Abstract
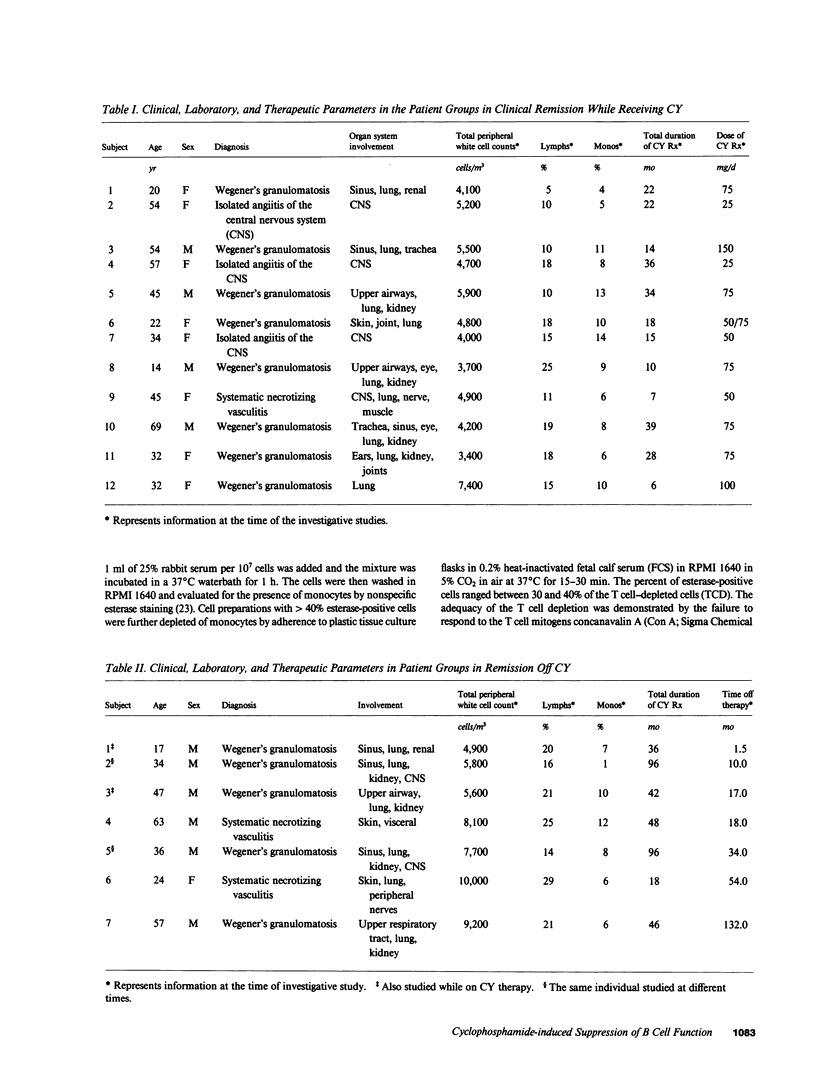
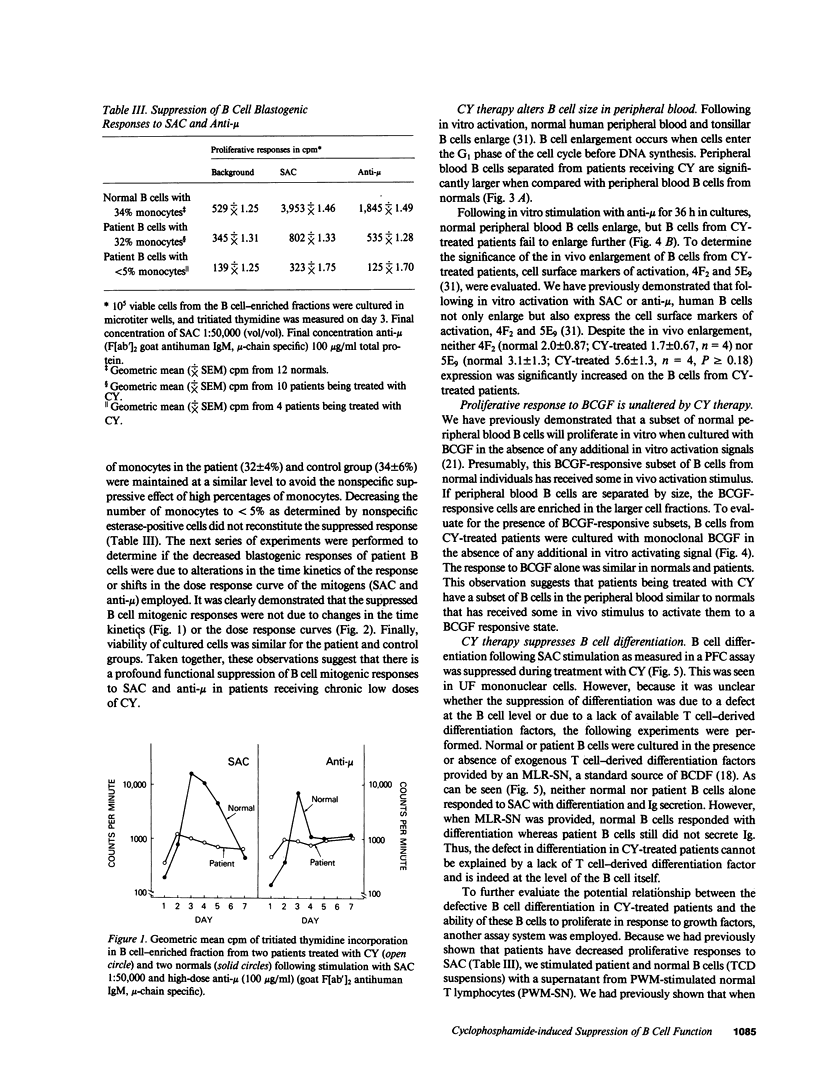
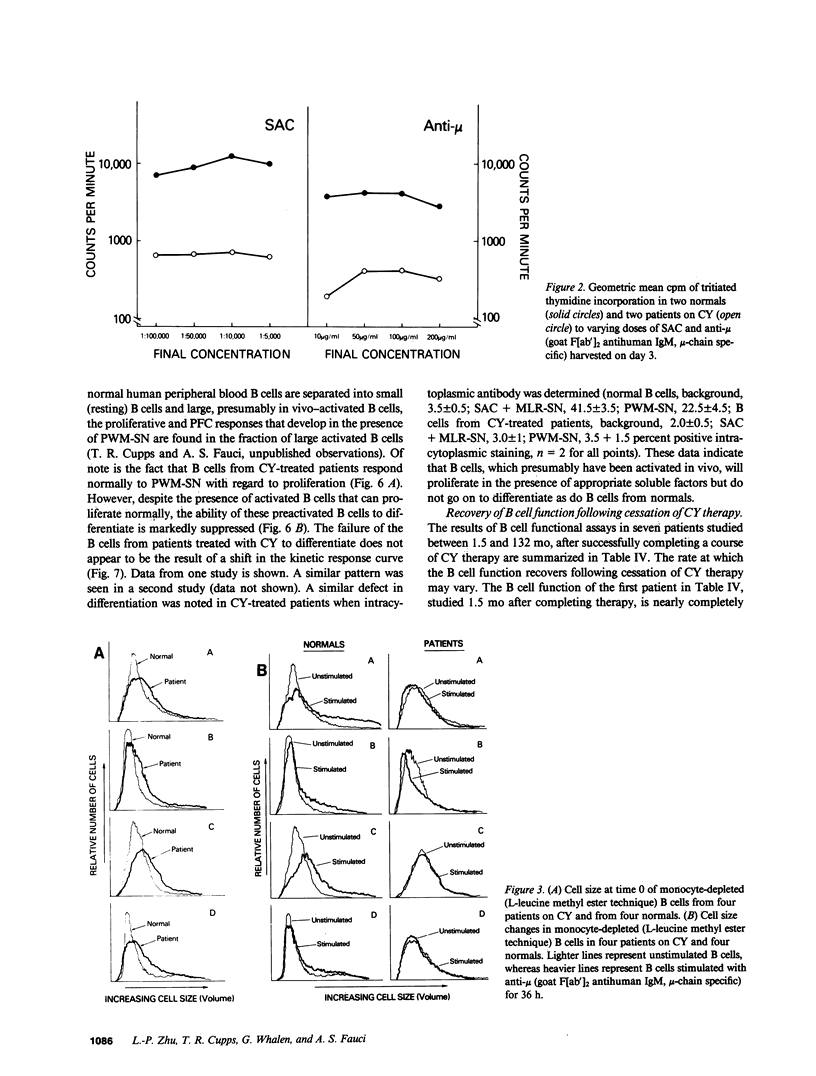
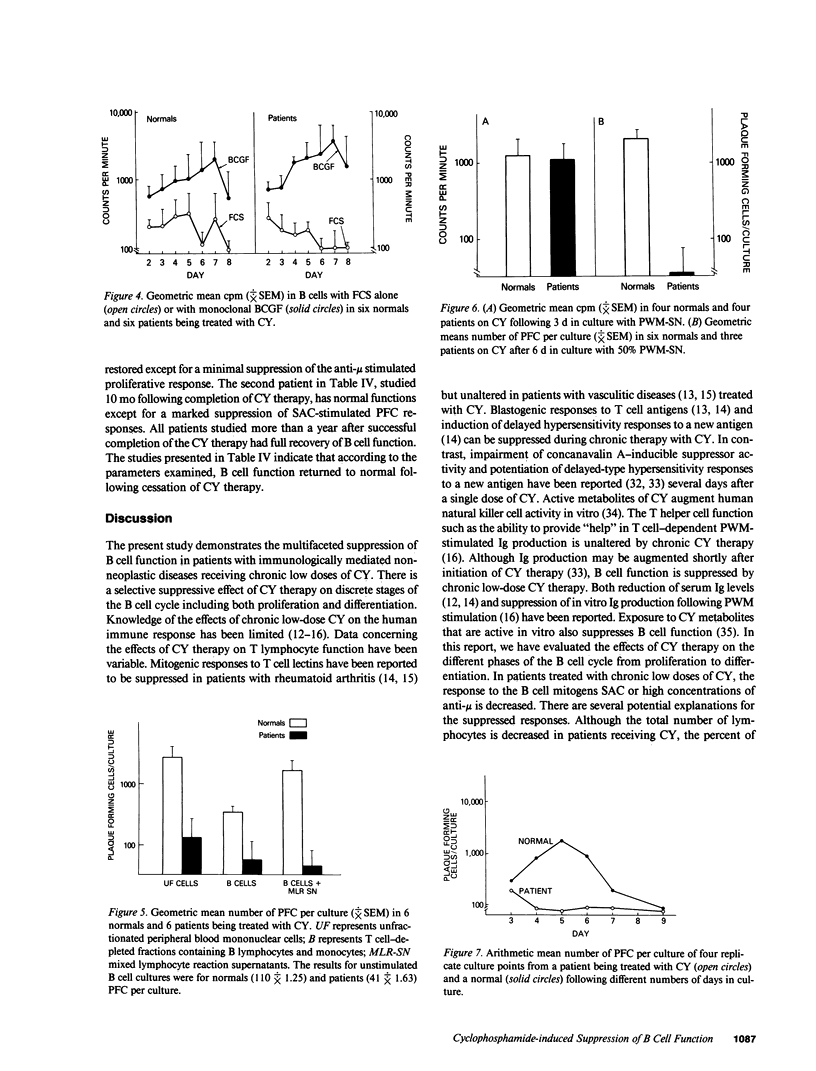
The immune function of B lymphocytes from 12 patients with nonneoplastic immune-mediated diseases receiving chronic low-dose (2 mg/kg per d) cyclophosphamide (CY) was evaluated. There was a selective and differential suppressive effect of CY therapy on the various stages of the B cell cycle including activation, proliferation, and differentiation. The proliferative responses to Staphylococcus aureus Cowan strain I (SAC) and mitogenic concentrations of anti-mu were suppressed. In contrast, B cells that have been presumably activated in vivo proliferated with a normal pattern when exposed to B cell growth factor in vitro. Chronic low-dose CY therapy also suppressed B cell differentiation. Secretion of immunoglobulin by B cells following in vitro triggering with SAC and a T cell supernatant was suppressed in CY-treated patients. Moreover, differentiation of the large in vivo-activated B cells (which do not require an in vitro activation signal) in the presence of appropriate T lymphocyte supernatant was also suppressed. This selective suppression of B cell function at multiple points in the B cell cycle may be responsible for the efficacy of CY therapy in certain antibody and immune complex-mediated diseases.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alepa F. P., Zvaifler N. J., Sliwinski A. J. Immunologic effects of cyclophosphamide treatment in rheumatoid arthritis. Arthritis Rheum. 1970 Nov-Dec;13(6):754–760. doi: 10.1002/art.1780130604. [DOI] [PubMed] [Google Scholar]
- Berd D., Maguire H. C., Jr, Mastrangelo M. J. Impairment of concanavalin A-inducible suppressor activity following administration of cyclophosphamide to patients with advanced cancer. Cancer Res. 1984 Mar;44(3):1275–1280. [PubMed] [Google Scholar]
- Berd D., Maguire H. C., Jr, Mastrangelo M. J. Potentiation of human cell-mediated and humoral immunity by low-dose cyclophosphamide. Cancer Res. 1984 Nov;44(11):5439–5443. [PubMed] [Google Scholar]
- Butler J. L., Muraguchi A., Lane H. C., Fauci A. S. Development of a human T-T cell hybridoma secreting B cell growth factor. J Exp Med. 1983 Jan 1;157(1):60–68. doi: 10.1084/jem.157.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cupps T. R., Edgar L. C., Fauci A. S. Suppression of human B lymphocyte function by cyclophosphamide. J Immunol. 1982 Jun;128(6):2453–2457. [PubMed] [Google Scholar]
- Cupps T. R., Fauci A. S. The vasculitides. Major Probl Intern Med. 1981;21:1–211. [PubMed] [Google Scholar]
- Diamantstein T., Willinger E., Reiman J. T-suppressor cells sensitive to cyclophosphamide and to its in vitro active derivative 4-hydroperoxycyclophosphamide control the mitogenic response of murine splenic B cells to dextran sulfate. A direct proof for different sensitivities of lymphocyte subsets to cyclophosphamide. J Exp Med. 1979 Dec 1;150(6):1571–1576. doi: 10.1084/jem.150.6.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenbarth G. S., Haynes B. F., Schroer J. A., Fauci A. S. Production of monoclonal antibodies reacting with peripheral blood mononuclear cell surface differentiation antigens. J Immunol. 1980 Mar;124(3):1237–1244. [PubMed] [Google Scholar]
- Falkoff R. J., Zhu L. P., Fauci A. S. Separate signals for human B cell proliferation and differentiation in response to Staphylococcus aureus: evidence for a two-signal model of B cell activation. J Immunol. 1982 Jul;129(1):97–102. [PubMed] [Google Scholar]
- Falkoff R. M., Peters M., Fauci A. S. T cell enrichment and depletion of human peripheral blood mononuclear cell preparations. Unexpected findings in the study of the functional activities of the separated populations. J Immunol Methods. 1982;50(1):39–49. doi: 10.1016/0022-1759(82)90302-7. [DOI] [PubMed] [Google Scholar]
- Fauci A. S., Dale D. C., Wolff S. M. Cyclophosphamide and lymphocyte subpopulations in Wegener's granulomatosis. Arthritis Rheum. 1974 Jul-Aug;17(4):355–361. doi: 10.1002/art.1780170404. [DOI] [PubMed] [Google Scholar]
- Fauci A. S., Haynes B. F., Costa J., Katz P., Wolff S. M. Lymphomatoid Granulomatosis. Prospective clinical and therapeutic experience over 10 years. N Engl J Med. 1982 Jan 14;306(2):68–74. doi: 10.1056/NEJM198201143060203. [DOI] [PubMed] [Google Scholar]
- Fauci A. S., Haynes B. F., Katz P., Wolff S. M. Wegener's granulomatosis: prospective clinical and therapeutic experience with 85 patients for 21 years. Ann Intern Med. 1983 Jan;98(1):76–85. doi: 10.7326/0003-4819-98-1-76. [DOI] [PubMed] [Google Scholar]
- Fauci A. S., Haynes B., Katz P. The spectrum of vasculitis: clinical, pathologic, immunologic and therapeutic considerations. Ann Intern Med. 1978 Nov;89(5 Pt 1):660–676. doi: 10.7326/0003-4819-89-5-660. [DOI] [PubMed] [Google Scholar]
- Fauci A. S., Katz P., Haynes B. F., Wolff S. M. Cyclophosphamide therapy of severe systemic necrotizing vasculitis. N Engl J Med. 1979 Aug 2;301(5):235–238. doi: 10.1056/NEJM197908023010503. [DOI] [PubMed] [Google Scholar]
- Fauci A. S., Whalen G., Burch C. Activation of human B lymphocytes XVI. Cellular requirements, interactions, and immunoregulation of pokeweed mitogen-induced total-immunoglobulin producing plaque-forming cells in peripheral blood. Cell Immunol. 1980 Aug 15;54(1):230–240. doi: 10.1016/0008-8749(80)90204-x. [DOI] [PubMed] [Google Scholar]
- Fauci A. S., Wolff S. M., Johnson J. S. Effect of cyclophosphamide upon the immune response in Wegener's granulomatosis. N Engl J Med. 1971 Dec 30;285(27):1493–1496. doi: 10.1056/NEJM197112302852701. [DOI] [PubMed] [Google Scholar]
- Gerrard T. L., Fauci A. S. Activation and immunoregulation of antigen-specific human b lymphocyte responses: multifaceted role of the monocyte. J Immunol. 1982 May;128(5):2367–2372. [PubMed] [Google Scholar]
- Gershwin M. E., Goetzl E. J., Steinberg A. D. Cyclophosphamide: use in practice. Ann Intern Med. 1974 Apr;80(4):531–540. doi: 10.7326/0003-4819-80-4-531. [DOI] [PubMed] [Google Scholar]
- Haynes B. F., Hemler M. E., Mann D. L., Eisenbarth G. S., Shelhamer J., Mostowski H. S., Thomas C. A., Strominger J. L., Fauci A. S. Characterization of a monoclonal antibody (4F2) that binds to human monocytes and to a subset of activated lymphocytes. J Immunol. 1981 Apr;126(4):1409–1414. [PubMed] [Google Scholar]
- Haynes B. F., Hemler M., Cotner T., Mann D. L., Eisenbarth G. S., Strominger J. L., Fauci A. S. Characterization of a monoclonal antibody (5E9) that defines a human cell surface antigen of cell activation. J Immunol. 1981 Jul;127(1):347–351. [PubMed] [Google Scholar]
- Hirano T., Kuritani T., Kishimoto T., Yamamura Y. In vitro immune response of human peripheral lymphocytes. I. The mechanism(s) involved in T cell helper functions in the pokeweed mitogen-induced differentiation and proliferation of B cells. J Immunol. 1977 Oct;119(4):1235–1241. [PubMed] [Google Scholar]
- Howard J. G., Shand F. L. The nature of drug-induced B cell tolerance. Immunol Rev. 1979;43:42–68. doi: 10.1111/j.1600-065x.1979.tb00417.x. [DOI] [PubMed] [Google Scholar]
- Hurme M. Differential cyclophosphamide sensitivity of precursor cells in allogeneic and H-2 restricted cytotoxic responses. J Exp Med. 1979 Jan 1;149(1):290–294. doi: 10.1084/jem.149.1.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehrl J. H., Muraguchi A., Fauci A. S. Differential expression of cell activation markers after stimulation of resting human B lymphocytes. J Immunol. 1984 Jun;132(6):2857–2861. [PubMed] [Google Scholar]
- Kishimoto T., Miyake T., Nishizawa Y., Watanabe T., Yamamura Y. Triggering mechanism of B lymphocytes. I. Effect of anti-immunoglobulin and enhancing soluble factor on differentiation and proliferation of B cells. J Immunol. 1975 Nov;115(5):1179–1184. [PubMed] [Google Scholar]
- Muraguchi A., Butler J. L., Kehrl J. H., Fauci A. S. Differential sensitivity of human B cell subsets to activation signals delivered by anti-mu antibody and proliferative signals delivered by a monoclonal B cell growth factor. J Exp Med. 1983 Feb 1;157(2):530–546. doi: 10.1084/jem.157.2.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraguchi A., Kasahara T., Oppenheim J. J., Fauci A. S. B cell growth factor and T cell growth factor produced by mitogen-stimulated normal human peripheral blood T lymphocytes are distinct molecules. J Immunol. 1982 Dec;129(6):2486–2489. [PubMed] [Google Scholar]
- Ozer H., Cowens J. W., Colvin M., Nussbaum-Blumenson A., Sheedy D. In vitro effects of 4-hydroperoxycyclophosphamide on human immunoregulatory T subset function. I. Selective effects on lymphocyte function in T-B cell collaboration. J Exp Med. 1982 Jan 1;155(1):276–290. doi: 10.1084/jem.155.1.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röllinghoff M., Starzinski-Powitz A., Pfizenmaier K., Wagner H. Cyclophosphamide-sensitive T lymphocytes suppress the in vivo generation of antigen-specific cytotoxic T lymphocytes. J Exp Med. 1977 Feb 1;145(2):455–459. doi: 10.1084/jem.145.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALVIN S. B., SMITH R. F. THE SPECIFICITY OF ALLERGIC REACTIONS. VII. IMMUNOLOGIC UNRESPONSIVENSSS, DELAYED HYPERSENSITIVITY, AND CIRCULATING ANTIBODY TO PROTEINS AND HAPTEN-PROTEIN CONJUGATES IN ADULT GUINEA PIGS. J Exp Med. 1964 May 1;119:851–868. doi: 10.1084/jem.119.5.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma B., Vaziri N. D. Augmentation of human natural killer cell activity by cyclophosphamide in vitro. Cancer Res. 1984 Aug;44(8):3258–3261. [PubMed] [Google Scholar]
- Sjöberg O., Kurnick J. Conditions for induction of specific and polyclonal antibody production by Cowan 1 bacteria and by pokeweed mitogen. Scand J Immunol. 1980;11(1):47–51. doi: 10.1111/j.1365-3083.1980.tb00207.x. [DOI] [PubMed] [Google Scholar]
- Thiele D. L., Kurosaka M., Lipsky P. E. Phenotype of the accessory cell necessary for mitogen-stimulated T and B cell responses in human peripheral blood: delineation by its sensitivity to the lysosomotropic agent, L-leucine methyl ester. J Immunol. 1983 Nov;131(5):2282–2290. [PubMed] [Google Scholar]
- Thomas M. R., Robinson W. A., Boyle D. J., Day J. F., Entringer M. A., Steigerwald J. F. Longterm effects of cyclophosphamide on granulocyte colony formation in patients with rheumatoid arthritis. J Rheumatol. 1983 Oct;10(5):778–783. [PubMed] [Google Scholar]
- Winkelstein A., Mikulla J. M., Nankin H. R., Pollock B. H., Stolzer B. L. Mechanisms of immunosuppression: effects of cyclophosphamide on lymphocytes. J Lab Clin Med. 1972 Oct;80(4):506–513. [PubMed] [Google Scholar]


