Abstract
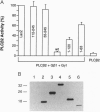
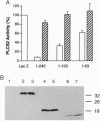
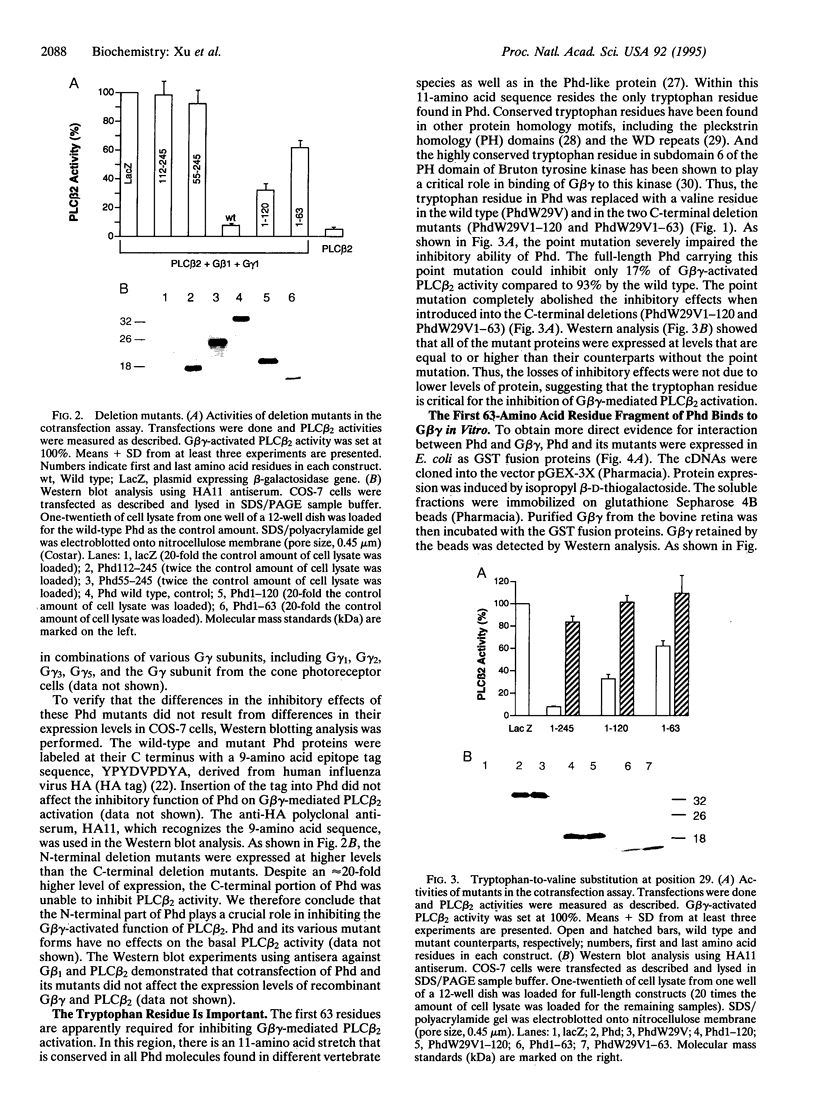
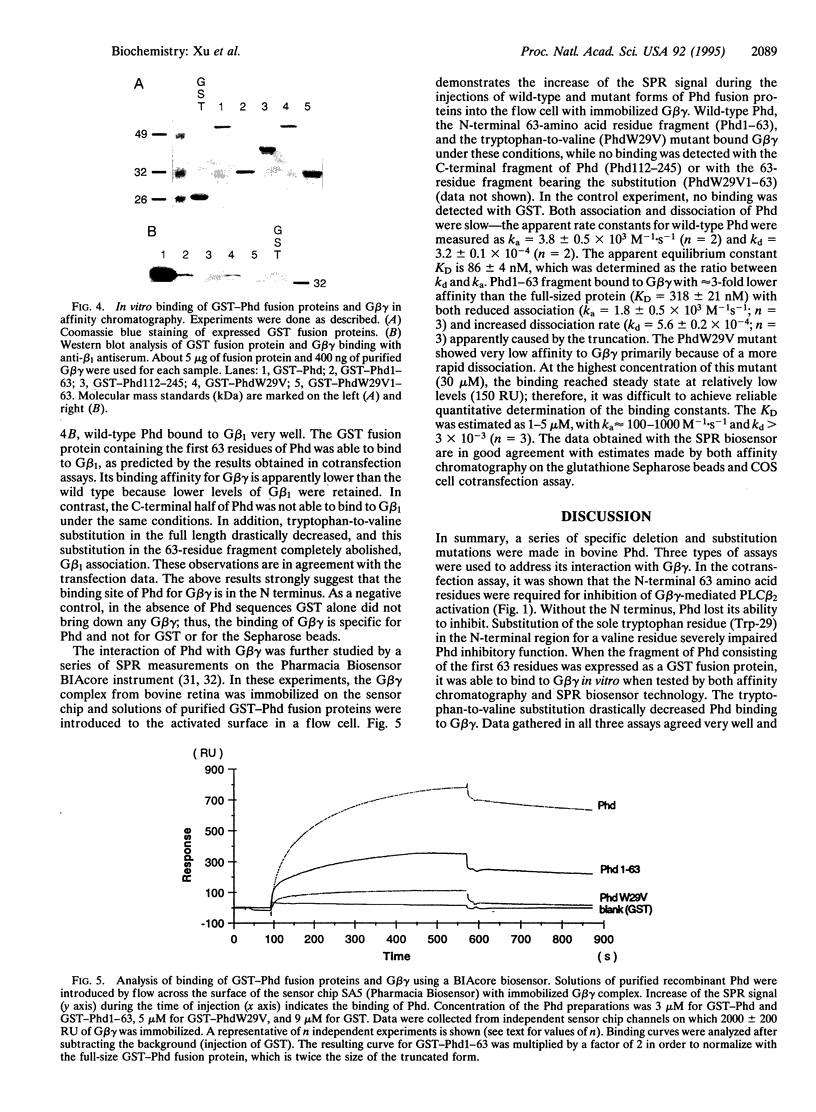
Phosducin is a soluble phosphoprotein found in retinal photoreceptor cells and in the pineal gland. It binds to the beta gamma subunits of guanine nucleotide-binding proteins (G proteins) (G beta gamma) and may regulate G-protein function. In this study, the ability of specific regions of phosducin to bind G beta gamma was characterized. A series of deletion mutants were made in bovine phosducin. They were tested in cotransfection assays for their ability to inhibit G beta gamma-mediated phospholipase C beta 2 isoform activation. Overexpression of the N-terminal half of phosducin showed inhibition, whereas overexpression of the C-terminal half did not. The first 63 amino acid residues were required for inhibition. A tryptophan-to-valine substitution at residue 29, which is part of a well conserved 11-amino acid sequence, severely impaired phosducin inhibitory function. Glutathione S-transferase-phosducin fusion proteins were expressed in Escherichia coli to study phosducin-G beta gamma interaction in vitro. The N-terminal 63-amino acid fragment was able to bind to G beta gamma. In contrast, the C-terminal half failed to bind to G beta gamma. The substitution mutants showed little or no binding. Furthermore, direct measurements of interaction between G beta gamma and fragments of phosducin, using surface plasmon resonance technology, confirmed the assignment of binding activity to the 63-amino acid fragment and the importance of the tryptophan residue.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer P. H., Müller S., Puzicha M., Pippig S., Obermaier B., Helmreich E. J., Lohse M. J. Phosducin is a protein kinase A-regulated G-protein regulator. Nature. 1992 Jul 2;358(6381):73–76. doi: 10.1038/358073a0. [DOI] [PubMed] [Google Scholar]
- Bigay J., Chabre M. Purification of T beta gamma subunit of transducin. Methods Enzymol. 1994;237:449–451. doi: 10.1016/s0076-6879(94)37081-8. [DOI] [PubMed] [Google Scholar]
- Camps M., Carozzi A., Schnabel P., Scheer A., Parker P. J., Gierschik P. Isozyme-selective stimulation of phospholipase C-beta 2 by G protein beta gamma-subunits. Nature. 1992 Dec 17;360(6405):684–686. doi: 10.1038/360684a0. [DOI] [PubMed] [Google Scholar]
- Clapham D. E., Neer E. J. New roles for G-protein beta gamma-dimers in transmembrane signalling. Nature. 1993 Sep 30;365(6445):403–406. doi: 10.1038/365403a0. [DOI] [PubMed] [Google Scholar]
- Craft C. M., Lolley R. N., Seldin M. F., Lee R. H. Rat pineal gland phosducin: cDNA isolation, nucleotide sequence, and chromosomal assignment in the mouse. Genomics. 1991 Jun;10(2):400–409. doi: 10.1016/0888-7543(91)90325-9. [DOI] [PubMed] [Google Scholar]
- Crespo P., Xu N., Simonds W. F., Gutkind J. S. Ras-dependent activation of MAP kinase pathway mediated by G-protein beta gamma subunits. Nature. 1994 Jun 2;369(6479):418–420. doi: 10.1038/369418a0. [DOI] [PubMed] [Google Scholar]
- Deng W. P., Nickoloff J. A. Site-directed mutagenesis of virtually any plasmid by eliminating a unique site. Anal Biochem. 1992 Jan;200(1):81–88. doi: 10.1016/0003-2697(92)90280-k. [DOI] [PubMed] [Google Scholar]
- Hawes B. E., Touhara K., Kurose H., Lefkowitz R. J., Inglese J. Determination of the G beta gamma-binding domain of phosducin. A regulatable modulator of G beta gamma signaling. J Biol Chem. 1994 Nov 25;269(47):29825–29830. [PubMed] [Google Scholar]
- Hekman M., Bauer P. H., Söhlemann P., Lohse M. J. Phosducin inhibits receptor phosphorylation by the beta-adrenergic receptor kinase in a PKA-regulated manner. FEBS Lett. 1994 Apr 25;343(2):120–124. doi: 10.1016/0014-5793(94)80302-1. [DOI] [PubMed] [Google Scholar]
- Hepler J. R., Gilman A. G. G proteins. Trends Biochem Sci. 1992 Oct;17(10):383–387. doi: 10.1016/0968-0004(92)90005-t. [DOI] [PubMed] [Google Scholar]
- Inglese J., Koch W. J., Caron M. G., Lefkowitz R. J. Isoprenylation in regulation of signal transduction by G-protein-coupled receptor kinases. Nature. 1992 Sep 10;359(6391):147–150. doi: 10.1038/359147a0. [DOI] [PubMed] [Google Scholar]
- Jönsson U., Fägerstam L., Ivarsson B., Johnsson B., Karlsson R., Lundh K., Löfås S., Persson B., Roos H., Rönnberg I. Real-time biospecific interaction analysis using surface plasmon resonance and a sensor chip technology. Biotechniques. 1991 Nov;11(5):620–627. [PubMed] [Google Scholar]
- Katz A., Wu D., Simon M. I. Subunits beta gamma of heterotrimeric G protein activate beta 2 isoform of phospholipase C. Nature. 1992 Dec 17;360(6405):686–689. doi: 10.1038/360686a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee R. H., Brown B. M., Lolley R. N. Protein kinase A phosphorylates retinal phosducin on serine 73 in situ. J Biol Chem. 1990 Sep 15;265(26):15860–15866. [PubMed] [Google Scholar]
- Lee R. H., Fowler A., McGinnis J. F., Lolley R. N., Craft C. M. Amino acid and cDNA sequence of bovine phosducin, a soluble phosphoprotein from photoreceptor cells. J Biol Chem. 1990 Sep 15;265(26):15867–15873. [PubMed] [Google Scholar]
- Lee R. H., Lieberman B. S., Lolley R. N. A novel complex from bovine visual cells of a 33,000-dalton phosphoprotein with beta- and gamma-transducin: purification and subunit structure. Biochemistry. 1987 Jun 30;26(13):3983–3990. doi: 10.1021/bi00387a036. [DOI] [PubMed] [Google Scholar]
- Lee R. H., Ting T. D., Lieberman B. S., Tobias D. E., Lolley R. N., Ho Y. K. Regulation of retinal cGMP cascade by phosducin in bovine rod photoreceptor cells. Interaction of phosducin and transducin. J Biol Chem. 1992 Dec 15;267(35):25104–25112. [PubMed] [Google Scholar]
- Logothetis D. E., Kurachi Y., Galper J., Neer E. J., Clapham D. E. The beta gamma subunits of GTP-binding proteins activate the muscarinic K+ channel in heart. Nature. 1987 Jan 22;325(6102):321–326. doi: 10.1038/325321a0. [DOI] [PubMed] [Google Scholar]
- Malmqvist M. Biospecific interaction analysis using biosensor technology. Nature. 1993 Jan 14;361(6408):186–187. doi: 10.1038/361186a0. [DOI] [PubMed] [Google Scholar]
- Miles M. F., Barhite S., Sganga M., Elliott M. Phosducin-like protein: an ethanol-responsive potential modulator of guanine nucleotide-binding protein function. Proc Natl Acad Sci U S A. 1993 Nov 15;90(22):10831–10835. doi: 10.1073/pnas.90.22.10831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musacchio A., Gibson T., Rice P., Thompson J., Saraste M. The PH domain: a common piece in the structural patchwork of signalling proteins. Trends Biochem Sci. 1993 Sep;18(9):343–348. doi: 10.1016/0968-0004(93)90071-t. [DOI] [PubMed] [Google Scholar]
- Neer E. J., Schmidt C. J., Nambudripad R., Smith T. F. The ancient regulatory-protein family of WD-repeat proteins. Nature. 1994 Sep 22;371(6495):297–300. doi: 10.1038/371297a0. [DOI] [PubMed] [Google Scholar]
- Pitcher J. A., Inglese J., Higgins J. B., Arriza J. L., Casey P. J., Kim C., Benovic J. L., Kwatra M. M., Caron M. G., Lefkowitz R. J. Role of beta gamma subunits of G proteins in targeting the beta-adrenergic receptor kinase to membrane-bound receptors. Science. 1992 Aug 28;257(5074):1264–1267. doi: 10.1126/science.1325672. [DOI] [PubMed] [Google Scholar]
- Reig J. A., Yu L., Klein D. C. Pineal transduction. Adrenergic----cyclic AMP-dependent phosphorylation of cytoplasmic 33-kDa protein (MEKA) which binds beta gamma-complex of transducin. J Biol Chem. 1990 Apr 5;265(10):5816–5824. [PubMed] [Google Scholar]
- Reuveny E., Slesinger P. A., Inglese J., Morales J. M., Iñiguez-Lluhi J. A., Lefkowitz R. J., Bourne H. R., Jan Y. N., Jan L. Y. Activation of the cloned muscarinic potassium channel by G protein beta gamma subunits. Nature. 1994 Jul 14;370(6485):143–146. doi: 10.1038/370143a0. [DOI] [PubMed] [Google Scholar]
- Simon M. I., Strathmann M. P., Gautam N. Diversity of G proteins in signal transduction. Science. 1991 May 10;252(5007):802–808. doi: 10.1126/science.1902986. [DOI] [PubMed] [Google Scholar]
- Stephens L., Smrcka A., Cooke F. T., Jackson T. R., Sternweis P. C., Hawkins P. T. A novel phosphoinositide 3 kinase activity in myeloid-derived cells is activated by G protein beta gamma subunits. Cell. 1994 Apr 8;77(1):83–93. doi: 10.1016/0092-8674(94)90237-2. [DOI] [PubMed] [Google Scholar]
- Tang W. J., Gilman A. G. Type-specific regulation of adenylyl cyclase by G protein beta gamma subunits. Science. 1991 Dec 6;254(5037):1500–1503. doi: 10.1126/science.1962211. [DOI] [PubMed] [Google Scholar]
- Tsukada S., Simon M. I., Witte O. N., Katz A. Binding of beta gamma subunits of heterotrimeric G proteins to the PH domain of Bruton tyrosine kinase. Proc Natl Acad Sci U S A. 1994 Nov 8;91(23):11256–11260. doi: 10.1073/pnas.91.23.11256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickman K. D., Iñiguez-Lluhl J. A., Davenport P. A., Taussig R., Krapivinsky G. B., Linder M. E., Gilman A. G., Clapham D. E. Recombinant G-protein beta gamma-subunits activate the muscarinic-gated atrial potassium channel. Nature. 1994 Mar 17;368(6468):255–257. doi: 10.1038/368255a0. [DOI] [PubMed] [Google Scholar]
- Wilson I. A., Niman H. L., Houghten R. A., Cherenson A. R., Connolly M. L., Lerner R. A. The structure of an antigenic determinant in a protein. Cell. 1984 Jul;37(3):767–778. doi: 10.1016/0092-8674(84)90412-4. [DOI] [PubMed] [Google Scholar]