Abstract
D1 and D2 dopamine receptors exist as heteromers in cells and brain tissue and are dynamically regulated and separated by agonist concentrations at the cell surface. We determined that these receptor pairs interact primarily through discrete amino acids in the cytoplasmic regions of each receptor, with no evidence of any D1-D2 receptor transmembrane interaction found. Specifically involved in heteromer formation we identified, in intracellular loop 3 of the D2 receptor, two adjacent arginine residues. Substitution of one of the arginine pair prevented heteromer formation. Also involved in heteromer formation we identified, in the carboxyl tail of the D1 receptor, two adjacent glutamic acid residues. Substitution of one of the glutamic acid pair prevented heteromer formation. These amino acid pairs in D1 and D2 receptors are oppositely charged, and presumably interact directly by electrostatic interactions.
Keywords: G protein coupled receptors, dopamine receptor, nuclear localization, protein structure, heteromer, interacting amino acids
1. Introduction
Family A G protein coupled receptors (GPCRs) form heteromers [1,2,3]. We reported that D1- D2 receptor heteromers exist in brain and cultured neurons [4,5]. We showed receptor activation within D1- D2 heteromers generated a Gq-mediated calcium signal [4,6,7]. We have determined that D1-D2 heteromers were subject to conformational changes and separation by dopamine or receptor-selective agonists [8]. We also reported that the D1 and D2 receptor heteromers reform at the cell surface when the agonist was removed [8]. These data provided evidence of the fate of a heteromer following agonist activation and demonstrated a unique regulation of GPCRs at the cell surface. However, many fine structural details of how D1-D2 heteromers dynamically interact remain unknown. In this report we have determined the precise amino acid interactions maintaining D1 and D2 receptors in a D1-D2 receptor complex. Our ultimate goal is the understanding of the physiological relevance of GPCR:GPCR heteromers, one of the leading questions in the GPCR field.
Progress in the fundamental area of GPCR oligomer structural investigation has been hampered by the lack of decisive methods for determining the interacting heteromer interface. We overcame technical challenges by the following process: a nuclear localization sequence (NLS) was inserted into the D2 receptor. Strategic placement of the NLS rendered this D2-NLS receptor conformationally sensitive, so that interacting ligands retained the receptor at the cell surface [9]. D2-NLS and the D1 receptors were coexpressed and following ligand removal, the D2-NLS receptor translocated with the D1 receptor from the cell surface. We demonstrated that as the D2-NLS receptor translocated with the D1 receptor this provided a tool to study receptor:receptor dynamic interactions in a cell [9]. By this strategy we sought to reveal the structural basis for the D1-D2 receptor interaction. By co-expressing D2-NLS and D1 receptors the contributions of various cytoplasmic regions of these receptors to heteromer formation was investigated.
In this report we have determined the precise amino acids in the cytoplasmic regions of both the D1 and D2 receptors involved in their heteromeric interactions. Activation of the heteromer contributes to conformational changes in the receptors within the oligomer. We have now identified these residues affected by agonist induced conformational changes. Also we identified that changing a single amino acid in the intracellular loop 3 of the D2 receptor or in the carboxyl tail of the D1 receptor prevented D1-D2 heteromer formation.
2. Materials and methods
2.1. Fluorescent proteins
cDNA sequences encoding GFP, RFP were obtained from Clontech (Palo Alto, CA), and the receptor constructs generated as described [9]. The YFP vector was obtained from BD Biosciences.
2.2. Cell culture
HEK cells grown to confluence on 60 mm plates in minimum essential medium (MEM), and were transfected with 0.5–2 µg cDNA using Lipofectamine (Life technologies, Rockville MD).
2.3. Microscopy
Live cells expressing GFP, RFP and YFP fusion proteins were visualized with a LSM510 Zeiss confocal laser microscope. In each experiment 5–8 fields, containing 50–80 cells per field were evaluated and the entire experiment was repeated several times (n=3–5).
2.4. DNA Constructs
All the DNA encoding the GPCRs were human origin. Sequences encoding GPCRs were cloned into plasmids pEGFP, as described previously [9]. The D1 carboxyl tail DNA PCR product, containing no stop codon was subcloned into vector pYFP-N1 (BD Biosciences) at EcoR1 and Kpn1 and inframe with the start codon of YFP.
2.5. Receptor Constructs
The D1 and D2 receptors were prepared using the Quickchange mutagenesis kit (Stratagene) according to the manufacturer’s instructions, and as described [9]. Receptor DNA was subjected to PCR as previously reported [9]. The reaction mixture consisted of: H2O (32 µl), 10x Pfu buffer (Stratagene) (5µl), dNTP (10mM, 5µl), DMSO (5µl), oligonucleotide primers (100ng, 1µl each), DNA template (100ng), Pfu enzyme (5U). Total volume 50µl. PCR conditions, one cycle at 94 °C for 2 min, 30–35 cycles at 94 °C for 30 sec, 55 °C for 30 sec, 72 °C for 1 min, per cycle, and then one cycle at 72 °C for 5 min. The NLS sequence was inserted into DNA encoding the D1 and D2 dopamine receptors by PCR [8].
2.6 Membrane Preparation
Cells expressing D2-NLS or D1-NLS were washed with phosphate-buffered saline, resuspended in hypotonic lysis buffer (5 mM Tris-HCl, 2 mM EDTA, 5 µg/ml leupeptin, 10 µg/ml benzamide, 5 µg/ml soybean trypsin inhibitor, pH 7.4), and homogenized by Polytron (Brinkmann Instruments). The homogenate was centrifuged to pellet unbroken cells and nuclei. The supernatant centrifuged at 40,000 × g to obtain a membrane pellet.
2.7 Radioligand Binding Assays
Competition binding assays were performed as described previously (1,3). Briefly, for competition experiments, 20–25 µg of membrane was incubated with 1nM [3H]-raclopride (for D2) or [3H]-SCH23390 (for D1) (NEN Life Science Products) and increasing concentrations of competing drug. The reaction volume was 0.5 ml, and the binding buffer consisted of 50 mM Tris-HCl, 5 mM EDTA, 1.5 mMCaCl2, 5 mMMgCl2, 5 mMKCl, and 120 mM NaCl, pH 7.4. Nonspecific binding was defined using 1 µM (+)-butaclamol (Research Biochemicals International, Hercules, CA). Binding reactions were incubated at room temperature for 2 h to reach equilibrium. Bound radioligand was then isolated from free by rapid filtration through a Brandel 48-well harvester using Whatman GF/C filters. Data were analyzed using nonlinear least squares regression equations on the curve-fitting computer program Prism (Graphpad).
3. Results
3.1. Binding and expression properties of the D2-NLS receptor and D1-NLS receptors
The incorporation of NLS into the D2 receptor did not alter the binding properties, with preserved agonist-detected high affinity and low affinity states, indicative of intact receptor-G protein coupling. The D2 receptor had a KHigh value of 1.51 × 10−9 M and KLow of 6.67 × 10−6 M for quinpirole. Similarly the D2-NLS receptor had a KHigh value of 3.22 × 10−9 M and KLow of 4.16 × 10 −6 M for quinpirole [9].
The incorporation of the NLS into the D1 receptor did not alter the binding pocket of the receptor, with preserved agonist- detected high affinity and low affinity states, indicative of intact receptor-G protein coupling and ligand affinities. The D1-NLS receptor had a Khigh value of 4.17 × 10−9 M and Klow of 1.19 × 10−7 M detected by agonist SKF 81297 not different from unmodified D1 receptor [9]
3.2. Identification of the D2 dopamine receptor amino acids involved in D1-D2 heteromer formation
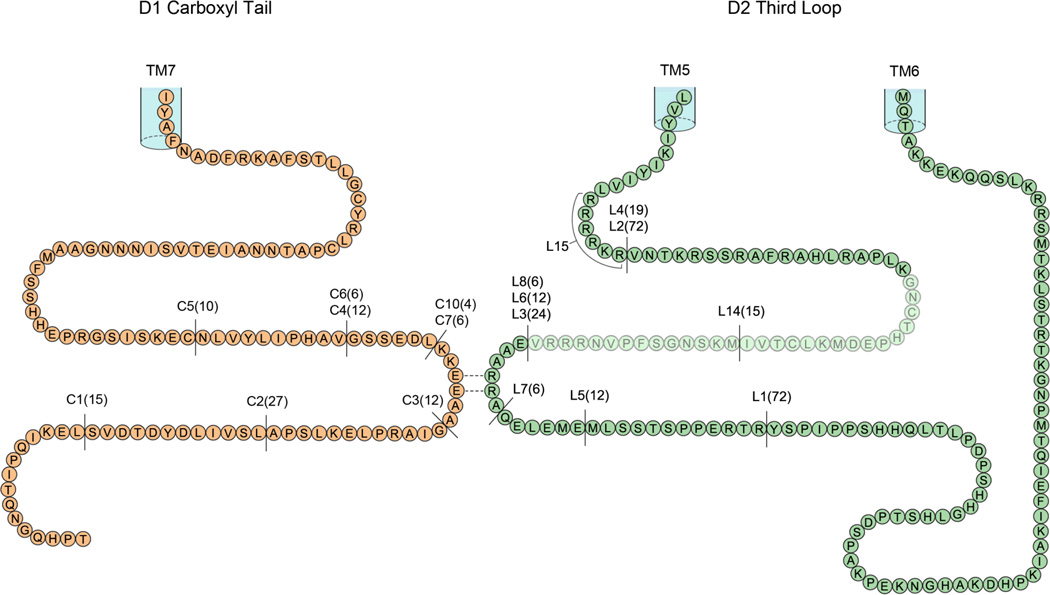
We wished to determine if amino acids located in the cytoplasmic loops of the D2 receptor were involved in forming heteromeric complexes with the D1 receptor. The D2 receptor has an unusual GPCR structure in having no significant carboxyl tail, as the carboxyl tail terminates with the palmitoylated cysteine [10]. There are two forms of the D2 dopamine receptor, namely D2 long and D2 short, differing by a 29 amino acid insert in ic3, located thirty amino acids from transmembrane 5 (TM5; Fig. 1) [11]. The very large intracellular D2 receptor third loop (intracellular loop 3, ic3) contains ~160 amino acids, this region comprises 40% of the total receptor structure, Fig 1. The D2-NLS long receptor with a fully intact ic3 and the D1 receptor are shown co-expressed in Fig. 2A, with significant co-translocation indicating robust heteromer formation.
Figure 1.
Representation of the of the primary amino acid sequence of the cytoplasmic intracellular tail of the D1 dopamine receptor and the primary amino acid sequence of the large cytoplasmic intracellular third loop of the D2 dopamine receptor. The locations of the various intracellular deletions constructs are shown, numbers in bracket indicate amino acids deleted. The position of the insert of 29 amino acids in the D2 long receptor is indicated by the shading.
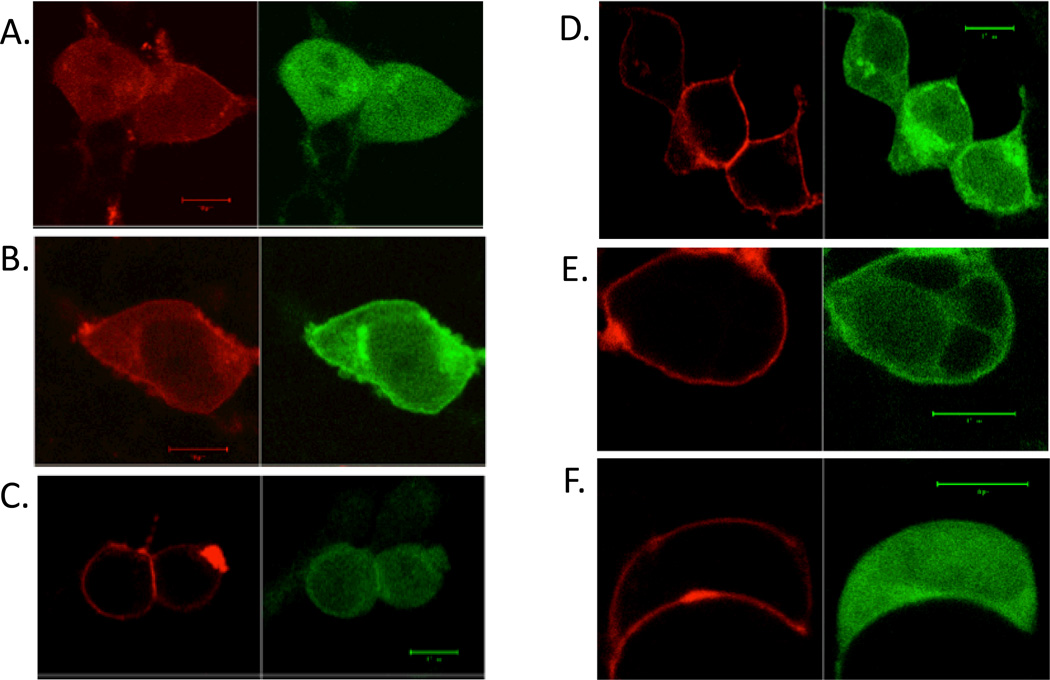
Figure 2.
Visualization of co-expression of D5 and D2-NLS dopamine receptors.
A. D5 (RFP) (red) and D2-NLS (GFP) (green) co-translocated to the cytoplasm and nucleus. B. C1 D5 (RFP) (red) and (D2-NLS) (GFP) (green) did not co-translocate to the nucleus. C. C2 D5 (RFP) (red) and (D2-NLS) (GFP) (green) did co-translocate. D. C3 D5 (RFP) (red) and (D2-NLS) (GFP) (green) and did not co-translocate. E. C4 D5 (RFP) (red) and (D2-NLS) (GFP) (green) co-translocated. F. C6 D5 (RFP) (red) and (D2-NLS) (GFP) (green) co-translocated.
Each size bar in figures showing cells indicates length of 10 µm.
In our strategy, initially working with the D2 long receptor, we prepared a series of D2 receptor constructs with deletions contained in this third loop (outlined in Table 1 and Fig. 1). Each of these ic3 receptor constructs of the D2-NLS receptor were co-expressed with the D1 receptor. In each case D1-D2 heteromerization was monitored by the ability of these D2-NLS receptors to enable transportation of the D1 receptor from the cell surface to the cytoplasm and nucleus. We first determined that a large deletion, L1, of 72 amino acids (Table 1 and Fig 1), from the carboxyl terminal half of D2 receptor ic3 had no effect on D1-D2 heteromer formation, these receptors translocated together (Fig. 2B). However, another D2 receptor construct, L2, with 72 amino acids deleted from the amino terminal half of ic3 (Table 1 and Fig. 1) failed to show D1-D2 receptor co-translocation, and hence failed to form D1-D2 receptor heteromers, Fig. 2C. Thus data from the L2 construct indicated that amino acids maintaining heteromer formation were likely contained in this region. To locate the critical amino acids, portions of this ic3 L2 region were serially deleted to identify regions involved in the interaction with the D1 receptor.
Table 1.
| D2 | Receptor constructs | Heteromer formation |
|---|---|---|
| L1 | …PPERTR (YSPIP…LSQQ) KEKK… | Yes |
| L2 | …RRRRKR (VNTK….ERTR) YSPI… | No |
| L3 | …VNRRRV (EAARRA….ERTR) YSPI … | No |
| L4 | …RRRRKR (VNTKR….APLK) GNCT… | Yes |
| L5 | …QELEME (MLSSTSPPERTR) YSPI … | Yes |
| L6 | …VNRRRV (EAARRAQELEME) MLSS… | No |
| L7 | …EAARRA (QELEME) MLSS … | Yes |
| L8 | …VNRRRV (EAARRA) QELE… | No |
| L9 | …VNRRRV EAAAAA QELE… | No |
| L10 | …VNRRRV AAARRA QELE… | Yes |
| L11 | …VNRRRV EAARAA QELE… | No |
| L12 | …VNRRRV EAAARA QELE… | No |
| L13 | …VNRRRV EAAKKA QELE… | No |
| L14 | …MKLCTVI (IMKSNGSFPVNRRRVE)AARR… | Yes |
| L15 | …KIYIVL AAAAAAVNTK… | Yes |
Amino acid sequence in italics, underlined and brackets indicates deletions.
Amino acid sequence in italics, and underlined indicates substitutions.
We divided the L2 region into two parts, L3 (24 amino acids) and L4 (19 amino acids, Fig. 1), not including the 29 amino acid insert of the D2 long receptor. The construct L4 formed D1- D2 receptor heteromers while construct L3 did not (L3 shown in Fig 2D), thus the region involved in heteromer formation was contained in the 24 amino acids of construct L3. The L3 region was divided in two equal parts, with constructs L5 and L6. Only construct L6 failed to form heteromers with the D1 receptor and this region of 12 amino acids was further divided in two equal parts, in constructs L7 and L8. Construct L8, with the sequence (271-EAARRA) deleted, also failed to form heteromers with the D1 receptor, Fig. 2E.
Thus as a result of following this systematic process we successfully narrowed the ic3 region of D2 receptor that was required for interacting with the D1 receptor to 6 amino acids (271 –EAARRA). The start of this sequence was located a distance of 59 amino acids from TM5 (Fig. 1), of the D2 long receptor. By substituting the three charged amino acids in this sequence, L9 (EAAAAA), Fig 2F, and L10 (AAARRA), we determined that the vicinal arginine residues alone (274-RR) were the key residues required for heteromerization with the D1 receptor. Substitution of the glutamic acid residue had no effect on the D1- D2 heteromerization, L10. We examined the role of each arginine residue separately, we prepared two D2-NLS constructs, namely L11 (EAARAA) and L12 (EAAARA), compared to wild type –EAARRA. Co-expression of L11 and L12 receptor constructs with the D1 receptor failed to show heteromerization, thus demonstrating that a single amino acid change prevented D1-D2 receptor heteromer formation, and demonstrating that both arginine residues were required for heteromer formation. Also we prepared a D2-NLS construct L13 (EAAKKA), where the vicinal arginines were replaced by similarly charged lysines. Co-expression of this construct failed to show heteromer formation with the D1 receptor. Thus from a total structure of the ~160 amino acids in ic3 loop of the D2 receptor only two specific charged amino acids (274-RR) were involved in forming heteromers with the D1 receptor.
3.3. Role of D2 long and D2 short dopamine receptors
As stated there are two forms of the D2 dopamine receptor, namely D2 long and D2 short, differing by a 29 amino acid insert in ic3, located thirty amino acids from TM5 (Fig. 1) [11]. A recent report in Nature Medicine [12] stated that D1 and D2 receptors interacted via a section of these 29 residues, thus implying that D2 short receptor could not form heteromers with the D1 receptor. To investigate we co-expressed the D1-NLS receptor with the D2 short receptor and showed them to be capable of forming heteromers, Fig. S1A. This result we expected as the significant region we identified in ic3 of D2 receptor (274-RR), was present in both the D2 long and D2 short receptors.
Specifically, it was pinpointed that a sequence of 15 amino acids in the carboxyl part of this 29 amino acids in ic3 of the D2 long receptor interacted directly with the D1 receptor [12]. Thus we prepared a D2-NLS receptor construct, L14, where we deleted these 15 amino acids (Table 1, Fig 1). However this D2-NLS receptor, L14, was also capable of forming D1-D2 heteromers, Fig. S1B.
3.4. Investigation of the role of the D2 receptor intracellular loop 3 region 217-RRRRKR
In several previous reports, the D2 ic3 region 217–RRRRKR was implicated as the possible heteromer interacting site, forming heteromers with either D1 dopamine [13], 5HT2A serotonin [14] or adenosine A2A receptors [15]. This highly charged amino sequence starts at a distance of six amino acids from TM5. We substituted this 217-RRRRKR region in D2-NLS receptor with alanines (construct L15, Table 1). Coexpression of L15 with the D1 receptor demonstrated co-translocation, and hence intact heteromer formation, Fig S1C.
3.5. Identification of the D1 dopamine receptor amino acids involved in D1-D2 heteromer formation
We wished to determine if amino acids located in any of the cytoplasmic loops or carboxyl tail of the D1 dopamine receptor were involved in forming heteromers with the D2 dopamine receptor. The D1 receptor has an extensive carboxyl tail, extending ~114 amino acids from the palmitoylated cysteine (26% of the total D1 receptor). Initially D1 and D2-NLS receptors were co-expressed with a construct containing the entire D1-carboxyl tail (Table 1). In the presence of the D1-carboxyl tail construct, the D1 and D2-NLS receptors did not form heteromers, indicating that the amino acids in the carboxyl tail were involved in heteromer formation, Fig S2A. Consequently we prepared a series of deletions constructs in the D1 carboxyl tail (C1 to C5), and each deletion construct was co-expressed with the D2-NLS receptor (Table 2 and Fig. 1). Of these D1 carboxyl tail deletion constructs only C4, failed to show receptor heteromerization, indicating the location of a 12 amino acid critical region involved in D1- D2 heteromer formation. In this sequence the deletion of 6 amino acids (GSSEDL; Fig 1), C6, had no effect on heteromer formation with the D2 receptor.
Table 2.
| D1 | Receptor constructs | Heteromer formation |
|---|---|---|
| C1 | …YDTDVS (LEKIQPITQNGQHPT) | Yes |
| C2 | …EKLSPA (LSVILDYDTDVSLEKIQPITQNGQHPT) | Yes |
| C3 | …KKEEAA (GIARPLEKLSPA) LSVI … | Yes |
| C4 | …IPHAV (GSSEDLKKEEAA) GIAR… | No |
| C5 | …SISKEC (NLVYLIPHAV) GSSE… | Yes |
| C6 | …IPHAV(GSSEDL) KKEE… | Yes |
| C7 | …GSSEDL (KKEEAA)GIAR… | No |
| C8 | …GSSEDL AAAAAA GIAR… | No |
| C9 | …GSSEDL KKEELL GIAR… | Yes |
| C10 | …GSSEDL (KKEE) AA GIAR… | No |
| C11 | …GSSEDL KKDDAA GIAR… | No |
| C12 | …GSSEDL AKEEAA GIAR… | Yes |
| C13 | …GSSEDL KAEEAA GIAR… | Yes |
| C14 | …GSSEDL KKEAAA GIAR… | No |
| C15 | …GSSEDL KKAEAA GIAR… | No |
| C16 | …GSSEDL KKAAAA GIAR… | -- |
| C17 | …GSSEDL AAEEAA GIAR… | -- |
| D1-Carboxyl tail: MAFSTLLGCYRLCP …‥ (86 amino acids)…‥ TQNGQHPT | ||
Amino acid sequence in italics, underlined and brackets indicates deletions.
Amino acid sequence in italics, and underlined indicates substitutions.
Thus, by this process we narrowed the critical sequence to 6 amino acids (402-KKEEAA), Fig. 1. The start of this discrete region of the D1 carboxyl tail is located 68 amino acids from TM7. In construct C7 this sequence was deleted and in construct C8 this sequence was substituted by alanines; in neither case was heteromer formation observed with the D2 receptor. Substitution of the alanine pair in this region, C9, had no effect on the heteromer formation. However the deletion of 4 amino acids (KKEE), C10, D2 receptor heteromer formation was not observed, Fig. S2B. By substituting the amino acids in this sequence (402-KKEE) we determined that the glutamic acid pair alone were the key residues required for D1-D2 heteromers. We prepared a construct with a similar charged pair of aspartic acid residues (DD) substituting for (EE), C11, and this receptor did not form D1-D2 heteromers, Fig. S2C. We prepared two single amino acid substitution D1 constructs, C12 (AKEE) and C13 (KAEE) and co-expressed each with the D2-NLS receptor, D1-D2 heteromer formation was observed, Fig. S2D and Fig. S2E. We prepared the receptors, C14 (KKEA) and C15 (KKAE), where one glutamic acid was substituted, in each case no D1-D2 heteromerization was observed. Thus from a total structure of 114 amino acids in this D1 carboxyl tail only a pair of glutamic acid residues were required for forming a heteromer with the D2 receptor.
Further analysis of the –KKEE- sequence with constructs C16 and C17 prepared with the two lysines (KK) deleted, or with the two glutamic acids deleted (EE) gave very poor expression, without any result.
4. Discussion
There are several significant and unique accomplishments regarding the oligomeric structures of the D1-D2 dopamine receptors reported here. (i) We determined that a pair of adjacent arginines of the D2 receptor, located in the third cytoplasmic loop, were involved in forming heteromers with the D1 receptor. (ii) We determined that both arginines were required, a D2 receptor with one of the arginines substituted did not form heteromers with the D1 receptor. (iii) We determined that the oppositely charged pair of glutamic acids located in the D1 receptor carboxyl tail was involved in forming heteromers. (iv) We determined that both glutamic acids were required, a D1 receptor construct with one of the glutamic acids substituted did not form heteromers with the D2 receptor. (v) We determined that a construct with the vicinal aspartic acids substituted for the glutamic acids in the D1 receptor carboxyl tail did not form heteromers. (vi) Both D2 long and D2 short dopamine receptors were capable of forming heteromers with the D1 receptor, and we found no evidence that any part of the 29 amino acid insert in D2 long receptor was involved in heteromer formation. (vii) We found no evidence that transmembrane interactions were required in D1-D2 heteromer formation.
We have previously shown that D1-D2 heteromers separated into D1 and D2 receptors by agonist treatment. Agonist binding alters the receptor conformation and resulted in the separation of the components of the heteromer. It appears likely that with dopamine activation of the D1-D2 heteromer there is a conformational change in the D2 intracellular third loop and D1 carboxyl tail which disengages the interaction between these receptors, perhaps disrupting a direct D1-D2 (EE:RR) electrostatic interaction. Receptor-selective agonists bind and alter the conformation of either D1 or D2 dopamine receptors and this change also was sufficient to disrupt the heteromer. Thus conformational change in either the ic3 or carboxyl tail can cause heteromer disruption.
Thus the NLS incorporation strategy has enabled precise elucidation of structural features of D1 and D2 receptor heteromers, aspects of GPCR oligomer structure that were not resolved previously. This method can be applied for other members of this rhodopsin related family of receptors.
A recent report also implicated cytoplasmic regions in the formation of M3-M5 muscarinic receptor heteromers. Co-expression of the M3 and M5 receptors with a peptide from ic3 of the M5 receptor reduced the degree of heteromerization [16].
The role of the D2 receptor ic3 217–RRRRKR sequence (Fig. 1) in heteromer formation with the D1 receptor and with the 5HT2A receptors was investigated [13,14]. These investigators concluded only that this region might be involved, their report contained caveats due to possible altered intracellular location of the D2 dopamine receptor with the removal of this arginine rich region. Ciruela et al. [15] also investigated the role of this D2 receptor (217–RRRRKR) region in heteromer formation with adenosine A2 receptors. They concluded that this ic3 region of the D2 dopamine receptor formed part of that heteromer [15]. However we found no evidence for a primary heteromer involvement as our deletion of 217–RRRRKR in the D2 receptor ic3, L15, had no effect on the intact D1-D2 heteromer formation. Although we do understand that GPCR:GPCR interactions are complex and likely involve multiple contact sites.
We are in agreement with [13] the role of vicinal glutamic acids (404 EE) present in the D1 carboxyl tail, as being involved in the D1-D2 heteromer. This glutamic acid pair is located 56 amino acid from the palmitoylated cysteine in the D1 receptor. Interestingly a glutamic acid pair located in the 5HT2A receptor carboxyl tail was identified as being involved in D2-5HT2A receptor heteromers [14]. This identified glutamic acid pair in the 5HT2A receptor was also located 56 amino acids from the palmitoylated cysteine. These results may indicate that the D1 and 5HT2A receptor carboxyl tails are required to extend a similar distance to interact directly with the arginine pair in ic3 of the D2 receptor.
The identified sites in the D2 ic3 and D1 carboxyl tail receptors as the heteromer forming site would require a proximity of these intracellular regions. These changes in cytoplasmic conformation will create other areas of heteromer contact. Formation of the D1-D2 heteromer likely changes the cytoplasmic architecture of these receptor pairs, due to this entanglement of the D1 and D2 cytoplasmic regions. These conformational changes enabled participation in G protein coupling of different signaling cascades by the D1-D2 heteromer [4].
Contrary to the implications of the data from [12] the D2 short receptor formed heteromers with the D1 receptor. Based on our data we did not find the ic3 29 amino acids, that differentiate D2 long from D2 short receptors, required for D1-D2 heteromer formation.
In summary, we used a novel approach to examine and elucidate the structure of D1-D2 receptor heteromers. As a result of the work described we are now in a position to prepare D1 and D2 receptor expressing cells engineered to be incapable of forming heteromers. The signaling properties of these unique cell lines will be of great interest, and this work will elucidate the true role of the heteromers in the physiology of receptor functioning as heteromers.
Supplementary Material
Acknowledgements
This work was partially supported by a Proof of Principle Grant from the Canadian Institutes for Health Research and National Institute on Drug Abuse Grant (DA007223). SRG holds a Canada Research Chair in Molecular Neuroscience. The authors thank Fan Hong Qian for preparation of Figure 1.
Footnotes
Appendix A. Supplementary data
Supplementary data associated with this article can be found in the online version.
References
- 1.George SR, O’Dowd BF, Lee SP. G-protein-coupled receptor oligomerization and its potential for drug discovery. Nat. Rev. Drug Discovery. 2002;1:808–820. doi: 10.1038/nrd913. [DOI] [PubMed] [Google Scholar]
- 2.Milligan G. G protein-coupled receptor dimerization: function and ligand pharmacology. Mol. Pharm. 2004;66:1–7. doi: 10.1124/mol.104.000497.. [DOI] [PubMed] [Google Scholar]
- 3.Ciruela F, Vallano A, Arnau JM, Sánchez S, Borroto-Escuela DO, Agnati LF, Fuxe K, Fernández-Dueñas V. G protein-coupled receptor oligomerization for what? J. Recept. Signal Transduct. Res. 2010;30:322–330. doi: 10.3109/10799893.2010.508166. [DOI] [PubMed] [Google Scholar]
- 4.Hasbi A, Fan T, Alijaniaram M, Nguyen T, Perreault ML, O'Dowd BF, George SR. Calcium signaling cascade links dopamine D1-D2 receptor heteromer to striatal BDNF production and neuronal growth. Proc. Natl. Acad. Sci. U S A. 2009;106:21377–21382. doi: 10.1073/pnas.0903676106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perreault ML, Hasbi A, Alijaniaram M, Fan T, Varghese G, Fletcher PJ, Seeman P, O'Dowd BF, George SR. The dopamine D1-D2 receptor heteromer localizes in dynorphin/enkephalin neurons: increased high affinity state following amphetamine and in schizophrenia. J. Biol. Chem. 2010;285:36625–36634. doi: 10.1074/jbc.M110.159954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee SP, So CH, Rashid AJ, Varghese G, Cheng R, Lança AJ, O'Dowd BF, George SR. Dopamine D1 and D2 receptor Co-activation generates a novel phospholipase C-mediated calcium signal. J. Biol. Chem. 2004;279:35671–35678. doi: 10.1074/jbc.M401923200. [DOI] [PubMed] [Google Scholar]
- 7.Rashid AJ, So CH, Kong MM, Furtak T, El-Ghundi M, Cheng R, O'Dowd BF, George SR. D1-D2 dopamine receptor heterooligomers with unique pharmacology are coupled to rapid activation of Gq/11 in the striatum. Proc. Natl. Acad. Sci. USA. 2007;104:654–659. doi: 10.1073/pnas.0604049104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Dowd BF, Ji X, Alijaniaram M, Nguyen T, George SR. Separation and reformation of cell surface dopamine receptor oligomers visualized in cells. Eur. J. Pharmacol. 2011;658:74–83. doi: 10.1016/j.ejphar.2011.02.030. [DOI] [PubMed] [Google Scholar]
- 9.O'Dowd BF, Ji X, Alijaniaram M, Rajaram RD, Kong MM, Rashid A, Nguyen T, George SR. Dopamine receptor oligomerization visualized in living cells. J. Biol. Chem. 2005;280:37225–37235. doi: 10.1074/jbc.M504562200. [DOI] [PubMed] [Google Scholar]
- 10.Bunzow JR, Van Tol HH, Grandy DK, Albert P, Salon J, Christie M, Machida CA, Neve KA, Civelli O. Cloning and expression of a rat D2 dopamine receptor cDNA. Nature. 1988;336:783–787. doi: 10.1038/336783a0. [DOI] [PubMed] [Google Scholar]
- 11.O'Dowd BF, Nguyen T, Tirpak A, Jarvie KR, Israel Y, Seeman P, Niznik HB. Cloning of two additional catecholamine receptors from rat brain. FEBS Lett. 1990;262:8–12. doi: 10.1016/0014-5793(90)80140-e. [DOI] [PubMed] [Google Scholar]
- 12.Pei L, Li S, Wang M, Diwan M, Anisman H, Fletcher PJ, Nobrega JN, Liu F. Uncoupling the dopamine D1-D2 receptor complex exerts antidepressant-like effects. Nat Med. 2010;16:1393–1395. doi: 10.1038/nm.2263. [DOI] [PubMed] [Google Scholar]
- 13.Łukasiewicz S, Faron-Górecka A, Dobrucki J, Polit A, Dziedzicka-Wasylewska M. Studies on the role of the receptor protein motifs possibly involved in electrostatic interactions on the dopamine D1 and D2 receptor oligomerization. FEBS J. 2009;276:760–775. doi: 10.1111/j.1742-4658.2008.06822.x. [DOI] [PubMed] [Google Scholar]
- 14.Lukasiewicz S, Polit A, Kędracka-Krok S, Wędzony K, Maćkowiak M, Dziedzicka-Wasylewska M. Hetero-dimerization of serotonin 5-HT(2A) and dopamine D(2) receptors. Biochim. Biophys. Acta. 2010;1803:1347–1358. doi: 10.1016/j.bbamcr.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 15.Ciruela F, Burgueño J, Casadó V, Canals M, Marcellino D, Goldberg SR, Bader M, Fuxe K, Agnati LF, Lluis C, Franco R, Ferré S, Woods AS. Combining mass spectrometry and pull-down techniques for the study of receptor heteromerization. Direct epitope-epitope electrostatic interactions between adenosine A2A and dopamine D2 receptors. Anal Chem. 2004;76:5354–5363. doi: 10.1021/ac049295f. [DOI] [PubMed] [Google Scholar]
- 16.Borroto-Escuela DO, García-Negredo G, Garriga P, Fuxe K, Ciruela F. The M(5) muscarinic acetylcholine receptor third intracellular loop regulates receptor function and oligomerization. Biochim. Biophys. Acta. 2010;1803:813–825. doi: 10.1016/j.bbamcr.2010.04.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.