Abstract
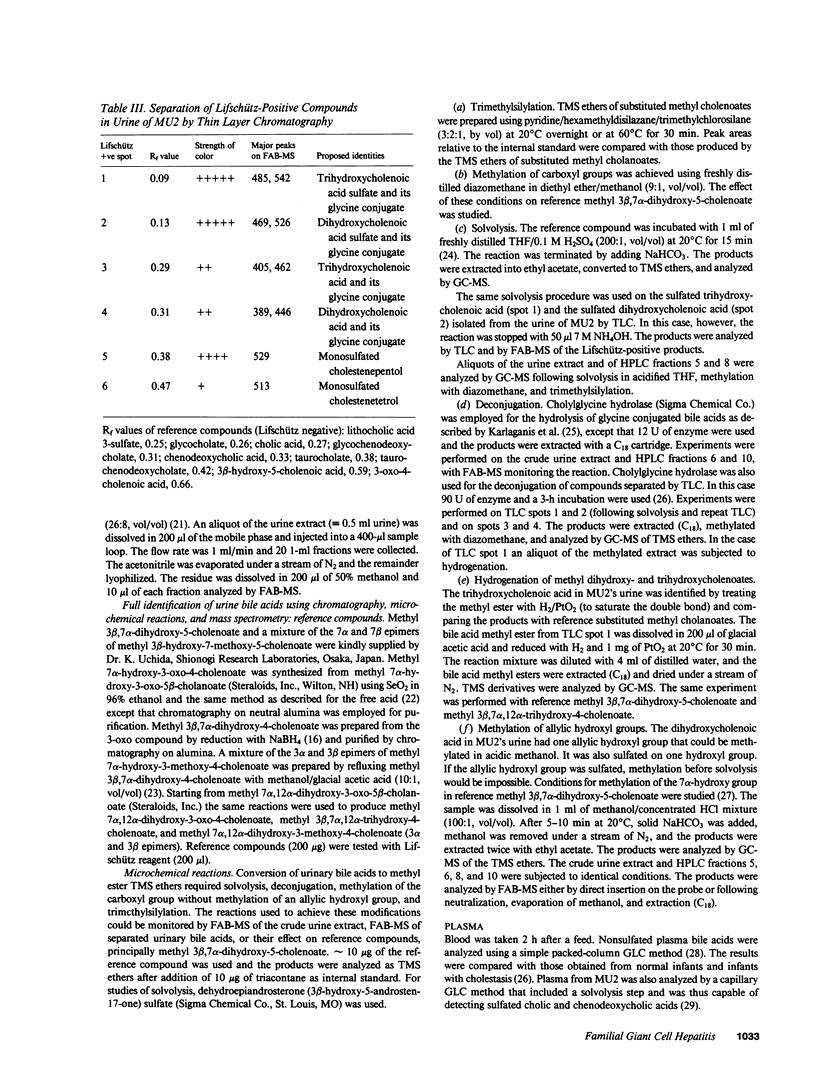
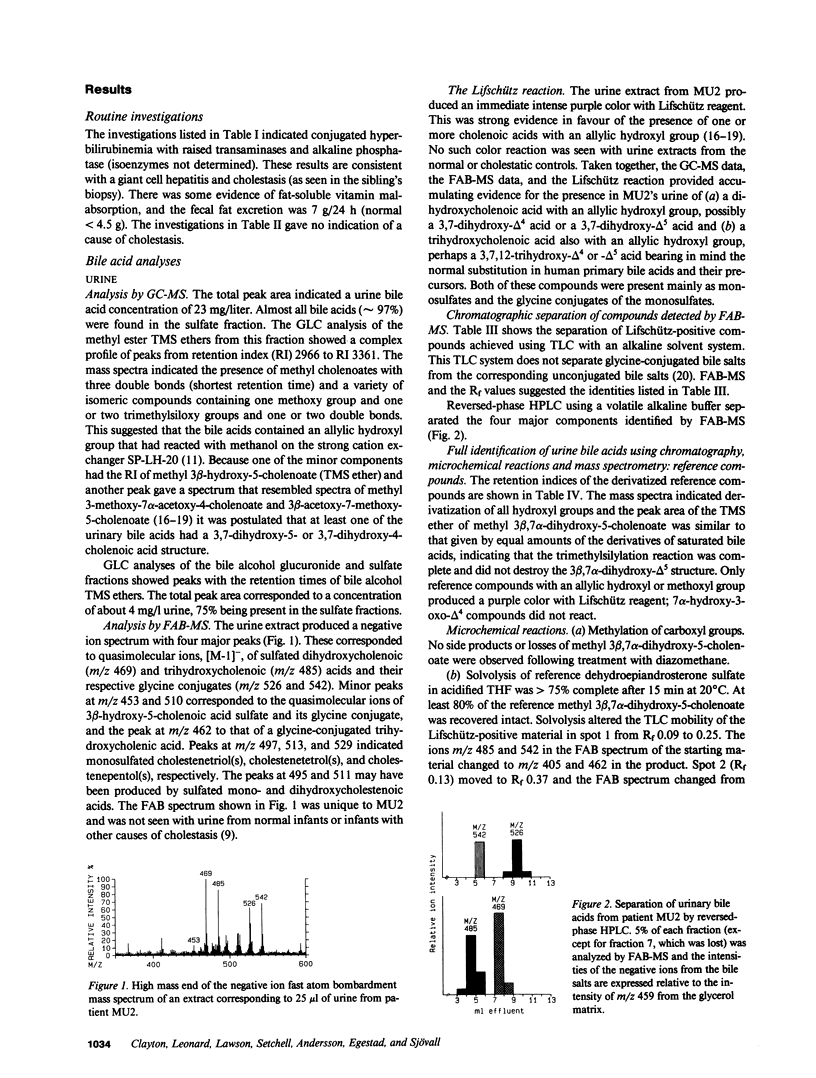
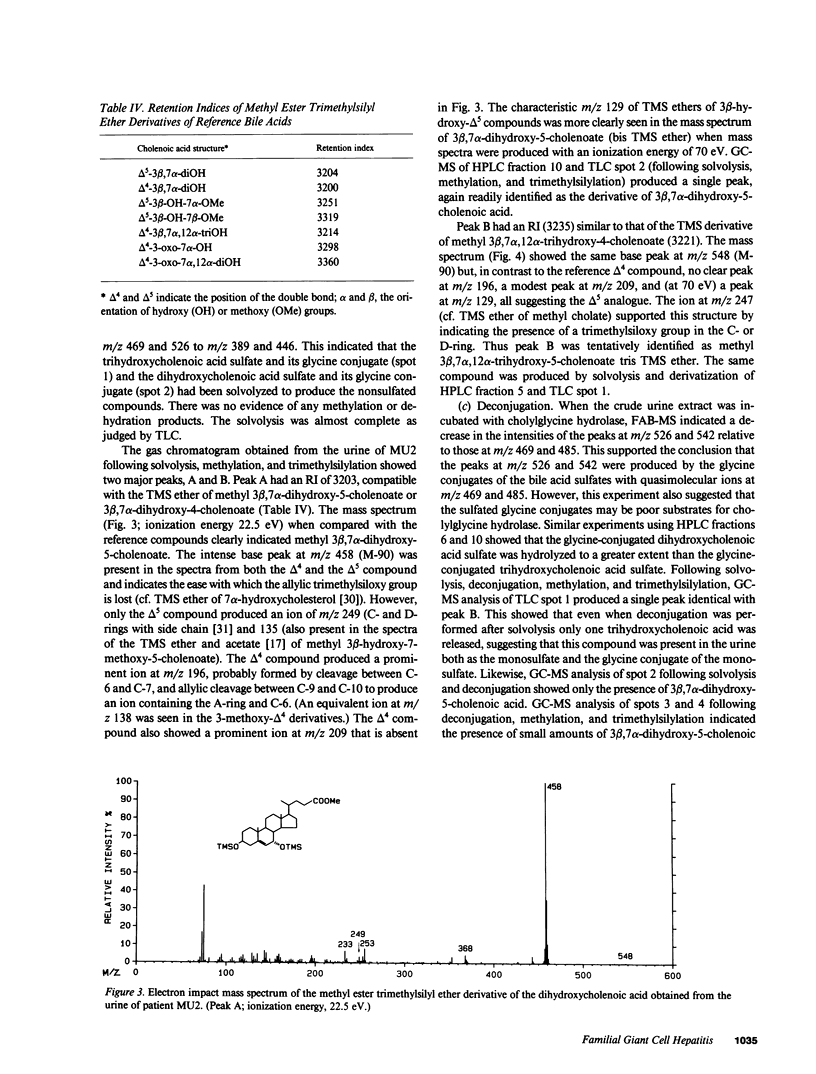
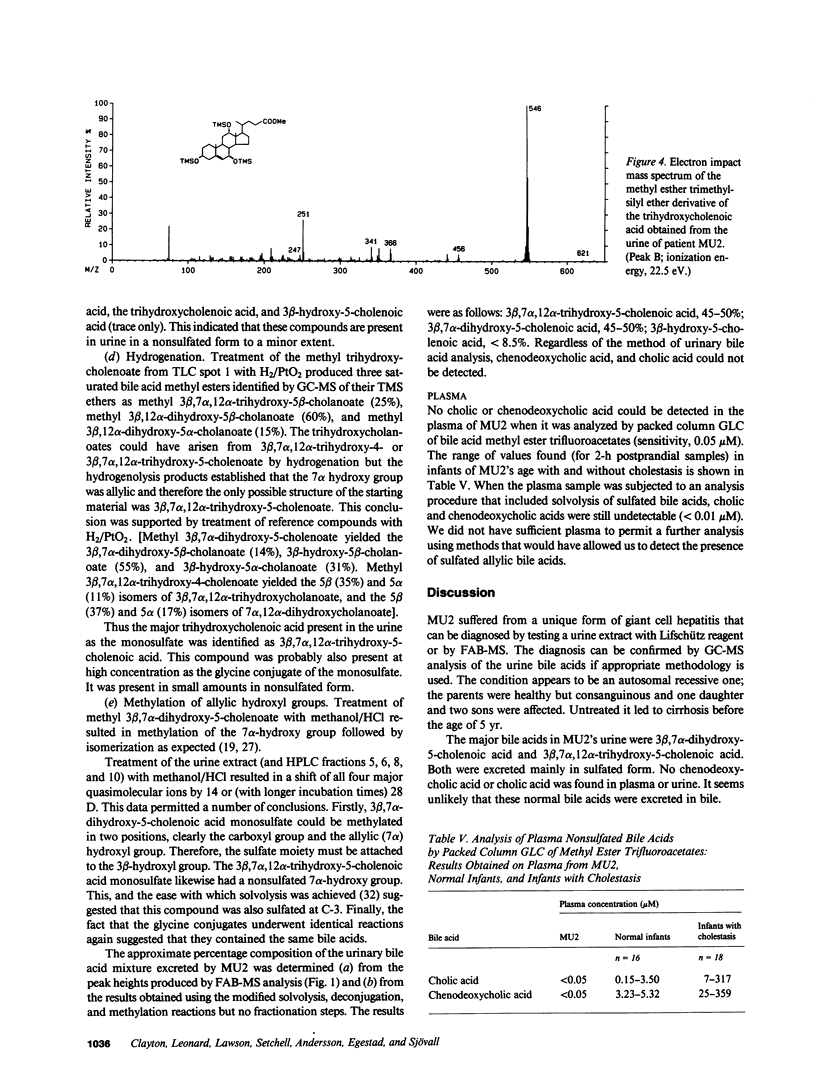
Urinary bile acids from a 3-mo-old boy with cholestatic jaundice were analyzed by ion exchange chromatography and gas chromatography-mass spectrometry (GC-MS). This suggested the presence of labile sulfated cholenoic acids with an allylic hydroxyl group, a conclusion supported by analysis using fast atom bombardment mass spectrometry (FAB-MS). The compounds detected by FAB-MS were separated by thin layer chromatography and high performance liquid chromatography. The sulfated bile acids could be solvolyzed in acidified tetrahydrofuran, and glycine conjugates were partially hydrolyzed by cholylglycine hydrolase. Following solvolysis, deconjugation, and methylation with diazomethane, the bile acids were identified by GC-MS of trimethylsilyl derivatives. The major bile acids in the urine were 3 beta,7 alpha-dihydroxy-5-cholenoic acid 3-sulfate, 3 beta,7 alpha,12 alpha-trihydroxy-5-cholenoic acid monosulfate, and their glycine conjugates. Chenodeoxycholic acid and cholic acid were undetectable in urine and plasma. The family pedigree suggested that abnormal bile acid synthesis was an autosomal recessive condition leading to cirrhosis in early childhood.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almé B., Bremmelgaard A., Sjövall J., Thomassen P. Analysis of metabolic profiles of bile acids in urine using a lipophilic anion exchanger and computerized gas-liquid chromatorgaphy-mass spectrometry. J Lipid Res. 1977 May;18(3):339–362. [PubMed] [Google Scholar]
- Anderson K. E., Kok E., Javitt N. B. Bile acid synthesis in man: metabolism of 7 -hydroxycholesterol- 14 C and 26-hydroxycholesterol- 3 H. J Clin Invest. 1972 Jan;51(1):112–117. doi: 10.1172/JCI106780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björkhem I., Einarsson K., Gustafsson J. A. 3 -Hydroxy- 5 -C 19 -and C 21 -steroid oxidoreductase activity in rat liver. Steroids. 1972 Apr;19(4):471–476. doi: 10.1016/s0039-128x(72)80015-1. [DOI] [PubMed] [Google Scholar]
- Bremmelgaard A., Sjövall J. Bile acid profiles in urine of patients with liver diseases. Eur J Clin Invest. 1979 Oct;9(5):341–348. doi: 10.1111/j.1365-2362.1979.tb00894.x. [DOI] [PubMed] [Google Scholar]
- Clayton P. T., Muller D. P. A simplified gas-liquid chromatographic methods for the estimation of non-sulphated plasma bile acids. Clin Chim Acta. 1980 Aug 19;105(3):401–405. doi: 10.1016/0009-8981(80)90122-9. [DOI] [PubMed] [Google Scholar]
- Cohen B. I., Budai K., Javitt N. B. Solvolysis of chenodeoxycholic acid sulfates. Steroids. 1981 Jun;37(6):621–626. doi: 10.1016/s0039-128x(81)90149-5. [DOI] [PubMed] [Google Scholar]
- Egestad B., Pettersson P., Skrede S., Sjövall J. Fast atom bombardment mass spectrometry in the diagnosis of cerebrotendinous xanthomatosis. Scand J Clin Lab Invest. 1985 Sep;45(5):443–446. doi: 10.3109/00365518509155241. [DOI] [PubMed] [Google Scholar]
- Goldfischer S., Collins J., Rapin I., Neumann P., Neglia W., Spiro A. J., Ishii T., Roels F., Vamecq J., Van Hoof F. Pseudo-Zellweger syndrome: deficiencies in several peroxisomal oxidative activities. J Pediatr. 1986 Jan;108(1):25–32. doi: 10.1016/s0022-3476(86)80764-8. [DOI] [PubMed] [Google Scholar]
- Gustafsson J. A., Shackleton C. H., Sjövall J. Steroids in newborns and infants. A semiquantitative analysis of steroids in faeces from infants. Acta Endocrinol (Copenh) 1970 Sep;65(1):18–28. [PubMed] [Google Scholar]
- Hanson R. F., Isenberg J. N., Williams G. C., Hachey D., Szczepanik P., Klein P. D., Sharp H. L. The metabolism of 3alpha, 7alpha, 12alpha-trihydorxy-5beta-cholestan-26-oic acid in two siblings with cholestasis due to intrahepatic bile duct anomalies. An apparent inborn error of cholic acid synthesis. J Clin Invest. 1975 Sep;56(3):577–587. doi: 10.1172/JCI108127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harano T., Harano K., Yamasaki K. Methoxylation of 3beta,7alpha-dihydroxychol-5-en-24-oic acid, a key intermediate of chenodeoxycholic acid biogenesis, compared with that of its 7beta-epimer. Steroids. 1978 Jul-Aug;32(1):73–83. doi: 10.1016/0039-128x(78)90101-0. [DOI] [PubMed] [Google Scholar]
- Ikeda M., Yamasaki K. Isolation of 3beta,7alpha-dihydroxychol-5-enoic acid, an intermediate of chenodeoxycholic acid biogenesis, and 3alpha,7alpha-dihydroxychol-4-enoic acid from bladder bile of hens. J Biochem. 1978 Mar;83(3):799–805. doi: 10.1093/oxfordjournals.jbchem.a131975. [DOI] [PubMed] [Google Scholar]
- Ikeda M., Yamasaki K. Methoxylation of methyl 3alpha,7alpha-dihydroxychol-4-en-24-oate and its 3beta-epimer. A contribution to chenodeoxycholic acid biogenesis. Steroids. 1978 Jul-Aug;32(1):85–93. doi: 10.1016/0039-128x(78)90102-2. [DOI] [PubMed] [Google Scholar]
- Javitt N. B., Emerman S. Effect of sodium taurolithocholate on bile flow and bile acid exeretion. J Clin Invest. 1968 May;47(5):1002–1014. doi: 10.1172/JCI105790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallner A. A method of synthesis of allocholanoic acids. Bile acids and steroids 182. Acta Chem Scand. 1967;21(2):322–328. doi: 10.3891/acta.chem.scand.21-0322. [DOI] [PubMed] [Google Scholar]
- Karlaganis G., Nemeth A., Hammarskjöld B., Strandvik B., Sjövall J. Urinary excretion of bile alcohols in normal children and patients with alpha 1-antitrypsin deficiency during development of liver disease. Eur J Clin Invest. 1982 Oct;12(5):399–405. doi: 10.1111/j.1365-2362.1982.tb00687.x. [DOI] [PubMed] [Google Scholar]
- Karlaganis G., Schwarzenbach R. P., Paumgartner G. Analysis of serum bile acids by capillary gas--liquid chromatography--mass spectrometry. J Lipid Res. 1980 Mar;21(3):377–381. [PubMed] [Google Scholar]
- Kok E., Burstein S., Javitt N. B., Gut M., Byon C. Y. Bile acid synthesis. Metabolism of 3 beta-hydroxy-5-cholenoic acid in the hamster. J Biol Chem. 1981 Jun 25;256(12):6155–6159. [PubMed] [Google Scholar]
- Krisans S. K., Thompson S. L., Pena L. A., Kok E., Javitt N. B. Bile acid synthesis in rat liver peroxisomes: metabolism of 26-hydroxycholesterol to 3 beta-hydroxy-5-cholenoic acid. J Lipid Res. 1985 Nov;26(11):1324–1332. [PubMed] [Google Scholar]
- Kulkarni B., Javitt N. B. Chenodeoxycholic acid synthesis in the hamster: a metabolic pathway via 3 beta, 7 alpha-dihydroxy-5-cholen-24-oic acid. Steroids. 1982 Nov;40(5):581–589. doi: 10.1016/0039-128x(82)90078-2. [DOI] [PubMed] [Google Scholar]
- Laatikainen T., Perheentupa J., Vihko R., Makino I., Sjövall J. Bile acids and hormonal steroids in bile of a boy with 3 -hyseoxysteroid dehydrogenase deficiency. J Steroid Biochem. 1972 Jun;3(4):715–719. doi: 10.1016/0022-4731(72)90062-3. [DOI] [PubMed] [Google Scholar]
- Lawson A. M., Madigan M. J., Shortland D., Clayton P. T. Rapid diagnosis of Zellweger syndrome and infantile Refsum's disease by fast atom bombardment--mass spectrometry of urine bile salts. Clin Chim Acta. 1986 Dec 15;161(2):221–231. doi: 10.1016/0009-8981(86)90215-9. [DOI] [PubMed] [Google Scholar]
- Oftebro H., Björkhem I., Skrede S., Schreiner A., Pederson J. I. Cerebrotendinous xanthomatosis: a defect in mitochondrial 26-hydroxylation required for normal biosynthesis of cholic acid. J Clin Invest. 1980 Jun;65(6):1418–1430. doi: 10.1172/JCI109806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salen G., Shefer S., Cheng F. W., Dayal B., Batta A. K., Tint G. S. Cholic acid biosynthesis: the enzymatic defect in cerebrotendinous xanthomatosis. J Clin Invest. 1979 Jan;63(1):38–44. doi: 10.1172/JCI109275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutgens R. B., Heymans H. S., Wanders R. J., van den Bosch H., Tager J. M. Peroxisomal disorders: a newly recognised group of genetic diseases. Eur J Pediatr. 1986 Feb;144(5):430–440. doi: 10.1007/BF00441734. [DOI] [PubMed] [Google Scholar]
- Setchell K. D., Almé B., Axelson M., Sjövall J. The multicomponent analysis of conjugates of neutral steroids in urine by lipophilic ion exchange chromatography and computerised gas chromatography-mass spectrometry. J Steroid Biochem. 1976 Aug;7:615–629. doi: 10.1016/0022-4731(76)90086-8. [DOI] [PubMed] [Google Scholar]
- Setchell K. D., Matsui A. Serum bile acid analysis. Clin Chim Acta. 1983 Jan 7;127(1):1–17. doi: 10.1016/0009-8981(83)90070-0. [DOI] [PubMed] [Google Scholar]
- Shefer S., Cheng F. W., Dayal B., Hauser S., Tint G. S., Salen G., Mosbach E. H. A 25-hydroxylation pathway of cholic acid biosynthesis in man and rat. J Clin Invest. 1976 Apr;57(4):897–903. doi: 10.1172/JCI108366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjövall J., Lawson A. M., Setchell K. D. Mass spectrometry of bile acids. Methods Enzymol. 1985;111:63–113. doi: 10.1016/s0076-6879(85)11006-2. [DOI] [PubMed] [Google Scholar]
- Subbiah M. T., Kuksis A. Alkaline solvent systems for thin-layer chromatography of bile acids. J Lipid Res. 1968 Mar;9(2):288–290. [PubMed] [Google Scholar]
- Wachtel N., Emerman S., Javitt N. B. Metabolism of cholest-5-ene-3 beta, 26-diol in the rat and hamster. J Biol Chem. 1968 Oct 10;243(19):5207–5212. [PubMed] [Google Scholar]
- Wikvall K. Purification and properties of a 3 beta-hydroxy-delta 5-C27-steroid oxidoreductase from rabbit liver microsomes. J Biol Chem. 1981 Apr 10;256(7):3376–3380. [PubMed] [Google Scholar]
- Yamasaki H., Yamasaki K. Natural occurrence of 3 , 7 -dihydroxychol-4-en-24-oic acid in hen bile. J Biochem. 1971 Aug;70(2):235–241. doi: 10.1093/oxfordjournals.jbchem.a129635. [DOI] [PubMed] [Google Scholar]


