Abstract
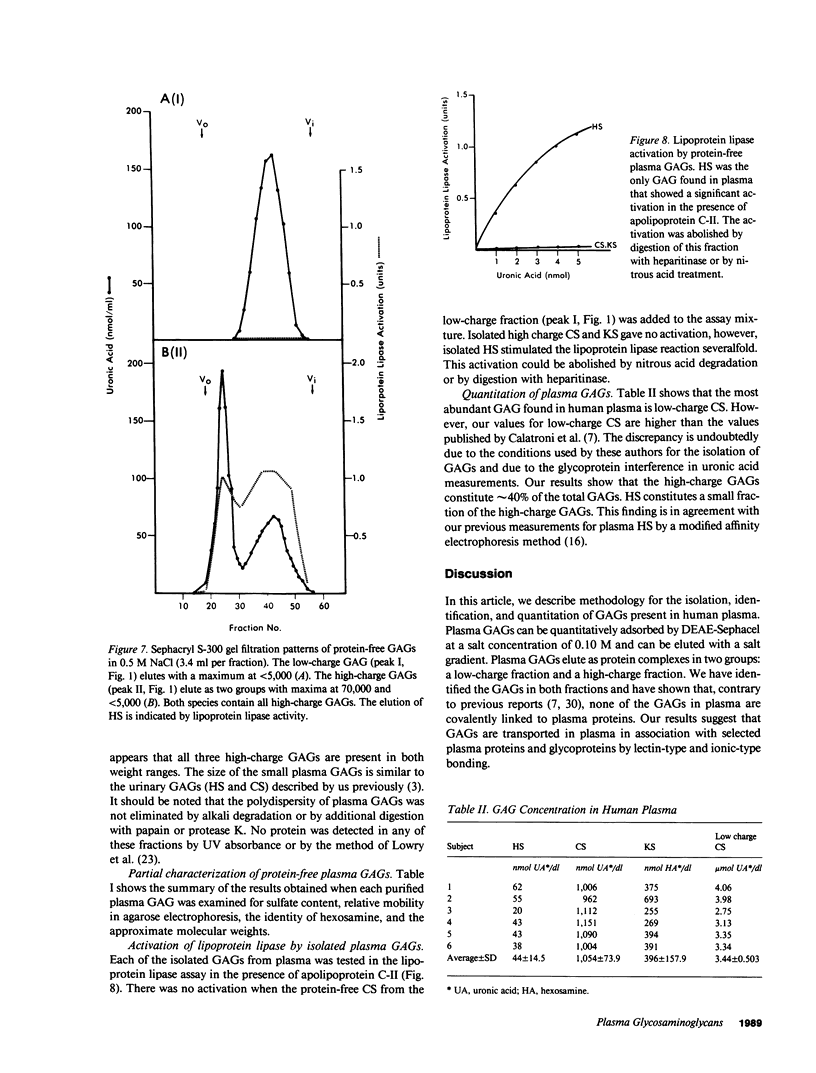
We have described methodology for the isolation and quantitation of glycosaminoglycans present in human plasma. Plasma glycosaminoglycans can be quantitatively adsorbed on a DEAE-Sephacel ion exchanger and eluted with a salt gradient as two groups: a low-charge fraction and a high-charge fraction. The low-charge fraction consists of chondroitin sulfate with a low sulfate content and the high-charge fraction consists of heparan sulfate, chondroitin sulfate, and keratan sulfate (type I). We have determined the plasma concentration of each of these glycosaminoglycans in six normal human subjects. We have established that none of the glycosaminoglycans in plasma are covalently linked to plasma proteins. All are isolated as complexes with plasma proteins in noncovalent linkages. The glycosaminoglycans in the low-charge fraction are bound with high affinity to a single plasma glycoprotein by a lectin-type bond that can be disrupted by a simple glycoside. The high-charge fraction contains three major proteins and several minor proteins associated with the glycosaminoglycans by both lectin-type and ionic bonding. The plasma proteins associated with glycosaminoglycans represent less than 0.5% of the total plasma proteins. Little is known about the physiologic role of the plasma glycosaminoglycans as components of metabolic processes. Because glycosaminoglycans have been implicated in lipid metabolism and atherosclerosis, we tested all of these compounds, isolated in free form, on the in vitro hydrolysis of triglycerides by lipoprotein lipase. Plasma heparan sulfate stimulated the rate of this reaction severalfold. All other plasma glycosaminoglycans were inactive. Thus, plasma heparan sulfate may play an important role in plasma lipoprotein metabolism.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BADIN J., SCHUBERT M., VOURAS M. Plasma polysaccharide fraction containing uronic acid, in normal subjects and in patients with rheumatoid arthritis. J Clin Invest. 1955 Aug;34(8):1317–1323. doi: 10.1172/JCI103178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- BOAS N. F. Method for the determination of hexosamines in tissues. J Biol Chem. 1953 Oct;204(2):553–563. [PubMed] [Google Scholar]
- BOLLET A. J., SERAYDARIAN M. W., SIMPSON W. F. Acid mucopolysaccharides in normal serum. J Clin Invest. 1957 Sep;36(9):1328–1332. doi: 10.1172/JCI103531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B. M., Baylis C., Deen W. M. Transport of molecules across renal glomerular capillaries. Physiol Rev. 1976 Jul;56(3):502–534. doi: 10.1152/physrev.1976.56.3.502. [DOI] [PubMed] [Google Scholar]
- Calatroni A., Donnelly P. V., Di Ferrante N. The glycosaminoglycans of human plasma. J Clin Invest. 1969 Feb;48(2):332–343. doi: 10.1172/JCI105989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOLE V. P. A relation between non-esterified fatty acids in plasma and the metabolism of glucose. J Clin Invest. 1956 Feb;35(2):150–154. doi: 10.1172/JCI103259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felts J. M., Staprans I., Gorman R. A. A glycosaminoglycan activator of lipoprotein lipase in human plasma. Life Sci. 1983 Apr 4;32(14):1659–1664. doi: 10.1016/0024-3205(83)90874-3. [DOI] [PubMed] [Google Scholar]
- Friman C., Juvani M., Skrifvars B. Acid glycosaminoglycans in plasma. II. Findings in rheumatoid arthritis. Scand J Rheumatol. 1977;6(3):177–182. doi: 10.3109/03009747709095445. [DOI] [PubMed] [Google Scholar]
- Horner A. A. Electrophoresis of acidic mucopolysaccharides in agarose gel. Can J Biochem. 1967 Jul;45(7):1009–1013. doi: 10.1139/o67-116. [DOI] [PubMed] [Google Scholar]
- Jansson L., Lindahl U. Evidence for the existence of a multichain proteoglycan of heparan sulphate. Biochem J. 1970 May;117(4):699–702. doi: 10.1042/bj1170699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juvani M., Friman C., Ranta H., Wegelius O. Isolation and characterization of undersulphated chondroitin-4-sulphate from normal human plasma. Biochim Biophys Acta. 1975 Nov 10;411(1):1–10. doi: 10.1016/0304-4165(75)90279-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Li Y. T. Studies on the glycosidases in jack bean meal. I. Isolation and properties of alpha-mannosidase. J Biol Chem. 1967 Dec 10;242(23):5474–5480. [PubMed] [Google Scholar]
- Linker A., Hovingh P. Structural studies of heparitin sulfates. Biochim Biophys Acta. 1975 Apr 7;385(2):324–333. doi: 10.1016/0304-4165(75)90360-8. [DOI] [PubMed] [Google Scholar]
- Martz M. D., Krivis A. F. Thin layer chromatography of hexosamines on copper impregnated sheets. Anal Chem. 1971 May;43(6):790–790. doi: 10.1021/ac60301a038. [DOI] [PubMed] [Google Scholar]
- Murata K., Horiuchi Y. Molecular weight-dependent distribution of acidic glycosaminoglycans in human plasma. Clin Chim Acta. 1977 Feb 15;75(1):59–69. doi: 10.1016/0009-8981(77)90500-9. [DOI] [PubMed] [Google Scholar]
- Nakashima Y., Ferrante N. D., Jackson R. L., Pownall H. J. The interaction of human plasma glycoaminoglycans with plasma lipoproteins. J Biol Chem. 1975 Jul 25;250(14):5386–5392. [PubMed] [Google Scholar]
- ROE J. H. The determination of sugar in blood and spinal fluid with anthrone reagent. J Biol Chem. 1955 Jan;212(1):335–343. [PubMed] [Google Scholar]
- Saito H., Yamagata T., Suzuki S. Enzymatic methods for the determination of small quantities of isomeric chondroitin sulfates. J Biol Chem. 1968 Apr 10;243(7):1536–1542. [PubMed] [Google Scholar]
- Staprans I., Felts J. M., Butts R. J. Quantitative determination of individual glycosaminoglycans in plasma by concanavalin A rocket electrophoresis. Anal Biochem. 1983 Oct 1;134(1):240–244. doi: 10.1016/0003-2697(83)90291-9. [DOI] [PubMed] [Google Scholar]
- Staprans I., Garon S. J., Hopper J., Jr, Felts J. M. Characterization of glycosaminoglycans in urine from patients with nephrotic syndrome and control subjects, and their effects on lipoprotein lipase. Biochim Biophys Acta. 1981 Dec 18;678(3):414–422. doi: 10.1016/0304-4165(81)90123-9. [DOI] [PubMed] [Google Scholar]
- TROUT D. L., ESTES E. H., Jr, FRIEDBERG S. J. Titration of free fatty acids of plasma: a study of current methods and a new modification. J Lipid Res. 1960 Apr;1:199–202. [PubMed] [Google Scholar]
- Terho T. T., Hartiala K. Method for determination of the sulfate content of glycosaminoglycans. Anal Biochem. 1971 Jun;41(2):471–476. doi: 10.1016/0003-2697(71)90167-9. [DOI] [PubMed] [Google Scholar]
- Yamada K., Fujita Y., Shimizu S. The effect of digestion with keratanase (Pseudomonas sp.) on certain histochemical reactions for glycosaminoglycans in cartilaginous and corneal tissues. Histochem J. 1982 Nov;14(6):897–910. doi: 10.1007/BF01005232. [DOI] [PubMed] [Google Scholar]