Abstract
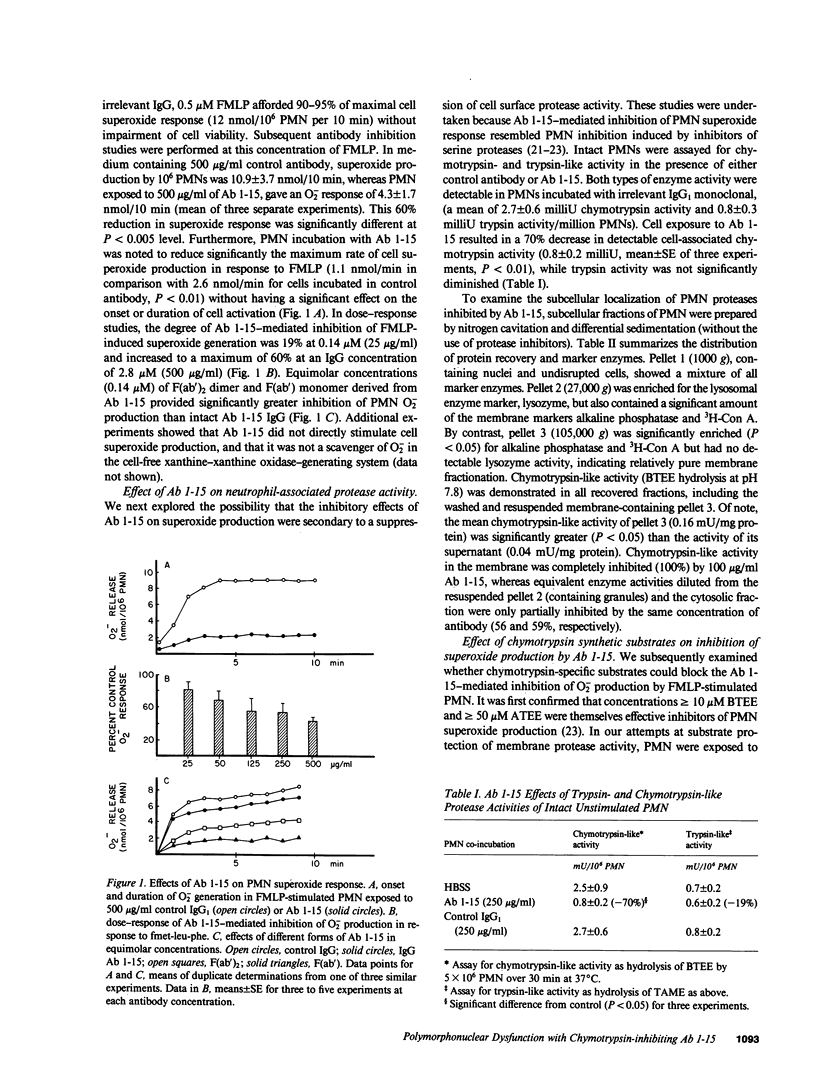
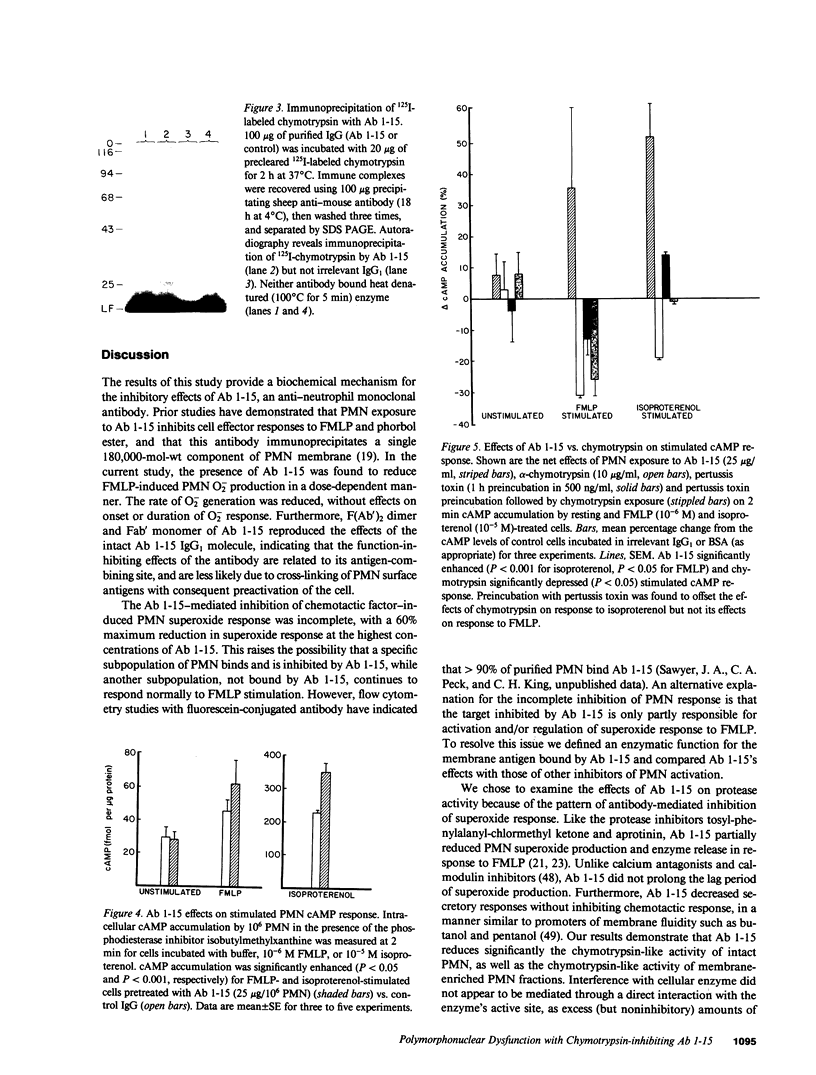
Monoclonal antibody 1-15 (Ab 1-15), is a murine anti-human neutrophil (PMN) IgG1 that inhibits PMN effector responses to N-formyl-met-leu-phe (FMLP) and phorbol myristate acetate. In this study, the effects of Ab 1-15 on PMN membrane-related functions were characterized: Ab 1-15 inhibited PMN superoxide (O-2) response to FMLP by 60% (P less than 0.005) without effect on the onset or duration of O-2 production. This inhibition of O-2 response was associated with a significant inhibition of PMN chymotrypsin-like, but not trypsin-like, protease activity. Cell fractionation studies indicated the presence of an Ab 1-15 inhibitable, chymotryptic neutral protease activity in PMN membranes. In studies of Ab 1-15 effects on membrane-related second messenger pathways, Ab 1-15 augmented both FMLP- and isoproterenol-induced intracellular cAMP accumulation, whereas alpha-chymotrypsin decreased PMN cAMP response to these stimuli. Our data suggest that the function-inhibiting, anti-PMN monoclonal Ab 1-15 defines a PMN chymotryptic enzyme on the membrane surface that is involved in regulation of two membrane-related functions, O-2 generation and cAMP generation.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramovitz A. S., Yavelow J., Randolph V., Troll W. Inhibition of superoxide production in human neutrophils by purified soybean polypeptides. Re-evaluation of the involvement of proteases. J Biol Chem. 1983 Dec 25;258(24):15153–15157. [PubMed] [Google Scholar]
- Arnaout M. A., Todd R. F., 3rd, Dana N., Melamed J., Schlossman S. F., Colten H. R. Inhibition of phagocytosis of complement C3- or immunoglobulin G-coated particles and of C3bi binding by monoclonal antibodies to a monocyte-granulocyte membrane glycoprotein (Mol). J Clin Invest. 1983 Jul;72(1):171–179. doi: 10.1172/JCI110955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano T., Katada T., Gilman A. G., Ross E. M. Activation of the inhibitory GTP-binding protein of adenylate cyclase, Gi, by beta-adrenergic receptors in reconstituted phospholipid vesicles. J Biol Chem. 1984 Aug 10;259(15):9351–9354. [PubMed] [Google Scholar]
- Aswanikumar S., Schiffmann E., Corcoran B. A., Wahl S. M. Role of a peptidase in phagocyte chemotaxis. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2439–2442. doi: 10.1073/pnas.73.7.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty P. G., Ledbetter J. A., Martin P. J., Price T. H., Hansen J. A. Definition of a common leukocyte cell-surface antigen (Lp95-150) associated with diverse cell-mediated immune functions. J Immunol. 1983 Dec;131(6):2913–2918. [PubMed] [Google Scholar]
- Becker E. L., Kermode J. C., Naccache P. H., Yassin R., Marsh M. L., Munoz J. J., Sha'afi R. I. The inhibition of neutrophil granule enzyme secretion and chemotaxis by pertussis toxin. J Cell Biol. 1985 May;100(5):1641–1646. doi: 10.1083/jcb.100.5.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker E. L., Ward P. A. Partial biochemical characterization of the activated esterase required in the complement-dependent chemotaxis of rabbit polymorphonuclear leukocytes. J Exp Med. 1967 Jun 1;125(6):1021–1030. doi: 10.1084/jem.125.6.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruck C., Portetelle D., Glineur C., Bollen A. One-step purification of mouse monoclonal antibodies from ascitic fluid by DEAE Affi-Gel blue chromatography. J Immunol Methods. 1982 Sep 30;53(3):313–319. doi: 10.1016/0022-1759(82)90178-8. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Cotter T. G., Keeling P. J., Henson P. M. A monoclonal antibody-inhibiting FMLP-induced chemotaxis of human neutrophils. J Immunol. 1981 Dec;127(6):2241–2245. [PubMed] [Google Scholar]
- DeChatelet L. R., Cooper M. R. A modified procedure for the determination of leukocyte alkaline phosphatase. Biochem Med. 1970 Aug;4(1):61–68. doi: 10.1016/0006-2944(70)90103-1. [DOI] [PubMed] [Google Scholar]
- Dewald B., Rindler-Ludwig R., Bretz U., Baggiolini M. Subcellular localization and heterogeneity of neutral proteases in neutrophilic polymorphonuclear leukocytes. J Exp Med. 1975 Apr 1;141(4):709–723. doi: 10.1084/jem.141.4.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque R. E., Phan S. H., Sulavik M. C., Ward P. A. Inhibition by tosyl-L-phenylalanyl chloromethyl ketone of membrane potential changes in rat neutrophils. Correlation with the inhibition of biological activity. J Biol Chem. 1983 Jul 10;258(13):8123–8128. [PubMed] [Google Scholar]
- Fleit H. B., Wright S. D., Unkeless J. C. Human neutrophil Fc gamma receptor distribution and structure. Proc Natl Acad Sci U S A. 1982 May;79(10):3275–3279. doi: 10.1073/pnas.79.10.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher M. P., Seligmann B. E., Gallin J. I. Correlation of human neutrophil secretion, chemoattractant receptor mobilization, and enhanced functional capacity. J Immunol. 1982 Feb;128(2):941–948. [PubMed] [Google Scholar]
- Goetzl E. J., Foster D. W., Goldman D. W. Isolation and partial characterization of membrane protein constituents of human neutrophil receptors for chemotactic formylmethionyl peptides. Biochemistry. 1981 Sep 29;20(20):5717–5722. doi: 10.1021/bi00523a013. [DOI] [PubMed] [Google Scholar]
- Goldstein B. D., Witz G., Amoruso M., Troll W. Protease inhibitors antagonize the activation of polymorphonuclear leukocyte oxygen consumption. Biochem Biophys Res Commun. 1979 Jun 13;88(3):854–860. doi: 10.1016/0006-291x(79)91487-6. [DOI] [PubMed] [Google Scholar]
- HUMMEL B. C. A modified spectrophotometric determination of chymotrypsin, trypsin, and thrombin. Can J Biochem Physiol. 1959 Dec;37:1393–1399. [PubMed] [Google Scholar]
- Hafeman D. G., Lewis J. T., McConnell H. M. Triggering of the macrophage and neutrophil respiratory burst by antibody bound to a spin-label phospholipid hapten in model lipid bilayer membranes. Biochemistry. 1980 Nov 11;19(23):5387–5394. doi: 10.1021/bi00564a037. [DOI] [PubMed] [Google Scholar]
- Hanoune J., Stengel D., Lacombe M. L. Proteolytic activation and solubilization of adenylate and guanylate cyclases. Mol Cell Endocrinol. 1983 Jul;31(1):21–41. doi: 10.1016/0303-7207(83)90028-x. [DOI] [PubMed] [Google Scholar]
- Hirata F., Corcoran B. A., Venkatasubramanian K., Schiffmann E., Axelrod J. Chemoattractants stimulate degradation of methylated phospholipids and release of arachidonic acid in rabbit leukocytes. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2640–2643. doi: 10.1073/pnas.76.6.2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King C. H., Peck C. A., Haimes C. S., Kazura J. W., Spagnuolo P. J., Sawyer J. A., Olds G. R., Mahmoud A. A. Modulation of human neutrophil effector functions by monoclonal antibodies against surface membrane molecules of 94,000 and 180,000 molecular weight. Blood. 1986 Jan;67(1):188–194. [PubMed] [Google Scholar]
- Kitagawa S., Takaku F., Sakamoto S. Evidence that proteases are involved in superoxide production by human polymorphonuclear leukocytes and monocytes. J Clin Invest. 1980 Jan;65(1):74–81. doi: 10.1172/JCI109662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa S., Takaku F., Sakamoto S. Possible involvement of proteases in superoxide production by human polymorphonuclear leukocytes. FEBS Lett. 1979 Mar 15;99(2):275–278. doi: 10.1016/0014-5793(79)80971-0. [DOI] [PubMed] [Google Scholar]
- Klempner M. S., Mikkelsen R. B., Corfman D. H., André-Schwartz J. Neutrophil plasma membranes. I. High-yield purification of human neutrophil plasma membrane vesicles by nitrogen cavitation and differential centrifugation. J Cell Biol. 1980 Jul;86(1):21–28. doi: 10.1083/jcb.86.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korchak H. M., Wilkenfeld C., Rich A. M., Radin A. R., Vienne K., Rutherford L. E. Stimulus response coupling in the human neutrophil. Differential requirements for receptor occupancy in neutrophil responses to a chemoattractant. J Biol Chem. 1984 Jun 25;259(12):7439–7445. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lad P. M., Olson C. V., Smiley P. A. Association of the N-formyl-Met-Leu-Phe receptor in human neutrophils with a GTP-binding protein sensitive to pertussis toxin. Proc Natl Acad Sci U S A. 1985 Feb;82(3):869–873. doi: 10.1073/pnas.82.3.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch G., Baudry M. The biochemistry of memory: a new and specific hypothesis. Science. 1984 Jun 8;224(4653):1057–1063. doi: 10.1126/science.6144182. [DOI] [PubMed] [Google Scholar]
- Martin L. S., Gordon D. S., Wilson M. E., Browning S. W., Fritz R. B. Monoclonal antibody to human granulocytes: cellular specificity and functional studies. J Leukoc Biol. 1984 Mar;35(3):265–279. doi: 10.1002/jlb.35.3.265. [DOI] [PubMed] [Google Scholar]
- Mason D. W., Williams A. F. The kinetics of antibody binding to membrane antigens in solution and at the cell surface. Biochem J. 1980 Apr 1;187(1):1–20. doi: 10.1042/bj1870001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murayama T., Ui M. Receptor-mediated inhibition of adenylate cyclase and stimulation of arachidonic acid release in 3T3 fibroblasts. Selective susceptibility to islet-activating protein, pertussis toxin. J Biol Chem. 1985 Jun 25;260(12):7226–7233. [PubMed] [Google Scholar]
- Niewiarowski S., Budzynski A. Z., Morinelli T. A., Brudzynski T. M., Stewart G. J. Exposure of fibrinogen receptor on human platelets by proteolytic enzymes. J Biol Chem. 1981 Jan 25;256(2):917–925. [PubMed] [Google Scholar]
- Odeberg H., Olsson I., Venge P. Cationic proteins of human granulocytes. IV. Esterase activity. Lab Invest. 1975 Jan;32(1):86–90. [PubMed] [Google Scholar]
- Painter R. G., Sklar L. A., Jesaitis A. J., Schmitt M., Cochrane C. G. Activation of neutrophils by N-formyl chemotactic peptides. Fed Proc. 1984 Sep;43(12):2737–2742. [PubMed] [Google Scholar]
- Parker P. J., Coussens L., Totty N., Rhee L., Young S., Chen E., Stabel S., Waterfield M. D., Ullrich A. The complete primary structure of protein kinase C--the major phorbol ester receptor. Science. 1986 Aug 22;233(4766):853–859. doi: 10.1126/science.3755547. [DOI] [PubMed] [Google Scholar]
- Record M., Laharrague P., Fillola G., Thomas J., Ribes G., Fontan P., Chap H., Corberand J., Douste-Blazy L. A rapid isolation procedure of plasma membranes from human neutrophils using self-generating Percoll gradients. Importance of pH in avoiding contamination by intracellular membranes. Biochim Biophys Acta. 1985 Sep 25;819(1):1–9. doi: 10.1016/0005-2736(85)90188-9. [DOI] [PubMed] [Google Scholar]
- SMOLELIS A. N., HARTSELL S. E. The determination of lysozyme. J Bacteriol. 1949 Dec;58(6):731–736. doi: 10.1128/jb.58.6.731-736.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedor J. R., Abboud H. E. Histamine modulates contraction and cyclic nucleotides in cultured rat mesangial cells. Differential effects mediated by histamine H1 and H2 receptors. J Clin Invest. 1985 May;75(5):1679–1689. doi: 10.1172/JCI111876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklar L. A., Hyslop P. A., Oades Z. G., Omann G. M., Jesaitis A. J., Painter R. G., Cochrane C. G. Signal transduction and ligand-receptor dynamics in the human neutrophil. Transient responses and occupancy-response relations at the formyl peptide receptor. J Biol Chem. 1985 Sep 25;260(21):11461–11467. [PubMed] [Google Scholar]
- Smith C. D., Cox C. C., Snyderman R. Receptor-coupled activation of phosphoinositide-specific phospholipase C by an N protein. Science. 1986 Apr 4;232(4746):97–100. doi: 10.1126/science.3006254. [DOI] [PubMed] [Google Scholar]
- Smith C. D., Lane B. C., Kusaka I., Verghese M. W., Snyderman R. Chemoattractant receptor-induced hydrolysis of phosphatidylinositol 4,5-bisphosphate in human polymorphonuclear leukocyte membranes. Requirement for a guanine nucleotide regulatory protein. J Biol Chem. 1985 May 25;260(10):5875–5878. [PubMed] [Google Scholar]
- Smolen J. E., Korchak H. M., Weissmann G. Increased levels of cyclic adenosine-3',5'-monophosphate in human polymorphonuclear leukocytes after surface stimulation. J Clin Invest. 1980 May;65(5):1077–1085. doi: 10.1172/JCI109760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolen J. E. Lag period for superoxide anion generation and lysosomal enzyme release from human neutrophils: effects of calcium antagonists and anion channel blockers. J Lab Clin Med. 1984 Jul;104(1):1–10. [PubMed] [Google Scholar]
- Speer C. P., Pabst M. J., Hedegaard H. B., Rest R. F., Johnston R. B., Jr Enhanced release of oxygen metabolites by monocyte-derived macrophages exposed to proteolytic enzymes: activity of neutrophil elastase and cathepsin G. J Immunol. 1984 Oct;133(4):2151–2156. [PubMed] [Google Scholar]
- TENNANT J. R. EVALUATION OF THE TRYPAN BLUE TECHNIQUE FOR DETERMINATION OF CELL VIABILITY. Transplantation. 1964 Nov;2:685–694. doi: 10.1097/00007890-196411000-00001. [DOI] [PubMed] [Google Scholar]
- Tsung P. K., Kegeles S. W., Becker E. L. The evidence for the existence of chymotrypsin-like esterase activity in the plasma membranes of rabbit neutrophils and the specific chemotactic peptide binding activity of the subcellular fractions. Biochim Biophys Acta. 1978 Jun 15;541(2):150–160. doi: 10.1016/0304-4165(78)90388-4. [DOI] [PubMed] [Google Scholar]
- Verghese M. W., Fox K., McPhail L. C., Snyderman R. Chemoattractant-elicited alterations of cAMP levels in human polymorphonuclear leukocytes require a Ca2+-dependent mechanism which is independent of transmembrane activation of adenylate cyclase. J Biol Chem. 1985 Jun 10;260(11):6769–6775. [PubMed] [Google Scholar]
- Vischer T. L., Bretz U., Baggiolini M. In vitro stimulation of lymphocytes by neutral proteinases from human polymorphonuclear leukocyte granules. J Exp Med. 1976 Oct 1;144(4):863–872. doi: 10.1084/jem.144.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpi M., Naccache P. H., Molski T. F., Shefcyk J., Huang C. K., Marsh M. L., Munoz J., Becker E. L., Sha'afi R. I. Pertussis toxin inhibits fMet-Leu-Phe- but not phorbol ester-stimulated changes in rabbit neutrophils: role of G proteins in excitation response coupling. Proc Natl Acad Sci U S A. 1985 May;82(9):2708–2712. doi: 10.1073/pnas.82.9.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh C. E., Waite B. M., Thomas M. J., DeChatelet L. R. Release and metabolism of arachidonic acid in human neutrophils. J Biol Chem. 1981 Jul 25;256(14):7228–7234. [PubMed] [Google Scholar]
- Yuli I., Tomonaga A., Synderman R. Chemoattractant receptor functions in human polymorphonuclear leukocytes are divergently altered by membrane fluidizers. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5906–5910. doi: 10.1073/pnas.79.19.5906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman G. A., McIntyre T. M., Prescott S. M. Thrombin stimulates the adherence of neutrophils to human endothelial cells in vitro. J Clin Invest. 1985 Dec;76(6):2235–2246. doi: 10.1172/JCI112232. [DOI] [PMC free article] [PubMed] [Google Scholar]