Abstract
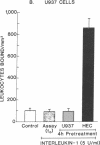
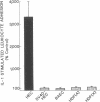
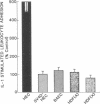
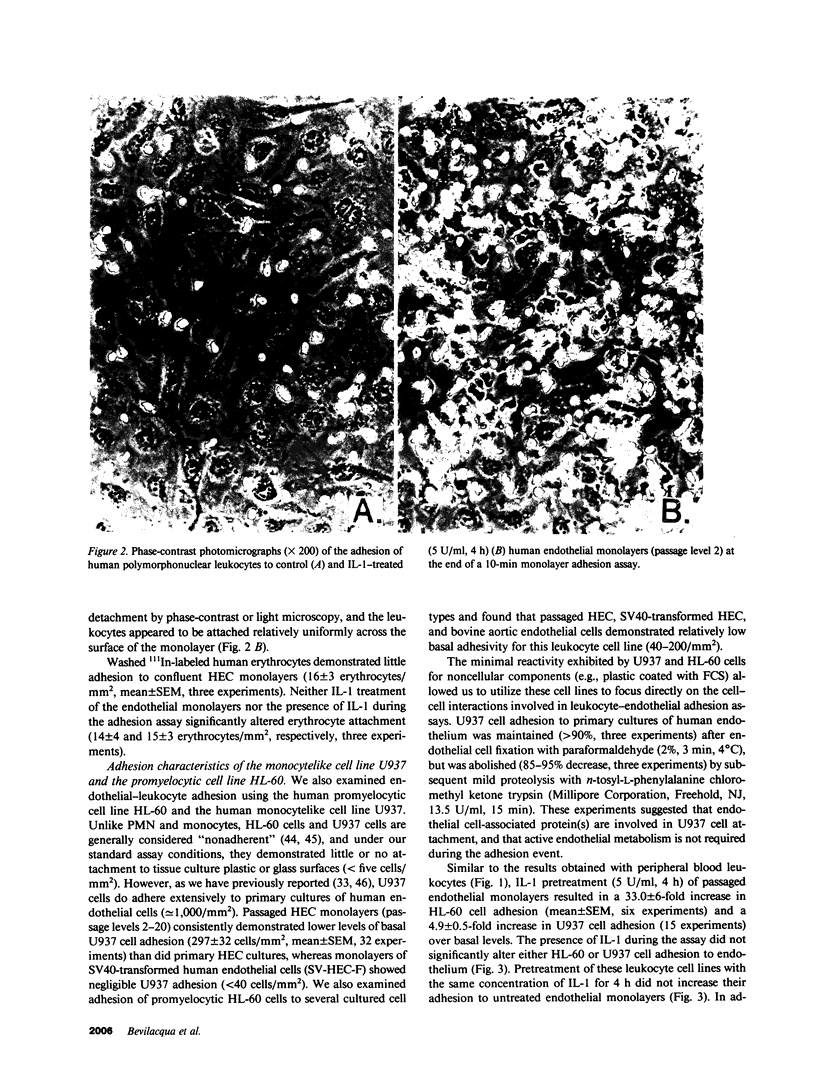
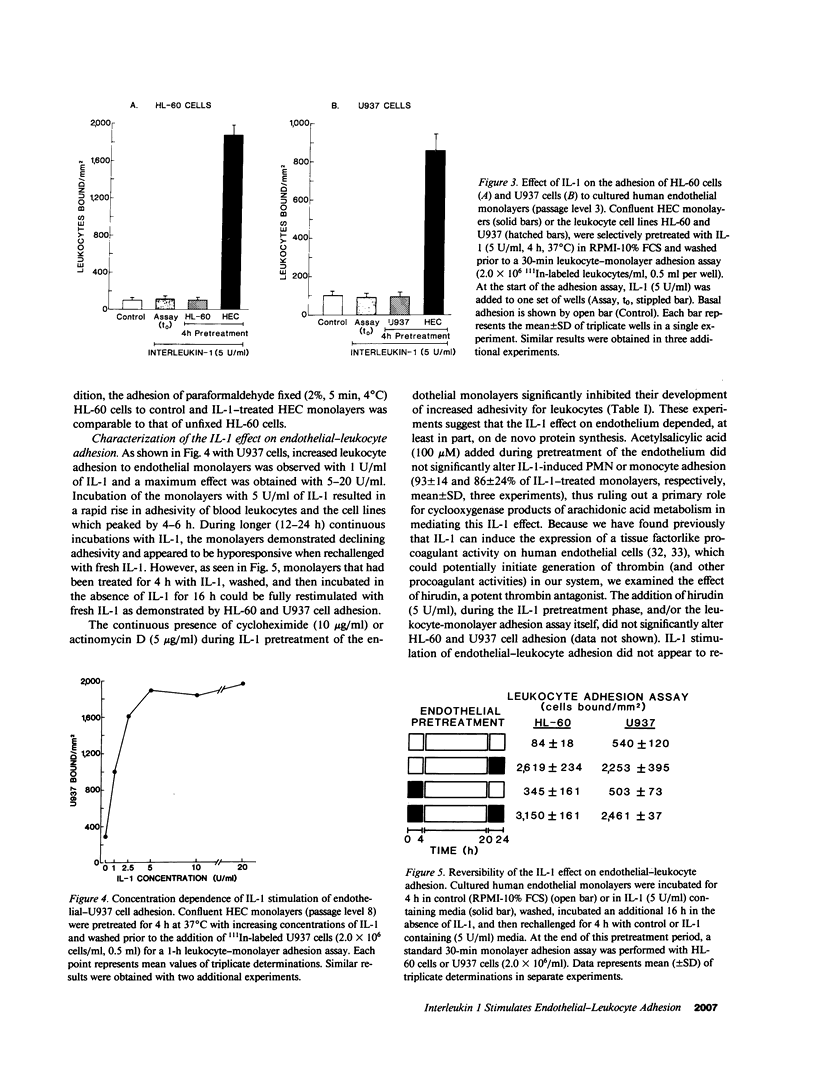
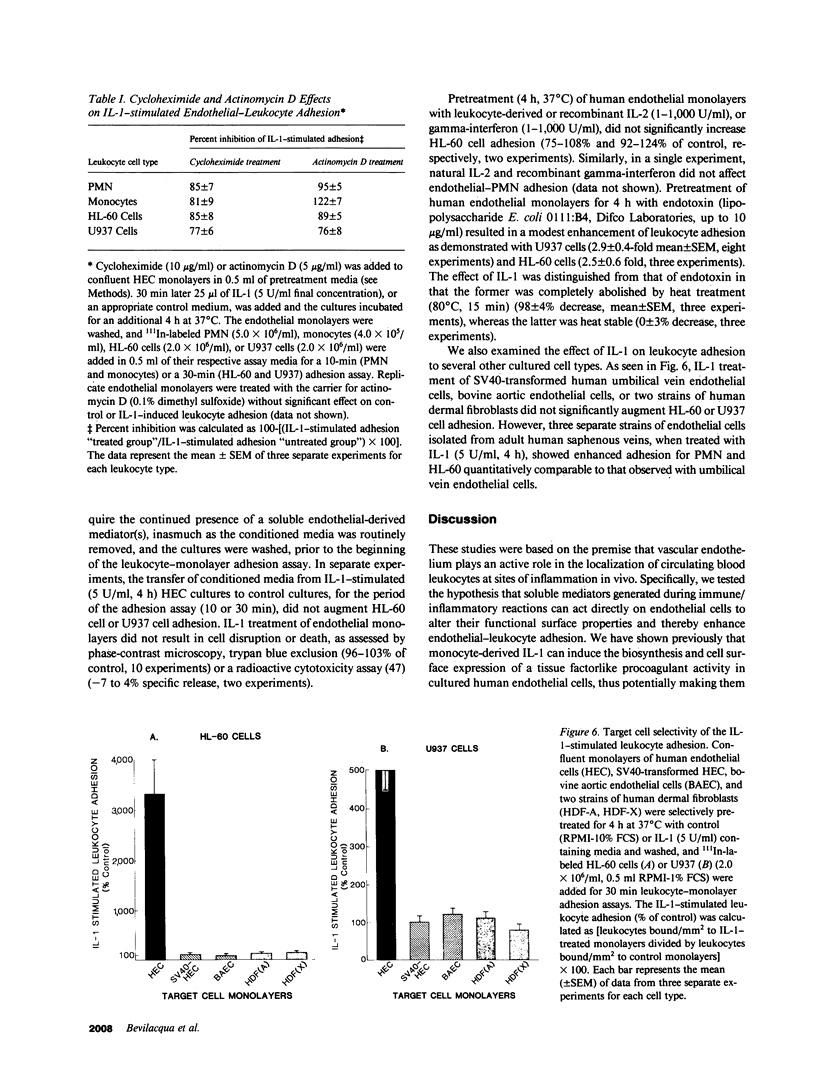
Increased leukocyte adhesion to the endothelial lining of blood vessels is an essential event in inflammation and the pathogenesis of certain vascular diseases. We have studied the effect of interleukin 1 (IL-1), an inflammatory/immune mediator, on endothelial-leukocyte adhesion using quantitative in vitro assays. Selective pretreatment of cultured human umbilical vein endothelial monolayers with IL-1 (5 U/ml, 4 h) resulted in an 18.3 +/- 2.6-fold increase in human peripheral blood polymorphonuclear leukocyte (PMN) adhesion (mean +/- SEM, n = 16) and a 2.6 +/- 0.3-fold increase in monocyte adhesion (n = 7) over basal levels. IL-1-treated endothelial monolayers also supported increased adhesion of the promyelocytic cell line HL-60 and the monocytelike cell line U937 (33.0 +/- 6.0-fold, n = 6 and 4.9 +/- 0.5-fold, n = 15, respectively). In contrast, selective IL-1 pretreatment of leukocytes, or the addition of IL-1 during the adhesion assay, did not alter endothelial-leukocyte adhesion. Conditioned medium from IL-1-treated endothelial cultures also did not promote leukocyte adhesion to untreated monolayers. IL-1 induction of endothelial adhesivity was concentration dependent (maximum, 10 U/ml), time dependent (peak, 4-6 h), and reversible, was blocked by cycloheximide (10 micrograms/ml) or actinomycin D (5 micrograms/ml) but not by acetylsalicylic acid (100 microM), and occurred without detectable endothelial cell damage. IL-1 treatment of SV40-transformed human endothelial cells and dermal fibroblasts did not increase their adhesivity for leukocytes. These data suggest that IL-1 can act selectively on human vascular endothelium to increase its adhesivity for circulating blood leukocytes, and thus to localize leukocyte-vessel wall interactions at sites of inflammation in vivo.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLISON F., Jr, SMITH M. R., WOOD W. B., Jr Studies on the pathogenesis of acute inflammation. I. The inflammatory reaction to thermal injury as observed in the rabbit ear chamber. J Exp Med. 1955 Dec 1;102(6):655–668. doi: 10.1084/jem.102.6.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auron P. E., Webb A. C., Rosenwasser L. J., Mucci S. F., Rich A., Wolff S. M., Dinarello C. A. Nucleotide sequence of human monocyte interleukin 1 precursor cDNA. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7907–7911. doi: 10.1073/pnas.81.24.7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Majeau G. R., Cotran R. S., Gimbrone M. A., Jr Interleukin 1 (IL-1) induces biosynthesis and cell surface expression of procoagulant activity in human vascular endothelial cells. J Exp Med. 1984 Aug 1;160(2):618–623. doi: 10.1084/jem.160.2.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan M. R., Crowley C. A., Rosin R. E., Gimbrone M. A., Jr, Babior B. M. Studies on the interaction between GP-18-0-deficient neutrophils and vascular endothelium. Blood. 1982 Jul;60(1):160–165. [PubMed] [Google Scholar]
- Buchanan M. R., Vazquez M. J., Gimbrone M. A., Jr Arachidonic acid metabolism and the adhesion of human polymorphonuclear leukocytes to cultured vascular endothelial cells. Blood. 1983 Oct;62(4):889–895. [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Charo I. F., Yuen C., Goldstein I. M. Adherence of human polymorphonuclear leukocytes to endothelial monolayers: effects of temperature, divalent cations, and chemotactic factors on the strength of adherence measured with a new centrifugation assay. Blood. 1985 Feb;65(2):473–479. [PubMed] [Google Scholar]
- Chin Y. H., Carey G. D., Woodruff J. J. Lymphocyte recognition of lymph node high endothelium. V. Isolation of adhesion molecules from lysates of rat lymphocytes. J Immunol. 1983 Sep;131(3):1368–1374. [PubMed] [Google Scholar]
- Collins S. J., Gallo R. C., Gallagher R. E. Continuous growth and differentiation of human myeloid leukaemic cells in suspension culture. Nature. 1977 Nov 24;270(5635):347–349. doi: 10.1038/270347a0. [DOI] [PubMed] [Google Scholar]
- Collins T., Krensky A. M., Clayberger C., Fiers W., Gimbrone M. A., Jr, Burakoff S. J., Pober J. S. Human cytolytic T lymphocyte interactions with vascular endothelium and fibroblasts: role of effector and target cell molecules. J Immunol. 1984 Oct;133(4):1878–1884. [PubMed] [Google Scholar]
- Curwen K. D., Kim H. Y., Vazquez M., Handin R. I., Gimbrone M. A., Jr Platelet adhesion to cultured vascular endothelial cells. A quantitative monolayer adhesion assay. J Lab Clin Med. 1982 Sep;100(3):425–436. [PubMed] [Google Scholar]
- Deuel T. F., Senior R. M., Huang J. S., Griffin G. L. Chemotaxis of monocytes and neutrophils to platelet-derived growth factor. J Clin Invest. 1982 Apr;69(4):1046–1049. doi: 10.1172/JCI110509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiCorleto P. E., Bowen-Pope D. F. Cultured endothelial cells produce a platelet-derived growth factor-like protein. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1919–1923. doi: 10.1073/pnas.80.7.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1. Rev Infect Dis. 1984 Jan-Feb;6(1):51–95. doi: 10.1093/clinids/6.1.51. [DOI] [PubMed] [Google Scholar]
- Faggiotto A., Ross R., Harker L. Studies of hypercholesterolemia in the nonhuman primate. I. Changes that lead to fatty streak formation. Arteriosclerosis. 1984 Jul-Aug;4(4):323–340. doi: 10.1161/01.atv.4.4.323. [DOI] [PubMed] [Google Scholar]
- Faggiotto A., Ross R. Studies of hypercholesterolemia in the nonhuman primate. II. Fatty streak conversion to fibrous plaque. Arteriosclerosis. 1984 Jul-Aug;4(4):341–356. doi: 10.1161/01.atv.4.4.341. [DOI] [PubMed] [Google Scholar]
- Gallatin W. M., Weissman I. L., Butcher E. C. A cell-surface molecule involved in organ-specific homing of lymphocytes. Nature. 1983 Jul 7;304(5921):30–34. doi: 10.1038/304030a0. [DOI] [PubMed] [Google Scholar]
- Gerrity R. G. The role of the monocyte in atherogenesis: I. Transition of blood-borne monocytes into foam cells in fatty lesions. Am J Pathol. 1981 May;103(2):181–190. [PMC free article] [PubMed] [Google Scholar]
- Gimbrone M. A., Jr, Brock A. F., Schafer A. I. Leukotriene B4 stimulates polymorphonuclear leukocyte adhesion to cultured vascular endothelial cells. J Clin Invest. 1984 Oct;74(4):1552–1555. doi: 10.1172/JCI111570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimbrone M. A., Jr, Buchanan M. R. Interactions of platelets and leukocytes with vascular endothelium: in vitro studies. Ann N Y Acad Sci. 1982;401:171–183. doi: 10.1111/j.1749-6632.1982.tb25716.x. [DOI] [PubMed] [Google Scholar]
- Gimbrone M. A., Jr Culture of vascular endothelium. Prog Hemost Thromb. 1976;3:1–28. [PubMed] [Google Scholar]
- Gimbrone M. A., Jr, Fareed G. C. Transformation of cultured human vascular endothelium by SV40 DNA. Cell. 1976 Dec;9(4 Pt 2):685–693. doi: 10.1016/0092-8674(76)90132-x. [DOI] [PubMed] [Google Scholar]
- Harlan J. M., Killen P. D., Senecal F. M., Schwartz B. R., Yee E. K., Taylor R. F., Beatty P. G., Price T. H., Ochs H. D. The role of neutrophil membrane glycoprotein GP-150 in neutrophil adherence to endothelium in vitro. Blood. 1985 Jul;66(1):167–178. [PubMed] [Google Scholar]
- Harlan J. M. Leukocyte-endothelial interactions. Blood. 1985 Mar;65(3):513–525. [PubMed] [Google Scholar]
- Harlan J. M., Schwartz B. R., Reidy M. A., Schwartz S. M., Ochs H. D., Harker L. A. Activated neutrophils disrupt endothelial monolayer integrity by an oxygen radical-independent mechanism. Lab Invest. 1985 Feb;52(2):141–150. [PubMed] [Google Scholar]
- Hoover R. L., Folger R., Haering W. A., Ware B. R., Karnovsky M. J. Adhesion of leukocytes to endothelium: roles of divalent cations, surface charge, chemotactic agents and substrate. J Cell Sci. 1980 Oct;45:73–86. doi: 10.1242/jcs.45.1.73. [DOI] [PubMed] [Google Scholar]
- Hoover R. L., Karnovsky M. J., Austen K. F., Corey E. J., Lewis R. A. Leukotriene B4 action on endothelium mediates augmented neutrophil/endothelial adhesion. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2191–2193. doi: 10.1073/pnas.81.7.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauchem J. R., Lopez M., Sprague E. A., Schwartz C. J. Mononuclear cell chemoattractant activity from cultured arterial smooth muscle cells. Exp Mol Pathol. 1982 Oct;37(2):166–174. doi: 10.1016/0014-4800(82)90033-8. [DOI] [PubMed] [Google Scholar]
- Joris I., Zand T., Nunnari J. J., Krolikowski F. J., Majno G. Studies on the pathogenesis of atherosclerosis. I. Adhesion and emigration of mononuclear cells in the aorta of hypercholesterolemic rats. Am J Pathol. 1983 Dec;113(3):341–358. [PMC free article] [PubMed] [Google Scholar]
- Lachman L. B. Human interleukin 1: purification and properties. Fed Proc. 1983 Jun;42(9):2639–2645. [PubMed] [Google Scholar]
- Lomedico P. T., Gubler U., Hellmann C. P., Dukovich M., Giri J. G., Pan Y. C., Collier K., Semionow R., Chua A. O., Mizel S. B. Cloning and expression of murine interleukin-1 cDNA in Escherichia coli. 1984 Nov 29-Dec 5Nature. 312(5993):458–462. doi: 10.1038/312458a0. [DOI] [PubMed] [Google Scholar]
- MARCHESI V. T., FLOREY H. W. Electron micrographic observations on the emigration of leucocytes. Q J Exp Physiol Cogn Med Sci. 1960 Oct;45:343–348. doi: 10.1113/expphysiol.1960.sp001489. [DOI] [PubMed] [Google Scholar]
- Mazzone T., Jensen M., Chait A. Human arterial wall cells secrete factors that are chemotactic for monocytes. Proc Natl Acad Sci U S A. 1983 Aug;80(16):5094–5097. doi: 10.1073/pnas.80.16.5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercandetti A. J., Lane T. A., Colmerauer M. E. Cultured human endothelial cells elaborate neutrophil chemoattractants. J Lab Clin Med. 1984 Sep;104(3):370–380. [PubMed] [Google Scholar]
- Pinkus G. S., Said J. W. Hodgkin's disease, lymphocyte predominance type, nodular--a distinct entity? Unique staining profile for L&H variants of Reed-Sternberg cells defined by monoclonal antibodies to leukocyte common antigen, granulocyte-specific antigen, and B-cell-specific antigen. Am J Pathol. 1985 Jan;118(1):1–6. [PMC free article] [PubMed] [Google Scholar]
- Recalde H. R. A simple method of obtaining monocytes in suspension. J Immunol Methods. 1984 Apr 13;69(1):71–77. doi: 10.1016/0022-1759(84)90278-3. [DOI] [PubMed] [Google Scholar]
- Rosenwasser L. J., Dinarello C. A. Ability of human leukocytic pyrogen to enhance phytohemagglutinin induced murine thymocyte proliferation. Cell Immunol. 1981 Sep 1;63(1):134–142. doi: 10.1016/0008-8749(81)90034-4. [DOI] [PubMed] [Google Scholar]
- Smith R. P., Lackie J. M., Wilkinson P. C. The effects of chemotactic factors on the adhesiveness of rabbit neutrophil granulocytes. Exp Cell Res. 1979 Aug;122(1):169–177. doi: 10.1016/0014-4827(79)90571-8. [DOI] [PubMed] [Google Scholar]
- Springer T. A., Davignon D., Ho M. K., Kürzinger K., Martz E., Sanchez-Madrid F. LFA-1 and Lyt-2,3, molecules associated with T lymphocyte-mediated killing; and Mac-1, an LFA-1 homologue associated with complement receptor function. Immunol Rev. 1982;68:171–195. doi: 10.1111/j.1600-065x.1982.tb01064.x. [DOI] [PubMed] [Google Scholar]
- Sundström C., Nilsson K. Establishment and characterization of a human histiocytic lymphoma cell line (U-937). Int J Cancer. 1976 May 15;17(5):565–577. doi: 10.1002/ijc.2910170504. [DOI] [PubMed] [Google Scholar]
- Thornton S. C., Mueller S. N., Levine E. M. Human endothelial cells: use of heparin in cloning and long-term serial cultivation. Science. 1983 Nov 11;222(4624):623–625. doi: 10.1126/science.6635659. [DOI] [PubMed] [Google Scholar]
- Tonnesen M. G., Smedly L. A., Henson P. M. Neutrophil-endothelial cell interactions. Modulation of neutrophil adhesiveness induced by complement fragments C5a and C5a des arg and formyl-methionyl-leucyl-phenylalanine in vitro. J Clin Invest. 1984 Nov;74(5):1581–1592. doi: 10.1172/JCI111574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente A. J., Fowler S. R., Sprague E. A., Kelley J. L., Suenram C. A., Schwartz C. J. Initial characterization of a peripheral blood mononuclear cell chemoattractant derived from cultured arterial smooth muscle cells. Am J Pathol. 1984 Dec;117(3):409–417. [PMC free article] [PubMed] [Google Scholar]
- Wagner C. R., Vetto R. M., Burger D. R. The mechanism of antigen presentation by endothelial cells. Immunobiology. 1984 Dec;168(3-5):453–469. doi: 10.1016/S0171-2985(84)80130-8. [DOI] [PubMed] [Google Scholar]
- Wilkinson P. C., Lackie J. M. The adhesion, migration and chemotaxis of leucocytes in inflammation. Curr Top Pathol. 1979;68:47–88. doi: 10.1007/978-3-642-67311-5_3. [DOI] [PubMed] [Google Scholar]
- Woloski B. M., Fuller G. M. Identification and partial characterization of hepatocyte-stimulating factor from leukemia cell lines: comparison with interleukin 1. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1443–1447. doi: 10.1073/pnas.82.5.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman G. A., Hill H. R. Inflammatory mediators stimulate granulocyte adherence to cultured human endothelial cells. Thromb Res. 1984 Jul 15;35(2):203–217. doi: 10.1016/0049-3848(84)90215-9. [DOI] [PubMed] [Google Scholar]