Abstract
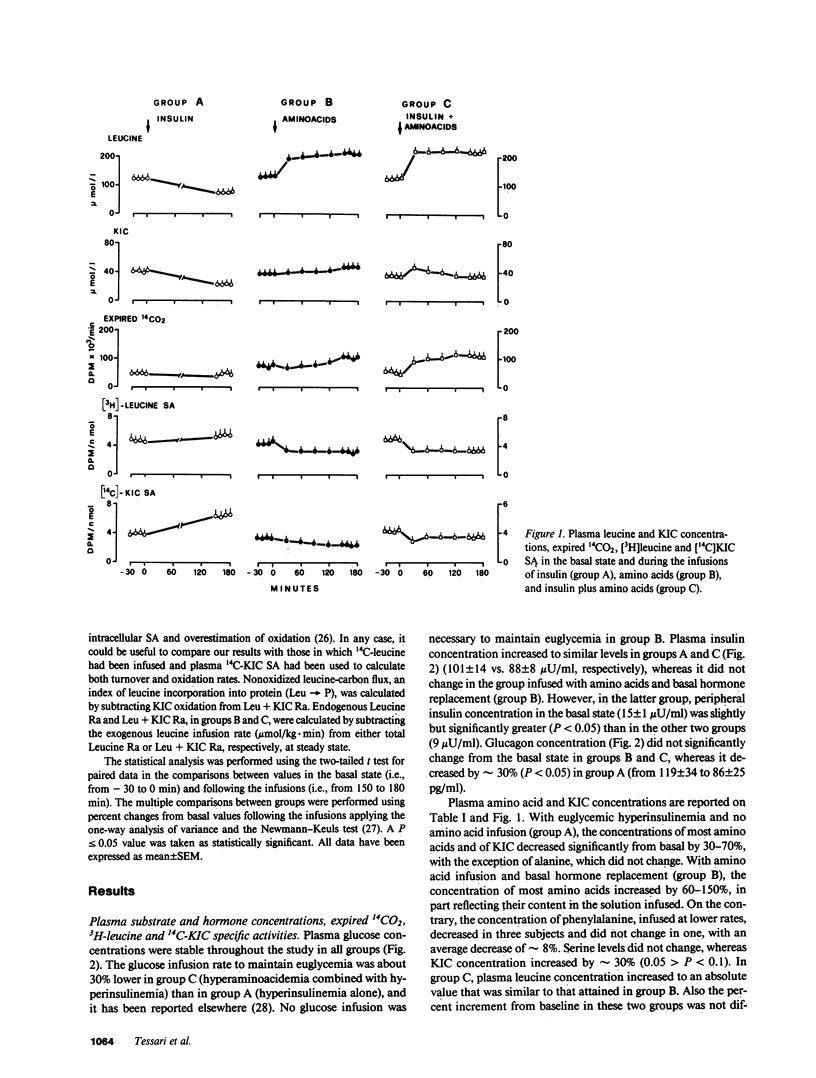
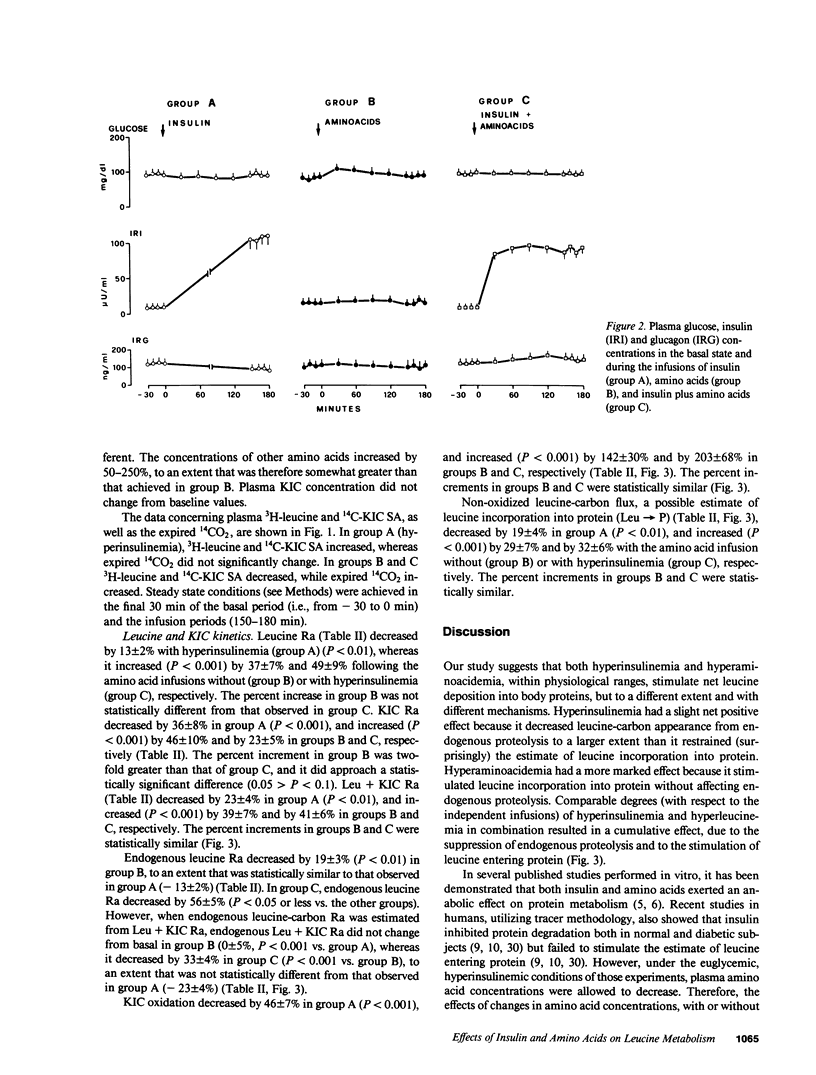
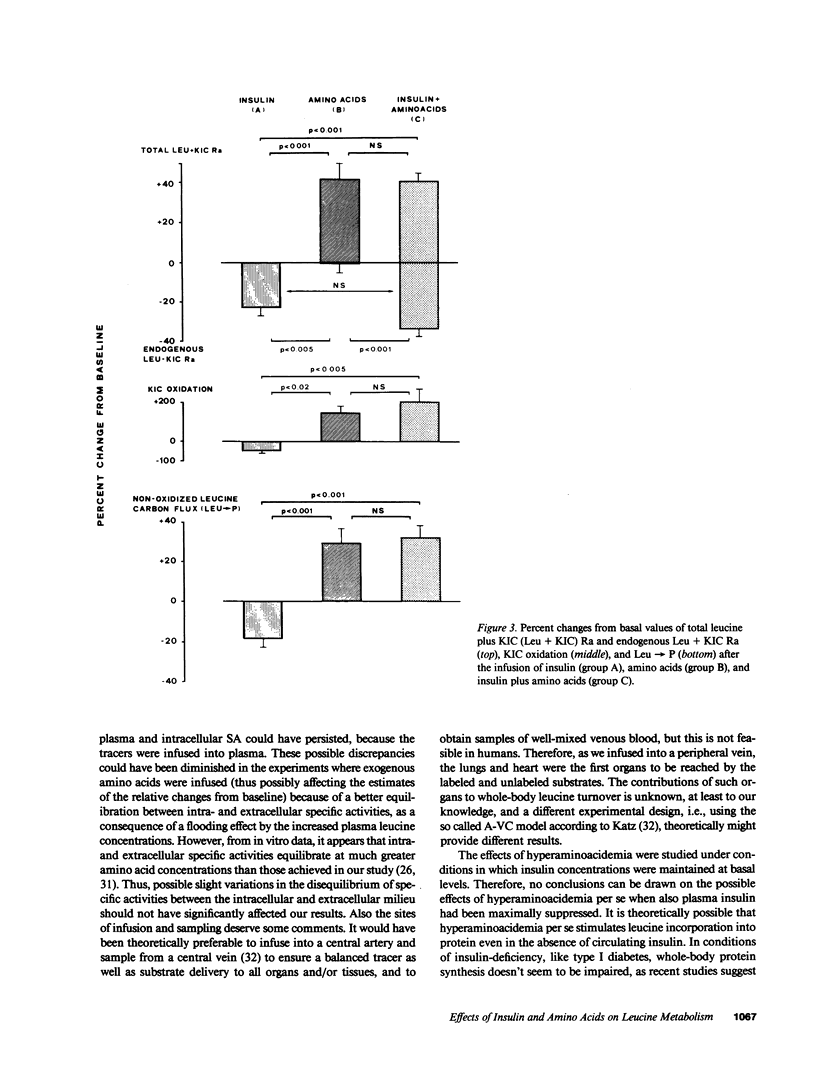
The effects of physiologic hyperinsulinemia and hyperaminoacidemia, alone or in combination, on leucine kinetics in vivo were studied in postabsorptive healthy subjects with primed-constant infusions of L-[4,5-3H]leucine and [1-14C]alpha-ketoisocaproate (KIC) under euglycemic conditions. Hyperinsulinemia (approximately 100 microU/ml) decreased (P less than 0.05 vs. baseline) steady state Leucine + KIC rates of appearance (Ra) from proteolysis, KIC (approximately leucine-carbon) oxidation, and nonoxidized leucine-carbon flux (leucine----protein). Hyperaminoacidemia (plasma leucine, 210 mumol/liter), with either basal hormone replacement or combined to hyperinsulinemia, resulted in comparable increases in leucine + KIC Ra, KIC oxidation, and leucine----protein (P less than 0.05 vs. baseline). However, endogenous leucine + KIC Ra was suppressed only with the combined infusion. Therefore, on the basis of leucine kinetic data, hyperinsulinemia and hyperaminoacidemia stimulated net protein anabolism in vivo by different mechanisms. Hyperinsulinemia decreased proteolysis but did not stimulate leucine----protein. Hyperaminoacidemia per se stimulated leucine----protein but did not suppress endogenous proteolysis. When combined, they had a cumulative effect on net leucine deposition into body protein.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abumrad N. N., Jefferson L. S., Rannels S. R., Williams P. E., Cherrington A. D., Lacy W. W. Role of insulin in the regulation of leucine kinetics in the conscious dog. J Clin Invest. 1982 Nov;70(5):1031–1041. doi: 10.1172/JCI110690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abumrad N. N., Rabin D., Diamond M. P., Lacy W. W. Use of a heated superficial hand vein as an alternative site for the measurement of amino acid concentrations and for the study of glucose and alanine kinetics in man. Metabolism. 1981 Sep;30(9):936–940. doi: 10.1016/0026-0495(81)90074-3. [DOI] [PubMed] [Google Scholar]
- Beaufrere B., Tessari P., Cattalini M., Miles J., Haymond M. W. Apparent decreased oxidation and turnover of leucine during infusion of medium-chain triglycerides. Am J Physiol. 1985 Aug;249(2 Pt 1):E175–E182. doi: 10.1152/ajpendo.1985.249.2.E175. [DOI] [PubMed] [Google Scholar]
- Buse M. G., Biggers J. F., Drier C., Buse J. F. The effect of epinephrine, glucagon, and the nutritional state on the oxidation of branched chain amino acids and pyruvate by isolated hearts and diaphragms of the rat. J Biol Chem. 1973 Jan 25;248(2):697–706. [PubMed] [Google Scholar]
- Buse M. G., Reid S. S. Leucine. A possible regulator of protein turnover in muscle. J Clin Invest. 1975 Nov;56(5):1250–1261. doi: 10.1172/JCI108201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill G. F., Jr The Banting Memorial Lecture 1971. Physiology of insulin in man. Diabetes. 1971 Dec;20(12):785–799. doi: 10.2337/diab.20.12.785. [DOI] [PubMed] [Google Scholar]
- Clemens A. H., Hough D. L., D'Orazio P. A. Development of the Biostator Glucose clamping algorithm. Clin Chem. 1982 Sep;28(9):1899–1904. [PubMed] [Google Scholar]
- DeFronzo R. A., Tobin J. D., Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979 Sep;237(3):E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- Freund H., Yoshimura N., Lunetta L., Fischer J. E. The role of the branched-chain amino acids in decreasing muscle catabolism in vivo. Surgery. 1978 Jun;83(6):611–618. [PubMed] [Google Scholar]
- Fukagawa N. K., Minaker K. L., Rowe J. W., Goodman M. N., Matthews D. E., Bier D. M., Young V. R. Insulin-mediated reduction of whole body protein breakdown. Dose-response effects on leucine metabolism in postabsorptive men. J Clin Invest. 1985 Dec;76(6):2306–2311. doi: 10.1172/JCI112240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden M. H., Waterlow J. C. Total protein synthesis in elderly people: a comparison of results with [15N]glycine and [14C]leucine. Clin Sci Mol Med. 1977 Sep;53(3):277–288. doi: 10.1042/cs0530277. [DOI] [PubMed] [Google Scholar]
- Greenberg G. R., Marliss E. B., Anderson G. H., Langer B., Spence W., Tovee E. B., Jeejeebhoy K. N. Protein-sparing therapy in postoperative patients. Effects of added hypocaloric glucose or lipid. N Engl J Med. 1976 Jun 24;294(26):1411–1416. doi: 10.1056/NEJM197606242942601. [DOI] [PubMed] [Google Scholar]
- Herbert V., Lau K. S., Gottlieb C. W., Bleicher S. J. Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab. 1965 Oct;25(10):1375–1384. doi: 10.1210/jcem-25-10-1375. [DOI] [PubMed] [Google Scholar]
- Hutson S. M., Zapalowski C., Cree T. C., Harper A. E. Regulation of leucine and alpha-ketoisocaproic acid metabolism in skeletal muscle. Effects of starvation and insulin. J Biol Chem. 1980 Mar 25;255(6):2418–2426. [PubMed] [Google Scholar]
- James W. P., Garlick P. J., Sender P. M., Waterlow J. C. Studies of amino acid and protein metabolism in normal man with L-[U-14C]tyrosine. Clin Sci Mol Med. 1976 Jun;50(6):525–532. doi: 10.1042/cs0500525. [DOI] [PubMed] [Google Scholar]
- Jefferson L. S., Li J. B., Rannels S. R. Regulation by insulin of amino acid release and protein turnover in the perfused rat hemicorpus. J Biol Chem. 1977 Feb 25;252(4):1476–1483. [PubMed] [Google Scholar]
- Katz J. Importance of sites of tracer administration and sampling in turnover studies. Fed Proc. 1982 Jan;41(1):123–128. [PubMed] [Google Scholar]
- Marchesini G., Zoli M., Dondi C., Bianchi G., Cirulli M., Pisi E. Anticatabolic effect of branched-chain amino acid-enriched solutions in patients with liver cirrhosis. Hepatology. 1982 Jul-Aug;2(4):420–425. doi: 10.1002/hep.1840020405. [DOI] [PubMed] [Google Scholar]
- Morgan H. E., Earl D. C., Broadus A., Wolpert E. B., Giger K. E., Jefferson L. S. Regulation of protein synthesis in heart muscle. I. Effect of amino acid levels on protein synthesis. J Biol Chem. 1971 Apr 10;246(7):2152–2162. [PubMed] [Google Scholar]
- Mortimore G. E., Mondon C. E. Inhibition by insulin of valine turnover in liver. Evidence for a general control of proteolysis. J Biol Chem. 1970 May 10;245(9):2375–2383. [PubMed] [Google Scholar]
- Mortimore G. E., Woodside K. H., Henry J. E. Compartmentation of free valine and its relation to protein turnover in perfused rat liver. J Biol Chem. 1972 May 10;247(9):2776–2784. [PubMed] [Google Scholar]
- Nair K. S., Garrow J. S., Ford C., Mahler R. F., Halliday D. Effect of poor diabetic control and obesity on whole body protein metabolism in man. Diabetologia. 1983 Nov;25(5):400–403. doi: 10.1007/BF00282518. [DOI] [PubMed] [Google Scholar]
- Nissen S. L., Van Huysen C., Haymond M. W. Measurement of branched chain amino acids and branched chain alpha-ketoacids in plasma by high-performance liquid chromatography. J Chromatogr. 1982 Oct 8;232(1):170–175. doi: 10.1016/s0378-4347(00)86021-1. [DOI] [PubMed] [Google Scholar]
- Robert J. J., Bier D. M., Zhao X. H., Matthews D. E., Young V. R. Glucose and insulin effects on the novo amino acid synthesis in young men: studies with stable isotope labeled alanine, glycine, leucine, and lysine. Metabolism. 1982 Dec;31(12):1210–1218. doi: 10.1016/0026-0495(82)90006-3. [DOI] [PubMed] [Google Scholar]
- Schneible P. A., Airhart J., Low R. B. Differential compartmentation of leucine for oxidation and for protein synthesis in cultured skeletal muscle. J Biol Chem. 1981 May 25;256(10):4888–4894. [PubMed] [Google Scholar]
- Schwenk W. F., Beaufrere B., Haymond M. W. Use of reciprocal pool specific activities to model leucine metabolism in humans. Am J Physiol. 1985 Dec;249(6 Pt 1):E646–E650. doi: 10.1152/ajpendo.1985.249.6.E646. [DOI] [PubMed] [Google Scholar]
- Tessari P., Inchiostro S., Biolo G., Duner E., Nosadini R., Tiengo A., Crepaldi G. Hyperaminoacidaemia reduces insulin-mediated glucose disposal in healthy man. Diabetologia. 1985 Nov;28(11):870–872. doi: 10.1007/BF00291080. [DOI] [PubMed] [Google Scholar]
- Tessari P., Nissen S. L., Miles J. M., Haymond M. W. Inverse relationship of leucine flux and oxidation to free fatty acid availability in vivo. J Clin Invest. 1986 Feb;77(2):575–581. doi: 10.1172/JCI112339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessari P., Nosadini R., Trevisan R., De Kreutzenberg S. V., Inchiostro S., Duner E., Biolo G., Marescotti M. C., Tiengo A., Crepaldi G. Defective suppression by insulin of leucine-carbon appearance and oxidation in type 1, insulin-dependent diabetes mellitus. Evidence for insulin resistance involving glucose and amino acid metabolism. J Clin Invest. 1986 Jun;77(6):1797–1804. doi: 10.1172/JCI112504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessari P., Trevisan R., Inchiostro S., Biolo G., Nosadini R., De Kreutzenberg S. V., Duner E., Tiengo A., Crepaldi G. Dose-response curves of effects of insulin on leucine kinetics in humans. Am J Physiol. 1986 Sep;251(3 Pt 1):E334–E342. doi: 10.1152/ajpendo.1986.251.3.E334. [DOI] [PubMed] [Google Scholar]
- Tessari P., Tsalikian E., Schwenk W. F., Nissen S. L., Haymond M. W. Effects of [15N]leucine infused at low rates on leucine metabolism in humans. Am J Physiol. 1985 Jul;249(1 Pt 1):E121–E130. doi: 10.1152/ajpendo.1985.249.1.E121. [DOI] [PubMed] [Google Scholar]
- Woodside K. H., Mortimore G. E. Suppression of protein turnover by amino acids in the perfused rat liver. J Biol Chem. 1972 Oct 25;247(20):6474–6481. [PubMed] [Google Scholar]