Abstract
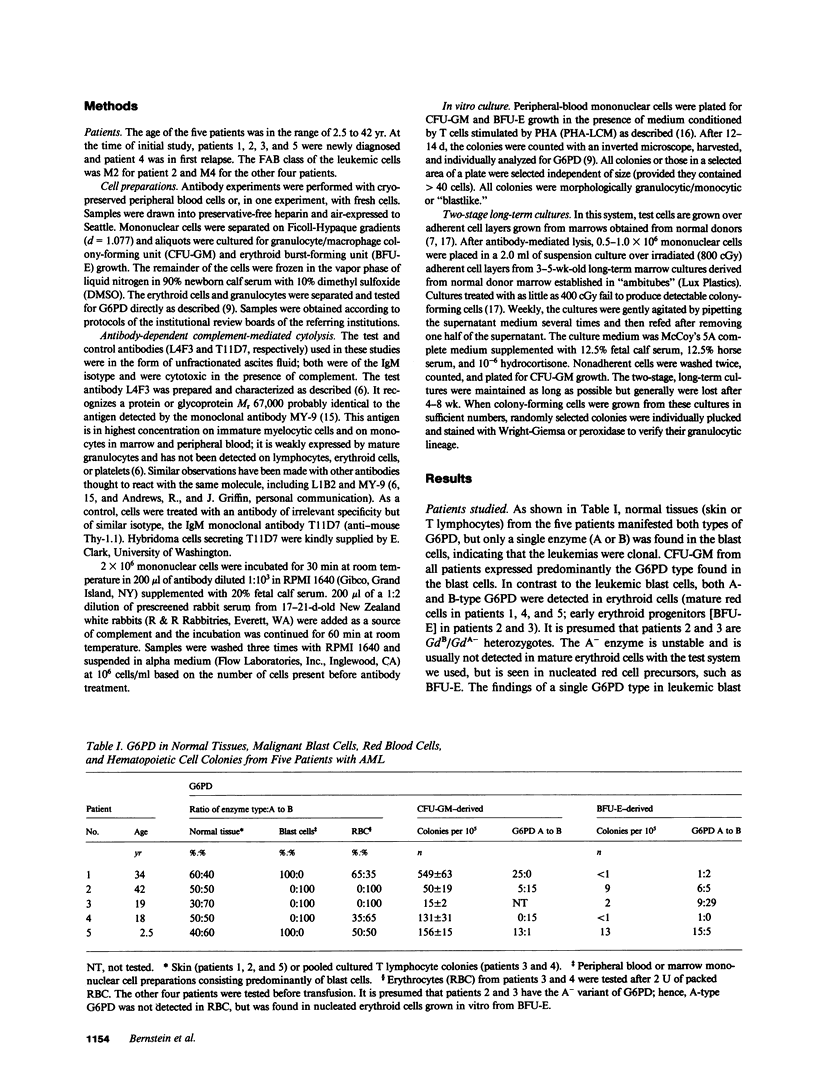
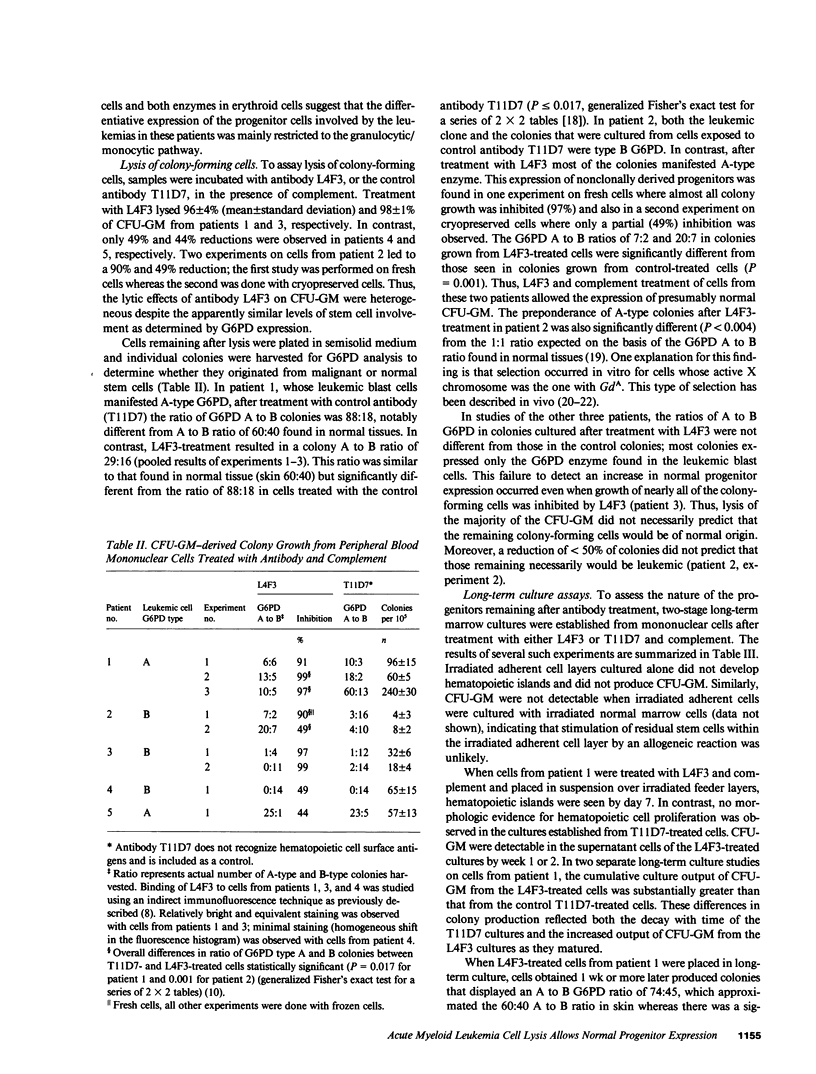
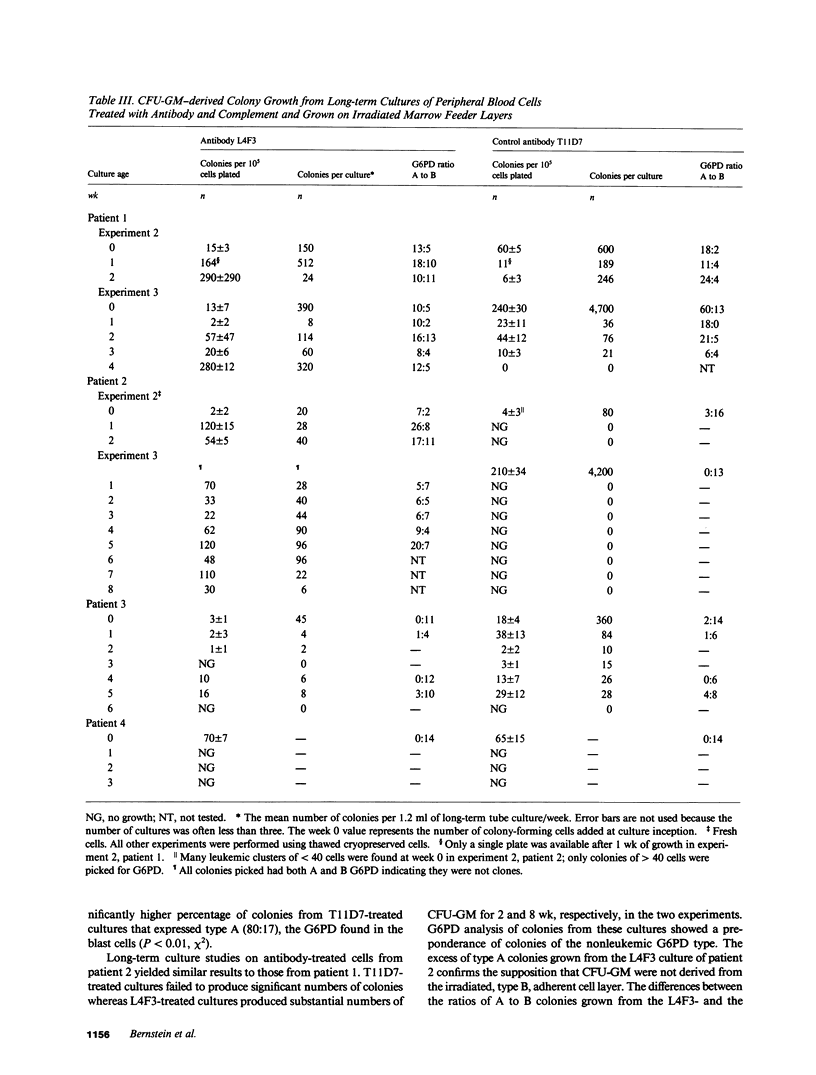
Monoclonal antibody L4F3 reacts with most acute myeloid leukemia (AML) cells and virtually all normal granulocyte/monocyte colony-forming cells (CFU-GM). Our objective was to determine whether lysis of AML cells with L4F3 and complement allowed expression of normal myeloid progenitors. The five glucose-6-phosphate dehydrogenase (G6PD) heterozygous patients with AML studied manifested only a single G6PD type in blast cells and in most or all granulocyte colony-forming cells, indicating that the leukemias developed clonally. The cells remaining after L4F3 treatment from two of the patients gave rise to granulocytic colonies that expressed the G6PD type not seen in the leukemic clone, indicating that they were derived from normal progenitors (CFU-GM). L4F3-treated cells from these two patients cultured over an irradiated adherent cell layer from normal long-term marrow cultures also gave rise to CFU-GM, which were shown by G6PD analysis to be predominantly nonleukemic. In the other three patients, the progenitor cells remaining after L4F3 treatment were derived mainly from the leukemic clone. The data suggest that in vitro cytolytic treatment with L4F3 of cells from certain patients with AML can enable normal, presumably highly immature progenitors to be expressed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews R. G., Takahashi M., Segal G. M., Powell J. S., Bernstein I. D., Singer J. W. The L4F3 antigen is expressed by unipotent and multipotent colony-forming cells but not by their precursors. Blood. 1986 Nov;68(5):1030–1035. [PubMed] [Google Scholar]
- Andrews R. G., Torok-Storb B., Bernstein I. D. Myeloid-associated differentiation antigens on stem cells and their progeny identified by monoclonal antibodies. Blood. 1983 Jul;62(1):124–132. [PubMed] [Google Scholar]
- Chang J., Coutinho L., Morgenstern G., Scarffe J. H., Deakin D., Harrison C., Testa N. G., Dexter T. M. Reconstitution of haemopoietic system with autologous marrow taken during relapse of acute myeloblastic leukaemia and grown in long-term culture. Lancet. 1986 Feb 8;1(8476):294–295. doi: 10.1016/s0140-6736(86)90828-7. [DOI] [PubMed] [Google Scholar]
- Coulombel L., Kalousek D. K., Eaves C. J., Gupta C. M., Eaves A. C. Long-term marrow culture reveals chromosomally normal hematopoietic progenitor cells in patients with Philadelphia chromosome-positive chronic myelogenous leukemia. N Engl J Med. 1983 Jun 23;308(25):1493–1498. doi: 10.1056/NEJM198306233082502. [DOI] [PubMed] [Google Scholar]
- Dinndorf P. A., Andrews R. G., Benjamin D., Ridgway D., Wolff L., Bernstein I. D. Expression of normal myeloid-associated antigens by acute leukemia cells. Blood. 1986 Apr;67(4):1048–1053. [PubMed] [Google Scholar]
- Ferraris A. M., Broccia G., Meloni T., Canepa L., Sessarego M., Gaetani G. F. Clonal origin of cells restricted to monocytic differentiation in acute nonlymphocytic leukemia. Blood. 1984 Oct;64(4):817–820. [PubMed] [Google Scholar]
- Ferraris A. M., Canepa L., Mareni C., Baule G., Meloni T., Salvidio E., Forteleoni G., Gaetani G. F. Reexpression of normal stem cells in erythroleukemia during remission. Blood. 1983 Jul;62(1):177–179. [PubMed] [Google Scholar]
- Fialkow P. J., Singer J. W., Adamson J. W., Vaidya K., Dow L. W., Ochs J., Moohr J. W. Acute nonlymphocytic leukemia: heterogeneity of stem cell origin. Blood. 1981 Jun;57(6):1068–1073. [PubMed] [Google Scholar]
- Gealy W. J., Dwyer J. M., Harley J. B. Allelic exclusion of glucose-6-phosphate dehydrogenase in platelets and T lymphocytes from a Wiskott-Aldrich syndrome carrier. Lancet. 1980 Jan 12;1(8159):63–65. doi: 10.1016/s0140-6736(80)90492-4. [DOI] [PubMed] [Google Scholar]
- Griffin J. D., Linch D., Sabbath K., Larcom P., Schlossman S. F. A monoclonal antibody reactive with normal and leukemic human myeloid progenitor cells. Leuk Res. 1984;8(4):521–534. doi: 10.1016/0145-2126(84)90001-8. [DOI] [PubMed] [Google Scholar]
- Jacobson R. J., Temple M. J., Singer J. W., Raskind W., Powell J., Fialkow P. J. A clonal complete remission in a patient with acute nonlymphocytic leukemia originating in a multipotent stem cell. N Engl J Med. 1984 Jun 7;310(23):1513–1517. doi: 10.1056/NEJM198406073102307. [DOI] [PubMed] [Google Scholar]
- Moore M. A., Broxmeyer H. E., Sheridan A. P., Meyers P. A., Jacobsen N., Winchester R. J. Continuous human bone marrow culture: Ia antigen characterization of probable pluripotential stem cells. Blood. 1980 Apr;55(4):682–690. [PubMed] [Google Scholar]
- Nyhan W. L., Bakay B., Connor J. D., Marks J. F., Keele D. K. Hemizygous expression of glucose-6-phosphate dehydrogenase in erythrocytes of heterozygotes for the Lesch-Nyhan syndrome. Proc Natl Acad Sci U S A. 1970 Jan;65(1):214–218. doi: 10.1073/pnas.65.1.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prchal J. T., Carroll A. J., Prchal J. F., Crist W. M., Skalka H. W., Gealy W. J., Harley J., Malluh A. Wiskott-Aldrich syndrome: cellular impairments and their implication for carrier detection. Blood. 1980 Dec;56(6):1048–1054. [PubMed] [Google Scholar]
- Ramsay N., LeBien T., Nesbit M., McGlave P., Weisdorf D., Kenyon P., Hurd D., Goldman A., Kim T., Kersey J. Autologous bone marrow transplantation for patients with acute lymphoblastic leukemia in second or subsequent remission: results of bone marrow treated with monoclonal antibodies BA-1, BA-2, and BA-3 plus complement. Blood. 1985 Sep;66(3):508–513. [PubMed] [Google Scholar]
- Ritz J., Sallan S. E., Bast R. C., Jr, Lipton J. M., Clavell L. A., Feeney M., Hercend T., Nathan D. G., Schlossman S. F. Autologous bone-marrow transplantation in CALLA-positive acute lymphoblastic leukemia after in-vitro treatment with J5 monoclonal antibody and complement. Lancet. 1982 Jul 10;2(8289):60–63. doi: 10.1016/s0140-6736(82)91686-5. [DOI] [PubMed] [Google Scholar]
- Singer J. W., Fialkow P. J., Dow L. W., Ernst C., Steinmann L. Unicellular or multicellular origin of human granulocyte-macrophage colonies in vitro. Blood. 1979 Dec;54(6):1395–1399. [PubMed] [Google Scholar]
- Takahashi M., Keating A., Singer J. W. A functional defect in irradiated adherent layers from chronic myelogenous leukemia long-term marrow cultures. Exp Hematol. 1985 Oct;13(9):926–931. [PubMed] [Google Scholar]


