Abstract
The muscular dystrophies collectively represent a major health challenge, as few significant treatment options currently exist for any of these disorders. Recent years have witnessed a proliferation of novel approaches to therapy, spanning increased testing of existing and new pharmaceuticals, DNA delivery (both anti-sense oligonucleotides and plasmid DNA), gene therapies and stem cell technologies. While none of these has reached the point of being used in clinical practice, all show promise for being able to impact different types of muscular dystrophies. Our group has focused on developing direct gene replacement strategies to treat recessively inherited forms of muscular dystrophy, particularly Duchenne and Becker muscular dystrophy (DMD/BMD). Both forms of dystrophy are caused by mutations in the dystrophin gene and all cases can in theory be treated by gene replacement using synthetic forms of the dystrophin gene. The major challenges for success of this approach are the development of a suitable gene delivery shuttle, generating a suitable gene expression cassette able to be carries by such a shuttle, and achieving safe and effective delivery. This review summarizes the current state of the art in terms of using adeno-associated viral vectors to deliver synthetic dystrophin genes for the purpose of developing gene therapy for DMD.
Keywords: Adeno-associated viral vector, Duchenne muscular dystrophy, Dystrophin, Gene Therapy, Immune response, Micro-dystrophin, Micro-utrophin
Introduction
Gene therapy for Duchenne muscular dystrophy [or the allelic Becker muscular dystrophy (DMD/BMD)] will require systemic delivery of a functional dystrophin expression cassette to the vast majority of striated (and possibly smooth) muscle cells of the human body. A major challenge is how to achieve such widespread delivery, but recent studies suggest that vectors derived from adeno-associated viruses may be suitable for this goal. However, AAV vectors have a limited cloning capacity, precluding delivery of the endogenous 2.1 MB dystrophin gene. The development of synthetic micro-dystrophin cDNAs regulated by muscle-specific promoter/enhancer cassettes show promise in overcoming this obstacle. The major barrier to applying these approaches in a therapeutic setting is the need to develop approaches to deliver these AAV/micro-dystrophin vectors systemically to patients without toxicity or elicitation of a harmful immune response against vector or dystrophin. In this article we summarize the key features related to development of this promising approach to therapy.
Mammalian Models of DMD
The extensive number of animal models that have been developed over the years supersede the scope of this review. However, we summarize here a number of the more commonly used models, which serves to give a general overview of the value of animal models of DMD for research and therapeutic development. Animal models for DMD have been indispensible for providing a more thorough understanding of disease progression, pathogenesis, and in evaluating new therapies. The most commonly used model of DMD is the mdx mouse which display some features of muscle degeneration but overall, with the exception of diaphragm, presents with a milder phenotype relative to the disease pathogenesis in humans. The mdx mouse contains a premature stop codon in exon 23 that leads to loss of full-length dystrophin, although smaller isoforms are still expressed [32; 86; 158]. Histological examination reveals that muscle fiber necrosis and cellular infiltration initiates at approximately 3 weeks of age. Shortly to follow, a ‘crises ‘period ensues that peaks at approximately 4–6 weeks of age and is characterized by the presence of extensive necrosis, regenerating muscle fibers with centrally located nuclei, and elevated levels of serum creatine kinase [32]. At approximately 12 weeks of age the cycles of necrosis and regeneration begin to dampen, though necrotic myofibers exist for the remainder of the mouse lifespan [37]. Four additional strains, mdx2cv–5cv, have been generated with chemical mutagenesis, resulting in point mutations which leads to loss of full-length dystrophin isoforms [39]. All five of these strains display nearly identical muscle pathology, though the mdx4cv and mdx5cv strains display a low background of revertent dystrophin-containing fibers, making them well suited in gene transfer studies exploring the feasibility of DMD therapy. An additional mutant mouse known as mdx52 was generated by a homologous recombination technique [6]. In this model, disruption of exon 52 of the dystrophin gene occurs, and dystrophin along with shorter dystrophin isoforms (Dp140 and Dp260) are ablated. This exon was targeted to replicate the most common out-of-frame deletion mutation that occurs in 13% of DMD patients [1; 80]. Recently, antisense-mediated skipping of exon 51 using antisense oligonucleotides (AOs) to enable restoration of the dystrophin mRNA open reading frame was found to be successful in some patients with DMD [35; 41; 68; 173]. Exon 51 skipping using AO in mdx52 mice successfully converted an out-of-frame mutation into an in-frame mutation, resulting in amelioration of the dystrophic pathology and improved muscle function [5]. In an effort to change the mdx muscle phenotype to one more similar to that of patients, several additional mutations have been crossed onto the mdx background to generate double knockout mice. The most widely used is a dystrophin:utrophin (mdx:utrn−/−) mouse strain that displays an earlier onset of dystrophy, more extensive muscle wasting, significant weight loss, joint contractures, and a considerably shortened lifespan [49; 71]. The severity of the phenotype supports the concept that utrophin upregulation in dystrophic muscles partially compensates for the absence of dystrophin, but one should also keep in mind the discrete function of utrophin. An mdx:telomerase double knockout has also been described recently, which after 2 generations on the telomerase-deficient background display s a severe muscle wasting phenotype and somewhat shortened lifespan [36; 150]. With regard to large animal models, spontaneous mutations causing dystrophinopathy have been identified in several breeds of dog [43; 152; 159; 172]. To date, the canine X-linked muscular dystrophy (cxmd) model is the most ideal representation of DMD in terms of disease progression and muscle pathology. The clinical manifestations of GRMD are progressive with muscle degeneration, necrosis, fibrosis, gradual loss of muscle mass, and formation of contractures that often lead to skeletal deformities. Similar to patients, respiratory failure or cardiomyopathy are frequently observed. The CXMD harbors a point mutation within the splicing acceptor site within intron 6, causing exon 7 to be skipped, resulting in a frameshift of downstream sequence, which brings a premature stop codon within exon 8 to be in frame. It should be recognized that the phenotype of the most widely used golden retriever (GRMD) model can be highly variable, making functional endpoints difficult to ascertain. Multiple colonies of the GRMD have been created but it is not the only species available. Due to previous use in research and its smaller size, the GRMD mutation has been bred onto the beagle background [157], where numerous studies have been conducted to eludicate pathogenesis and the development of therapeutic approaches with this model. Overall, even though each mammalian model may have limitations, they have been critical for the development of several treatment strategies for DMD.
Adeno-associated viruses
Human adeno-associated virus (AAV) was first discovered as a contaminant of adenovirus cultures in 1965 [11]. AAV is a small, non-enveloped icosahedral shaped virus ~22 nm in diameter [191], within which is encapsidated a linear, single-stranded DNA genome that is ~4.7 kb long. AAV belongs to the family Parvoviridae and is placed in the genus Dependovirus because infection by AAV occurs only in the presence of a helper virus, such as adenovirus or herpesvirus. AAV has not been associated to any human illness, and because of its wide range of infectivity and ability to establish long-term transgene expression, AAV has rapidly gained popularity in gene therapy applications.
The AAV genome is made up of two open reading frames (ORF) known as rep and cap, which are flanked by two 145-bp inverted terminal repeats (ITRs) (Figure 1A). The rep ORF (located on the 5’-half of the AAV genome) codes for the proteins necessary for replication and packaging, i.e. non-structural proteins, while the cap ORF codes for three capsid proteins (VP1, VP2, VP3), i.e. the structural or virion proteins, and is located on the 3’-half of the AAV genome.
Figure 1.
Schematic of A) the structure of adeno-associated virus (AAV) and B) AAV DNA replication. Rep and cap genes are flanked by inverted terminal repeats (ITR) and are transcribed by the promoters p5, p19 (rep) and p40 (cap) to produce the Rep proteins and viral capsid proteins, respectively. The ITRs (shown enlarged in A) serves as the origin of replication and is composed of two arm palindromes (B-B’) and (C-C’) embedded in a larger stem palindrome (A-A’). The ITR also contains the Rep-binding element (RBE), which consists of a tetranucleotide repeat with the consensus sequence 5’-GNGC-3’. The Rep78 and Rep68 proteins bind to the ITR; the DNA helicase activities of the Rep proteins remodel the A-A’ region to generate a stem-loop, allowing for ITR primed DNA polymerization, while the strand- and site-specific endonuclease catalytic domain of the Rep proteins introduces a nick at the terminal resolution site (trs). An additional RBE (RBE’) at the apex of the T-shaped structure stabilizes the association between the Rep proteins and the ITR.
The rep ORF is regulated by the 5’-terminus promoter (p5) and an internal promoter (p19). Both p5 and p19 transcripts utilize a common splice donor and acceptor, which results in two mRNAs from each promoter. The p5 promoter produces the larger of the Rep proteins (Rep78, Rep68), while the smaller Rep proteins (Rep52, Rep40) are produced from transcripts using the p19 promoter. All four Rep proteins possess helicase and ATPase activity necessary for DNA replication [21; 42; 85; 160], but one p5 and one p19 protein alone are sufficient for AAV production.
The cap genes encode for VP1, 2 and 3 and are regulated by the p40 promoter. Alternative splicing of the p40 transcript results in the three viral proteins from two transcripts due to the presence of two splice acceptors: VP1 (87 kDa) is produced from the unspliced transcript, while the spliced transcript produces VP2 (72 kDa) and VP3 (62 kDa). The start codon for VP3 is produced using the more downstream, conventional start codon (AUG), while VP2 is produced using the non-canonical start codon (ACG). As most ribosomes scan the mRNA until encountering the first AUG, this accounts for the relative over-abundance of VP3. In AAV serotype 2 (AAV2), the capsid proteins are present in a 1:1:10 molar ratio.
The ITRs that flank the two rep and cap ORFs are the only parts of the genome that must be maintained for vector production. The first 125 nucleotides of the ITR constitute a palindrome that allows it to elegantly fold back upon itself to form a T-shaped hairpin structure. The remaining 20 bases are unpaired and form the D-sequence. The ITRs are important cis-active sequences in AAV biology and serves as the origin of replication and as a primer for second-strand synthesis by DNA polymerase present within the cell.
Critical to the replication process are the Rep binding elements (RBEs) and the terminal resolution site (TRS), which are located within the ITR. Rep78 and Rep68 proteins participate in the AAV DNA replication process via binding at the RBE and by strand- and site-specific ‘nicking’ at the TRS [29; 161]. Site-specific endonuclease activity by the Rep proteins requires the presence of the secondary structure element, as well as a specific sequence at the TRS [161].
Serotypes
To date, numerous AAV serotypes and over 110 new AAV isolates have been acquired from adenovirus stocks or from human and non-human primate tissues [60; 62; 148; 153]. AAV serotypes share a common genome structure but show variation in cell and tissue tropism due to differences in their capsid proteins that lead to recognition by different cell surface receptors. (This will be described in greater detail in the following section).
By definition, a serotype is a newly isolated virus that does not efficiently cross-react with neutralizing sera specific for all other existing and characterized serotype. Under this definition, only AAV1-AAV5, and AAV7-AAV9 can be defined as real serotypes. AAV6, 10 and 11 do not fit into this definition, since the serology of AAV6 is similar to that of AAV1 [60; 62; 76], while AAV10 and 11 are not well characterized [122]. AAV6 is thought to be a hybrid recombinant between AAV1 and 2 since its left ITR and p5 promoter are virtually identical to those of AAV2, while the rest of its genome is nearly identical to AAV1 [148; 189].
At present, a consensus of opinion has not been reached in regards to which serotype shows the best transduction efficiency for which tissue. However, a number of studies have shown that high transduction efficiency in striated muscle can be achieved with AAV1, 6, 7, 8 and 9 [38; 62; 74; 87; 124; 129; 130; 179; 196; 197], which will be advantageous in the treatment of DMD.
AAV Biology
AAV vectors replicate and infect target cells through a complex series of processes (for detailed review, see [154]). The process of AAV DNA replication is illustrated in Figure 1B. Briefly, DNA replication is initiated at the 3’ hairpin primer and leads to the formation of linear double-stranded molecules that terminate by site-specific nicking at the TRS by Rep proteins [20]. The newly generated 3’ hydroxyl groups then provide a substrate for Rep-mediated unwinding of the terminal hairpin, allowing for the ITR to be copied. Finally, the linear duplex termini renaturate into terminal hairpins, putting the 3’ hydroxyl groups in position for single-strand displacement synthesis. The smaller Rep proteins Rep52 and Rep40 are then involved in the accumulation of single-stranded viral DNA for packaging within AAV capsids.
AAV2 virions utilize heparin sulfate proteoglycans as primary attachment receptors [165], and internalization is aided by co-receptors such as αvβ5-integrin heterodimers [164], fibroblast growth factor receptor type 1 [141], the hepatocyte growth factor receptor c-Met [92] as well as α5β1-integrin [10]. In addition, the 37/67 kDa laminin receptor has also been shown to play a role in transduction by AAV2, 3, 8 and 9 [3]. AAV4 and AAV5 have been found to bind to charged carbohydrate moieties in the form of O- and N-linked sialic acids, respectively [91]; AAV5 also uses platelet-derived growth factor receptor as another cellular determinant for infection [50]. Similarly, AAV1, AAV5 and AAV6 also utilize N-linked sialic acid for transduction, though for AAV6 this was dependent on the target cells tested [155; 188]. Recently, comparative gene analysis also identified a positive correlation between AAV6 transduction and epidermal growth factor receptor (EFGR) expression, suggesting that EGFR may also act as a co-receptor for AAV6 [183].
Upon cellular surface binding, AAV is thought to be endocytosed into the cells primarily via clathrin-coated pits [18; 52], and are actively transported along microtubules and microfilaments [156]. Internalized virions then escape from endosomes into the cytosol by a low pH-dependent process [156]. The conserved phospholipase A2 motif in the N terminus of the VP1 protein also play a crucial role during AAV trafficking in maintaining capsid stability [25]. Moreover, there is evidence to suggest that endosomal cysteine proteases, cathepsins B and L, may also participate in AAV trafficking and capsid disassembly in a serotype-specific manner [4]. Specific inhibition of cathepsins decreases AAV2 and AAV8 transduction in a dose-dependent manner [4], suggesting that endosomal processing of capsids may be important for downstream events such as viral uncoating and nuclear entry.
Upon entering the nucleus, AAV particles shed their viral coating and the AAV genome becomes double-stranded in order to be transcribed. This is achieved either by second strand DNA synthesis from the hairpin at the 3’ end of the genome, or via annealing of single-stranded molecules with opposing polarities [125]. These genomes first appear as monomer circular intermediates, but over time, there is a decline in these small circular genomes in favor of high-molecular-weight circular concatamers [53; 192]. In this organization, the AAV genome can stably persist as episomes or be integrated [192] into the host cell genome. In cultured cells wild-type AAV can integrate into a specific site of human chromosome 19 (19q13.3q-ter) called AAVS1 [101], and remains latent unless the host cell is co-infected by a helper virus. It remains unclear whether this site-specific integration can also occur in vivo. The rate of viral uncoating is thought to be the rate-limiting step of transduction, and is serotype and cell-specific.
Recombinant AAV vectors
Recombinant AAV vectors (rAAV) contain only the AAV 145bp ITRs flanking the cassette with the transgene of interest and its promoter. All the replication and cap genes are removed and are provided by a packaging plasmid to grow vectors [134]. The loss of these genes makes rAAV vectors inefficient at integrating their genome into that of the host cell, therefore the engineered vector genome exists as a plasmid inside the nucleus (episomal chromatin). The presence of ITRs at each end of the AAV genome allows the plasmid to form supercoiled monomers and concatemers [132], which are very stable and hence accounts for their persistence in the host cell. Vectors can be retained for years in non-dividing cells, but are rapidly lost during mitosis.
Systemic delivery approaches
Since Duchenne muscular dystrophy affects the heart and all skeletal muscles, and to a lesser degree some smooth muscles, the goal in gene replacement therapies is to target all striated muscles in the body by a route of administration that is least likely to elicit an immune response and which is translatable to the clinic. To facilitate targeting of the therapy, much research is currently focused on identifying the AAV serotype with highest striated muscle tropism [87; 129; 136; 179]. Systemic delivery of rAAV6/micro-dystrophins (see below) into neonatal or adult mdx mice (a mouse model for DMD) via intravenous injection had been effective in achieving body-wide transduction; a dose of 1×1013 vector genomes was sufficient to result in truncated dystrophin expression throughout the limb and torso muscles at levels comparable to that found in wild-type mice [74]. Similarly, intra-arterial infusion via the femoral artery also resulted in widespread expression in skeletal muscle in mice [59]. Body-wide transduction in rodents using AAV8 and AAV9 carrying reporter genes has also been demonstrated [66; 129; 179].
In contrast, systemic delivery into canine models by a single intravascular infusion has proved to be a greater challenge. Firstly, due to the larger size of the animal, there is a need for much higher quantities of vector. This daunting task of generating sufficient vector has been compensated in part by the ability to scale up rAAV production by drastically increasing adherent cell cultivation using cell culture plates or roller bottles with large surface areas. For example, a 100 layered CellCube® unit typically has the potential to generate >1010 cells and yielding 1014–1015 rAAV particles [100]. Another hurdle to successful systemic delivery into large animal models is the heightened immune response to viral vectors resulting in lack of transgene expression [75; 99]. Transient immune suppression has been effective in suppressing immune responses to rAAV vectors administered dogs, leading to long-term gene expression [177; 178]. The immunological challenges to systemic gene therapy are addressed in greater detail later in this review. Given the many challenges still existing, current clinical trials have not progressed beyond phase I, and much of the gene delivery is via direct intramuscular or regional intravenous routes to determine the efficacy of the AAV cassette in delivering the transgene to muscle and to assess the presence of immune response [27; 82; 113–115].
Micro-dystrophins in rAAV-mediated gene therapy
Gene replacement therapies for DMD are conceptually simple since the disorder is inherited in a recessive X-linked manner where the dystrophin gene is typically not expressed in males[48; 83; 102; 103; 143]. Afflicted patients suffer from progressive muscle impairment, resulting in a shortened lifespan. In the case of the milder disorder Becker muscular dystrophy, deletion mutations in the dystrophin gene can result in the expression of truncated, yet partially functional, dystrophin [19; 69; 94]. This observation led to the idea of using truncated dystrophins for therapeutic use, which has already enabled body-wide amelioration of the dystrophic phenotype in mdx mice via rAAV vector-mediated gene transfer [24]. The limited rAAV vector carrying capacity requires the use of truncated dystrophins that conserve crucial functional domains. Therefore, splicing ~14 Kb of coding DNA down to ~5 Kb stresses the diligence needed in designing micro-dystrophin constructs [24; 96]. Their optimization requires not only specific goals for improvement of transduced muscles, but also a sufficiently sensitive testing system.
The full-length dystrophin isoform, found in neurons, cardiac and skeletal muscle, has four major domains that allow dystrophin to mediate a link between the cytoskeleton and a protein complex situated at the sarcolemma [95; 186]. The N-terminal region has three sub-domains that bind to F-actin [56; 81; 131; 181]. The rod domain is the largest and has 24 repeats that share homology with spectrin family proteins. Two hinges flank the rod domain, and two others are interspersed among the spectrin-like repeats (SRs). Collectively, the central rod offers the flexibility and elasticity that is crucial for dystrophin mechanical function [186]. Among the repeats are distinct binding domains that direct interaction with multiple proteins, including F-actin[149], the scaffolding protein dystrobrevin[116], and the signaling protein neuronal nitric oxide synthase (nNOS)[7; 28]. The cysteine-rich and C-terminal domains are required for assembly, stabilization, and signaling of the full dystrophin-glycoprotein complex (DGC), and facilitate linkage of the actin cytoskeleton to the sarcolemma [23; 88]. The role of dystrophin is believed to be primarily structural, transmitting contractile forces through the sarcolemma to the extracellular matrix. In doing so, fibers can generate contractile forces while remaining protected from contraction-induced injury. Stability of the sarcolemma is greatly improved by cooperative binding of dystrophin and the multiple components of the DGC [33]. Various signaling functions are also conferred by the DGC, but to date only the aforementioned NOS generation has been well characterized.
Several labs have explored the functionality of micro-dystrophins encoded by various deletions within the dystrophin coding sequence. They have also demonstrated that engineered constructs can be small enough to be packaged within the rAAV capsid while maintaining crucial functional domains. A continuous connection between the cytoskeletal actin filaments to the integral membrane protein β-dystroglycan is an essential feature of any potential construct for both the stability of the protein and to provide mechanical function and enable assembly of the DGC [46; 63; 72; 78]. Truncation of the N-terminal actin-binding domain severely disrupts the stability and function of dystrophin [14; 45; 180]. However, the C-terminal domain has been found to be nonessential in properly localizing the majority of the DGC. Analysis of the correlation between deletions and phenotypic severity of BMD patients revealed that deletion of the entire C-terminal region results in a mild phenotype [112]. Transgenic mdx mice expressing a C-terminal truncated dystrophin displayed no obvious impairment of DGC assembly, muscle morphology or muscle mechanics [47; 54; 70]. However, further truncation within the dystroglycan-binding domain completely ablated stable expression and localization of dystrophin [19; 45; 94]. Therefore, the amino-terminal and cysteine-rich domains are necessary for generation of a functional dystrophin.
Analysis of various in-frame deletions in the rod domain revealed a high degree of functionality and stability even when spanning several SRs and internal hinges [78]. These studies suggest that, secondarily to the C-terminal domain, the central rod may be the most tolerant region for deletions. However, there is a correlation between the number of SRs and the degree of functionality in skeletal muscle. Constructs with less than four SRs exhibit minimal functionality, at best, while those with four or more exhibit high functionality [78; 175]. Transgenic dystrophic lines expressing dystrophins with eight SRs (mini-dystrophins) restore all the DGC components and increase specific force to near wild-type levels [78]. Other studies have allocated the binding domains for other structural proteins within the rod domain including microtubules [139], nNOS [104], and synemin [22]. Apart from the previously mentioned interactions, keratin 19 and plectin have been shown to interact with dystrophin at the N- and C-terminal, respectively [145; 163]. With all these domains spanning across the entire gene, the design of micro-dystrophins must find a balance between conserving the critical functional domains, recruiting associated proteins, and maintaining the structural stability of micro-dystrophin itself as they are alternatively spliced and truncated.
Despite efforts to increase the packaging capacity of AAV capsids, the carrying capacity of these vectors has remained limited to approximately 5 Kb. This space must incorporate a promoter, a polyadenylation signal, and a transgene between the ITRs of the recombinant AAV genome. The first two factors have been successfully reduced in size without dramatically compromising the efficacy of expression activity, allowing the micro-dystrophin encoding portion to increase in size [8 in press]. However, larger (mini-) dystrophin constructs that span beyond the capacity of a single rAAV genome vector can be split amongst two that overlap in order to recombine in vivo [126]. This study highlighted the advantage of delivering larger constructs by rAAV recombination with significant improvements in the mdx phenotype. However, it was noted that this method was confronted with two uncontrollable variables; undesirable concatemerization of the constructs and the inability to ensure that each nuclei received both halves of the split mini-dystrophin gene.
The daunting task of systemically delivering a therapeutic gene to striated muscle has been compensated in part by the ability to scale up rAAV production on an industrial scale. A multi-plasmid transfection of either HEK 293 cells or baculovirus insect (Sf9) cells allows the capsid proteins of a particular serotype to be expressed while allowing the vector genome to be processed for packaging into the rAAV capsid. Purification strategies for striated muscle-tropic serotypes typically involve affinity and/or ion exchange chromatography. For increased purification, rAAV can be isolated by size-exclusion chromatography or gradient centrifugation [100]. Although a critical threshold of vector genomes is required to achieve transduction [74], this dosage could be decreased by codon-optimization of the transgene. This principle has been addressed by codon-optimizing a previously established micro-dystrophin [78] (See “Hinge 2 micro-dystrophin” in Figure 2), along with a novel construct [97], and delivering into mdx mice.
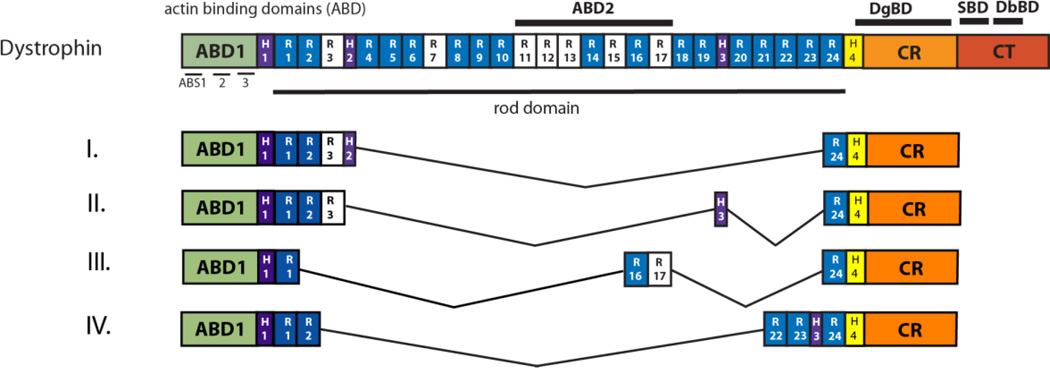
Figure 2.
Structure of dystrophin. The full length coding sequence of dystrophin can be spliced down to approximately a third of its original size to yield a highly truncated, yet functional, micro-dystrophin that could be delivered by rAAV. This was first shown with the “Hinge 2 micro-dystrophin” (I) [73; 78]. This construct was refined to create “Hinge 3 micro-dystrophin” (II) in order to correct abnormalities seen under closer examination [13; 15]. The binding domain for signaling molecule nNOS was allocated and incorporated into micro-dystrophin designs (III), resulting in improved muscle performance [15; 104]. A five spectrin-like repeat construct (IV) was used in a clinical trial; its functionality remained inconclusive due to immune responses in patients [113].
Although high expression and improvement in several dystrophic aspects were observed, no direct comparison of this exact construct was made between codon-optimized and non-codon-optimized dystrophins [58]. Additionally, it is difficult to compare the results of this experiment with those of similar experiments due to differences in AAV serotype, the dystrophic mouse model used, and the age of treatment. It is not yet clear if codon-optimizing constructs will lead to significant differences in expression, but future studies should address this issue.
It is important to recognize that improvement of micro-dystrophin design is an on-going story in DMD research and creating novel junctions in the rod domain may have adverse effects that are not obvious. An instance is the creation of the R4–23/ΔCT construct (“Hinge 2 micro-dystrophin”) discussed above. Morphology data from cross-sections of the muscle belly were encouraging, along with the increased physiological performance compared to untreated mdx mice. However, electron microscopy at the myotendinous junctions revealed that this particular micro-dystrophin was causing an abnormal formation of ringbinden, where a subset of muscle myofibrils reorient themselves orthogonally to the rest of the muscle and encircle the muscle [13]. Though this was found to reduce susceptibility to contraction-induced injury, the ringbinden was also obstructing muscle growth. The internal hinge 2 was replaced with hinge 3 in a novel micro-dystrophin (See Figure 2) and no ringbinden developed in “hinge 3 micro-dystrophin” rAAV-treated mdx mice. It is suspected that the higher amount of proline amino acids in hinge 2, compared to hinge 3, was the cause of the ringbinden as well as the alteration of neuromuscular synapse structure [12; 15]. This is an additional concern that must be addressed in proposed therapeutic constructs.
Apart from the design of micro-dystrophins, the choice and production method of the rAAV serotype, the amount of vector genomes delivered, which mouse model to use, variable methods of delivery, time of evaluation, and assays used to monitor function are all important parameters that must be clearly in order to create a rubric with the intention on improving existing and future constructs. It is expected that deviation will occur as improvements in one or more parameters are made, yet it must be recognized that even one variable can lead to dramatically different results and add ambiguity to the field. One additional parameter that heavily influences the outcome of assessing micro-dystrophins is the animal age of treatment. For example, treating mdx mice before or after the onset of the dystrophic phenotype with rAAV-micro-dystrophin vectors results in different morphological outcome as shown with the degree of degeneration/regeneration (centrally nucleated fibers) [15; 73; 175].
Immunological considerations for rAAV gene therapy
Evading an immune response will be critical for safe vector administration and robust transgene expression in the gene therapy setting. While rAAV has minimal immunogenicity compared with other vectors such as adenovirus [195], both AAV capsid proteins and the delivered transgene have the potential to incite immunotoxicity in target tissues, thereby reducing or eliminating transgene expression. Initial studies in immunocompetent mice found no cellular immune responses to viral capsid proteins or the transgene [57; 93; 190], with robust and long-term expression of the delivered transgene, in contrast with immune clearance of transduced cells following adenoviral delivery [90]. Phase I clinical trials using rAAV2 also reported relative safety, with no adverse events after intramuscular injections, and pre-existing antibodies to the serotype having little effect on myofiber transduction [108]. However, it has since become clear that in many settings the immune response can strongly diminish the efficacy of AAV-mediated gene delivery.
Recent studies have found cytotoxic T cell and humoral immune responses to the viral capsid of various AAV serotypes in murine as well as canine models, nonhuman primates, and humans [40; 77; 89; 107; 109; 117; 119; 146; 176]. In addition, the magnitude of muscle-specific capsid T cell response in humans appears dose-dependent [118]. Transient immune suppression might avoid a cytotoxic T cell response by allowing time for clearance of viral capsids from transduced cells and professional antigen presenting cells. It may also prevent activation of CD4+ T cells involved in stimulating the development of neutralizing antibodies to capsid proteins [144]. The presence of neutralizing antibodies to capsid, whether from prior administration or natural infection [26], can diminish transgene expression, though in some cases may not entirely prevent transduction [9; 30; 40; 57; 89; 107; 123; 146]. Several studies have shown that transient immune suppression during the initial vector administration effectively allows readministration in murine and canine models as well as nonhuman primates. Methods include blocking T cell activation with antibodies that interfere with immune priming [40; 77; 107] and chemical suppressants such as cyclosporine, tacrolimus, and mycophenolate mofetil [89; 176]. These methods may prevent formation of neutralizing antibodies, but additional strategies are required to evade pre-existing immunity. One example is the use of alternative serotypes for readministration [107; 146]. Furthermore, capsid engineering has generated useful variants through rational design, for example to avoid immunogenic epitopes, and directed evolution, where diverse libraries can be screened for immunological advantages [17; 106; 123; 133]. Other approaches for evading pre-existing immunity include the application of biomaterials to shield antibody recognition of capsid proteins and plasmapheresis to reduce the presence of neutralizing factors [16; 121].
Gene therapy for DMD is further complicated by an immune response against dystrophin. Transgenes delivered via AAV undergo MHC class I processing and surface presentation [30], and can therefore be detected by immune effector cells. Furthermore, several studies have identified dystrophin as a neoantigen [67; 84; 128; 193], either through transduced cell surface presentation or release from degenerating muscle fibers and contact with antigen presenting cells [111; 184]. CD4+ and CD8+ T cell responses to truncated dystrophin were observed in a subset of patients following intramuscular delivery of rAAV in a recent clinical trial, as well as the unexpected discovery of dystrophin-specific T cells prior to vector administration [113]. Delivery route can also affect the immune response [30], and intravascular administration, in addition to the advantage of reaching all striated muscle [74], appears to reduce transgene immunogenicity compared to intramuscular delivery [118; 147; 171]. Blocking more than one pathway involved in activation of a cytotoxic T cell response against transgene-carrying cells encourages tolerance and has shown promise for AAV-mediated delivery to muscle [2].
The many strategies under development to reduce immunotoxicity improve the clinical potential of rAAV-mediated gene therapy. Considering the complexity and wide range of immune responses to AAV-mediated gene delivery observed within and between species [61; 178], translational studies in nonhuman primates and humans that include immunomodulation should undergo careful design and evaluation. Efficient targeting of vectors to muscle or restricting expression to muscle should further limit the immune response. Using AAV serotypes with natural tropism for muscle [74; 87; 179; 194] lowers off-target transduction, while muscle specific promoters reduce ectopic expression in cells that can be involved in T cell priming [44; 79; 90; 98; 111; 177; 193]. Another recent strategy for restricting transgene expression to desired tissues is to use microRNAs to target the transgene itself via post-transcriptional silencing in off-target tissues [31; 65]. MicroRNAs can be incorporated into the 3’UTRs of transgene cassettes and have effectively reduced off-target transgene expression in the liver [64; 140]. This is an important finding considering that an effective therapy may depend on systemic delivery, where a range of tissues become exposed to the therapeutic transgene. Finally, a promising alternative for avoiding the immune response to dystrophin is to either upregulate the utrophin gene or deliver a truncated utrophin (see below)[127]. As a highly similar protein in structure and function, utrophin has the potential to compensate for the loss of dystrophin [110; 120; 182; 187].
Micro-utrophins
Utrophin, an autosomal paralog of dystrophin, was originally named for its ubiquitous expression and high degree of sequence similarity to dystrophin, particularly in regards to critical protein binding domains located at the amino and carboxyl terminal portions of the protein [105; 167]. The discovery of utrophin occurred through screening of cDNA-libraries with a fragment from the 3’ end of the dystrophin cDNA and hence was termed dystrophin-related protein (DRP) or DMD-like (DMDL)[105]. As utrophin shares many structural and functional similarities with dystrophin, it has long been thought to be capable of substituting for dystrophin [142; 168]. An additional advantage stems from the fact that utrophin is widely expressed in DMD patients and therefore genetic delivery would avoid destructive cellular immune responses against dystrophin. This is an important consideration in view of the aforementioned observation that many patients are immune reactive to dystrophin, perhaps through low-level dystrophin expression in revertant fibers [27; 113]. Thus, given immune response concerns directed against dystrophin, the development of utrophin as an alternative to dystrophin gene transfer in certain immunocompetent DMD patients appears to offer a significant therapeutic advantage.
Evidence of the ability of utrophin to compensate and therefore alleviate the consequences of the lack of dystrophin is provided in the distal toe and extraocular muscles, where utrophin expression is normally high and whose muscles are resistant to dystrophy in DMD patients [51; 137; 138]. In mdx mice the sparing of extraocular muscles is also observed with a coincident pronounced upregulation of utrophin being in sharp contrast to dystrophin−/−:utrophin−/− double knock-out (mdx:utrn−/−) mice whose extraocular muscles are not spared from disease initiation and progression [138]. Additionally, in mdx:utrn−/− mice, a mouse model whose phenotype appears more representative of the pathological manifestations of DMD, transgenic expression of utrophin or somatic mini-utrophin (ΔR4–R17), modeled on a mildly affected BMD patient deletion [135; 185] (Figure 3) prevented the dystrophic phenotype [142; 174]. Further, ectopic expression of mini-utrophin in the mdx mouse markedly reduces the dystrophic phenotype [162; 166; 170] with major improvements in mechanical deficits of skeletal muscle, suggesting that a truncated yet highly functional mini-utrophin could be therapeutic in DMD muscle. In the highly relevant canine X-linked muscular dystrophy model (cxmd) amelioration of the dystrophic phenotype is observed with adenoviral-mediated gene transfer of mini-utrophin [34]. A direct comparison between dystrophin and utrophin gene transfer in mdx mice revealed an essentially equivalent performance with significant improvements in muscle histopathology, force generation, and resistance to contraction-induced injury [55]. The question remained whether a highly truncated utrophin, capable of being efficiently packaged within rAAV vectors, could be therapeutic in a dystrophic mouse model. Our lab has since pioneered the use of micro-utrophin cassettes (Figure 3) delivered by rAAV6 using a construct analogous in design to a micro-dystrophin (ΔR4–R23/CT). When administered intravenously to mdx:utrn−/− mice a profound amelioration of the dystrophic phenotype was observed. Muscles expressing micro-utrophin further displayed restoration of the dystrophin-glycoprotein complex, increased myofiber size, and considerably improved physiological performance compared with untreated mdx:utrn−/− mice [127]. Hence, gene therapy for DMD could potentially be achieved by delivery of micro-utrophin vectors using rAAV if dystrophin is found to be eliminated via immunological mechanisms in some patients. More recently, pharmacological upregulation of utrophin in mdx mice has been shown to reduce pathology and improve physiological performance [169], supporting the hypothesis put forth by Davies and colleagues [170] regarding the use of small molecules that can act to increase endogenous levels of utrophin to compensate for the absence of dystrophin.
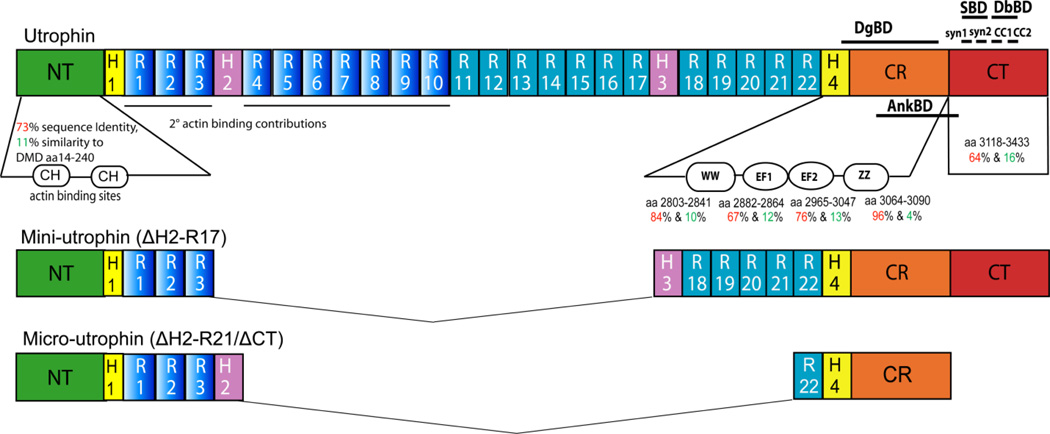
Figure 3.
Structure of Utrophin. Shown is an illustration depicting the major functional domains of the full-length, 395 kDa isoform of utrophin-A, mini-utrophin (222 kDa), and micro-utrophin (130 kDa). Like dystrophin, at left is an amino-terminal (NT) actin-binding domain, formed by 2 adjacent calponin homology (CH) domains. The central rod domain is composed of 22 spectrin-like triple-helical elements or repeats (R), the first 10 of which also contribute to actin binding (unlike dystrophin), further separated internally by 2 proline-rich hinge (H) domains. Two additional hinges separate the rod domain from the remainder of the protein. Toward the carboxy-terminus is a dystroglycan-binding domain (DgBD), composed of a WW domain, 2 EF-hand-like structures, and a ZZ-type zinc-finger domain. The EF-hands and ZZ domain are found within the cysteine-rich region (CR), i.e. 13 cysteines (dystrophin has 15) within 280 amino acids. The carboxy-terminal (CT) domain is composed of syntrophin- and dystrobrevin-binding domains (SBD and DbBD, respectively) and does not show sequence identity to any known protein other than those in the dystrophin family. Amino acid sequence identity and similarity is shown relative to dystrophin, noted in red and green percentage values respectively. Abbreviations: CH, calponin homology domain; R, spectrin-like repeats; H, hinge domain; DgBD, dystroglycan-binding domain; CR, cysteine-rich region; CT, carboxy-terminal domain; SBD, syntrophin binding domain; DbBD, dystrobrevin binding domain; AnkBD, ankyrin binding domain; aa, amino acids.
Clinical trial of AAV and micro-dystrophin
To date there has been one clinical trial conducted testing AAV vectors to deliver a mini-dystrophin construct to muscles of boys with DMD (REF). The trial was a phase 1 trial designed to assess the safety of intramuscular injection of an AAV vector expressing a human micro-dystrophin into muscles of DMD boys. Six boys between the ages of 5 and 11 were injected in one biceps muscle with either 2×1010 vector genomes/kg or 1×1011 vector genomes/kg (3 boys with each dose). Each patient also received an injection of methylprednisone 4 hours before vector administration to blunt any potential immune response. The primary outcome measurements were to test for mini-dystrophin expression in biopsies from the injected muscles, to monitor blood chemistries and to examine for an immune response against dystrophin or the AAV capsid proteins. The AAV vector was pseudotyped with a hybrid capsid between AAV2 and AAV1, in which 5 amino acids from AAV1 were incorporated into the AAV2 capsid in an attempt to increase muscle transduction (REF). The dystrophic cassette consisted of a micro-dystrophin incorporating 5 spectrin-like repeats (Figure 1), and was controlled by a ubiquitously active CMV promoter (REF). Unfortunately, little if any vector-derived dystrophin was detected in any of the 6 injected patients, and a cellular immune response was observed against mini-dystrophin in 2 patients, and against the AAV capsid in 5 patients. Thus, it appears that gene expression may have been eliminated due to an immune response against AAV capsid, dystrophin, or both. Potential ways to overcome these immune-mediated barriers to transgene expression might be to incorporate into the vector a muscle-restricted promoter cassette to prevent gene expression in immune effector cells [151], to deliver a micro-utrophin cassette rather than a micro-dystrophin cassette [127], to test alternate AAV capsid types [98] or to apply immune blocking strategies as discussed above [178].
Summary and Conclusions
Developing a gene therapy approach for a disorder such as DMD, which requires widespread gene transfer, remains a daunting challenge. Nonetheless, the identification of rAAV vectors as a method for systemic gene delivery, the development of highly functional micro-dystrophins and utrophins, and the improvement of methods to minimize immunological consequences of vector mediated gene transfer (via use of muscle-specific promoters and application of transient immune suppression) make the implementation of this technology a realistic proposition. A compelling argument for developing rAAV/dystrophin delivery technologies is that they appear applicable to all patents with DMD or BMD, i.e. they are not mutation-specific or tissue-restricted technologies. The major significant obstacle to a gene therapy treatment remains the development of optimal delivery regimens for intravascular delivery of rAAV vectors to human muscle. These include vector dose, delivery route and number of sites for vector infusion. Minimizing any toxicity and controlling immunological consequences of delivery will be the critical outcomes guiding such studies. In the meantime, improvements in optimal micro-dystrophin structure and development of stronger muscles-specific promoters will enhance the efficacy of any vector delivery protocol. With diligence it is hoped that these methods will have a major impact on the health and quality of life for patients with DMD and BMD.
Acknowledgements
Supported by grants R37AR40864 and R01AG33610 from the National Institutes for Health (to JSC) and by a grant from the Muscular Dystrophy Association (USA, to GLO). JTS is supported by the Early Career Fellowship from the NHMRC of Australia (1036656). JNR was supported in part by PHS NRSA T32 GM07270 from NIGMS.
List of abbreviations
- DMD
Duchenne muscular dystrophy
- DGC
Dystrophin-glycoprotein complex
- ITR
inverted terminal repeat
- ORF
open-reading frame
- rAAV
recombinant adeno-associated virus
- RBE
Rep binding elements
- SR
spectrin-like repeat
- TRS
terminal resolution site
- VP
viral capsid protein
Footnotes
Conflict of Interest
The authors declare no conflicts of interest.
References
- 1.Aartsma-Rus A, Fokkema I, Verschuuren J, Ginjaar I, van Deutekom J, van Ommen GJ, den Dunnen JT. Theoretic applicability of antisense-mediated exon skipping for Duchenne muscular dystrophy mutations. Hum Mutat. 2009;30:293–299. doi: 10.1002/humu.20918. [DOI] [PubMed] [Google Scholar]
- 2.Adriouch S, Franck E, Drouot L, Bonneau C, Jolinon N, Salvetti A, Boyer O. Improved immunological tolerance following combination therapy with CTLA-4/Ig and AAV-mediated PD-L1/2 muscle gene transfer. Frontiers in Microbiology. 2011;2 doi: 10.3389/fmicb.2011.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akache B, Grimm D, Pandey K, Yant SR, Xu H, Kay MA. The 37/67-kilodalton laminin receptor is a receptor for adeno-associated virus serotypes 8, 2, 3, and 9. J Virol. 2006;80:9831–9836. doi: 10.1128/JVI.00878-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akache B, Grimm D, Shen X, Fuess S, Yant SR, Glazer DS, Park J, Kay MA. A two-hybrid screen identifies cathepsins Ban d L as uncoating factors for adeno-associated virus 2 and 8. Molecular Therapy. 2007;15:330–339. doi: 10.1038/sj.mt.6300053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aoki Y, Nakamura A, Yokota T, Saito T, Okazawa H, Nagata T, Takeda S. In-frame dystrophin following exon 51-skipping improves muscle pathology and function in the exon 52-deficient mdx mouse. Mol Ther. 2010;18:1995–2005. doi: 10.1038/mt.2010.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Araki E, Nakamura K, Nakao K, Kameya S, Kobayashi O, Nonaka I, Kobayashi T, Katsuki M. Targeted disruption of exon 52 in the mouse dystrophin gene induced muscle degeneration similar to that observed in Duchenne muscular dystrophy. Biochem Biophys Res Commun. 1997;238:492–497. doi: 10.1006/bbrc.1997.7328. [DOI] [PubMed] [Google Scholar]
- 7.Arnett AL, Chamberlain JR, Chamberlain JS. Therapy for neuromuscular disorders. Curr Opin Genet Dev. 2009;19:290–297. doi: 10.1016/j.gde.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 8.Arnett AL, Ramos JN, Chamberlain JS. Viral Gene Therapy in Skeletal Muscle. Muscle: Fundamental Biology and Mechanisms of DiseaseJoseph Hill. 2011 in press. [Google Scholar]
- 9.Arnett ALH, Garikipati D, Wang Z, Tapscott SJ, Chamberlain JS. Immune responses to rAAV6: The influence of canine parvovirus vaccination and neonatal administration of viral vector. Frontiers in Microbiology. 2011;2 doi: 10.3389/fmicb.2011.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asokan A, Hamra JB, Govindasamy L, Agbandje-McKenna M, Samulski RJ. Adeno-associated virus type 2 contains an integrin alpha 5 beta 1 binding domain essential for viral cell entry. J Virol. 2006;80:8961–8969. doi: 10.1128/JVI.00843-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Atchison RW, Casto BC, Hammon WM. Adenovirus-Associated Defective Virus Particles. Science. 1965;149:754–756. doi: 10.1126/science.149.3685.754. [DOI] [PubMed] [Google Scholar]
- 12.Banks GB, Chamberlain JS, Froehner SC. Truncated dystrophins can influence neuromuscular synapse structure. Mol Cell Neurosci. 2009;40:433–441. doi: 10.1016/j.mcn.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Banks GB, Combs AC, Chamberlain JR, Chamberlain JS. Molecular and cellular adaptations to chronic myotendinous strain injury in mdx mice expressing a truncated dystrophin. Hum Mol Genet. 2008;17:3975–3986. doi: 10.1093/hmg/ddn301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Banks GB, Gregorevic P, Allen JM, Finn EE, Chamberlain JS. Functional capacity of dystrophins carrying deletions in the N-terminal actin-binding domain. Hum Mol Genet. 2007;16:2105–2113. doi: 10.1093/hmg/ddm158. [DOI] [PubMed] [Google Scholar]
- 15.Banks GB, Judge LM, Allen JM, Chamberlain JS. The polyproline site in hinge 2 influences the functional capacity of truncated dystrophins. PLoS Genet. 2010;6:e1000958. doi: 10.1371/journal.pgen.1000958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bartel M, Schaffer D, Büning H. Enhancing the clinical potential of AAV vectors by capsid engineering to evade pre-existing immunity. Frontiers in Microbiology. 2011;2 doi: 10.3389/fmicb.2011.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bartel MA, Weinstein JR, Schaffer DV. Directed evolution of novel adeno-associated viruses for therapeutic gene delivery. Gene Ther. 2012 doi: 10.1038/gt.2012.20. [DOI] [PubMed] [Google Scholar]
- 18.Bartlett JS, Wilcher R, Samulski RJ. Infectious entry pathway of adeno-associated virus and adeno-associated virus vectors. J Virol. 2000;74:2777–2785. doi: 10.1128/jvi.74.6.2777-2785.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beggs AH, Hoffman EP, Snyder JR, Arahata K, Specht L, Shapiro F, Angelini C, Sugita H, Kunkel LM. Exploring the molecular basis for variability among patients with Becker muscular dystrophy: dystrophin gene and protein studies. Am J Hum Genet. 1991;49:54–67. [PMC free article] [PubMed] [Google Scholar]
- 20.Berns KI. Parvovirus replication. Microbiological reviews. 1990;54:316–329. doi: 10.1128/mr.54.3.316-329.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berns KI, Giraud C. Biology of adeno-associated virus. Current topics in microbiology and immunology. 1996;218:1–23. doi: 10.1007/978-3-642-80207-2_1. [DOI] [PubMed] [Google Scholar]
- 22.Bhosle RC, Michele DE, Campbell KP, Li Z, Robson RM. Interactions of intermediate filament protein synemin with dystrophin and utrophin. Biochem Biophys Res Commun. 2006;346:768–777. doi: 10.1016/j.bbrc.2006.05.192. [DOI] [PubMed] [Google Scholar]
- 23.Blake DJ, Weir A, Newey SE, Davies KE. Function and genetics of dystrophin and dystrophin-related proteins in muscle. Physiol Rev. 2002;82:291–329. doi: 10.1152/physrev.00028.2001. [DOI] [PubMed] [Google Scholar]
- 24.Blankinship M, Gregorevic P, Chamberlain J. Chamberlain J, Rando T, editors. Gene Therapy of Muscular Dystrophy Using Adeno-Associated Viral Vectors: Promises and Limitations. Duchenne Muscular Dystrophy: Advances in Therapeutics Informa Healthcare. 2006:413–438. [Google Scholar]
- 25.Bleker S, Sonntag F, Kleinschmidt JA. Mutational analysis of narrow pores at the fivefold symmetry axes of adeno-associated virus type 2 capsids reveals a dual role in genome packaging and activation of phospholipase A2 activity. J Virol. 2005;79:2528–2540. doi: 10.1128/JVI.79.4.2528-2540.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boutin S, Monteilhet V, Veron P, Leborgne C, Benveniste O, Françoise Montus M, Masurier C. Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types, 1, 2, 5, 6, 8, and 9 in the healthy population: implications for gene therapy using AAV vectors. Human Gene Therapy. 2010;21:704–712. doi: 10.1089/hum.2009.182. [DOI] [PubMed] [Google Scholar]
- 27.Bowles DE, McPhee SW, Li C, Gray SJ, Samulski JJ, Camp AS, Li J, Wang B, Monahan PE, Rabinowitz JE, et al. Phase 1 gene therapy for Duchenne muscular dystrophy using a translational optimized AAV vector. Mol Ther. 2012;20:443–455. doi: 10.1038/mt.2011.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brenman JE, Chao DS, Xia H, Aldape K, Bredt DS. Nitric oxide synthase complexed with dystrophin and absent from skeletal muscle sarcolemma in Duchenne muscular dystrophy. Cell. 1995;82:743–752. doi: 10.1016/0092-8674(95)90471-9. [DOI] [PubMed] [Google Scholar]
- 29.Brister JR, Muzyczka N. Mechanism of Rep-mediated adeno-associated virus origin nicking. J Virol. 2000;74:7762–7771. doi: 10.1128/jvi.74.17.7762-7771.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brockstedt DG, Podsakoffc GM, Fonga L, Kurtzmanc G, Mueller-Ruchholtzb W, Englemana EG. Induction of immunity to antigens expressed by recombinant adeno-associated virus depends on the route of administration. Clinical Immunology. 1999;92:67–75. doi: 10.1006/clim.1999.4724. [DOI] [PubMed] [Google Scholar]
- 31.Brown BD, Gentner B, Cantore A, Colleoni S, Amendola M, Zingale A, Baccarini A, Lazzari G, Galli C, Naldini L. Endogenous microRNA can be broadly exploited to regulate transgene expression according to tissue, lineage and differentiation state. Nat Biotech. 2007;25:1457–1467. doi: 10.1038/nbt1372. [DOI] [PubMed] [Google Scholar]
- 32.Bulfield G, Siller WG, Wight PA, Moore KJ. X chromosome-linked muscular dystrophy (mdx) in the mouse. Proc Natl Acad Sci U S A. 1984;81:1189–1192. doi: 10.1073/pnas.81.4.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Campbell KP, Knudson CM, Imagawa T, Leung AT, Sutko JL, Kahl SD, Raab CR, Madson L. Identification and characterization of the high affinity [3H]ryanodine receptor of the junctional sarcoplasmic reticulum Ca2+ release channel. J Biol Chem. 1987;262:6460–6463. [PubMed] [Google Scholar]
- 34.Cerletti M, Negri T, Cozzi F, Colpo R, Andreetta F, Croci D, Davies KE, Cornelio F, Pozza O, Karpati G, et al. Dystrophic phenotype of canine X-linked muscular dystrophy is mitigated by adenovirus-mediated utrophin gene transfer. Gene Ther. 2003;10:750–757. doi: 10.1038/sj.gt.3301941. [DOI] [PubMed] [Google Scholar]
- 35.Chamberlain JR, Chamberlain JS. Muscling in: Gene therapies for muscular dystrophy target RNA. Nat Med. 2010;16:170–171. doi: 10.1038/nm0210-170. [DOI] [PubMed] [Google Scholar]
- 36.Chamberlain JS. Duchenne muscular dystrophy models show their age. Cell. 2010;143:1040–1042. doi: 10.1016/j.cell.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chamberlain JS, Metzger J, Reyes M, Townsend D, Faulkner JA. Dystrophin-deficient mdx mice display a reduced life span and are susceptible to spontaneous rhabdomyosarcoma. FASEB Journal. 2007;21:2195–2204. doi: 10.1096/fj.06-7353com. [DOI] [PubMed] [Google Scholar]
- 38.Chao H, Liu Y, Rabinowitz J, Li C, Samulski RJ, Walsh CE. Several log increase in therapeutic transgene delivery by distinct adeno-associated viral serotype vectors. Molecular therapy : the journal of the American Society of Gene Therapy. 2000;2:619–623. doi: 10.1006/mthe.2000.0219. [DOI] [PubMed] [Google Scholar]
- 39.Chapman VM, Miller DR, Armstrong D, Caskey CT. Recovery of induced mutations for X chromosome-linked muscular dystrophy in mice. Proc Natl Acad Sci U S A. 1989;86:1292–1296. doi: 10.1073/pnas.86.4.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chirmule N, Xiao W, Truneh A, Schnell MA, Hughes JV, Zoltick P, Wilson JM. Humoral immunity to adeno-associated virus type 2 vectors following administration to murine and nonhuman primate muscle. J Virol. 2000;74:2420–2425. doi: 10.1128/jvi.74.5.2420-2425.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cirak S, Arechavala-Gomeza V, Guglieri M, Feng L, Torelli S, Anthony K, Abbs S, Garralda ME, Bourke J, Wells DJ, et al. Exon skipping and dystrophin restoration in patients with Duchenne muscular dystrophy after systemic phosphorodiamidate morpholino oligomer treatment: an open-label, phase 2, dose-escalation study. Lancet. 2011;378:595–605. doi: 10.1016/S0140-6736(11)60756-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Collaco RF, Kalman-Maltese V, Smith AD, Dignam JD, Trempe JP. A biochemical characterization of the adeno-associated virus Rep40 helicase. J Biol Chem. 2003;278:34011–34017. doi: 10.1074/jbc.M301537200. [DOI] [PubMed] [Google Scholar]
- 43.Cooper BJ, Winand NJ, Stedman H, Valentine BA, Hoffman EP, Kunkel LM, Scott MO, Fischbeck KH, Kornegay JN, Avery RJ, et al. The Homolog of the Duchenne Locus Is Defective in X-Linked Muscular-Dystrophy of Dogs. Nature. 1988;334:154–156. doi: 10.1038/334154a0. [DOI] [PubMed] [Google Scholar]
- 44.Cordier L, Gao GP, Hack AA, McNally EM, Wilson JM, Chirmule N, HL S. Muscle-specific promoters may be necessary for adeno-associated virus-mediated gene transfer in the treatment of muscular dystrophies. Hum Gene Ther. 2001;12:205–215. doi: 10.1089/104303401750061267. [DOI] [PubMed] [Google Scholar]
- 45.Corrado K, Rafael JA, Mills PL, Cole NM, Faulkner JA, Wang K, Chamberlain JS. Transgenic mdx mice expressing dystrophin with a deletion in the actin-binding domain display a "mild Becker" phenotype. J Cell Biol. 1996;134:873–884. doi: 10.1083/jcb.134.4.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cox GA, Sunada Y, Campbell KP, Chamberlain JS. Dp71 can restore the dystrophin-associated glycoprotein complex in muscle but fails to prevent dystrophy. Nat Genet. 1994;8:333–339. doi: 10.1038/ng1294-333. [DOI] [PubMed] [Google Scholar]
- 47.Crawford GE, Faulkner JA, Crosbie RH, Campbell KP, Froehner SC, Chamberlain JS. Assembly of the dystrophin-associated protein complex does not require the dystrophin COOH-terminal domain. J Cell Biol. 2000;150:1399–1410. doi: 10.1083/jcb.150.6.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Davies KE, Pearson PL, Harper PS, Murray JM, O'Brien T, Sarfarazi M, Williamson R. Linkage analysis of two cloned DNA sequences flanking the Duchenne muscular dystrophy locus on the short arm of the human X chromosome. Nucleic Acids Res. 1983;11:2303–2312. doi: 10.1093/nar/11.8.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Deconinck AE, Rafael JA, Skinner JA, Brown SC, Potter AC, Metzinger L, Watt DJ, Dickson JG, Tinsley JM, Davies KE. Utrophin-dystrophin-deficient mice as a model for Duchenne muscular dystrophy. Cell. 1997;90:717–727. doi: 10.1016/s0092-8674(00)80532-2. [DOI] [PubMed] [Google Scholar]
- 50.Di Pasquale G, Davidson BL, Stein CS, Martins IS, Scudiero D, Monks A, Chiorini JA. Identification of PDGFR as a receptor for AAV-5 transduction. Nat Med. 2003;9:1306–1312. doi: 10.1038/nm929. [DOI] [PubMed] [Google Scholar]
- 51.Dowling P, Culligan K, Ohlendieck K. Distal mdx muscle groups exhibiting up-regulation of utrophin and rescue of dystrophin-associated glycoproteins exemplify a protected phenotype in muscular dystrophy. Naturwissenschaften. 2002;89:75–78. doi: 10.1007/s00114-001-0289-4. [DOI] [PubMed] [Google Scholar]
- 52.Duan DS, Li Q, Kao AW, Yue YP, Pessin JE, Engelhardt JF. Dynamin is required for recombinant adeno-associated virus type 2 infection. J Virol. 1999;73:10371–10376. doi: 10.1128/jvi.73.12.10371-10376.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Duan DS, Sharma P, Yang JS, Yue YP, Dudus L, Zhang YL, Fisher KJ, Engelhardt JF. Circular intermediates of recombinant adeno-associated virus have defined structural characteristics responsible for long-term episomal persistence in muscle tissue. J Virol. 1998;72:8568–8577. doi: 10.1128/jvi.72.11.8568-8577.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dunckley MG, Love DR, Davies KE, Walsh FS, Morris GE, Dickson G. Retroviral-mediated transfer of a dystrophin minigene into mdx mouse myoblasts in vitro. FEBS Lett. 1992;296:128–134. doi: 10.1016/0014-5793(92)80363-l. [DOI] [PubMed] [Google Scholar]
- 55.Ebihara S, Guibinga GH, Gilbert R, Nalbantoglu J, Massie B, Karpati G, Petrof BJ. Differential effects of dystrophin and utrophin gene transfer in immunocompetent muscular dystrophy (mdx) mice. Physiological genomics. 2000;3:133–144. doi: 10.1152/physiolgenomics.2000.3.3.133. [DOI] [PubMed] [Google Scholar]
- 56.Fabbrizio E, Bonet-Kerrache A, Leger JJ, Mornet D. Actin-dystrophin interface. Biochemistry. 1993;32:10457–10463. doi: 10.1021/bi00090a023. [DOI] [PubMed] [Google Scholar]
- 57.Fisher KJ, Jooss K, Alston J, Yang Y, Haecker SE, High K, Pathak R, Raper SE, Wilson JM. Recombinant adeno-associated virus for muscle directed gene therapy. Nat Med. 1997;3:306–312. doi: 10.1038/nm0397-306. [DOI] [PubMed] [Google Scholar]
- 58.Foster H, Sharp PS, Athanasopoulos T, Trollet C, Graham IR, Foster K, Wells DJ, Dickson G. Codon and mRNA sequence optimization of microdystrophin transgenes improves expression and physiological outcome in dystrophic mdx mice following AAV2/8 gene transfer. Mol Ther. 2008;16:1825–1832. doi: 10.1038/mt.2008.186. [DOI] [PubMed] [Google Scholar]
- 59.Fougerousse F, Bartoli M, Poupiot J, Arandel L, Durand M, Guerchet N, Gicquel E, Danos O, Richard I. Phenotypic correction of alpha-sarcoglycan deficiency by intra-arterial injection of a muscle-specific serotype 1 rAAV vector. Molecular therapy : the journal of the American Society of Gene Therapy. 2007;15:53–61. doi: 10.1038/sj.mt.6300022. [DOI] [PubMed] [Google Scholar]
- 60.Gao G, Vandenberghe LH, Alvira MR, Lu Y, Calcedo R, Zhou X, Wilson JM. Clades of Adeno-associated viruses are widely disseminated in human tissues. J Virol. 2004;78:6381–6388. doi: 10.1128/JVI.78.12.6381-6388.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gao G, Wang Q, Calcedo R, Mays L, Bell P, Wang L, Vandenberghe LH, Grant R, Sanmiguel J, Furth EE, Wilson JM. Adeno-associated virus-mediated gene transfer to nonhuman primate liver can elicit destructive transgene-specific T cell responses. Human Gene Therapy. 2009;20:930–942. doi: 10.1089/hum.2009.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gao GP, Alvira MR, Wang L, Calcedo R, Johnston J, Wilson JM. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc Natl Acad Sci U S A. 2002;99:11854–11859. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gardner KL, Kearney JA, Edwards JD, Rafael-Fortney JA. Restoration of all dystrophin protein interactions by functional domains in trans does not rescue dystrophy. Gene Ther. 2006;13:744–751. doi: 10.1038/sj.gt.3302686. [DOI] [PubMed] [Google Scholar]
- 64.Geisler A, Jungmann A, Kurreck J, Poller W, Katus HA, Vetter R, Fechner H, Muller OJ. microRNA122-regulated transgene expression increases specificity of cardiac gene transfer upon intravenous delivery of AAV9 vectors. Gene Ther. 2011;18:199–209. doi: 10.1038/gt.2010.141. [DOI] [PubMed] [Google Scholar]
- 65.Gentner B, Schira G, Giustacchini A, Amendola M, Brown BD, Ponzoni M, Naldini L. Stable knockdown of microRNA in vivo by lentiviral vectors. Nat Meth. 2009;6:63–66. doi: 10.1038/nmeth.1277. [DOI] [PubMed] [Google Scholar]
- 66.Ghosh A, Yue Y, Long C, Bostick B, Duan D. Efficient whole-body transduction with trans-splicing adeno-associated viral vectors. Mol Ther. 2007;15:750–755. doi: 10.1038/sj.mt.6300081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gilchrist SC, Ontell MP, Kochanek S, Clemens PR. Immune response to full-length dystrophin delivered to DMD muscle by a high-capacity adenoviral vector. Molecular Therapy. 2002;6:359–368. doi: 10.1006/mthe.2002.0675. [DOI] [PubMed] [Google Scholar]
- 68.Goemans NM, Tulinius M, van den Akker JT, Burm BE, Ekhart PF, Heuvelmans N, Holling T, Janson AA, Platenburg GJ, Sipkens JA, et al. Systemic administration of PRO051 in Duchenne's muscular dystrophy. N Engl J Med. 2011;364:1513–1522. doi: 10.1056/NEJMoa1011367. [DOI] [PubMed] [Google Scholar]
- 69.Goldberg LR, Hausmanowa-Petrusewicz I, Fidzianska A, Duggan DJ, Steinberg LS, Hoffman EP. A dystrophin missense mutation showing persistence of dystrophin and dystrophin-associated proteins yet a severe phenotype. Ann Neurol. 1998;44:971–976. doi: 10.1002/ana.410440619. [DOI] [PubMed] [Google Scholar]
- 70.Grady RM, Grange RW, Lau KS, Maimone MM, Nichol MC, Stull JT, Sanes JR. Role for alpha-dystrobrevin in the pathogenesis of dystrophin-dependent muscular dystrophies. Nat Cell Biol. 1999;1:215–220. doi: 10.1038/12034. [DOI] [PubMed] [Google Scholar]
- 71.Grady RM, Teng H, Nichol MC, Cunningham JC, Wilkinson RS, Sanes JR. Skeletal and cardiac myopathies in mice lacking utrophin and dystrophin: a model for Duchenne muscular dystrophy. Cell. 1997;90:729–738. doi: 10.1016/s0092-8674(00)80533-4. [DOI] [PubMed] [Google Scholar]
- 72.Greenberg DS, Sunada Y, Campbell KP, Yaffe D, Nudel U. Exogenous Dp71 restores the levels of dystrophin associated proteins but does not alleviate muscle damage in mdx mice. Nat Genet. 1994;8:340–344. doi: 10.1038/ng1294-340. [DOI] [PubMed] [Google Scholar]
- 73.Gregorevic P, Allen JM, Minami E, Blankinship MJ, Haraguchi M, Meuse L, Finn E, Adams ME, Froehner SC, Murry CE, Chamberlain JS. rAAV6-microdystrophin preserves muscle function and extends lifespan in severely dystrophic mice. Nat Med. 2006;12:787–789. doi: 10.1038/nm1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gregorevic P, Blankinship MJ, Allen JM, Crawford RW, Meuse L, Miller DG, Russell DW, Chamberlain JS. Systemic delivery of genes to striated muscles using adeno-associated viral vectors. Nat Med. 2004;10:828–834. doi: 10.1038/nm1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gregorevic P, Schultz BR, Allen JM, Halldorson JB, Blankinship MJ, Meznarich NA, Kuhr CS, Doremus C, Finn E, Liggitt D, Chamberlain JS. Evaluation of vascular delivery methodologies to enhance rAAV6-mediated gene transfer to canine striated musculature. Mol Ther. 2009;17:1427–1433. doi: 10.1038/mt.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Grimm D, Kay MA. From virus evolution to vector revolution: use of naturally occurring serotypes of adeno-associated virus (AAV) as novel vectors for human gene therapy. Curr Gene Ther. 2003;3:281–304. doi: 10.2174/1566523034578285. [DOI] [PubMed] [Google Scholar]
- 77.Halbert CL, Standaert TA, Wilson CB, Miller AD. Successful readministration of adeno-associated virus vectors to the mouse lung requires transient immunosuppression during the initial exposure. Journal of Virology. 1998;72:9795–9805. doi: 10.1128/jvi.72.12.9795-9805.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Harper SQ, Hauser MA, DelloRusso C, Duan D, Crawford RW, Phelps SF, Harper HA, Robinson AS, Engelhardt JF, Brooks SV, Chamberlain JS. Modular flexibility of dystrophin: implications for gene therapy of Duchenne muscular dystrophy. Nat Med. 2002;8:253–261. doi: 10.1038/nm0302-253. [DOI] [PubMed] [Google Scholar]
- 79.Hartigan-O'Connor D, Kirk CJ, Crawford R, Mule JJ, Chamberlain JS. Immune evasion by muscle-specific gene expression in dystrophic muscle. Molecular Therapy. 2001;4:525–533. doi: 10.1006/mthe.2001.0496. [DOI] [PubMed] [Google Scholar]
- 80.Heemskerk H, de Winter CL, van Ommen GJ, van Deutekom JC, Aartsma-Rus A. Development of antisense-mediated exon skipping as a treatment for duchenne muscular dystrophy. Ann N Y Acad Sci. 2009;1175:71–79. doi: 10.1111/j.1749-6632.2009.04973.x. [DOI] [PubMed] [Google Scholar]
- 81.Hemmings L, Kuhlman PA, Critchley DR. Analysis of the actin-binding domain of alpha-actinin by mutagenesis and demonstration that dystrophin contains a functionally homologous domain. J Cell Biol. 1992;116:1369–1380. doi: 10.1083/jcb.116.6.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Herson S, Hentati F, Rigolet A, Behin A, Romero NB, Leturcq F, Laforet P, Maisonobe T, Amouri R, Haddad H, et al. A phase I trial of adeno-associated virus serotype 1-gamma-sarcoglycan gene therapy for limb girdle muscular dystrophy type 2C. Brain. 2012;135:483–492. doi: 10.1093/brain/awr342. [DOI] [PubMed] [Google Scholar]
- 83.Hoffman EP, Brown RH, Jr, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51:919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- 84.Huard J, Roy R, Bouchard J, Malouin F, Richards C, JP T. Human myoblast transplantation between immunohistocompatible donors and recipients produces immune reactions. Transplant Proceedings. 1992;24:3049–3051. [PubMed] [Google Scholar]
- 85.Im DS, Muzyczka N. The AAV origin binding protein Rep68 is an ATP-dependent site-specific endonuclease with DNA helicase activity. Cell. 1990;61:447–457. doi: 10.1016/0092-8674(90)90526-k. [DOI] [PubMed] [Google Scholar]
- 86.Im WB, Phelps SF, Copen EH, Adams EG, Slightom JL, Chamberlain JS. Differential expression of dystrophin isoforms in strains of mdx mice with different mutations. Hum Mol Genet. 1996;5:1149–1153. doi: 10.1093/hmg/5.8.1149. [DOI] [PubMed] [Google Scholar]
- 87.Inagaki K, Fuess S, Storm TA, Gibson GA, McTiernan CF, Kay MA, Nakai H. Robust systemic transduction with AAV9 vectors in mice: efficient global cardiac gene transfer superior to that of AAV8. Molecular therapy : the journal of the American Society of Gene Therapy. 2006;14:45–53. doi: 10.1016/j.ymthe.2006.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ishikawa-Sakurai M, Yoshida M, Imamura M, Davies KE, Ozawa E. ZZ domain is essentially required for the physiological binding of dystrophin and utrophin to beta-dystroglycan. Hum Mol Genet. 2004;13:693–702. doi: 10.1093/hmg/ddh087. [DOI] [PubMed] [Google Scholar]
- 89.Jiang H, Couto LB, Patarroyo-White S, Liu T, Nagy D, Vargas JA, Zhou S, Scallan CD, Sommer J, Vijay S, et al. Effects of transient immunosuppression on adeno-associated virus-mediated liver-directed gene transfer in rhesus macaques and implications for human gene therapy. Blood. 2006;108:3321–3328. doi: 10.1182/blood-2006-04-017913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jooss K, Yang Y, Fisher KJ, Wilson JM. Transduction of dendritic cells by DNA viral vectors directs the immune response to transgene products in muscle fibers. Journal of Virology. 1998;72:4212–4223. doi: 10.1128/jvi.72.5.4212-4223.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kaludov N, Brown KE, Walters RW, Zabner J, Chiorini JA. Adeno-associated virus serotype 4 (AAV4) and AAV5 both require sialic acid binding for hemagglutination and efficient transduction but differ in sialic acid linkage specificity. J Virol. 2001;75:6884–6893. doi: 10.1128/JVI.75.15.6884-6893.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kashiwakura Y, Tamayose K, Iwabuchi K, Hirai Y, Shimada T, Matsumoto K, Nakamura T, Watanabe M, Oshimi K, Daida H. Hepatocyte growth factor receptor is a coreceptor for adeno-associated virus type 2 infection. J Virol. 2005;79:609–614. doi: 10.1128/JVI.79.1.609-614.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kessler PD, Podsakoff GM, Chen X, McQuiston SA, Colosi PC, Matelis LA, Kurtzman GJ, Byrne BJ. Gene delivery to skeletal muscle results in sustained expression and systemic delivery of a therapeutic protein. Proceedings of the National Academy of Sciences. 1996;93:14082–14087. doi: 10.1073/pnas.93.24.14082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Koenig M, Beggs AH, Moyer M, Scherpf S, Heindrich K, Bettecken T, Meng G, Muller CR, Lindlof M, Kaariainen H, et al. The molecular basis for Duchenne versus Becker muscular dystrophy: correlation of severity with type of deletion. Am J Hum Genet. 1989;45:498–506. [PMC free article] [PubMed] [Google Scholar]
- 95.Koenig M, Hoffman EP, Bertelson CJ, Monaco AP, Feener C, Kunkel LM. Complete cloning of the Duchenne muscular dystrophy (DMD) cDNA and preliminary genomic organization of the DMD gene in normal and affected individuals. Cell. 1987;50:509–517. doi: 10.1016/0092-8674(87)90504-6. [DOI] [PubMed] [Google Scholar]
- 96.Koenig M, Monaco AP, Kunkel LM. The complete sequence of dystrophin predicts a rod-shaped cytoskeletal protein. Cell. 1988;53:219–228. doi: 10.1016/0092-8674(88)90383-2. [DOI] [PubMed] [Google Scholar]
- 97.Koo T, Malerba A, Athanasopoulos T, Trollet C, Boldrin L, Ferry A, Popplewell L, Foster H, Foster K, Dickson G. Delivery of AAV2/9-microdystrophin genes incorporating helix 1 of the coiled-coil motif in the C-terminal domain of dystrophin improves muscle pathology and restores the level of alpha1-syntrophin and alpha-dystrobrevin in skeletal muscles of mdx mice. Hum Gene Ther. 2011;22:1379–1388. doi: 10.1089/hum.2011.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Koo T, Okada T, Athanasopoulos T, Foster H, Takeda Si, Dickson G. Long-term functional adeno-associated virus-microdystrophin expression in the dystrophic CXMDj dog. The Journal of Gene Medicine. 2011;13:497–506. doi: 10.1002/jgm.1602. [DOI] [PubMed] [Google Scholar]
- 99.Kornegay JN, Li J, Bogan JR, Bogan DJ, Chen C, Zheng H, Wang B, Qiao C, Howard JF, Jr, Xiao X. Widespread muscle expression of an AAV9 human mini-dystrophin vector after intravenous injection in neonatal dystrophin-deficient dogs. Mol Ther. 2010;18:1501–1508. doi: 10.1038/mt.2010.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kotin RM. Large-scale recombinant adeno-associated virus production. Hum Mol Genet. 2011;20:R2–R6. doi: 10.1093/hmg/ddr141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kotin RM, Menninger JC, Ward DC, Berns KI. Mapping and Direct Visualization of a Region-Specific Viral-DNA Integration Site on Chromosome-19q13-Qter. Genomics. 1991;10:831–834. doi: 10.1016/0888-7543(91)90470-y. [DOI] [PubMed] [Google Scholar]
- 102.Kunkel LM, Hejtmancik JF, Caskey CT, Speer A, Monaco AP, Middlesworth W, Colletti CA, Bertelson C, Muller U, Bresnan M, et al. Analysis of deletions in DNA from patients with Becker and Duchenne muscular dystrophy. Nature. 1986;322:73–77. doi: 10.1038/322073a0. [DOI] [PubMed] [Google Scholar]
- 103.Kunkel LM, Monaco AP, Middlesworth W, Ochs HD, Latt SA. Specific cloning of DNA fragments absent from the DNA of a male patient with an X chromosome deletion. Proc Natl Acad Sci U S A. 1985;82:4778–4782. doi: 10.1073/pnas.82.14.4778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lai Y, Thomas GD, Yue Y, Yang HT, Li D, Long C, Judge L, Bostick B, Chamberlain JS, Terjung RL, Duan D. Dystrophins carrying spectrin-like repeats 16 and 17 anchor nNOS to the sarcolemma and enhance exercise performance in a mouse model of muscular dystrophy. J Clin Invest. 2009;119:624–635. doi: 10.1172/JCI36612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Love DR, Hill DF, Dickson G, Spurr NK, Byth BC, Marsden RF, Walsh FS, Edwards YH, Davies KE. An autosomal transcript in skeletal muscle with homology to dystrophin. Nature. 1989;339:55–58. doi: 10.1038/339055a0. [DOI] [PubMed] [Google Scholar]
- 106.Maheshri N, Koerber JT, Kaspar BK, Schaffer DV. Directed evolution of adeno-associated virus yields enhanced gene delivery vectors. Nat Biotech. 2006;24:198–204. doi: 10.1038/nbt1182. [DOI] [PubMed] [Google Scholar]
- 107.Manning WC, Zhou S, Bland MP, Escobedo JA, Dwarki V. Transient immunosuppression allows transgene expression following readministration of adeno-associated viral vectors. Human Gene Therapy. 1998;9:477–485. doi: 10.1089/hum.1998.9.4-477. [DOI] [PubMed] [Google Scholar]
- 108.Manno CS. AAV-mediated factor IX gene transfer to skeletal muscle in patients with severe hemophilia, B. Blood. 2003;101:2963–2972. doi: 10.1182/blood-2002-10-3296. [DOI] [PubMed] [Google Scholar]
- 109.Manno CS, Arruda VR, Pierce GF, Glader B, Ragni M, Rasko J, Ozelo MC, Hoots K, Blatt P, Konkle B, et al. Successful transduction of liver in hemophilia by AAV-Factor, IX and limitations imposed by the host immune response. Nat Med. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- 110.Matsumura K, Ervasti JM, Ohlendieck K, Kahl SD, Campbell KP. Association of dystrophin-related protein with dystrophin-associated proteins in mdx mouse muscle. Nature. 1992;360:588–591. doi: 10.1038/360588a0. [DOI] [PubMed] [Google Scholar]
- 111.Mays LE, Wilson JM. The complex and evolving story of T cell activation to AAV vector-encoded transgene products. Mol Ther. 2011;19:16–27. doi: 10.1038/mt.2010.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.McCabe ER, Towbin J, Chamberlain J, Baumbach L, Witkowski J, van Ommen GJ, Koenig M, Kunkel LM, Seltzer WK. Complementary DNA probes for the Duchenne muscular dystrophy locus demonstrate a previously undetectable deletion in a patient with dystrophic myopathy, glycerol kinase deficiency, and congenital adrenal hypoplasia. J Clin Invest. 1989;83:95–99. doi: 10.1172/JCI113890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mendell JR, Campbell K, Rodino-Klapac L, Sahenk Z, Shilling C, Lewis S, Bowles D, Gray S, Li C, Galloway G, et al. Dystrophin immunity in Duchenne's muscular dystrophy. N Engl J Med. 2010;363:1429–1437. doi: 10.1056/NEJMoa1000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mendell JR, Rodino-Klapac LR, Rosales XQ, Coley BD, Galloway G, Lewis S, Malik V, Shilling C, Byrne BJ, Conlon T, et al. Sustained alpha-sarcoglycan gene expression after gene transfer in limb-girdle muscular dystrophy, type 2D. Ann Neurol. 2010;68:629–638. doi: 10.1002/ana.22251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mendell JR, Rodino-Klapac LR, Rosales-Quintero X, Kota J, Coley BD, Galloway G, Craenen JM, Lewis S, Malik V, Shilling C, et al. Limb-girdle muscular dystrophy type 2D gene therapy restores alpha-sarcoglycan and associated proteins. Ann Neurol. 2009;66:290–297. doi: 10.1002/ana.21732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Metzinger L, Blake DJ, Squier MV, Anderson LV, Deconinck AE, Nawrotzki R, Hilton-Jones D, Davies KE. Dystrobrevin deficiency at the sarcolemma of patients with muscular dystrophy. Hum Mol Genet. 1997;6:1185–1191. doi: 10.1093/hmg/6.7.1185. [DOI] [PubMed] [Google Scholar]
- 117.Mingozzi F, High K. Immune responses to AAV in clinical trials. Current Gene Therapy. 2007;7:316–324. doi: 10.2174/156652307782151425. [DOI] [PubMed] [Google Scholar]
- 118.Mingozzi F, High K. Immune responses to AAV in clinical trials. Current Gene Therapy. 2011;11:321–330. doi: 10.2174/156652311796150354. [DOI] [PubMed] [Google Scholar]
- 119.Mingozzi F, Maus MV, Hui DJ, Sabatino DE, Murphy SL, Rasko JEJ, Ragni MV, Manno CS, Sommer J, Jiang H, et al. CD8+ T-cell responses to adeno-associated virus capsid in humans. Nat Med. 2007;13:419–422. doi: 10.1038/nm1549. [DOI] [PubMed] [Google Scholar]
- 120.Mizuno Y, Nonaka I, Hirai S, Ozawa E. Reciprocal expression of dystrophin and utrophin in muscles of Duchenne muscular dystrophy patients, female DMD-carriers and control subjects. J Neurol Sci. 1993;119:43–52. doi: 10.1016/0022-510x(93)90190-a. [DOI] [PubMed] [Google Scholar]
- 121.Monteilhet V, Saheb S, Boutin S, Leborgne C, Veron P, Montus M-F, Moullier P, Benveniste O, Masurier C. A 10 patient case report on the impact of plasmapheresis upon neutralizing factors against adeno-associated virus (AAV) types, 1, 2, 6, and 8. Mol Ther. 2011;19:2084–2091. doi: 10.1038/mt.2011.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mori S, Wang L, Takeuchi T, Kanda T. Two novel adeno-associated viruses from cynomolgus monkey: pseudotyping characterization of capsid protein. Virology. 2004;330:375–383. doi: 10.1016/j.virol.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 123.Moskalenko M, Chen L, van Roey M, Donahue BA, Snyder RO, McArthur JG, Patel SD. Epitope mapping of human anti-adeno-associated virus type 2 neutralizing antibodies: implications for gene therapy and virus structure. Journal of Virology. 2000;74:1761–1766. doi: 10.1128/jvi.74.4.1761-1766.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nakai H, Fuess S, Storm TA, Muramatsu S, Nara Y, Kay MA. Unrestricted hepatocyte transduction with adeno-associated virus serotype 8 vectors in mice. J Virol. 2005;79:214–224. doi: 10.1128/JVI.79.1.214-224.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nakai H, Storm TA, Kay MA. Recruitment of single-stranded recombinant adeno-associated virus vector genomes and intermolecular recombination are responsible for stable transduction of liver in vivo. J Virol. 2000;74:9451–9463. doi: 10.1128/jvi.74.20.9451-9463.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Odom GL, Gregorevic P, Allen JM, Chamberlain JS. Gene therapy of mdx mice with large truncated dystrophins generated by recombination using rAAV6. Mol Ther. 2011;19:36–45. doi: 10.1038/mt.2010.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Odom GL, Gregorevic P, Allen JM, Finn E, Chamberlain JS. Microutrophin delivery through rAAV6 increases lifespan and improves muscle function in dystrophic dystrophin/utrophin-deficient mice. Mol Ther. 2008;16:1539–1545. doi: 10.1038/mt.2008.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ohtsuka Y, Udaka K, Yamashiro Y, Yagita H, Okumura K. Dystrophin acts as a transplantation rejection antigen in dystrophin-deficient mice: implication for gene therapy. J Immunol. 1998;160:4635–4640. [PubMed] [Google Scholar]
- 129.Pacak CA, Mah CS, Thattaliyath BD, Conlon TJ, Lewis MA, Cloutier DE, Zolotukhin I, Tarantal AF, Byrne BJ. Recombinant adeno-associated virus serotype 9 leads to preferential cardiac transduction in vivo. Circ Res. 2006;99:e3–e9. doi: 10.1161/01.RES.0000237661.18885.f6. [DOI] [PubMed] [Google Scholar]
- 130.Palomeque J, Chemaly ER, Colosi P, Wellman JA, Zhou S, Del Monte F, Hajjar RJ. Efficiency of eight different AAV serotypes in transducing rat myocardium in vivo. Gene Ther. 2007;14:989–997. doi: 10.1038/sj.gt.3302895. [DOI] [PubMed] [Google Scholar]
- 131.Pavalko FM, Otey CA. Role of adhesion molecule cytoplasmic domains in mediating interactions with the cytoskeleton. Proc Soc Exp Biol Med. 1994;205:282–293. doi: 10.3181/00379727-205-43709. [DOI] [PubMed] [Google Scholar]
- 132.Penaud-Budloo M, Le Guiner C, Nowrouzi A, Toromanoff A, Cherel Y, Chenuaud P, Schmidt M, von Kalle C, Rolling F, Moullier P, Snyder RO. Adeno-associated virus vector genomes persist as episomal chromatin in primate muscle. J Virol. 2008;82:7875–7885. doi: 10.1128/JVI.00649-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Perabo L, Endell J, King S, Lux K, Goldnau D, Hallek M, Büning H. Combinatorial engineering of a gene therapy vector: directed evolution of adeno-associated virus. The Journal of Gene Medicine. 2006;8:155–162. doi: 10.1002/jgm.849. [DOI] [PubMed] [Google Scholar]
- 134.Pfeifer A, Verma IM. Gene therapy: promises and problems. Annual review of genomics and human genetics. 2001;2:177–211. doi: 10.1146/annurev.genom.2.1.177. [DOI] [PubMed] [Google Scholar]
- 135.Phelps SF, Hauser MA, Cole NM, Rafael JA, Hinkle RT, Faulkner JA, Chamberlain JS. Expression of full-length and truncated dystrophin mini-genes in transgenic mdx mice. Hum Mol Genet. 1995;4:1251–1258. doi: 10.1093/hmg/4.8.1251. [DOI] [PubMed] [Google Scholar]
- 136.Phillips JL, Hegge J, Wolff JA, Samulski RJ, Asokan A. Systemic gene transfer to skeletal muscle using reengineered AAV vectors. Methods Mol Biol. 2011;709:141–151. doi: 10.1007/978-1-61737-982-6_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Porter JD. Commentary: extraocular muscle sparing in muscular dystrophy: a critical evaluation of potential protective mechanisms. Neuromuscul Disord. 1998;8:198–203. doi: 10.1016/s0960-8966(98)00015-7. [DOI] [PubMed] [Google Scholar]
- 138.Porter JD, Rafael JA, Ragusa RJ, Brueckner JK, Trickett JI, Davies KE. The sparing of extraocular muscle in dystrophinopathy is lost in mice lacking utrophin and dystrophin. J Cell Sci. 1998;111(Pt 13):1801–1811. doi: 10.1242/jcs.111.13.1801. [DOI] [PubMed] [Google Scholar]
- 139.Prins KW, Humston JL, Mehta A, Tate V, Ralston E, Ervasti JM. Dystrophin is a microtubule-associated protein. J Cell Biol. 2009;186:363–369. doi: 10.1083/jcb.200905048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Qiao C, Yuan Z, Li J, He B, Zheng H, Mayer C, Xiao X. Liver-specific microRNA-122 target sequences incorporated in AAV vectors efficiently inhibits transgene expression in the liver. Gene Ther. 2011;18:403–410. doi: 10.1038/gt.2010.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Qing K, Mah C, Hansen J, Zhou S, Dwarki V, Srivastava A. Human fibroblast growth factor receptor 1 is a co-receptor for infection by adeno-associated virus 2. Nat Med. 1999;5:71–77. doi: 10.1038/4758. [DOI] [PubMed] [Google Scholar]
- 142.Rafael JA, Tinsley JM, Potter AC, Deconinck AE, Davies KE. Skeletal muscle-specific expression of a utrophin transgene rescues utrophin-dystrophin deficient mice. Nat Genet. 1998;19:79–82. doi: 10.1038/ng0598-79. [DOI] [PubMed] [Google Scholar]
- 143.Ray PN, Belfall B, Duff C, Logan C, Kean V, Thompson MW, Sylvester JE, Gorski JL, Schmickel RD, Worton RG. Cloning of the breakpoint of an X;21 translocation associated with Duchenne muscular dystrophy. Nature. 1985;318:672–675. doi: 10.1038/318672a0. [DOI] [PubMed] [Google Scholar]
- 144.Reis e Sousa C, Hieny S, Scharton-Kersten T, Jankovic D, Charest H, Germain RN, Sher A. In vivo microbial stimulation induces rapid CD40 ligand–independent production of interleukin 12 by dendritic cells and their redistribution to T cell areas. The Journal of Experimental Medicine. 1997;186:1819–1829. doi: 10.1084/jem.186.11.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rezniczek GA, Konieczny P, Nikolic B, Reipert S, Schneller D, Abrahamsberg C, Davies KE, Winder SJ, Wiche G. Plectin 1f scaffolding at the sarcolemma of dystrophic (mdx) muscle fibers through multiple interactions with beta-dystroglycan. J Cell Biol. 2007;176:965–977. doi: 10.1083/jcb.200604179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Rivière C, Danos O, Douar AM. Long-term expression and repeated administration of AAV type, 1, 2 and 5 vectors in skeletal muscle of immunocompetent adult mice. Gene Ther. 2006;13:1300–1308. doi: 10.1038/sj.gt.3302766. [DOI] [PubMed] [Google Scholar]
- 147.Rodino-Klapac LR, Montgomery CL, Bremer WG, Shontz KM, Malik V, Davis N, Sprinkle S, Campbell KJ, Sahenk Z, Clark KR, et al. Persistent Expression of FLAG-tagged Micro dystrophin in Nonhuman Primates Following Intramuscular and Vascular Delivery. Mol Ther. 2010;18:109–117. doi: 10.1038/mt.2009.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Rutledge EA, Halbert CL, Russell DW. Infectious clones and vectors derived from adeno-associated virus (AAV) serotypes other than AAV type 2. J Virol. 1998;72:309–319. doi: 10.1128/jvi.72.1.309-319.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Rybakova IN, Amann KJ, Ervasti JM. A new model for the interaction of dystrophin with F-actin. J Cell Biol. 1996;135:661–672. doi: 10.1083/jcb.135.3.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sacco A, Mourkioti F, Tran R, Choi J, Llewellyn M, Kraft P, Shkreli M, Delp S, Pomerantz JH, Artandi SE, Blau HM. Short telomeres and stem cell exhaustion model Duchenne muscular dystrophy in mdx/mTR mice. Cell. 2010;143:1059–1071. doi: 10.1016/j.cell.2010.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Salva MZ, Himeda CL, Tai PW, Nishiuchi E, Gregorevic P, Allen JM, Finn EE, Nguyen QG, Blankinship MJ, Meuse L, et al. Design of tissue-specific regulatory cassettes for high-level rAAV-mediated expression in skeletal and cardiac muscle. Mol Ther. 2007;15:320–329. doi: 10.1038/sj.mt.6300027. [DOI] [PubMed] [Google Scholar]
- 152.Schatzberg SJ, Olby NJ, Breen M, Anderson LV, Langford CF, Dickens HF, Wilton SD, Zeiss CJ, Binns MM, Kornegay JN, et al. Molecular analysis of a spontaneous dystrophin 'knockout' dog. Neuromuscul Disord. 1999;9:289–295. doi: 10.1016/s0960-8966(99)00011-5. [DOI] [PubMed] [Google Scholar]
- 153.Schmidt M, Voutetakis A, Afione S, Zheng C, Mandikian D, Chiorini JA. Adeno-associated virus type 12 (AAV12): a novel AAV serotype with sialic acid- and heparan sulfate proteoglycan-independent transduction activity. J Virol. 2008;82:1399–1406. doi: 10.1128/JVI.02012-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Schultz BR, Chamberlain JS. Recombinant adeno-associated virus transduction and integration. Mol Ther. 2008;16:1189–1199. doi: 10.1038/mt.2008.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Seiler MP, Miller AD, Zabner J, Halbert CL. Adeno-associated virus types 5 and 6 use distinct receptors for cell entry. Hum Gene Ther. 2006;17:10–19. doi: 10.1089/hum.2006.17.10. [DOI] [PubMed] [Google Scholar]
- 156.Seisenberger G, Ried MU, Endress T, Buning H, Hallek M, Brauchle C. Real-time single-molecule imaging of the infection pathway of an adeno-associated virus. Science. 2001;294:1929–1932. doi: 10.1126/science.1064103. [DOI] [PubMed] [Google Scholar]
- 157.Shimatsu Y, Katagiri K, Furuta T, Nakura M, Tanioka Y, Yuasa K, Tomohiro M, Kornegay JN, Nonaka I, Takeda S. Canine X-linked muscular dystrophy in Japan (CXMDJ) Exp Anim. 2003;52:93–97. doi: 10.1538/expanim.52.93. [DOI] [PubMed] [Google Scholar]
- 158.Sicinski P, Geng Y, Ryder-Cook AS, Barnard EA, Darlison MG, Barnard PJ. The molecular basis of muscular dystrophy in the mdx mouse: a point mutation. Science. 1989;244:1578–1580. doi: 10.1126/science.2662404. [DOI] [PubMed] [Google Scholar]
- 159.Smith BF, Yue Y, Woods PR, Kornegay JN, Shin JH, Williams RR, Duan D. An intronic LINE-1 element insertion in the dystrophin gene aborts dystrophin expression and results in Duchenne-like muscular dystrophy in the corgi breed. Lab Invest. 2011;91:216–231. doi: 10.1038/labinvest.2010.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Smith RH, Kotin RM. The Rep52 gene product of adeno-associated virus is a DNA helicase with 3'-to-5' polarity. J Virol. 1998;72:4874–4881. doi: 10.1128/jvi.72.6.4874-4881.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Snyder RO, Im DS, Ni T, Xiao X, Samulski RJ, Muzyczka N. Features of the adeno-associated virus origin involved in substrate recognition by the viral Rep protein. J Virol. 1993;67:6096–6104. doi: 10.1128/jvi.67.10.6096-6104.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Squire S, Raymackers JM, Vandebrouck C, Potter A, Tinsley J, Fisher R, Gillis JM, Davies KE. Prevention of pathology in mdx mice by expression of utrophin: analysis using an inducible transgenic expression system. Hum Mol Genet. 2002;11:3333–3344. doi: 10.1093/hmg/11.26.3333. [DOI] [PubMed] [Google Scholar]
- 163.Stone MR, O'Neill A, Catino D, Bloch RJ. Specific interaction of the actin-binding domain of dystrophin with intermediate filaments containing keratin 19. Mol Biol Cell. 2005;16:4280–4293. doi: 10.1091/mbc.E05-02-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Summerford C, Bartlett JS, Samulski RJ. AlphaVbeta5 integrin: a co-receptor for adeno-associated virus type 2 infection. Nat Med. 1999;5:78–82. doi: 10.1038/4768. [DOI] [PubMed] [Google Scholar]
- 165.Summerford C, Samulski RJ. Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J Virol. 1998;72:1438–1445. doi: 10.1128/jvi.72.2.1438-1445.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Tinsley J, Deconinck N, Fisher R, Kahn D, Phelps S, Gillis JM, Davies K. Expression of full-length utrophin prevents muscular dystrophy in mdx mice. Nat Med. 1998;4:1441–1444. doi: 10.1038/4033. [DOI] [PubMed] [Google Scholar]
- 167.Tinsley JM, Blake DJ, Roche A, Fairbrother U, Riss J, Byth BC, Knight AE, Kendrick-Jones J, Suthers GK, Love DR, et al. Primary structure of dystrophin-related protein. Nature. 1992;360:591–593. doi: 10.1038/360591a0. [DOI] [PubMed] [Google Scholar]
- 168.Tinsley JM, Davies KE. Utrophin: a potential replacement for dystrophin? Neuromuscul Disord. 1993;3:537–539. doi: 10.1016/0960-8966(93)90111-v. [DOI] [PubMed] [Google Scholar]
- 169.Tinsley JM, Fairclough RJ, Storer R, Wilkes FJ, Potter AC, Squire SE, Powell DS, Cozzoli A, Capogrosso RF, Lambert A, et al. Daily treatment with SMTC1100, a novel small molecule utrophin upregulator, dramatically reduces the dystrophic symptoms in the mdx mouse. PLoS One. 2011;6:e19189. doi: 10.1371/journal.pone.0019189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Tinsley JM, Potter AC, Phelps SR, Fisher R, Trickett JI, Davies KE. Amelioration of the dystrophic phenotype of mdx mice using a truncated utrophin transgene. Nature. 1996;384:349–353. doi: 10.1038/384349a0. [DOI] [PubMed] [Google Scholar]
- 171.Toromanoff A, Adjali O, Larcher T, Hill M, Guigand L, Chenuaud P, Deschamps J-Y, Gauthier O, Blancho G, Vanhove B, et al. Lack of immunotoxicity after regional intravenous (RI) delivery of rAAV to nonhuman primate skeletal muscle. Mol Ther. 2010;18:151–160. doi: 10.1038/mt.2009.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Valentine BA, Cooper BJ, de Lahunta A, O'Quinn R, Blue JT. Canine X-linked muscular dystrophy. An animal model of Duchenne muscular dystrophy: clinical studies. Journal of the Neurological Sciences. 1988;88:69–81. doi: 10.1016/0022-510x(88)90206-7. [DOI] [PubMed] [Google Scholar]
- 173.van Deutekom JC, Janson AA, Ginjaar IB, Frankhuizen WS, Aartsma-Rus A, Bremmer-Bout M, den Dunnen JT, Koop K, van der Kooi AJ, Goemans NM, et al. Local dystrophin restoration with antisense oligonucleotide PRO051. N Engl J Med. 2007;357:2677–2686. doi: 10.1056/NEJMoa073108. [DOI] [PubMed] [Google Scholar]
- 174.Wakefield PM, Tinsley JM, Wood MJ, Gilbert R, Karpati G, Davies KE. Prevention of the dystrophic phenotype in dystrophin/utrophin-deficient muscle following adenovirus-mediated transfer of a utrophin minigene. Gene Ther. 2000;7:201–204. doi: 10.1038/sj.gt.3301066. [DOI] [PubMed] [Google Scholar]
- 175.Wang B, Li J, Xiao X. Adeno-associated virus vector carrying human minidystrophin genes effectively ameliorates muscular dystrophy in mdx mouse model. Proc Natl Acad Sci U S A. 2000;97:13714–13719. doi: 10.1073/pnas.240335297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wang Z, Allen JM, Riddell SR, Gregorevic P, Storb R, Tapscott SJ, Chamberlain JS, Kuhr CS. Immunity to adeno-associated virus-mediated gene transfer in a random-bred canine model of Duchenne muscular dystrophy. Hum Gene Ther. 2007;18:18–26. doi: 10.1089/hum.2006.093. [DOI] [PubMed] [Google Scholar]
- 177.Wang Z, Kuhr CS, Allen JM, Blankinship M, Gregorevic P, Chamberlain JS, Tapscott SJ, Storb R. Sustained AAV-mediated dystrophin expression in a canine model of Duchenne muscular dystrophy with a brief course of immunosuppression. Mol Ther. 2007;15:1160–1166. doi: 10.1038/sj.mt.6300161. [DOI] [PubMed] [Google Scholar]
- 178.Wang Z, Tapscott SJ, Chamberlain JS, Storb R. Immunity and AAV-Mediated Gene Therapy for Muscular Dystrophies in Large Animal Models and Human Trials. Front Microbiol. 2011;2:201. doi: 10.3389/fmicb.2011.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wang Z, Zhu T, Qiao C, Zhou L, Wang B, Zhang J, Chen C, Li J, Xiao X. Adeno-associated virus serotype 8 efficiently delivers genes to muscle and heart. Nat Biotechnol. 2005;23:321–328. doi: 10.1038/nbt1073. [DOI] [PubMed] [Google Scholar]
- 180.Warner LE, DelloRusso C, Crawford RW, Rybakova IN, Patel JR, Ervasti JM, Chamberlain JS. Expression of Dp260 in muscle tethers the actin cytoskeleton to the dystrophin-glycoprotein complex and partially prevents dystrophy. Hum Mol Genet. 2002;11:1095–1105. doi: 10.1093/hmg/11.9.1095. [DOI] [PubMed] [Google Scholar]
- 181.Way M, Pope B, Cross RA, Kendrick-Jones J, Weeds AG. Expression of the N-terminal domain of dystrophin in E. coli and demonstration of binding to F-actin. FEBS Lett. 1992;301:243–245. doi: 10.1016/0014-5793(92)80249-g. [DOI] [PubMed] [Google Scholar]
- 182.Weir AP, Morgan JE, Davies KE. A-utrophin up-regulation in mdx skeletal muscle is independent of regeneration. Neuromuscular Disorders. 2004;14:19–23. doi: 10.1016/j.nmd.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 183.Weller ML, Amornphimoltham P, Schmidt M, Wilson PA, Gutkind JS, Chiorini JA. Epidermal growth factor receptor is a co-receptor for adeno-associated virus serotype 6. Nat Med. 2010;16:662–664. doi: 10.1038/nm.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Wells DJ, Ferrer A, Wells KE. Immunological hurdles in the path to gene therapy for Duchenne muscular dystrophy. Expert Reviews in Molecular Medicine. 2002;4:1–23. doi: 10.1017/S146239940200515X. [DOI] [PubMed] [Google Scholar]
- 185.Wells DJ, Wells KE, Asante EA, Turner G, Sunada Y, Campbell KP, Walsh FS, Dickson G. Expression of human full-length and minidystrophin in transgenic mdx mice: implications for gene therapy of Duchenne muscular dystrophy. Hum Mol Genet. 1995;4:1245–1250. doi: 10.1093/hmg/4.8.1245. [DOI] [PubMed] [Google Scholar]
- 186.Winder SJ. The membrane-cytoskeleton interface: the role of dystrophin and utrophin. J Muscle Res Cell Motil. 1997;18:617–629. doi: 10.1023/a:1018627705273. [DOI] [PubMed] [Google Scholar]
- 187.Winder SJ, Hemmings L, Maciver SK, Bolton SJ, Tinsley JM, Davies KE, Critchley DR, Kendrick-Jones J. Utrophin actin binding domain: analysis of actin binding and cellular targeting. J Cell Sci. 1995;108:63–71. doi: 10.1242/jcs.108.1.63. [DOI] [PubMed] [Google Scholar]
- 188.Wu ZJ, Miller E, Agbandje-McKenna M, Samulski RJ. alpha 2,3 and alpha 2,6 N-linked sialic acids facilitate efficient binding and transduction by adeno-associated virus types 1 and 6. J Virol. 2006;80:9093–9103. doi: 10.1128/JVI.00895-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Xiao W, Chirmule N, Berta SC, McCullough B, Gao G, Wilson JM. Gene therapy vectors based on adeno-associated virus type 1. J Virol. 1999;73:3994–4003. doi: 10.1128/jvi.73.5.3994-4003.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Xiao X, Li J, Samulski RJ. Efficient long-term gene transfer into muscle tissue of immunocompetent mice by adeno-associated virus vector. Journal of Virology. 1996;70:8098–8108. doi: 10.1128/jvi.70.11.8098-8108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Xie Q, Bu W, Bhatia S, Hare J, Somasundaram T, Azzi A, Chapman MS. The atomic structure of adeno-associated virus (AAV-2), a vector for human gene therapy. Proc Natl Acad Sci U S A. 2002;99:10405–10410. doi: 10.1073/pnas.162250899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Yang JS, Zhou WH, Zhang YL, Zidon T, Ritchie T, Engelhardt JF. Concatamerization of adeno-associated virus circular genomes occurs through intermolecular recombination. J Virol. 1999;73:9468–9477. doi: 10.1128/jvi.73.11.9468-9477.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Yuasa K, Sakamoto M, Miyagoe-Suzuki Y, Tanouchi A, Yamamoto H, Li J, Chamberlain J, Xiao X, Takeda S. Adeno-associated virus vector-mediated gene transfer into dystrophin-deficient skeletal muscles evokes enhanced immune response against the transgene product. Gene Ther. 2002;9:1576–1588. doi: 10.1038/sj.gt.3301829. [DOI] [PubMed] [Google Scholar]
- 194.Yue Y, Ghosh A, Long C, Bostick B, Smith BF, Kornegay JN, Duan D. A single intravenous injection of adeno-associated virus serotype-9 leads to whole body skeletal muscle transduction in dogs. Mol Ther. 2008;16:1944–1952. doi: 10.1038/mt.2008.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Zaiss A-K, Liu Q, Bowen GP, Wong NCW, Bartlett JS, Muruve DA. Differential activation of innate immune responses by adenovirus and adeno-associated virus vectors. Journal of Virology. 2002;76:4580–4590. doi: 10.1128/JVI.76.9.4580-4590.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Zincarelli C, Soltys S, Rengo G, Koch WJ, Rabinowitz JE. Comparative cardiac gene delivery of adeno-associated virus serotypes 1–9 reveals that AAV6 mediates the most efficient transduction in mouse heart. Clin Transl Sci. 2010;3:81–89. doi: 10.1111/j.1752-8062.2010.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Zincarelli C, Soltys S, Rengo G, Rabinowitz JE. Analysis of AAV serotypes 1–9 mediated gene expression and tropism in mice after systemic injection. Mol Ther. 2008;16:1073–1080. doi: 10.1038/mt.2008.76. [DOI] [PubMed] [Google Scholar]