma membranes and characterization of a calcium-dependent glycoprotein IIb-III complex. J Biol Chem. 1982 Sep 10;257(17):10458–10466. [PubMed] [Google Scholar]
- Kunicki T. J., Pidard D., Rosa J. P., Nurden A. T. The formation of Ca++-dependent complexes of platelet membrane glycoproteins IIb and IIIa in solution as determined by crossed immunoelectrophoresis. Blood. 1981 Aug;58(2):268–278. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liu F. T., Bohn J. W., Ferry E. L., Yamamoto H., Molinaro C. A., Sherman L. A., Klinman N. R., Katz D. H. Monoclonal dinitrophenyl-specific murine IgE antibody: preparation, isolation, and characterization. J Immunol. 1980 Jun;124(6):2728–2737. [PubMed] [Google Scholar]
- Marguerie G. A., Edgington T. S., Plow E. F. Interaction of fibrinogen with its platelet receptor as part of a multistep reaction in ADP-induced platelet aggregation. J Biol Chem. 1980 Jan 10;255(1):154–161. [PubMed] [Google Scholar]
- Mason D. W., Williams A. F. The kinetics of antibody binding to membrane antigens in solution and at the cell surface. Biochem J. 1980 Apr 1;187(1):1–20. doi: 10.1042/bj1870001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masri M. A., Masri S. A., Boyd N. D. Isolation o human fibrinogen of high purity and in high yield using polyethylene glycol 1000. Thromb Haemost. 1983 Apr 28;49(2):116–119. [PubMed] [Google Scholar]
- McEver R. P., Bennett E. M., Martin M. N. Identification of two structurally and functionally distinct sites on human platelet membrane glycoprotein IIb-IIIa using monoclonal antibodies. J Biol Chem. 1983 Apr 25;258(8):5269–5275. [PubMed] [Google Scholar]
- McKee P. A., Rogers L. A., Marler E., Hill R. L. The subunit polypeptides of human fibrinogen. Arch Biochem Biophys. 1966 Sep 26;116(1):271–279. doi: 10.1016/0003-9861(66)90033-6. [DOI] [PubMed] [Google Scholar]
- Montgomery R. R., Kunicki T. J., Taves C., Pidard D., Corcoran M. Diagnosis of Bernard-Soulier syndrome and Glanzmann's thrombasthenia with a monoclonal assay on whole blood. J Clin Invest. 1983 Feb;71(2):385–389. doi: 10.1172/JCI110780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NISONOFF A., DIXON D. J. EVIDENCE FOR LINKAGE OF UNIVALENT FRAGMENTS OR HALF-MOLECULES OF RABBIT GAMMA-GLOBULIN BY THE SAME DISULFIDE BOND. Biochemistry. 1964 Sep;3:1338–1342. doi: 10.1021/bi00897a025. [DOI] [PubMed] [Google Scholar]
- Nachman R. L., Leung L. L. Complex formation of platelet membrane glycoproteins IIb and IIIa with fibrinogen. J Clin Invest. 1982 Feb;69(2):263–269. doi: 10.1172/JCI110448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman J., Johnson A. J., Karpatkin M. H., Puszkin S. Methods for the production of clinically effective intermediate- and high-purity factor-VIII concentrates. Br J Haematol. 1971 Jul;21(1):1–20. doi: 10.1111/j.1365-2141.1971.tb03413.x. [DOI] [PubMed] [Google Scholar]
- PORTER R. R. The hydrolysis of rabbit y-globulin and antibodies with crystalline papain. Biochem J. 1959 Sep;73:119–126. doi: 10.1042/bj0730119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parham P. On the fragmentation of monoclonal IgG1, IgG2a, and IgG2b from BALB/c mice. J Immunol. 1983 Dec;131(6):2895–2902. [PubMed] [Google Scholar]
- Pidard D., Montgomery R. R., Bennett J. S., Kunicki T. J. Interaction of AP-2, a monoclonal antibody specific for the human platelet glycoprotein IIb-IIIa complex, with intact platelets. J Biol Chem. 1983 Oct 25;258(20):12582–12586. [PubMed] [Google Scholar]
- Piétu G., Cherel G., Marguerie G., Meyer D. Inhibition of von Willebrand factor-platelet interaction by fibrinogen. Nature. 1984 Apr 12;308(5960):648–649. doi: 10.1038/308648a0. [DOI] [PubMed] [Google Scholar]
- Plow E. F., McEver R. P., Coller B. S., Woods V. L., Jr, Marguerie G. A., Ginsberg M. H. Related binding mechanisms for fibrinogen, fibronectin, von Willebrand factor, and thrombospondin on thrombin-stimulated human platelets. Blood. 1985 Sep;66(3):724–727. [PubMed] [Google Scholar]
- Plow E. F., Srouji A. H., Meyer D., Marguerie G., Ginsberg M. H. Evidence that three adhesive proteins interact with a common recognition site on activated platelets. J Biol Chem. 1984 May 10;259(9):5388–5391. [PubMed] [Google Scholar]
- Reynolds J. A. Interaction of divalent antibody with cell surface antigens. Biochemistry. 1979 Jan 23;18(2):264–269. doi: 10.1021/bi00569a004. [DOI] [PubMed] [Google Scholar]
- Ruggeri Z. M., Bader R., de Marco L. Glanzmann thrombasthenia: deficient binding of von Willebrand factor to thrombin-stimulated platelets. Proc Natl Acad Sci U S A. 1982 Oct;79(19):6038–6041. doi: 10.1073/pnas.79.19.6038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggeri Z. M., De Marco L., Gatti L., Bader R., Montgomery R. R. Platelets have more than one binding site for von Willebrand factor. J Clin Invest. 1983 Jul;72(1):1–12. doi: 10.1172/JCI110946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schullek J., Jordan J., Montgomery R. R. Interaction of von Willebrand factor with human platelets in the plasma milieu. J Clin Invest. 1984 Feb;73(2):421–428. doi: 10.1172/JCI111228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Switzer M. E., McKee P. A. Studies on human antihemophilic factor. Evidence for a covalently linked subunit structure. J Clin Invest. 1976 Apr;57(4):925–937. doi: 10.1172/JCI108369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura G. S., Dailey M. O., Gallatin W. M., McGrath M. S., Weissman I. L., Pillemer E. A. Isolation of molecules recognized by monoclonal antibodies and antisera: the solid phase immunoisolation technique. Anal Biochem. 1984 Feb;136(2):458–464. doi: 10.1016/0003-2697(84)90244-6. [DOI] [PubMed] [Google Scholar]
- Timmons S., Kloczewiak M., Hawiger J. ADP-dependent common receptor mechanism for binding of von Willebrand factor and fibrinogen to human platelets. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4935–4939. doi: 10.1073/pnas.81.15.4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh P. N., Mills D. C., White J. G. Metabolism and function of human platelets washed by albumin density gradient separation. Br J Haematol. 1977 Jun;36(2):287–296. doi: 10.1111/j.1365-2141.1977.tb00649.x. [DOI] [PubMed] [Google Scholar]
- Zimmerman T. S., Ruggeri Z. M., Fulcher C. A. Factor VIII/von Willebrand factor. Prog Hematol. 1983;13:279–309. [PubMed] [Google Scholar]
- Zucker M. B., Varon D., Masiello N. C., Karpatkin S. The combining ability of glycoproteins IIb, IIIa and Ca2+ in EDTA-treated nonaggregable platelets. Thromb Haemost. 1983 Dec 30;50(4):848–851. [PubMed] [Google Scholar]