Abstract
Matrix metalloproteinases (MMPs) regulate tissue remodeling, inflammation, and disease progression. Some soluble MMPs are inexplicably active near cell surfaces. Here, we demonstrate binding of MMP-12 directly to bilayers and cellular membranes using paramagnetic NMR and fluorescence. Opposing sides of the catalytic domain engage spin-labeled membrane mimics. Loops project from the β-sheet interface to contact the phospholipid bilayer with basic and hydrophobic residues. The distal membrane interface comprises loops on the other side of the catalytic cleft. Both interfaces mediate MMP-12 association with vesicles and cell membranes. MMP-12 binds plasma membranes and is internalized to hydrophobic perinuclear features, the nuclear membrane, and inside the nucleus within minutes. While binding of TIMP-2 to MMP-12 hinders membrane interactions beside the active site, TIMP-2-inhibited MMP-12 binds vesicles and cells, suggesting compensatory rotation of its membrane approaches. MMP-12 association with diverse cell membranes may target its activities to modulate innate immune responses and inflammation.
Keywords: peripheral membrane protein, protein-bilayer interactions, pericellular proteolysis, NMR, paramagnetic relaxation enhancement, live cell imaging, metzincin, metalloproteinase
Macrophages use macrophage elastase (matrix metalloproteinase-12; MMP-12) to overcome bacterial infections1, regulate antiviral immunity2, and resolve inflammation3. The therapeutic potential of MMP-12 stems from its roles in smoking-induced emphysema4, abdominal aortic aneurysms5, atherosclerotic plaques6, and viral infections2. More generally MMPs modulate inflammation3, 7, wound healing8, tumor microenvironments9, arthritis10, cardiovascular disease11, brain injury, and synaptic remodeling12. Hence, understanding of MMP mechanisms of action is critical for development of targeted interventions.
Among the 23 human MMPs, MMP-12 is one of the best understood and illustrates the conundrum of the compartmentalization of several of the water-soluble enzymes12, 13. After MMP-12 is secreted by macrophages, it diffuses through extracellular spaces, where the protease can be inhibited, en route to virus-infected cells2. Yet MMP-12 activity was not observed in the aqueous milieu around activated macrophages, but rather near the cells’ surface14. Pericellular activity is consistent with MMP-12 shedding of TNF-α from leukocytes in lungs exposed to smoke15. Remarkably, MMP-12 enters virus-infected cells and nuclei where it activates transcription to respond to viral infection2.
Proteolysis by soluble MMP-8 and -9 occurs on neutrophil surfaces and surprisingly resists inhibition by TIMPs16, 17. Purified plasma membranes harbor MMP-817. MMP-7 binds epithelial and cancer cell surfaces and remains proteolytically active even with TIMPs present18. The findings that these soluble MMPs are active near cells, even when high affinity TIMP inhibitors are present, range from being partly understood in the case of MMP-7 to completely mysterious in the case of MMP-12.
Diverse mechanisms may recruit metalloproteinases to cell surfaces. MMP-7 is anchored to cells by heparan sulfate proteoglycans (CD44) and the cholesterol sulfate component of lipid rafts18, 19, 20, as well as binding vesicles21. Some soluble MMPs bind transmembrane proteins including αvβ3 and α2β1 integrins, tetraspanin CD151, CD44, and low-density lipoprotein receptor-related protein13, implying many means of localization.
We set out to discover whether the well-characterized MMP-12 22, 23, 24, 25, 26 binds directly to lipid bilayers and plasma membranes of living cells. Our results take on unexpected relevance to the recent question of how active MMPs enter and move through cells2, 27, 28. To provide mechanistic insights, spin-labeled phospholipids in micelles were used to probe interactions with peripheral membrane proteins via paramagnetic NMR relaxation enhancements (PREs)29. Such PREs provide distance estimates that are useful for calculating realistic structures of the complexes. Disk-like, isotropic bicelles (bilayered micelles) are well-characterized30 and offer a central bilayer and relatively fast tumbling for resolving NMR peaks. These membrane models yielded quantitative results with a peripheral membrane protein31 and an integral membrane protein32, and are employed here. A polarity-sensitive fluorophore for detecting membrane interactions33, 34 and live cell imaging are applied as well. These strategies are integrated here to generate validated structures of complexes, revealing that MMP-12 consistently interacts in an “ambidextrous” manner with bicelles, vesicles, and various cellular membranes, using two non-contiguous surfaces. The unprecedented pair of membrane-binding surfaces position the active site and substrate access nearby, providing explanations for its pericellular proteolytic activities14, 15, and potentially for the trafficking of this protease towards perinuclear and intranuclear compartments to shape innate immune and inflammatory responses2.
Results
Discovery of membrane-binding sites and complexes of MMP-12
In order to predict potential novel membrane binding sites, we applied an experimentally trained algorithm called ‘membrane optimal docking area’ (MODA), which is now freely available at moda.ucsd.edu35, 36. Application to the crystal structure of MMP-12 (PDB ID 1jk3)37 predicted direct membrane binding by Gly167 to His172 of the III-IV loop and Gly188 to Gly192 of the IV-V loop due to the exposure of bilayer-interactive groups, particularly of Phe171 and Ile191 (Fig. 1a).
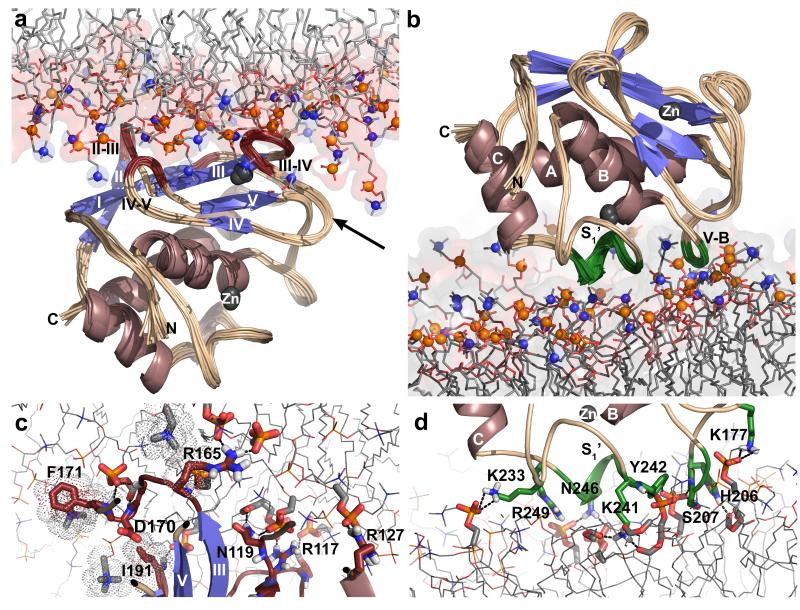
Figure 1. MMP-12 associates with bilayer-mimicking bicelles via two distinct interfaces.
(a) The MODA tool predicts membrane association with the residues of the IV-V and S-shaped III-IV loops which are marked with yellow or orange ribbon and side chains. The proximities of MMP-12 groups to bicelles were measured using isotropic bicelles doped with nitroxide spin-labeled phosphatidylcholine (PC). This induces large NMR peak broadenings (PREs) to residues in two clusters highlighted in dark red/orange or green, respectively, on the NMR structure23. The 5-doxyl PC broadens the NMR line widths of amide protons marked with dots where Γ2 > 14 s−1 and methyl groups with side chains plotted where Γ2 > 10 s−1. The more deeply buried spin label of 14-doxyl PC broadens the lines of amide protons plotted with spheres where Γ2 > 14 s−1. Orange marks residues with both PRE evidence and MODA prediction of bilayer interactions. Black spheres indicate zinc ions. (b) Close approaches of the protease to the bicelles are observed as PREs induced in amide and methyl protons by addition of nitroxide spin-labeled PC to one per leaflet of the isotropic bicelles. The PREs are plotted as Γ2 relaxation rates. The colors correspond to the regions encompassing the two clusters indicated in (a), with red representing the β-face and green the α-face. (c) Examples of methyl proton relaxation at the β-interface (upper panels) and α-interface (lower panels) with the membrane mimics are plotted with black circles for the diamagnetic control without addition or using colored triangles with the addition of paramagnetic 5-doxyl PC.
We tested these hypothetical sites using the catalytic domain, expressed without the pro-domain and containing a stabilizing E219A mutation (“MMP-12”), to represent the physiological state formed by rapid autolytic removal of the C-terminal domain38. The proximity of MMP-12 residues to bicelles that mimic phospholipid membranes was measured by paramagnetic NMR. The isotope-labeled protease was introduced to bicelles containing phosphatidylcholine (PC) with dimyristoyl (DM) and dihexanoyl (DH) chains. Relaxation of amide and methyl NMR signals of MMP-12 was compared without and with addition of nitroxide spin-labeled PC to the bicelles mixed with the enzyme. The PC with either shallow 5-doxyl or deep 14-doxyl probe selectively broadens NMR peaks of Ala167, His168, Asp170, Phe171, and Ala173 with PREs, indicating that part of the III-IV loop to interact directly with bicelles (Fig. 1). Likewise, the PREs of Gly190 and Ile191 indicate that the IV-V loop binds bicelles, again confirming the prediction. Accompanying bicelle-induced chemical shift perturbations further confirm these binding sites (Supplementary Fig. 1a). Neighboring II-III loop residues Leu152, Thr154, and Met156 as well as Arg117 and Asn119 at the end of β-strand I also experience PREs from spin-labeled lipids in the bicelles (Fig. 1). On the opposite side of the catalytic domain, the V-B loop at His206 to Gly208 and Asn211 and the S1’ loop at Lys233, Val235, Lys241 to Asp244, and Thr247 also exhibit PREs. Surprisingly, this suggests two non-overlapping sites that can both bind bilayers, with one involving four loops around the β-sheet and the other comprising two loops on the opposite side of the active site cleft.
The proximity of the bicelle binding surfaces to either flank of the active site (Fig. 1a) and the proposed occlusion of MMP-7 and -26 active sites by non-physiological cationic liposomes21 or detergent micelles39, respectively, raised the question of membrane occlusion of the MMP-12 active site. However, small unilamellar vesicles (SUVs) of zwitterionic DMPC that bind MMP-12 did not alter its peptidase activity (Supplementary Fig. 1b), indicating that the active site remains intact and accessible to peptide substrates. The open active site separated the PREs into two functionally significant sets, designated the β-interface for proximity to the β-sheet or the α-interface nearer the α-helices. The larger β-interface comprises Thr115, Arg117, Asn119, Thr154, Met156, Leu160, Val162, Ala167, His168, Asp170, Phe171, Ala173, Gly190, and Ile191 (Fig. 1). The α-interface includes Thr205 to Gly208, Asn211, Lys233, Val235, Lys241 to Ile245, Thr247, Leu250, and Asp254. Addition of the spin-labeled PC induced rapid relaxation of only around half of the NMR signal at either interface (Fig. 1c). This suggested nearly equal populations of enzyme bound by way of the α- and β-interfaces to bicelles, i.e., around half in each orientation.
Due to the intrinsic uncertainties in the locations visited by a spin-labeled PC probe in a bicelle, the PRE-based distances were ambiguously restrained to eight alternative DMPC molecules within a bilayer model available40. Each significant PRE demonstrated proximity of that chemical group to the bicelle (Fig. 1b) and was converted using eq. 1 into a group of distance restraints to the depth in both acyl chains in the eight DMPC molecules corresponding to the position of the nitroxide spin label. The structural calculations sought to satisfy any single restraint among each 16-member group of ambiguous depth restraints. Rigid body docking of the β-face of the MMP-12 solution structure23 to DMPC bilayer coordinates employed 352 ambiguous distance restraints between them, while the docking to the α-face used a non-overlapping set of 384 ambiguous restraints. These resulted in the distinct and well-converged starting structures of the complexes with DMPC bilayers that were refined by molecular dynamics (MD). Both orientations of MMP-12 stably converged on the fluid model bilayer (Supplementary Movies 1 and 2). Ensembles of 14 representative structures were selected for best agreement with the PRE-derived depth restraints (Fig. 2a,b) with minimal violations (Table 1). The backbone RMSD values to the mean structures of the β and α ensembles average 0.49 Å and 0.42 Å, respectively. The two structural ensembles differ in their orientations on the bilayer by 137°, and thus bind membranes via opposite sides of the enzyme.
Figure 2. PRE-NMR structural ensembles and interfacial contacts of DMPC bilayer bound to opposite sides of catalytic domain.
(a) The ensemble of 14 structures of MMP-12 positioned with its β-face on the bilayer was selected from the MD trajectory of Supplementary Movie 1. Backbone segments that contact the bicelles are colored dark red. The view is illustrated as a stereo pair in Supplementary Fig. 9a. (b) The ensemble of 14 structures of MMP-12 bound with its α-face engaging the bilayer with green segments of the S1’ and V-B loops was selected from the MD trajectory of Supplementary Movie 2. This view is illustrated as a stereo pair in Supplementary Fig. 9b. (c) A structure from panel a (viewed along the arrow in a) is marked with interfacial contacts which are populated in at least 64% of the final structures. Hydrophobic contacts are plotted with dot surfaces. Hydrogen bonds and salt bridges are plotted with broken lines. Nitrogen, phosphorous, and oxygen atoms are colored blue, gold, and red respectively. See Supplementary Fig. 2 for a 3D, rotatable view. (d) A structure from panel b in the α orientation is marked with interfacial contacts reproduced in at least 64% of the final structures. See Supplementary Fig. 3 for a 3D, rotatable view.
Table 1. Structural statistics of PRE-based structural ensembles.
| restraints for β-interface | restraints for α-interface at S1’ , V-B loops | |
|
| ||
| Restraints$ to 5-Doxyl position | ||
|
| ||
| HN 8a aa *8 DMPC lipids *2 acyl chains = 128 | HN 9e aa *8 DMPC lipids *2 acyl chains = 144 | |
|
| ||
| HCH3 8b aa *8 DMPC lipids *2 acyl chains = 128 | HCH3 7f aa *8 DMPC lipids *2 acyl chains =112 | |
|
| ||
| Restraints$ to 14-Doxyl position | ||
|
| ||
| HN 4c aa *8 DMPC lipids *2 acyl chains = 64 | HN 6g aa *8 DMPC lipids *2 acyl chains = 96 | |
|
| ||
| Restraints$ to fluor-tagged position | ||
|
| ||
| CA 2d aa *8 DMPC lipids *2 acyl chains = 32 | CA 2h aa *8 DMPC lipids *2 acyl chains = 32 | |
|
| ||
| Total PRE-based distance restraints = 352 | Total PRE-based distance restraints = 384 | |
|
| ||
| aR117, N119, T154, A167, H168, D170, G190, I191 | eH206, S207, G208, K233, K241, Y242, D244, T247, D254 | |
| bT115, T154, M156, L160, V162, A167, A173, I191 | fT205, V235, V243(2), I245, T247, L250 | |
| cV162, H168, F171, I191 | gH206, G208, N211, K241, D244, T247 | |
| dG155C, G190C | hS207C, A252C | |
|
| ||
| 56 φ + 57 ψ dihedral angle restraints§ = 113 | 56 φ + 57 ψ dihedral angle restraints§ = 113 | |
| Total NMR solution structural restraints = 465 | Total NMR solution structural restraints = 497 | |
|
| ||
| Distances to metals¶:17 to Zn++ + 19 to Ca++ = 36 | Distances to metals¶:: 17 to Zn++ + 19 to Ca++ = 36 | |
|
| ||
|
RMS deviations£
|
||
| β-interface | α-interface | |
|
| ||
| Average pairwise r.m.s deviation (Å) | ||
| All Backbone atoms | 0.79 ± 0.08 | 0.75 ± 0.06 |
| All Heavy atoms | 1.25 ± 0.09 | 1.18 ± 0.07 |
| Backbone to MMP-12 NMR structure (2POJ) (Å) | 1.85 ± 0.13 | 1.71 ± 0.09 |
| RMSD from PRE-based distance restraints (Å) | 0.96 ± 0.19 | 0.93 ± 0.13 |
| Q-factor for PRE-based distance restraints | 0.06 ± 0.01 | 0.06 ± 0.01 |
| Deviations from idealized bond lengths (Å) | 0.011 ± 0.001 | 0.01 ± 0.001 |
| Deviations from idealized bond angles (°) | 3.61 ± 0.05 | 3.57 ± 0.06 |
|
|
||
| Statistics of overall structural quality |
Mean Score
|
|
| Procheck G-factor (all dihedrals) | −0.59 | −0.64 |
| Molprobity ¢ | 1.49 | 1.59 |
| Residues in most favored regions of Ramachandran plot€ | 89.8% | 92.7% |
| Residues in additional allowed regions | 8.8% | 5.2 % |
ambiguous with respect to location of spin-labeled phospholipid; each restraint is satisfied by proximity to any of 8 phospholipids in either acyl chain at the depth corresponding to the position of the label
Dihedral restraints of Bhaskaran et al. 23
Based on distances measured by Lang et al. 37
RMSD calculated from the ensemble of 14 lowest energy structures.
Molprobity score combines the clash score, rotamers, and Ramachandran evaluation into a single score.
from Procheck-NMR
The breadth of interfaces and shallow contacts with lipid head groups (Table 2) suggest reversible association with the bilayer. The α- and β-interfaces bury 2070 and 2530 Å2 of surface area, respectively, making them larger than most interfaces of phospholipid recognition domains41. The interacting loops of MMP-12 generally are positioned with backbone among the choline head groups (Fig. 2a,b) and side chains slightly deeper near the phosphodiester linkages, but not so deep as to reach the acyl chains (Fig. 2c,d and Supplementary Figs. 2, 3). Reversible MMP-12 association with membranes is corroborated by the rotational correlation time (τc) of bicelle-associated MMP-12 of 13.4 ns, which is only 54% larger than the 8.7 ns τc of the free state25, despite the slower tumbling of the bicelles.
Table 2. Interfacial contacts of phospholipid head groups with amino acids and their occupancy in the structural ensembles.
| Type of contact, participating DMPC group, and frequency in each ensemble of 14 structures | |||
|
| |||
| Site in β-interface | Hydrogen-bonding | Salt bridge | Hydrophobic |
| Arg117 2HH1 | OC¥(51 DMP) 100% | ||
| 2HH2 | OC¥( 51 DMP) 36% | ||
| Asn119 2HD2 | OH^(51 DMP) 71% | ||
| 2HD2 | OB£(50 DMP) 36% | ||
| Arg127 2HH1 | OB£(33 DMP) 64% | ||
| 2HH2 | OB£(33 DMP) 50% | ||
| Thr154 HG1 | OC¥(26 DMP) 64% | ||
| Arg165 1HH1 | OB£(49 DMP) 100% | ||
| 2HH1 | OB£(41 DMP) 93% | ||
| HB# or HD# | CN#ɦ (51 DMP) 86% | ||
| Gly169 NH | OC¥(2 DMP) 86% | ||
| Asp170 NH | OF®(2 DMP) 100% | ||
| Phe171 NH | OD€(2 DMP) 78% | ||
| CZ | CN#ɦ (59 DMP) 64% | ||
| Ile191 HD# | C1B-D (9 DMP) 79% | ||
| #HG2 | CA (44 DMP) 86% | ||
| Site in α-interface | |||
| Lys177 HZ1, HZ2 | OB£(49 DMP) 78.5 % | ||
| His206 HE2 | OH^(2 DMP) 100% | ||
| Ser207 NH | OC¥(9 DMP) 100% | ||
| HG | OC¥(9 DMP) 93% | ||
| Lys233 HZ2 | OB£(26 DMP) 71% | ||
| Lys241 HZ3 | OF®(41 DMP) 100% | ||
| HZ2 | OF®(57 DMP) 86% | ||
| HZ1 | 0F®(50 DMP) 78% | ||
| Tyr242 NH | OB£(57 DMP) 100% | ||
| HH | OB£(9 DMP) 64 % | ||
| Asn246 1HD2 | OA¢(44 DMP) 100% | ||
| 2HD2 | OC¥(46 DMP) 93% | ||
Phosphate oxygen
Phosphate oxygen
Phosphate oxygen
Phosphate oxygen
Ester carbonyl oxygen
Ester carbonyl oxygen
Choline head group
The peripheral bilayer engagement by MMP-12 features hydrogen bonding, salt bridges, and hydrophobic contacts with the lipid head groups (Table 2). In the β-interface, Arg117 (β-strand I) and Arg127 (helix A) each form a salt bridge with a phosphate group of DMPC, while Arg165 (III-IV loop) forms salt bridges with two phosphates (Fig. 2c, Table 2, and Supplementary Fig. 2). The side chains of Arg165, Phe171, and Ile191 each form hydrophobic contacts with a choline head group while their backbone amide groups donate hydrogen bonds to a DMPC phosphate group or ester linkage. Arg127 and Phe171 fluctuate in and out of contact with phospholipid head groups in the MD trajectories (Supplementary Movie 1). The side chains of Thr154 (II-III loop) and Asn119 (I-A loop) each donate a hydrogen bond to a phosphate group and Asn119 to an ester linkage as well (Fig. 2c, Table 2, and Supplementary Fig. 2).
The α-interface is dominated by electrostatic interactions, displaying salt bridges between Lys177 and Lys233 and phosphate groups of DMPC molecules (Fig. 2d, Table 2, and Supplementary Fig. 3). The Lys241 side chain donates hydrogen bonds to three ester linkages, and His206 to one ester linkage. The side chain and amide of Ser207 both donate hydrogen bonds to the same phosphate group. In contrast, the side chain and amide groups of Tyr242 donate hydrogen bonds to different DMPC phosphates. The side chain amide of Asn246 donates hydrogen bonds to two DMPC phosphate groups (Fig. 2d, Table 2, and Supplementary Fig. 3). Thus, the α- and β-interfaces differ in hydrophobicity and polarity.
Both sides of MMP-12 bind vesicles
We tested whether both modes of MMP-12 binding of bicelles (Fig. 1, 2) occur with vesicles. Membrane insertion was detected using the polarity-sensitive fluor IANBD, which increases dramatically in fluorescence emission when inserted33. The IANBD reporter was conjugated to single cysteine residues, designated here site-directed fluor labeling (SDFL), with labeling yields of 94 to 100% (Supplementary Table 1). Excellent NMR spectra imply native-like structure with minimal perturbation by either representative cysteine substitutions or the fluor labeling (Supplementary Fig. 4), in agreement with surface mutations preserving MMP-12 intact22, 24, 26. As a negative control, IANBD was conjugated to Q139C away from both bicelle-binding sites, resulting in no significant increase of fluorescence (Fig. 3a,b). Greater than two-fold increases (p < 0.05 by one-tailed Student’s t-test) in fluorescence (FDMPC/F0) were induced by DMPC vesicles interacting with IANBD attached to any of the four bilayer-binding loops in the β-interface, namely at N119C, G155C, A173C, or G190C (Fig. 3a,c). The Met156 and Phe171 side chains insert among the choline head groups (Figs. 2c, 3c and Supplementary Fig. 2) and their alanine substitutions reduced the fluorescence to 0.57-fold and 0.78-fold the levels of the wild-type sequence (p < 0.05, by Student’s t-test), respectively, as reported by the fluor linked to G155C (Fig. 3a). Thus, the four loops around the β-sheet and hydrophobic residues therein do engage membranes.
Figure 3. Both α and β-interfaces of MMP-12 bind vesicles and cells.
(a) Addition of DMPC vesicles (SUVs, 400 μM in monomers) increased the fluorescence of MMP-12 forms (5 nM) labeled with IANBD at the single cysteine mutations indicated. The resulting fluorescence emission, FDMPC, is plotted relative to no addition of SUVs, F0. The error bars mark the s.d. of the mean among quadruplicate samples. Differences from the Q139C control represent p < 0.005 by **, p < 0.0005 by ***, and p < 0.00005 by **** from two-tailed Student’s t-testing. Blue asterisks (****) mark p-values for differences with and without the alanine mutation. The dashed line indicates significant proximity to a liposome according to elevated F/F0 ratios, judged by previous IANBD probing of the membrane-binding face of a peripheral membrane protein33. Results with bicelles are shown in Supplementary Fig. 5. (b) Addition of murine RAW 264.7 macrophages or human HeLa cells (5 million cells per well; quadruplicate samples) increased the fluorescence of MMP-12 variants labeled with IANBD where indicated. Labeling at Q139C outside the α- and β-interfaces serves as a negative control. (c) The exposed residues which were mutated and conjugated to the fluorochrome in the β- and α-sites are colored dark red and green, respectively. The negative control site, Q139C, and zinc ions are colored gray. (d, j) Confocal fluorescence images for cells (located by differential interference contrast) with no added protein are shown. (e-i) RAW 264.7 macrophages were incubated with IANBD-conjugated MMP-12 single cysteine variants and imaged by confocal microscopy. The scale bars represent 20 μm. (k-o) HeLa cells were incubated with the labeled MMP-12 variants and imaged.
To validate the α-interface, IANBD was conjugated to a position in the V-B loop or to one of three residues after helix B in the long S1’ loop. The fluor at each site underwent large fluorescence increase upon addition of DMPC liposomes. Significant enhancements of at least 2.8, 1.6, 2.5, or 3.3-fold with p < 0.05 by one-tailed Student’s t-test were observed for labeling at S207C, S230C, K241C, or A252C, respectively (Fig. 3a). This suggests that the IANBD group at each of these four sites inserted directly into vesicular membranes. However, the insertion was shallower for the S230C label, suggesting greater distance from the vesicle. The Ala substitution of Lys241 decreased the fluorescence to 0.81-fold (Fig. 3a, p < 0.05 by Student’s t-test), consistent with hydrogen bonding and charge complementarity with phospholipid head groups (Fig. 2d). Together this confirms that the S1’ and V-B loops of the α-face bind lipid bilayers.
MMP-12 binds cells by the α- and β-faces
We investigated whether MMP-12 binds cells by the α and β-interfaces using a cell-based SDFL approach34. We compared HeLa cells with RAW 264.7 macrophages which secrete MMP-12. The IANBD probe emits dramatically brighter fluorescence in hydrophobic compartments, vesicles and cells33, 34, 42, but did not insert itself into plasma membranes when free (Supplementary Fig. 6) and only minimally, if at all, when linked to MMP-12(Q139C) (Figs. 3b,e,k and 4). This implies that IANBD does not promote cell membrane binding. The two most bilayer-responsive sites were tagged with IANBD in each of the two membrane interfaces: G155C or G190C in the β-interface or S207C or A252C in the α-interface (Fig. 3c). Addition of either cell type markedly brightened the cellular fluorescence emission of the fluor-conjugated MMP-12, in contrast to the Q139C-labeled negative control (Fig. 3b). This strongly suggests that the catalytic domain of MMP-12 binds the cells and that this occurs via both the membrane-binding α- and β-interfaces.
Figure 4. MMP-12 binds plasma membranes, is internalized to hydrophobic compartments including the nuclear envelope, and enters the nucleus.
The cellular distribution of MMP-12 was visible by confocal microscopy using the enzyme labeled with IANBD on its β-face at G155C or α-face at A252C, but not when labeled outside the membrane-binding surfaces at Q139C. HeLa cells were labeled to color the outside of plasma membranes red with wheat germ agglutinin-Alexa Fluor 594 (WGA-AF 594) and the nuclei blue with Hoechst 33342 (HT33342) using Image-IT™ live cell labeling kit. The images shown were taken 15 min after applying MMP-12 to cells at room temperature (left images) or to cells pre-cooled on ice for 20 min to slow down endocytosis (right images). In the images of the pre-cooled samples, the brightness of the green channel was increased uniformly in order to see the much weaker green signal and internalization. The scale bars represent 20 μm.
Confocal images of RAW 264.7 macrophages and HeLa cells were acquired after 2 h of incubation at 4 °C with MMP-12 conjugated to IANBD at the β or α-surface. The addition of MMP-12 labeled at G155C, G190C, S207C, or A252C shows much brighter fluorescence than Q139C-labeled outside the interfaces (Fig. 3e-i, k-o). The fluorescence is distributed broadly throughout both cell types (Fig. 3f-i, l-o). The dramatic brightening of IANBD label at the α- and β-faces, but not at the remote Q139C control site, implicates membrane bilayer binding by MMP-12 in its cellular distribution.
Rapid internalization of membrane-binding MMP-12
We investigated where MMP-12 is localized in HeLa cells with the aid of markers of the plasma membrane and nucleus, namely red labeling of carbohydrates decorating the cell surface and blue Hoechst 33342 staining of the nucleus. Live cells were imaged in order to monitor time-dependent changes in the distribution of exogenous MMP-12 catalytic domain across intact cells. The presence of the MMP-12 catalytic domain on the cytoplasmic membrane is suggested by its ringing of cells and its co-localization with carbohydrates that decorate the extracellular surface (Fig. 4). The MMP-12 tagged at the α- or β-face is distributed across cytoplasmic regions with concentration in the perinuclear compartment. The consistently bright green fluorescence emission of these constructs indicates membrane insertion of IANBD emanating from either the α- or β-face. The likelihood of this being insertion into membranes is underscored by the contrasting absence of such signal from the control of MMP-12 labeled at Q139C. The MMP-12 labeled at the α- or β-interface also appears at the nuclear envelope where the polarity-sensitive dye probably inserts in the nuclear membrane. The appearance of the protease within the nucleus might suggest its association with lipids or hydrophobic species there. Bright, punctate MMP-12 staining within the nucleus is especially evident using chilled cells (Fig. 4). The internalization is rapid, with the MMP-12 tagged at G155C evident inside HeLa cells within 90 s after adding the protease exogenously (Fig. 5). Major accumulation of MMP-12 on the plasma membrane, in regions around the nucleus, on the nuclear envelope, and within the nucleus was apparent within 4.5 min, based on the intense fluorescence emitted by the protease labeled on its β-face. The accumulation of MMP-12 on the cell and internal organelle surfaces continued through 12 min, as evidenced by the increased orange and green brightness, respectively (Fig. 5).
Figure 5. Time-lapse confocal imaging shows IANBD-tagged MMP-12(G155C) appearing at the plasma membrane, internalized, in perinuclear regions and within the nuclei.

The frames were collected through 12 min of incubation of live HeLa cells at room temperature with the labeled MMP-12 (green when associated with a membrane). The cytoplasmic membrane is stained red using WGA-AF 594. The arrowheads mark one section of plasma membrane where labeled MMP-12 appears. The dashed lines outline the cell nuclei detected by Hoechst 33342 (nuclear staining not shown).
Stable binding of active MMP-12 to the outside of the plasma membrane after repeated washing of cells is suggested by its proteolytic activity toward a linear peptide substrate (Supplementary Fig. 7). This suggests that the active site remains mostly accessible while the protease is bound to the cell surface. However, several-fold lower activity of MMP-12 bound to cells is observed using a collagen V-derived substrate43, suggesting impaired access of the bulky, rigid substrate to the catalytic cleft. These observations are consistent with the accessibility of the central active site of the membrane-bound enzyme and the bilayer passing near the flanks of the active site (Fig. 2).
Effect of glycosaminoglycans on MMP-12 binding to cells
The possibility of MMP-12 association with cells by binding heparan sulfate chains from proteoglycans was investigated, as these molecules are known to anchor MMP-7 18, 19, 44. Whenoctasaccharide lengths of heparan sulfate were added up to 1.5-fold excess over 15N-labeled MMP-12 at low ionic strength, several small chemical shift perturbations were observed. The largest of these peak shifts occur at Glu128, Tyr132, and Lys136 which form an exposed strip along helix A (Supplementary Fig. 8a,b). This surface corresponds to the middle of the positively charged swath on the back of MMP-7 that was hypothesized to bind heparan sulfate44. Since this putative GAG binding site lies outside the bilayer interfaces (Figs. 1-3), we investigated the possibility of bilayers and GAGs jointly anchoring MMP-12 to cells. Addition of full-length heparan sulfate to 2.5 μg/ml preserved at least 80% of the fluorescence signature of MMP-12 binding to RAW 264.7 macrophages (p < 0.05 by one-tailed Student’s t-test) and had no effect on MMP-12 binding to HeLa cells (Supplementary Fig. 8c,d). The absence of heparan sulfate competition with MMP-12 binding of HeLa cells appears consistent with the rapid internalization of a large fraction of MMP-12 (Figs. 4, 5) sequestering it from exposure to the exogenous heparan sulfate. The slight competition in binding macrophages suggests that heparan sulfate proteolglycans play only a limited role in anchoring MMP-12 to macrophages. With competing heparan sulfate present, the fluorescence changes (Supplementary Fig. 8c,d) imply that the polarity-sensitive probe attached to the α- or β-faces continues to insert into cellular membrane bilayers. The dominant means of binding is probably directly to lipid bilayers. The modes of bilayer binding by MMP-12 permit simultaneous anchoring by GAGs, but this appears to be minor in importance with macrophages and inconsequential with HeLa cells (Supplementary Fig. 8).
Electrostatics and unsaturation enhance vesicle binding
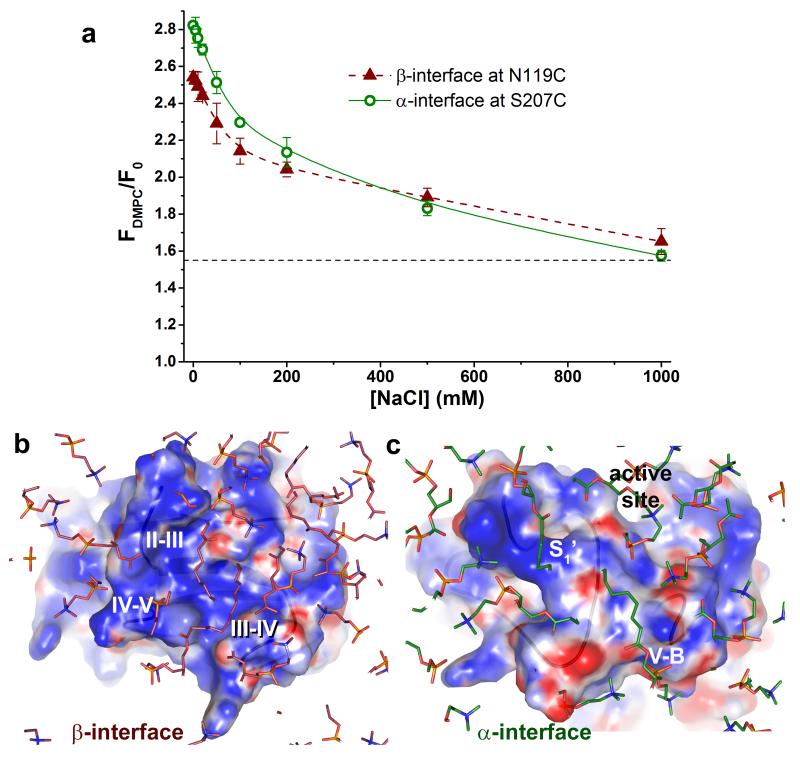
We explored the properties that could influence membrane association. The importance of interfacial salt bridges (Fig. 2c,d and Table 2) to membrane association is evident from effects of salt on vesicle binding. Physiological saline decreased the vesicle-induced FDMPC/F0 signature of binding at both α- and β-interfaces to 0.7-fold that at 0 mM NaCl. Addition of 1 M NaCl decreased this signature of association via the α- and β-interfaces to 0.3-fold and 0.4-fold the levels without NaCl, respectively (Fig. 6a). This corroborates the structures implicating electrostatic attraction in membrane-binding via both sites (Fig. 2c,d). It also strongly suggests that salt frees some MMP-12 to the aqueous compartment, in equilibrium with membrane-bound forms. The slightly lower sensitivity of the β-interface to high salt is consistent with its higher proportion of buried hydrophobic surface. After correcting for the charge effects of the five cations bound to MMP-12, its electrostatic potential was calculated. This indicates positive electrostatic potential across nearly all of the β-interface and much of the α-interface (Fig. 6b,c), consistent with predominant attraction for the phospholipid head groups.
Figure 6. Favorable electrostatics support MMP-12 association with vesicles.
(a) Binding at both α- and β-interfaces is weakened by increasing salt concentration. MMP-12 was conjugated to the polarity-sensitive fluorophore IANBD at a single cysteine in the α- or β-interface. DMPC vesicle-induced increases of the fluorescence of the tagged variants (5 nM) with FDMPC/F0 > 1.55 imply insertion of the fluorophore into the vesicles33. B-spline curves are fitted through the points. (b) The β-interface is electropositive overall. (c) Key parts of the α-interface are electropositive. The electrostatic potentials were calculated after first simulating the partial charges of the atoms surrounding the two zinc and three calcium atoms, as described in Methods. Dark blue represents +3 kT/e while red represents −3 kT/e. The region containing DMPC head groups is shown in the foreground in the complexes.
The electrostatic interactions mediated by Arg117, Arg127, and Arg165 in the β-interface and Lys241 and His206 in the α-interface (Fig. 2c,d) were also tested by adding negatively charged lipids found in plasma membranes. The SDFL vesicle-binding assay used MMP-12 labeled with IANBD at G190C or S207C to respond to binding near the β or α-site, respectively. Additions of negatively charged lipids to 8% (w/w) consistently boosted the fluorescence evidence of insertion into DMPC or POPC vesicles by the reporter placed at either the β- or α-face (Fig. 7). Cholesterol sulfate typically introduces the largest of these fluorescence increases, which are ≥ 1.07-fold (p < 0.05 by one-tailed Student’s t-test). The increases from addition of phosphatidyl serine are also statistically significant (Fig. 7). The most significant increases of ≥ 1.2-fold (p < 0.05 by Student’s t-test) are from the CS and PS introduced to the DMPC vesicles and reported by IANBD in the α-interface at S207C (Fig. 7a). Together these results suggest that MMP-12 affinity for bilayers may be dominated by electrostatic complementarity rather than affinity for a specific lipid ligand.
Figure 7. MMP-12 association with vesicles is enhanced by unsaturated acyl chains and negatively charged lipids.
(a) Vesicles of pure DMPC or mixed with another lipid were incubated with MMP-12 conjugated to IANBD at G190C (red columns) to respond to membrane binding at the β-interface or at S207C (green, cross-hatched columns) at the α-interface. The resulting fluorescence is reported relative to no addition of SUVs, F0. The negatively charged lipid additions were cholesterol sulfate (CS), phosphatidic acid (PA), or phosphatidyl serine (PS). The zwitterionic additions were phosphatidyl ethanolamine (PE), brain sphingomyelin (BSM), and POPC. The means and s.d. of triplicate measurements are shown. The p-values from two-tailed Student’s t-testing are calculated for differences from the control without addition, i.e. DMPC or POPC only, at left. (b) Binding of the tagged forms of MMP-12 to SUVs of POPC with the same lipid additions used in panel (a). The blue asterisks refer to the differences from DMPC.
Given the large areas of the interfaces, the effect of membrane fluidity on insertion was examined. Addition of zwitterionic phosphatidyl ethanolamine and brain sphingomyelin from plasma membranes preserved the levels of fluorescence from the labeled MMP-12 associated with vesicles (Fig. 7). POPC, however, which contains a 9-cis double bond in its oleic acid chain at sn-2, introduced the largest enhancement of binding-induced fluorescence increase. POPC mixed 1:1 with DMPC increased Fliposomes/F0 by ≥ 1.25-fold with p < 0.05 (Fig. 7a by one-tailed Student’s t-test). Vesicles entirely of POPC boosted the fluorescence over DMPC vesicles by ≥ 1.3-fold (Fig. 7, p < 0.05 by Student’s t-test). Either the greater membrane fluidity or the 13% larger effective area of the POPC head group45 may allow MMP-12 better access to its shallow mode of binding or deeper insertion of the fluor among the fatty acyl chains. Thus, cell membranes containing negative charge and unsaturated lipids like POPC appear to be especially attractive to MMP-12.
TIMP-2 competes with membrane bilayers for MMP-12
The resistance of MMP-7, -8, and -9 to inhibition by TIMPs when bound to cells16, 17, 18 prompted us to investigate the possibility of steric conflict between the membrane association and TIMP-2 binding of MMP-12. More than 70% of the activity of MMP-12 catalytic domain on the cell surface is, however, sensitive to inhibition by TIMP-2 (Supplementary Fig. 7). Both structural modes of bilayer binding by MMP-12 were superimposed on the crystal structure of MMP-13 bound to TIMP-2 46. This overlay suggests that the AB loop of TIMP-2 that reaches between the IV-V and III-IV loops of MMPs would clash sterically with the bound bilayer, as these two loops are integral to the β-interface (Fig. 8a). The C-terminal domain of TIMP-2 reaches beyond the S1’ loop of the MMP to approach the adjoining V-B loop closely to clash sterically with bilayer binding across this part of the α-interface (Fig. 8b). Moreover, pre-incubation of fluor-tagged MMP-12 variants with TIMP-2 interfered in the vesicle-induced increase of the fluorescence of IANBD placed at S207C and G190C. The bulky C-terminal domain of TIMP-2 next to the V-B loop (Fig. 8b) disrupted most of the fluorescence increase from bilayer penetration by the fluor linked to S207C (Fig. 8c), conservatively falling to 0.48-fold of the unperturbed level (p ≤ 0.05 by one-tailed Student’s t-test). The next largest steric clash predicted, i.e. from the AB loop of TIMP-2 adjacent to the IV-V loop of MMP-12 (Fig. 8a), halved the vesicle-induced fluorescence of the fluor at G190C (Fig. 8c), conservatively falling to 0.67-fold the level without TIMP-2 (p ≤ 0.05 by Student’s t-test). Hence, the bilayer and TIMP-2 sites are structurally overlapping and functionally competitive.
Figure 8. The inhibitor TIMP-2 competes with membranes for binding beside the catalytic cleft while MMP-12 remains bound to the bilayers.
(a) The structure of MMP-12 (gray) bound to the bilayer model at the β-interface (dark red loops) is superimposed on the crystal structure of the complex of TIMP-2 (purple) with MMP-13 (PDB ID 2e2d)46. The TIMP-2 surface in steric conflict is transparent purple. (b) The rotated structure of MMP-12 bound to the bilayer model at the α-interface (green loops) is superimposed on the TIMP-2-MMP-13 crystal structure. The surface of the TIMP-2 domain in steric conflict with the bilayer is transparent purple. (c) TIMP-2 competes with vesicles for binding contacts at either end of the catalytic cleft of MMP-12, while MMP-12 remains bound to the vesicles. Fluorescence increases of each MMP-12 variant (5 nM) conjugated to IANBD upon incubation with DMPC SUVs are plotted, without or with pre-incubation with 15 nM of the tight-binding TIMP-2 inhibitor (striped bars). The means and s.d. of triplicate measurements are plotted. The p-values marked are from two-tailed Student’s t-testing. (d) TIMP-2 complexes with MMP-12 labeled with IANBD bind RAW 264.7 macrophages robustly. Each MMP-12 variant (20 nM) was incubated 6 h with TIMP-2 (50 nM) at 23 °C before 30 min of incubation with cells on ice and detection at room temperature. The means and s.d. of quadruplicate measurements are shown. (e) TIMP-2 complexes with labeled MMP-12 (n=4) persist in binding HeLa cells as well.
Much less competition was observed when probing membrane-binding loops that do not meet TIMP-2. The TIMP-2-induced attenuation of fluorescence of IANBD at G155C and A252C, which are outside the area of direct steric clash with TIMP-2 binding, are much smaller (Fig. 8c). TIMP-2 attenuates the vesicle-induced fluorescence of IANBD at G155C to 0.89-fold conservatively (p ≤ 0.05 by one-tailed Student’s t-test). Thus, TIMP-2 competition with membrane binding is minimal at the G155C or A252C sites remote from the TIMP-2 binding interface, medium where there is moderate steric conflict (G190C of the IV-V loop), and large where the steric conflict is also large (S207C of the V-B loop). This suggests that MMP-12 remains bound to the vesicle, but rotates on the membrane to accommodate the TIMP-2 obstacle spanning the active site.
TIMP-2 competition with MMP-12 binding to cells was examined. The patterns of cell-induced fluorescence of the IANBD-labeled MMP-12 variants, without and with pre-incubation with TIMP-2 (Fig. 8d,e), are similar to the patterns of vesicle-induced fluorescence. After pre-incubation of TIMP-2 with MMP-12, the high and continuing cell-induced fluorescence of the labels at G155C, G190C, and A252C (Fig. 8d,e) implies that TIMP-2-inhibited MMP-12 continues to bind macrophages and HeLa cells. The fluorescence response of the label at G190C or S207C to cells appears to be less sensitive to TIMP-2 than the response to vesicles (Fig. 8c-e). For example, TIMP-2 bound to MMP-12 attenuates the fluorescence of IANBD at G190C induced by RAW264.7 macrophages only to 0.88-fold (p < 0.05 by one-tailed Student’s t-test) and of IANBD at S207C only to 0.72-fold (p < 0.05 by Student’s t-test). This suggests the persistence of cell binding by MMP-12 despite steric competition from TIMP-2, and further suggests compensatory adjustments of MMP-12 binding to cellular membranes, such as rotations to decrease steric conflict with the TIMP-2 bound.
Discussion
MMP-12 has been observed to bind diverse membrane bilayers consistently via two opposing surfaces of its catalytic domain, i.e., using bicelles, vesicles, plasma membranes, and nuclear membranes of cells. The modes of binding are well-defined by NMR and fluorescence spectroscopic methods, revealing orientations that leave the active site open enough for peptide substrates to enter (Figs. 2, 3 and Supplementary Fig. 1) and that overlap at the α-face with binding of a hydrophobic, non-competitive inhibitor 47. This offers an unprecedented basis to account for the pericellular activity of MMP-12 around activated pulmonary macrophages14 and HeLa cells (Supplementary Fig. 7). Binding of MMP-12 directly to the plasma membranes of macrophages (Fig. 3b, f-i, l-o) provides a mechanism for positioning MMP-12 for juxtamembrane proteolytic release of TNF-α to stimulate inflammation in smokers’ lungs15.
MMP-12 attachment to cellular bilayers ranging from plasma membranes, perinuclear networks, and the nuclear membrane (Fig. 4), as well as the time course of its appearance at these compartments (Fig. 5), suggest a trafficking pathway. The progression of the trafficking in minutes and its slowing and reduction upon cooling are consistent with a hypothesis of endocytosis of MMP-12 bound to membranes (Fig. 4). This could account for the presence of MMP-12 in phagolysozomes of macrophages where its C-terminal domain kills ingested bacteria1. IANBD-tagged MMP-12 accumulates in hydrophobic, presumably membranous, regions near the nucleus, according to the brightness of the fluorescence. The inward transit of MMP-12 catalytic domain to this network and the nuclear envelope is rapid and could involve lipid-dependent trafficking pathways that help deliver MMP-12 towards the nucleus. The bright punctate staining of a few particles in the nucleus by MMP-12 labeled with IANBD in either of its membrane interfaces (Fig. 4) corroborates the report of MMP-12 entry into the nucleus to act as a transcription factor2. These particles, whether containing chromatin or otherwise, must have hydrophobic surfaces into which the polarity-sensitive fluor on MMP-12 inserts. Indeed, the nucleus contains diverse lipids48. The intracellular transit of MMP-12 in HeLa cells and macrophages (Fig. 3, 4) suggests that the trafficking might occur in cases of inflammation beyond the antiviral response2. Numerous reports of MMP-1, -2, -3, -9, -13, -14, and -26 in nuclei 27, as well as putative nuclear localization signals noted in MMP-1, -2, -3, -8, -10, -13, -14, -16, -17, -19, -20, -23, and -24 49 suggest nuclear localization by more MMPs.
The electrostatic attraction of MMP-12 for bilayers is analogous to well-characterized peripheral membrane proteins. Cytochrome c shuttles between redox partners embedded in the membrane and alternates between planar diffusion on the membrane and 3D diffusion in the nearby solution where it is concentrated50, similar to intracellular lipid binding proteins41. MMP-12 may behave similarly, although with opportunity for the 137° flipping between the α and β orientations when it detaches and rebinds. While detached, MMP-12 is freed to hydrolyze its many soluble substrates being identified2, 3. For perspective on surface mobility, the Rheb GTPase rotates freely about its deeply inserted farnesyl tether between modes of bilayer binding51. The two shallow modes of MMP-12 binding may increase its local concentration and residence time on membranes in order to increase the chance of encountering nearby partners and protein substrates. The shallow insertion of MMP-12 among the head groups (Fig. 2) should reduce its frictional drag for faster translational diffusion across the membrane52. This could hasten collisions of MMP-12 with substrates or potential partners on membrane surfaces. This pattern is consistent with the halo of MMP-12 activity observed around activated macrophages14.
The membrane-binding mechanisms described herein could be relevant to some other MMPs. For example, MMP-7 binds vesicles21, and MODA predicts that some loops of MMP-7 and -9 corresponding to the β-face of MMP-12 should bind membranes. The affinity of MMP-7 for bilayers21 potentially enhances its association with uterine epithelial and cancer cells18, 19, 20. Direct binding of bilayers might also aid MMP-8 and -9 interactions with neutrophils16,17. If so, an MMP-12-like mode of binding membranes could explain why cell-bound MMP-7, -8, and -9 retain proteolytic activity yet modestly resist TIMP inhibition17, 18. This hypothesis assumes that the catalytic cleft of these MMPs is also accessible to small substrates without obstruction by the bilayer (Fig. 2 and Supplementary Figs. 1b, 7), which is consistent with the activity of MMP-7, -8, and -9 bound to cells16, 17, 19. Binding of MMP-7, -8, or -9 to plasma membranes with sufficient affinity might sterically hinder binding of TIMP to the ends of the catalytic cleft (Fig. 8a). The fluorescence evidence (Fig. 8c-e) suggests that TIMP-inhibited MMP-12 adjusts its modes of binding to vesicles and cell membranes in order to avoid the steric clashes depicted in Fig. 8a,b.
In summary, the MMP-12 catalytic domain binds to plasma and intracellular membranes, using two complementary structural orientations found on opposite sides of the catalytic cleft. This alternative positioning can promote its almost continuous localization near membranes and invites further studies of MMP trafficking and compartmentalized activities, for example, during inflammatory processes. The broad membrane-binding interfaces of MMP-12 are defined by favorable electrostatic and hydrophobic contacts with phospholipid head groups. The dual positioning of MMP-12 on membranes could be strategically important for activities near the membranes including pericellular shedding of TNF-α to promote inflammation15 and entering and trafficking through cells to reach the nucleus to activate transcription2.
Methods
Preparations of enzyme and lipid assemblies
The inactivated catalytic domain of MMP-12(E219A) was expressed in E. coli without pro-domain from Phe100 to Gly263 and with 15N and 13C labeling of NMR samples23. The catalytic domain was purified with cation exchange chromatography 53 on S-sepharose and folded with decreasing steps of urea while withholding 0.1 mM ZnCl2 until the final dialysis 54. To prepare SUVs of DMPC (Avanti Polar Lipids) at 100 mM, the DMPC was dissolved with vortexing at 42 °C, homogenized with around seven cycles of freezing and thawing, pelleted, redissolved, sonicated three times for 15 min in a bath sonicator to reach a transparent blue appearance, and centrifuged to remove large unilamellar and multilamellar vesicles55. Bicelles were prepared by dissolving large unilamellar vesicles of DMPC with DHPC32 with three cycles of incubation at 42 °C, followed by freezing in liquid nitrogen.
NMR paramagnetic relaxation enhancements in bicelles
NMR samples of 0.5 mM 15N/13C-labeled MMP-12 bearing an E219A point mutation to stabilize the protease were studied at 300 K in 20 mM imidazole (pH 6.6), 10 mM CaCl2, and 20 μM ZnCl2. The NMR peak assignments used in measuring PREs were those of BioMagResBank entry 7089 56. Bicelles (1 DMPC: 2 DHPC; q=0.5; non-orienting) were used at total phospholipid concentration of 80 mM and exhibited hydrodynamic radius of 4.8 nm (with 110 μM MMP-12 present) by dynamic light scattering using a DynaPro MSTC. The implied MW of 144 kDa suggests approximately 86 DMPC molecules per bicelle and a bicelle concentration of approximately 310 μM. PRE experiments introduced 5-doxyl-PC or 14-doxyl-PC (Avanti Polar Lipids) to 620 μM, which is about 1.0 molecule per leaflet of the bicelle. Apparent 1H R2 relaxation rate constants were measured with and without doping with the spin-labeled phospholipid in order to estimate the PRE as Γ2 = R2,paramag-R2,diamag. The relaxation rate constants were measured using a CPMG sequence that suppresses 1H-1H J-couplings 57. Two-point fitting 58 of TROSY-detected, amide proton relaxation used relaxation periods of 0 and 8 ms. HMQC detection of methyl relaxation used CPMG periods of 0, 4, 8, 12, and 16 ms. Distance restraints were obtained from the PREs (Γ2) using an equation of ref59 simplified by ωHτc >> 1 to the form:
| (eq.1) |
where K is 1.23·10−44 m6s−2. The rotational correlation time τc of protein-bicelle complexes was obtained from amide cross-correlation rates ηxy60 interpreted by the TRACT approach61.
NMR-restrained rigid body docking with DMPC bilayer
The PRE-derived depth restraints were divided into separate sets at the α- and β-interfaces for separate structural calculations. The NMR structure of MMP-12(E219A) (PDB code 2POJ)23 was used in rigid body docking using either set of restraints. The coordinates of the bilayer with 128 DMPC molecules were those of Wohlert and Edholm 40. A topology file for the bilayer was prepared with DMPC molecules linked by dummy atoms to permit fast torsion angle dynamics in HADDOCK62. PREs introduced by the doxyl-PC added to bicelles were represented by distance restraints to atoms at a similar depth as the doxyl group in the acyl chains of eight DMPC molecules nearest the MMP-12. These were C1D and C2D atoms for 5-doxyl PC and C1M and C2M atoms for 14-doxyl spin labeling. Implementing rigid body docking using HADDOCK 2.1 63 required raising the limit on lipid molecules to 150 using MAXTREE.
Molecular dynamics refinement of PRE-docked complexes
The HADDOCK results provided starting structures for MD simulations using GROMACS 4.5.5 64. The OPLS force field was used with the Berger lipid parameters65. The DMPC bilayer coordinates with 128 DMPC molecules40 were downloaded from the Automated Force Field Topology Builder (ATB)66, which was also used to generate the topology file for the DMPC bilayer. The complexes at the α- and β-interfaces were solvated with 9619 or 9256 TIP3P water molecules, respectively. The solvated systems were energy minimized, eight Cl− counter ions added to neutralize the charge of MMP-12, and minimized again. The first 200 ps of equilibration used the NVT ensemble and maintained 298 K by velocity scaling (the Berendsen thermostat) with a temperature coupling constant (tau_t) of 0.1 ps and coupling of each group to the temperature bath. The next 1 ns of equilibration used the NPT ensemble. During the equilibration period of 200 ps of the NVT ensemble and 1 ns of the NPT ensemble, positional restraints (spring constant of Kpr = 1000 kJ·mol−1·nm−2) were maintained on the backbone of MMP-12. The linear constraint solver method constrained all bond lengths, with a 2 fs integration step.
The 15 ns production phases of the simulations avoided positional restraints and used the NPT ensemble. Pressure regulation to 1.0 bar in each direction used semi-isotropic coupling and a pressure coupling constant (tau_p) of 5.0 ps. The loose dihedral restraints used in determining the NMR structure of MMP-12 23 and distance restraints to Zn2+ and Ca2+ (Table 1) were applied during the 15 ns production phase of the trajectories. Analyses were performed using GROMACS tools. Fourteen snapshots in best agreement with the PRE-derived distance restraints were drawn from 2 ns segments of the trajectories to represent the experimentally most probable ensembles.
Site-directed fluorchrome labeling
Single cysteine mutations were prepared with the QuikChange approach and using PCR master mix (Stratagene). Addition of 2-mercaptoethanol to the folding protocol54 boosted the yield. The single cysteine variants of MMP-12(E219A) at 1 μM (in fluorescence buffer of 20 mM Tris, pH 7.2, 5 mM CaCl2, 0.1 mM ZnCl2) were conjugated at 4°C overnight with 10 μM IANBD (Invitrogen)33 according to the manufacturer’s protocol with excess 2-mercaptoethanol in the dark in an anaerobic chamber under vacuum. The derivatization of each cysteine variant proceeded to 94 to 100% completion (Supplementary Table 1). The unreacted IANBD was readily removed by desalting on Sephadex G-25 (Sigma)67.
Fluorescence changes detected by plate reader
Fluorescence assays of interactions used quadruplicate samples of 5 nM of IANBD-labeled MMP-12 at 25 °C in 20 mM Tris, pH 7.2, 5 mM CaCl2, and 0.1 mM ZnCl2. Excitation and detection wavelengths were 478 and 541 nm, respectively33, monitored with a BioTek Synergy MX plate reader. Assays using liposomes added them to 400 μM concentrations of lipid monomers. Assays using bicelles (1 DMPC: 2 DHPC) at 55 mM total phospholipid were estimated to be around 100 μM in bicelles using the revised bicelle model of Glover et al.30. Excess TIMP-2 (Syd Labs, Boston) was added using several hours of pre-incubation with MMP-12 variants in order for the slow binding to equilibrate. For plate-based assays of cell-increased fluorescence of IANBD-labeled MMP-12(E219A), approximately 5 million mammalian cells were added per well. 20 nM MMP-12 variant was pre-incubated 6 h with 50 nM TIMP-2 at 23 °C before incubating 30 min with the cells on ice and detecting fluorescence at room temperature.
The data points plotted in the bar charts are means +/− s.d. of triplicate or quadruplicate measurements. The process of statistically comparing the significance of differences in membrane or cell-increased fluorescence of IANBD-labeled MMP-12 first applied F-testing for equal or unequal variances. Then the data were analyzed by Student’s t-test with equal or unequal variance accordingly, using Microsoft Excel. The significance of differences in measurements was evaluated using two-tailed Student’s t-tests. One-tailed Student’s t-tests established the size of significant changes in fluorescence due to the treatment.
Culture of RAW 264.7 and HeLa cell lines
The mouse monocyte/macrophage cell line RAW 264.7 and the human HeLa cervical adenocarcinoma cell line were obtained from the American Type Culture Collection (Manassas, VA) and were free of mycoplasma. Cells were maintained in Dulbecco’s modified Eagle’s high glucose medium (DMEM, Life Technologies, Grand Island, NY) supplemented with 10% (v/v) fetal bovine serum (FBS, Sigma) and 50 μg/ml gentamicin (APP Pharmaceuticals, Schaumburg, IL)) and cultured at 37 °C in a humidified atmosphere with 5% CO2. Cells were harvested with TrypLE (Life Technologies) and DMEM media was added to the suspension. Cells were resuspended in 20 mM Tris pH 7.2, 5 mM CaCl2, 0.1 mM ZnCl2 following centrifugation, counted, and adjusted to the appropriate concentration.
Fluorescent imaging of MMP-12 association with mammalian cells
Cells to be fixed were grown overnight to 80% confluency on sterile glass cover slips. The cells were placed on ice to impede membrane trafficking. After washing three times with ice-cold DMEM without phenol red, the cells were incubated with staining agents. For the images of Fig. 3, cultures of RAW 264.7 macrophages and HeLa cells were incubated on ice with 100 nM MMP-12 variants in DMEM without phenol red for 2 h, followed by washing five times with phosphate-buffered saline on ice, fixing for 10 min with 4% paraformaldehyde, and imaging with a Zeiss LSM 510 Meta confocal microscope.
To examine the time course of MMP-12 internalization in living cells, HeLa cells were grown overnight to 60-80% confluency on sterile MatTek (Ashland, MA) glass bottom culture dishes. These cells were washed three times with DMEM without phenol red and incubated 10 min (at 37 °C, 5% CO2) with the Image-It™ kit (Life Technologies) comprising the Alexa Fluor 594 conjugate of wheat germ agglutinin (WGA-AF 594) at 5 μg/ml to label the outer face of the plasma membranes red and 2 μM of Hoechst 33342 to label double-stranded DNA and nuclei blue. The cells were then washed three times with 20 mM Tris, pH 7.2, 5 mM CaCl2, 0.1 mM ZnCl. A culture dish was then placed on the stage of a Leica TCP SP8 MP inverted spectral confocal microscope equipped with 405 nm and tunable white light lasers. The excitation/emission bandpass wavelengths used to detect Hoechst 33342, IANBD, and Alexa Fluor 594 WGA were set to 405/415-470, 472/485-525, and 594/610-680 nm, respectively. After taking the first multichannel image (time point 0), 100 nM of the IANBD-labeled MMP-12 variant was added and images collected at 21-22 °C every 90 s over a period of ~20 min using the settings mentioned above. In order to slow down MMP-12 trafficking, cultured HeLa cells were placed on ice for 20 min and then subjected to the same staining and time-lapse imaging protocols.
Electrostatic calculations
The MMP-12 crystal structure (PDB code 1JK3)37 was used for calculating partial charges on atoms surrounding the two zinc ions and three calcium ions. At the active site, the inhibitor was removed from 1JK3 and two bound waters introduced where two the oxygen ligands from the inhibitor had been. The charges and radii at the two zinc and three calcium binding sites were parameterized by combined application of Ambertools module MCPB/MTKPP68 and Gaussian 09 (Revision B.01) at the B3LYP/6-31G* level. The partial charges surrounding these five cations were transferred to the coordinates (PQR files) of the α- and β-complexes of MMP-12 with bilayer models. Elsewhere, the Amber f99SB69 radii and charges were applied to the assemblies. Electrostatic potentials were calculated using the Adaptive Poisson-Boltzmann Solver (APBS)70 at 298 K and ionic strength of 30 mM. The protein and solvent dielectric constants were set to 2.0 and 78.54, respectively. The protonation state was set to pH 7.
Supplementary Material
Acknowledgments
We are grateful to Ruben Abagyan and Irina Kufareva for sharing the MODA software, Kathy Schreiber and the Cell and Immunobiology Core (U. of Missouri) for cell cultures, Alexandre Bonvin for discussion of HADDOCK configuration, Robert Linhardt and Fuming Zhang for heparan sulfate fragments, Linda Randall for critically reading the manuscript, and the U. of Missouri NMR facility for 800 MHz access and Bioinformatics Consortium for parallel computing access. This work was supported by NIH grants R01 GM057289 and S10 RR022341 toward the 800 MHz system. MO and ML were funded by the BBSRC and Cancer Research UK.
Abbreviations
- COPD
chronic pulmonary obstructive disease
- DHPC
dihexanoyl phosphatidyl choline
- DMPC
dimyristoyl phosphatidylcholine
- GAG
glycosaminoglycan
- IANBD
N,N′-dimethyl-N-(iodoacetyl)-N′-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)ethylenediamine
- MD
molecular dynamics
- MMP
matrix metalloproteinase
- MODA
membrane optimal docking area software
- PC
phosphatidylcholine
- POPC
1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine
- PRE
paramagnetic NMR relaxation enhancement
- RMSD
root mean-squared deviation
- SDFL
site-directed fluor labeling
- SUVs
small unilamellar vesicles
- TIMP
tissue inhibitor of metalloproteinases
- TNF-α
tissue necrosis factor-α
Footnotes
Supplementary Information accompanies this paper at http://www.nature.com/naturecommunications
Competing financial interests
The authors declare no competing financial interests.
Accession codes
The experimental restraints and structural ensembles of the MMP-12 complexes with DMPC bilayer have been deposited in the Protein Data Bank under accession numbers 2MLR and 2MLS for the α and β-interfaces, respectively.
References
- 1.Houghton AM, Hartzell WO, Robbins CS, Gomis-Ruth FX, Shapiro SD. Macrophage elastase kills bacteria within murine macrophages. Nature. 2009;460:637–641. doi: 10.1038/nature08181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marchant DJ, et al. A new transcriptional role for matrix metalloproteinase-12 in antiviral immunity. Nat Med. 2014;20:493–502. doi: 10.1038/nm.3508. [DOI] [PubMed] [Google Scholar]
- 3.Dean RA, Cox JH, Bellac CL, Doucet A, Starr AE, Overall CM. Macrophage-specific metalloelastase (MMP-12) truncates and inactivates ELR+ CXC chemokines and generates CCL2, −7, −8, and −13 antagonists: potential role of the macrophage in terminating polymorphonuclear leukocyte influx. Blood. 2008;112:3455–3464. doi: 10.1182/blood-2007-12-129080. [DOI] [PubMed] [Google Scholar]
- 4.Hautamaki RD, Kobayashi DK, Senior RM, Shapiro SD. Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice. Science. 1997;277:2002–2004. doi: 10.1126/science.277.5334.2002. [DOI] [PubMed] [Google Scholar]
- 5.Curci JA, Liao S, Huffman MD, Shapiro SD, Thompson RW. Expression and localization of macrophage elastase (matrix metalloproteinase-12) in abdominal aortic aneurysms. J Clin Invest. 1998;102:1900–1910. doi: 10.1172/JCI2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson JL, George SJ, Newby AC, Jackson CL. Divergent effects of matrix metalloproteinases 3, 7, 9, and 12 on atherosclerotic plaque stability in mouse brachiocephalic arteries. Proc Natl Acad Sci U S A. 2005;102:15575–15580. doi: 10.1073/pnas.0506201102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parks WC, Wilson CL, Lopez-Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol. 2004;4:617–629. doi: 10.1038/nri1418. [DOI] [PubMed] [Google Scholar]
- 8.Gill SE, Parks WC. Metalloproteinases and their inhibitors: Regulators of wound healing. The International Journal of Biochemistry & Cell Biology. 2008;40:1334–1347. doi: 10.1016/j.biocel.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kessenbrock K, Plaks V, Werb Z. Matrix Metalloproteinases: Regulators of the Tumor Microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Troeberg L, Nagase H. Proteases involved in cartilage matrix degradation in osteoarthritis. Biochim Biophys Acta. 2012;1824:133–145. doi: 10.1016/j.bbapap.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koenig GC, et al. MT1-MMP-Dependent Remodeling of Cardiac Extracellular Matrix Structure and Function Following Myocardial Infarction. Am J Pathol. 2012;180:1863–1878. doi: 10.1016/j.ajpath.2012.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huntley GW. Synaptic circuit remodelling by matrix metalloproteinases in health and disease. Nat Rev Neurosci. 2012;13:743–757. doi: 10.1038/nrn3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murphy G, Nagase H. Localizing matrix metalloproteinase activities in the pericellular environment. FEBS J. 2011;278:2–15. doi: 10.1111/j.1742-4658.2010.07918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cobos-Correa A, Trojanek JB, Diemer S, Mall MA, Schultz C. Membrane-bound FRET probe visualizes MMP12 activity in pulmonary inflammation. Nat Chem Biol. 2009;5:628–630. doi: 10.1038/nchembio.196. [DOI] [PubMed] [Google Scholar]
- 15.Churg A, et al. Macrophage metalloelastase mediates acute cigarette smoke-induced inflammation via tumor necrosis factor-alpha release. Am J Respir Crit Care Med. 2003;167:1083–1089. doi: 10.1164/rccm.200212-1396OC. [DOI] [PubMed] [Google Scholar]
- 16.Owen CA, Hu Z, Barrick B, Shapiro SD. Inducible Expression of Tissue Inhibitor of Metalloproteinases-Resistant Matrix Metalloproteinase-9 on the Cell Surface of Neutrophils. Am J Respir Cell Mol Biol. 2003;29:283–294. doi: 10.1165/rcmb.2003-0034OC. [DOI] [PubMed] [Google Scholar]
- 17.Owen CA, Hu Z, Lopez-Otin C, Shapiro SD. Membrane-Bound Matrix Metalloproteinase-8 on Activated Polymorphonuclear Cells Is a Potent, Tissue Inhibitor of Metalloproteinase-Resistant Collagenase and Serpinase. J Immunol. 2004;172:7791–7803. doi: 10.4049/jimmunol.172.12.7791. [DOI] [PubMed] [Google Scholar]
- 18.Berton A, et al. Binding of matrilysin-1 to human epithelial cells promotes its activity. Cell Mol Life Sci. 2007;64:610–620. doi: 10.1007/s00018-007-6415-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu WH, Woessner JF, Jr., McNeish JD, Stamenkovic I. CD44 anchors the assembly of matrilysin/MMP-7 with heparin-binding epidermal growth factor precursor and ErbB4 and regulates female reproductive organ remodeling. Genes Dev. 2002;16:307–323. doi: 10.1101/gad.925702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamamoto K, Higashi S, Kioi M, Tsunezumi J, Honke K, Miyazaki K. Binding of active matrilysin to cell surface cholesterol sulfate is essential for its membrane-associated proteolytic action and induction of homotypic cell adhesion. J Biol Chem. 2006;281:9170–9180. doi: 10.1074/jbc.M510377200. [DOI] [PubMed] [Google Scholar]
- 21.Ganguly B, Banerjee J, Elegbede AI, Klocke DJ, Mallik S, Srivastava DK. Intrinsic selectivity in binding of matrix metalloproteinase-7 to differently charged lipid membranes. FEBS Lett. 2007;581:5723–5726. doi: 10.1016/j.febslet.2007.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bertini I, et al. Conformational variability of matrix metalloproteinases: beyond a single 3D structure. Proc Natl Acad Sci U S A. 2005;102:5334–5339. doi: 10.1073/pnas.0407106102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhaskaran R, Palmier MO, Bagegni NA, Liang X, Van Doren SR. Solution structure of inhibitor-free human metalloelastase (MMP-12) indicates an internal conformational adjustment. J Mol Biol. 2007;374:1333–1344. doi: 10.1016/j.jmb.2007.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palmier MO, Fulcher YG, Bhaskaran R, Duong VQ, Fields GB, Van Doren SR. NMR and bioinformatics discovery of exosites that tune metalloelastase specificity for solubilized elastin and collagen triple helices. J Biol Chem. 2010;285:30918–30930. doi: 10.1074/jbc.M110.136903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liang X, et al. Apparent tradeoff of higher activity in MMP-12 for enhanced stability and flexibility in MMP-3. Biophys J. 2010;99:273–283. doi: 10.1016/j.bpj.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fulcher YG, Van Doren SR. Remote Exosites of the Catalytic Domain of Matrix Metalloproteinase-12 Enhance Elastin Degradation. Biochemistry. 2011;50:9488–9499. doi: 10.1021/bi2009807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cauwe B, Opdenakker G. Intracellular substrate cleavage: a novel dimension in the biochemistry, biology and pathology of matrix metalloproteinases. Crit Rev Biochem Mol Biol. 2010;45:351–423. doi: 10.3109/10409238.2010.501783. [DOI] [PubMed] [Google Scholar]
- 28.Eguchi T, et al. Novel Transcription Factor-Like Function of Human Matrix Metalloproteinase 3 Regulating the CTGF/CCN2 Gene. Mol Cell Biol. 2008;28:2391–2413. doi: 10.1128/MCB.01288-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kutateladze TG, et al. Multivalent mechanism of membrane insertion by the FYVE domain. J Biol Chem. 2004;279:3050–3057. doi: 10.1074/jbc.M309007200. [DOI] [PubMed] [Google Scholar]
- 30.Glover KJ, et al. Structural Evaluation of Phospholipid Bicelles for Solution-State Studies of Membrane-Associated Biomolecules. Biophys J. 2001;81:2163–2171. doi: 10.1016/s0006-3495(01)75864-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Y, Kahn RA, Prestegard JH. Dynamic structure of membrane-anchored Arf*GTP. Nat Struct Mol Biol. 2010;17:876–881. doi: 10.1038/nsmb.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morrison EA, et al. Antiparallel EmrE exports drugs by exchanging between asymmetric structures. Nature. 2012;481:45–50. doi: 10.1038/nature10703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schulz TA, et al. Lipid-regulated sterol transfer between closely apposed membranes by oxysterol-binding protein homologues. J Cell Biol. 2009;187:889–903. doi: 10.1083/jcb.200905007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim YE, Chen J, Chan JR, Langen R. Engineering a polarity-sensitive biosensor for time-lapse imaging of apoptotic processes and degeneration. Nat Meth. 2010;7:67–73. doi: 10.1038/nmeth.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bissig C, et al. Viral Infection Controlled by a Calcium-Dependent Lipid-Binding Module in ALIX. Dev Cell. 2013;25:364–373. doi: 10.1016/j.devcel.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kufareva I, et al. Discovery of novel membrane binding structures and functions. Biochem Cell Biol. 2014 doi: 10.1139/bcb-2014-0074. doi: 10.1139/bcb-2014-0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lang R, et al. Substrate specificity determinants of human macrophage elastase (MMP-12) based on the 1.1 A crystal structure. J Mol Biol. 2001;312:731–742. doi: 10.1006/jmbi.2001.4954. [DOI] [PubMed] [Google Scholar]
- 38.Shapiro SD, et al. Molecular cloning, chromosomal localization, and bacterial expression of a murine macrophage metalloelastase. J Biol Chem. 1992;267:4664–4671. [PubMed] [Google Scholar]
- 39.Park HI, Lee S, Ullah A, Cao Q, Sang Q-XA. Effects of detergents on catalytic activity of human endometase/matrilysin 2, a putative cancer biomarker. Anal Biochem. 2010;396:262–268. doi: 10.1016/j.ab.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wohlert J, Edholm O. Dynamics in atomistic simulations of phospholipid membranes: Nuclear magnetic resonance relaxation rates and lateral diffusion. J Chem Phys. 2006;125:204703. doi: 10.1063/1.2393240. [DOI] [PubMed] [Google Scholar]
- 41.Moravcevic K, Oxley Camilla L, Lemmon Mark A. Conditional Peripheral Membrane Proteins: Facing up to Limited Specificity. Structure. 2012;20:15–27. doi: 10.1016/j.str.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haugland RP. Myosin structure. Proximity measurements by fluorescence energy transfer. J Supramol Struct. 1975;3:338–347. doi: 10.1002/jss.400030405. [DOI] [PubMed] [Google Scholar]
- 43.Bhaskaran R, Palmier MO, Lauer-Fields JL, Fields GB, Van Doren SR. MMP-12 Catalytic Domain Recognizes Triple Helical Peptide Models of Collagen V with Exosites and High Activity. J Biol Chem. 2008;283:21779–21788. doi: 10.1074/jbc.M709966200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu WH, Woessner JF., Jr. Heparan sulfate proteoglycans as extracellular docking molecules for matrilysin (matrix metalloproteinase 7) J Biol Chem. 2000;275:4183–4191. doi: 10.1074/jbc.275.6.4183. [DOI] [PubMed] [Google Scholar]
- 45.Kučerka N, Tristram-Nagle S, Nagle J. Structure of Fully Hydrated Fluid Phase Lipid Bilayers with Monounsaturated Chains. J Membrane Biol. 2006;208:193–202. doi: 10.1007/s00232-005-7006-8. [DOI] [PubMed] [Google Scholar]
- 46.Maskos K, Lang R, Tschesche H, Bode W. Flexibility and variability of TIMP binding: X-ray structure of the complex between collagenase-3/MMP-13 and TIMP-2. J Mol Biol. 2007;366:1222–1231. doi: 10.1016/j.jmb.2006.11.072. [DOI] [PubMed] [Google Scholar]
- 47.Udi Y, et al. Unraveling Hidden Regulatory Sites in Structurally Homologous Metalloproteases. J Mol Biol. 2013;425:2330–2346. doi: 10.1016/j.jmb.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 48.Irvine RF. Nuclear lipid signalling. Nat Rev Mol Cell Biol. 2003;4:349–361. doi: 10.1038/nrm1100. [DOI] [PubMed] [Google Scholar]
- 49.Si-Tayeb K, et al. Matrix Metalloproteinase 3 Is Present in the Cell Nucleus and Is Involved in Apoptosis. The American Journal of Pathology. 2006;169:1390–1401. doi: 10.2353/ajpath.2006.060005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gupte SS, Hackenbrock CR. Multidimensional diffusion modes and collision frequencies of cytochrome c with its redox partners. J Biol Chem. 1988;263:5241–5247. [PubMed] [Google Scholar]
- 51.Mazhab-Jafari MT, et al. Membrane-Dependent Modulation of the mTOR Activator Rheb: NMR Observations of a GTPase Tethered to a Lipid-Bilayer Nanodisc. J Am Chem Soc. 2013;135:3367–3370. doi: 10.1021/ja312508w. [DOI] [PubMed] [Google Scholar]
- 52.Ziemba BP, Falke JJ. Lateral diffusion of peripheral membrane proteins on supported lipid bilayers is controlled by the additive frictional drags of (1) bound lipids and (2) protein domains penetrating into the bilayer hydrocarbon core. Chem Phys Lipids. 2013;172-173:67–77. doi: 10.1016/j.chemphyslip.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Parkar AA, et al. Large-scale expression, refolding, and purification of the catalytic domain of human macrophage metalloelastase (MMP-12) in Escherichia coli. Protein Expr Purif. 2000;20:152–161. doi: 10.1006/prep.2000.1280. [DOI] [PubMed] [Google Scholar]
- 54.Zheng X, Ou L, Tong X, Zhu J, Wu H. Over-expression and refolding of isotopically labeled recombinant catalytic domain of human macrophage elastase (MMP-12) for NMR studies. Protein Expr Purif. 2007;56:160–166. doi: 10.1016/j.pep.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 55.Sommer LAM, Meier MA, Dames SA. A fast and simple method for probing the interaction of peptides and proteins with lipids and membrane-mimetics using GB1 fusion proteins and NMR spectroscopy. Protein Sci. 2012;21:1566–1570. doi: 10.1002/pro.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bhaskaran R, Van Doren SR. (1)H, (13)C, and (15)N peak assignments and secondary structure of human macrophage metalloelastase (MMP-12) in its inhibitor-free state. J Biomol NMR. 2006;36(Suppl 1):55. doi: 10.1007/s10858-006-9035-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aguilar JA, Nilsson M, Bodenhausen G, Morris GA. Spin echo NMR spectra without J modulation. Chem Commun. 2012;48:811–813. doi: 10.1039/c1cc16699a. [DOI] [PubMed] [Google Scholar]
- 58.Iwahara J, Tang C, Clore GM. Practical aspects of H-1 transverse paramagnetic relaxation enhancement measurements on macromolecules. J Magn Reson. 2007;184:185–195. doi: 10.1016/j.jmr.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Battiste JL, Wagner G. Utilization of site-directed spin labeling and high-resolution heteronuclear nuclear magnetic resonance for global fold determination of large proteins with limited nuclear overhauser effect data. Biochemistry. 2000;39:5355–5365. doi: 10.1021/bi000060h. [DOI] [PubMed] [Google Scholar]
- 60.Liu Y, Prestegard JH. Direct measurement of dipole-dipole/CSA cross-correlated relaxation by a constant-time experiment. J Magn Reson. 2008;193:23–31. doi: 10.1016/j.jmr.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee D, Hilty C, Wider G, Wüthrich K. Effective rotational correlation times of proteins from NMR relaxation interference. J Magn Reson. 2006;178:72–76. doi: 10.1016/j.jmr.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 62.Dancea F, Kami K, Overduin M. Lipid interaction networks of peripheral membrane proteins revealed by data-driven micelle docking. Biophys J. 2008;94:515–524. doi: 10.1529/biophysj.107.115923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dominguez C, Boelens R, Bonvin AM. HADDOCK: a protein-protein docking approach based on biochemical or biophysical information. J Am Chem Soc. 2003;125:1731–1737. doi: 10.1021/ja026939x. [DOI] [PubMed] [Google Scholar]
- 64.Pronk S, et al. GROMACS 4.5: a high-throughput and highly parallel open source molecular simulation toolkit. Bioinformatics. 2013;29:845–854. doi: 10.1093/bioinformatics/btt055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Berger O, Edholm O, Jähnig F. Molecular dynamics simulations of a fluid bilayer of dipalmitoylphosphatidylcholine at full hydration, constant pressure, and constant temperature. Biophys J. 1997;72:2002–2013. doi: 10.1016/S0006-3495(97)78845-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Malde AK, et al. An Automated Force Field Topology Builder (ATB) and Repository: Version 1.0. J Chem Theory and Comput. 2011;7:4026–4037. doi: 10.1021/ct200196m. [DOI] [PubMed] [Google Scholar]
- 67.Dunn SMJ, Raftery MA. Multiple binding sites for agonists on Torpedo californica acetylcholine receptor. Biochemistry. 1982;21:6264–6272. doi: 10.1021/bi00267a035. [DOI] [PubMed] [Google Scholar]
- 68.Peters MB, Yang Y, Wang B, Fusti-Molnar L, Weaver MN, Merz KM., Jr. Structural Survey of Zinc Containing Proteins and the Development of the Zinc AMBER Force Field (ZAFF) J Chem Theory Comput. 2010;6:2935–2947. doi: 10.1021/ct1002626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hornak V, Abel R, Okur A, Strockbine B, Roitberg A, Simmerling C. Comparison of multiple Amber force fields and development of improved protein backbone parameters. Proteins: Structure, Function, and Bioinformatics. 2006;65:712–725. doi: 10.1002/prot.21123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Baker NA, Sept D, Joseph S, Holst MJ, McCammon JA. Electrostatics of nanosystems: application to microtubules and the ribosome. Proc Natl Acad Sci U S A. 2001;98:10037–10041. doi: 10.1073/pnas.181342398. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.