Abstract
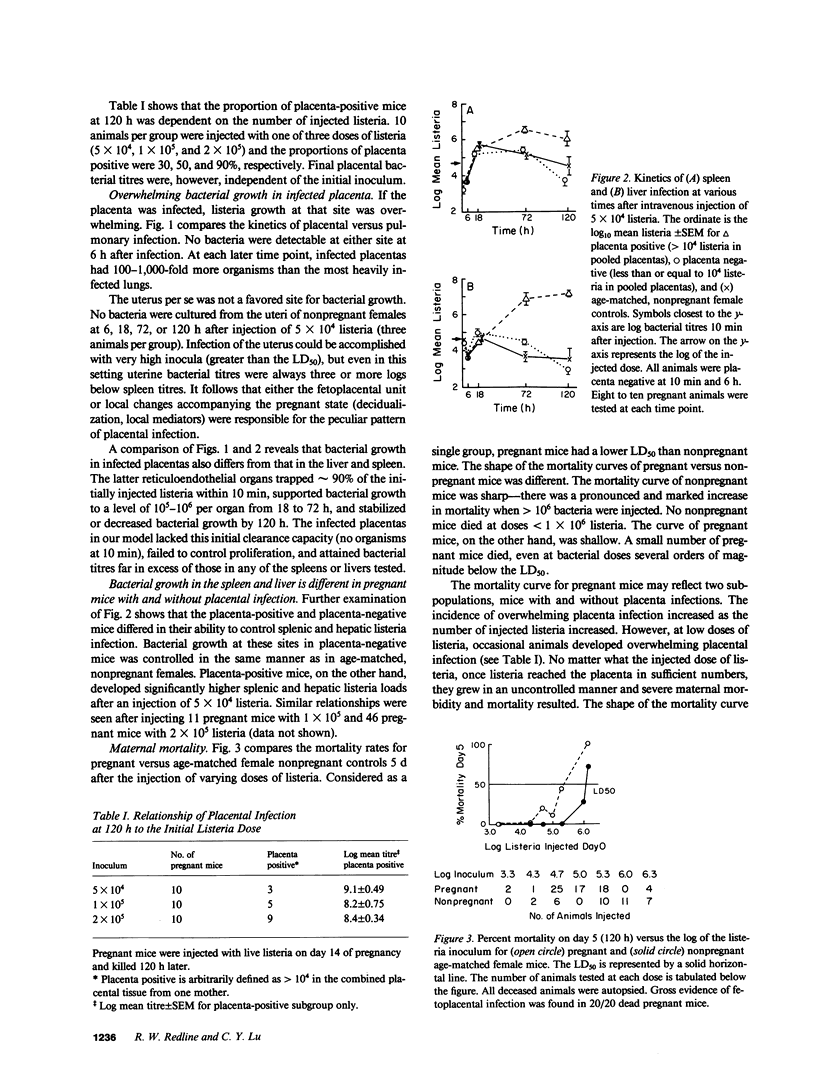
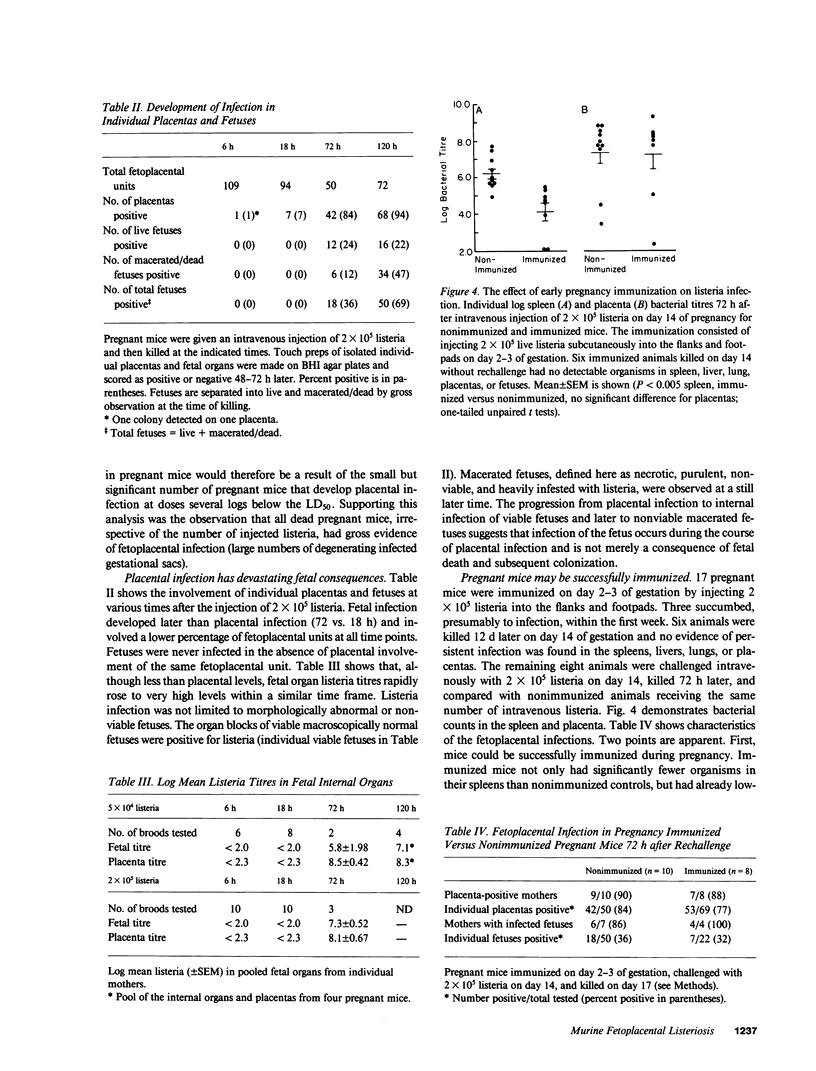
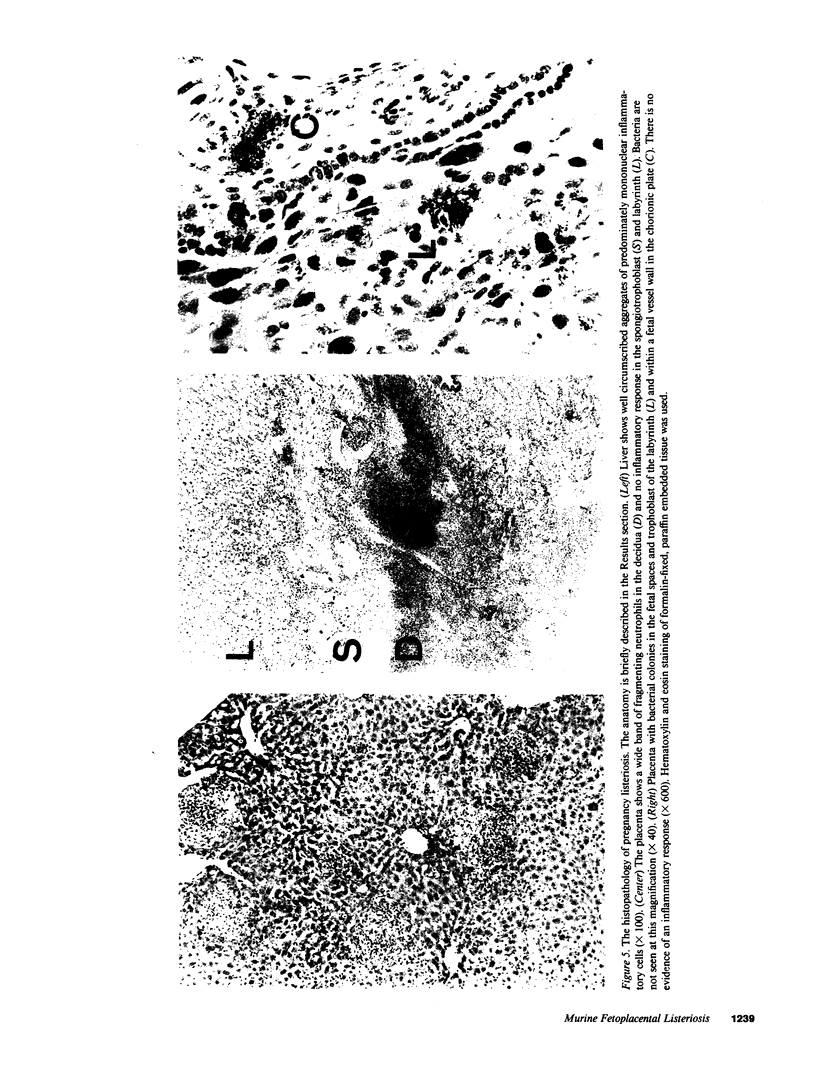
Recent evidence suggests that local immunoregulation may prevent rejection of the placenta by the mother. This local immunoregulation may also compromise the response to placental infection. Listeria monocytogenes infection in 121 pregnant mice and 1,050 fetoplacental units was examined and the kinetics of bacterial growth in various maternal and fetal tissues were determined. A subset of pregnant mice developed overwhelming placental listeria infections. Pregnancy did not impair the maternal immune response in the liver and spleen. Pregnant mice without placental infection had numbers of listeria equivalent to nonpregnant controls and mice immunized during pregnancy had significantly less listeria than nonimmunized controls. The secondary response in immunized pregnant mice had no effect on the development of placental infection and the histologic features of placental infection were distinct from those in other organs. Our data suggest that an ineffective local immune response may contribute to the pathogenicity of listeria for the placenta.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. J., Berkowitz R. S. Gamma-interferon enhances expression of Class I MHC antigens in the weakly HLA+ human choriocarcinoma cell line BeWo, but does not induce MHC expression in the HLA- choriocarcinoma cell line Jar. J Immunol. 1985 Oct;135(4):2498–2501. [PubMed] [Google Scholar]
- BILLINGHAM R. E. TRANSPLANTATION IMMUNITY AND THE MATERNAL-FETAL RELATION. N Engl J Med. 1964 Mar 26;270:667–CONTD. doi: 10.1056/NEJM196403262701306. [DOI] [PubMed] [Google Scholar]
- Badet M. T., Bell S. C., Billington W. D. Immunoregulatory activity of supernatants from short-term cultures of mouse decidual tissue. J Reprod Fertil. 1983 Jul;68(2):351–358. doi: 10.1530/jrf.0.0680351. [DOI] [PubMed] [Google Scholar]
- Beer A. E., Sio J. O. Placenta as an immunological barrier. Biol Reprod. 1982 Feb;26(1):15–27. doi: 10.1095/biolreprod26.1.15. [DOI] [PubMed] [Google Scholar]
- Bortolussi R., Campbell N., Krause V. Dynamics of Listeria monocytogenes type 4b infection in pregnant and infant rats. Clin Invest Med. 1984;7(4):273–279. [PubMed] [Google Scholar]
- Chaouat G., Kolb J. P. Immunoactive products of placenta. IV. Impairment by placental cells and their products of CTL function at effector stage. J Immunol. 1985 Jul;135(1):215–222. [PubMed] [Google Scholar]
- Chatterjee-Hasrouni S., Lala P. K. MHC antigens on mouse trophoblast cells: paucity of Ia antigens despite the presence of H-2K and D. J Immunol. 1981 Nov;127(5):2070–2073. [PubMed] [Google Scholar]
- Cheers C., McKenzie I. F. Resistance and susceptibility of mice to bacterial infection: genetics of listeriosis. Infect Immun. 1978 Mar;19(3):755–762. doi: 10.1128/iai.19.3.755-762.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D. A., Chaput A., Tutton D. Active suppression of host-vs-graft reaction in pregnant mice. VII. Spontaneous abortion of allogeneic CBA/J x DBA/2 fetuses in the uterus of CBA/J mice correlates with deficient non-T suppressor cell activity. J Immunol. 1986 Mar 1;136(5):1668–1675. [PubMed] [Google Scholar]
- Clark D. A., Chaput A., Walker C., Rosenthal K. L. Active suppression of host-vs-graft reaction in pregnant mice. VI. Soluble suppressor activity obtained from decidua of allopregnant mice blocks the response to IL 2. J Immunol. 1985 Mar;134(3):1659–1664. [PubMed] [Google Scholar]
- Croy B. A., Rossant J., Clark D. A. Histological and immunological studies of post implantation death of Mus caroli embryos in the Mus musculus uterus. J Reprod Immunol. 1982 Sep;4(5):277–293. doi: 10.1016/0165-0378(82)90003-1. [DOI] [PubMed] [Google Scholar]
- DRISCOLL S. G., GORBACH A., FELDMAN D. Congenital listeriosis: diagnosis from placental studies. Oncologia. 1962 Aug;20:216–220. [PubMed] [Google Scholar]
- ENDERS A. C. A COMPARATIVE STUDY OF THE FINE STRUCTURE OF THE TROPHOBLAST IN SEVERAL HEMOCHORIAL PLACENTAS. Am J Anat. 1965 Jan;116:29–67. doi: 10.1002/aja.1001160103. [DOI] [PubMed] [Google Scholar]
- Giraud J. R., Denis F., Gargot F., Fizazi T., Babin P., Rautlin de la Roy Y D. E., Hoppeler A., Brisou J., de Tourris H. La listériose. Incidence dans les interruptions spontanées de la grossesse. Nouv Presse Med. 1973 Jan 27;2(4):215–218. [PubMed] [Google Scholar]
- Hahn H., Kaufmann S. H. The role of cell-mediated immunity in bacterial infections. Rev Infect Dis. 1981 Nov-Dec;3(6):1221–1250. doi: 10.1093/clinids/3.6.1221. [DOI] [PubMed] [Google Scholar]
- Hamada M., Kuroiwa A., Matsumoto T., Nomoto K., Takeya K. Modification of protective mechanisms against Listeria monocytogenes during pregnancy. J Clin Lab Immunol. 1981 Sep;6(2):169–173. [PubMed] [Google Scholar]
- Hunziker R. D., Wegmann T. G. Placental immunoregulation. Crit Rev Immunol. 1986;6(3):245–285. [PubMed] [Google Scholar]
- Lane F. C., Unanue E. R. Requirement of thymus (T) lymphocytes for resistance to listeriosis. J Exp Med. 1972 May 1;135(5):1104–1112. doi: 10.1084/jem.135.5.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C. Y., Changelian P. S., Unanue E. R. Alpha-fetoprotein inhibits macrophage expression of Ia antigens. J Immunol. 1984 Apr;132(4):1722–1727. [PubMed] [Google Scholar]
- Luft B. J., Remington J. S. Effect of pregnancy on resistance to Listeria monocytogenes and Toxoplasma gondii infections in mice. Infect Immun. 1982 Dec;38(3):1164–1171. doi: 10.1128/iai.38.3.1164-1171.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel T. E., Cheers C. Resistance and susceptibility of mice to bacterial infection: histopathology of listeriosis in resistant and susceptible strains. Infect Immun. 1980 Dec;30(3):851–861. doi: 10.1128/iai.30.3.851-861.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchmore A. V., Decker J. M. Uromodulin: a unique 85-kilodalton immunosuppressive glycoprotein isolated from urine of pregnant women. Science. 1985 Aug 2;229(4712):479–481. doi: 10.1126/science.2409603. [DOI] [PubMed] [Google Scholar]
- Rocklin R. E., Kitzmiller J. L., Carpenter C. B., Garovoy M. R., David J. R. Maternal-fetal relation. Absence of an immunologic blocking factor from the serum of women with chronic abortions. N Engl J Med. 1976 Nov 25;295(22):1209–1213. doi: 10.1056/NEJM197611252952201. [DOI] [PubMed] [Google Scholar]
- Skamene E., Kongshavn P. A., Sachs D. H. Resistance to Listeria monocytogenes in mice: genetic control by genes that are not linked to the H-2 complex. J Infect Dis. 1979 Feb;139(2):228–231. doi: 10.1093/infdis/139.2.228. [DOI] [PubMed] [Google Scholar]
- Snyder D. S., Beller D. I., Unanue E. R. Prostaglandins modulate macrophage Ia expression. Nature. 1982 Sep 9;299(5879):163–165. doi: 10.1038/299163a0. [DOI] [PubMed] [Google Scholar]
- Vawter G. F. Perinatal listeriosis. Perspect Pediatr Pathol. 1981;6:153–166. [PubMed] [Google Scholar]





