Abstract
The contribution of p53 to kidney dysfunction, inflammation, and tubular cell death, hallmark features of ischemic renal injury (IRI), remains undefined. Here, we studied the role of proximal tubule cell (PTC)-specific p53 activation on the short- and long-term consequences of renal ischemia/reperfusion injury in mice. After IRI, mice with PTC-specific deletion of p53 (p53 knockout [KO]) had diminished whole-kidney expression levels of p53 and its target genes, improved renal function, which was shown by decreased plasma levels of creatinine and BUN, and attenuated renal histologic damage, oxidative stress, and infiltration of neutrophils and macrophages compared with wild-type mice. Notably, necrotic cell death was attenuated in p53 KO ischemic kidneys as well as oxidant-injured p53-deficient primary PTCs and pifithrin-α–treated PTC lines. Reduced oxidative stress and diminished expression of PARP1 and Bax in p53 KO ischemic kidneys may account for the decreased necrosis. Apoptosis and expression of proapoptotic p53 targets, including Bid and Siva, were also significantly reduced, and cell cycle arrest at the G2/M phase was attenuated in p53 KO ischemic kidneys. Furthermore, IRI-induced activation of TGF-β and the long-term development of inflammation and interstitial fibrosis were significantly reduced in p53 KO mice. In conclusion, specific deletion of p53 in the PTC protects kidneys from functional and histologic deterioration after IRI by decreasing necrosis, apoptosis, and inflammation and modulates the long-term sequelae of IRI by preventing interstitial fibrogenesis.
AKI is a clinical syndrome characterized by a rapid decline in GFR over a period of hours to days, leading to retention of metabolic waste products and disrupted fluid, electrolyte, and acid–base balance.1 Ischemic renal injury (IRI), which results from compromised perfusion of renal tissues, is the leading cause of AKI.2,3 Persistent perfusion deficit in the medulla, limited anaerobic glycolytic capacity, and targeted inhibition of glycolysis in the proximal tubule cell (PTC) make the proximal straight tubule the most vulnerable tubular segment to ischemic injury.4–7 Pathologically, IRI is characterized by apoptotic/necrotic cell death and inflammation in the outer medulla, which are proportional to the severity of renal ischemia.8,9 Pro- and antiapoptotic signaling pathways in the PTC are precisely regulated by essential factors, such as p53 and its proapoptotic targets, BCL2 family proteins and caspases.9–14 Therefore, it is crucial to understand the roles of these molecules in regulating tubular cell apoptosis in the pathogenesis of IRI.
The transcription factor p53, which was first identified as a tumor suppressor, performs many essential cell functions, such as halting the cell cycle, promoting senescence and apoptosis, and regulating cell metabolism.15 In response to various cell stresses and DNA damage, p53 controls the transcription of target genes that are usually key factors in cellular stress pathways.16 Although the transcriptional activation of p53 was considered the major mechanism by which p53 responds to cell stress, several studies suggest that transcription-independent activity of p53 can potentiate apoptosis by directly interacting with members of BCL2 family proteins.12,17
The involvement of p53 has been reported in nephrotoxic injury and IRI.18–20 Although previous studies showed that p53 levels are significantly increased in the medulla after IRI,9 the contribution of p53 to tubular cell death and kidney dysfunction is still unclear.8,9,20–22 Thus, considering the importance of p53 in promoting apoptotic/necrotic cell death, we hypothesized that knockout (KO) of p53 in the proximal tubule significantly reduces tubular cell death and kidney dysfunction after IRI. We tested this hypothesis using KO mice with the p53 gene specifically deleted in the proximal tubule.
Results
Deletion of p53 in the Proximal Tubule Reduced p53 Expression after IRI
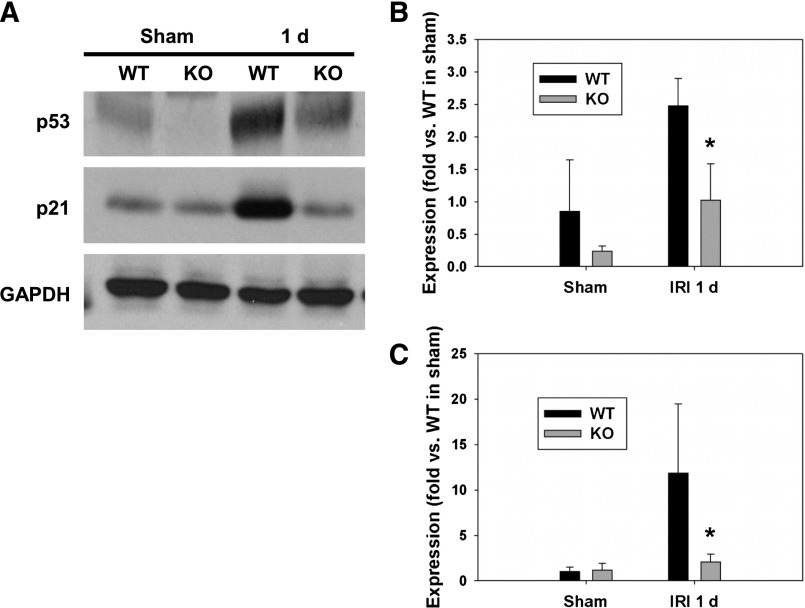
We analyzed the expression of p53 and one of its target genes, p21, after injury in whole-kidney tissues. Indeed, 24 hours after IRI, the expression levels of p53 (Figure 1, A and B) and p21 (Figure 1, A and C) were significantly increased in wild-type (WT) male littermates compared with sham-operated mice kidneys. However, the expression level of p53 was only slightly increased and p21 expression was not altered in p53 KO mice compared with sham-operated mice after IRI. The slightly increased levels of p53 in IRI-induced p53 KO may be because of p53 expression in cells other than the proximal tubules (PTs). Because of the difficulty in immunodetection of p53 in renal tissue, we carried out Western blot analysis for p53 in PTs isolated from p53 KO mice. Our data further confirmed successful deletion of p53 in the PT (Supplemental Figure 1). These data suggest that p53 is induced and involved in transcriptional regulation after IRI.
Figure 1.
PT-specific KO of p53 reduced p53 and p21 expression after IRI. (A) Representative images of Western blot analysis showing expression levels of p53 and p21 at 24 hours (1 d) after IRI. (B and C) The expression levels of p53 and p21 in sham and IRI-induced kidneys were quantified (n=4 in each group). *P<0.05 compared with WT IRI. GAPDH served as a loading control. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
PT-Specific KO of p53 Reduced Deterioration of Renal Function after IRI
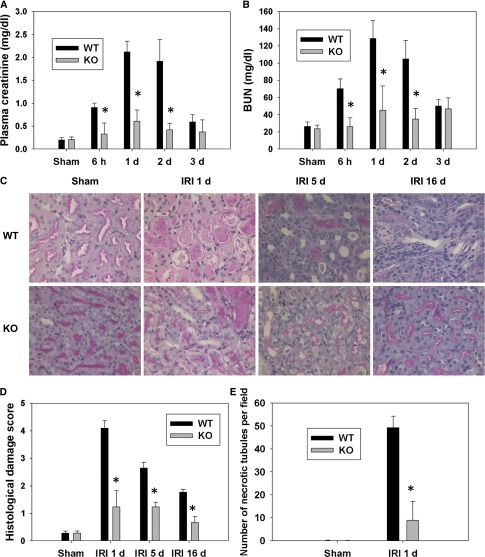
To test whether the absence of p53 in the PTC changes the course of IRI, kidney function after IRI in p53 KO mice and WT littermates was assessed. BUN levels were increased in WT mice 6 hours after IRI and peaked during 24–48 hours compared with WT sham-operated mice. The increase in BUN level was significantly less in p53 KO mice 6–48 hours after IRI compared with WT mice (Figure 2A). Serum creatinine levels were also increased in WT mice 6–48 hours after IRI compared with WT sham-operated mice. Similar to the BUN levels, the increase of serum creatinine was less in p53 KO compared with WT at 6, 24, and 48 hours after IRI (Figure 2B). No significant difference in renal function occurred among sham-operated WT and KO mice (Figure 2, A and B). Moreover, there were no significant differences in BUN or serum creatinine values between WT and heterozygote mice after IRI (data not shown). These data suggest that specific deletion of the p53 gene in the PT leads to renal functional protection after IRI in mice.
Figure 2.
PT-specific KO of p53 reduced renal function deterioration and histologic damage after IRI. (A) Plasma creatinine and (B) BUN levels from WT and p53 KO mice (n=6) at 6 hours (h) and 1, 2, and 3 days after IRI. *P<0.05 compared with WT IRI. (C) WT or p53 KO mice underwent sham surgery or IRI. Renal histologic changes in the outer medulla after IRI were assessed by Periodic acid–Schiff (PAS) staining at 1, 5, and 16 days after IRI. Original magnification, ×400. (D) Histologic damage in the outer medulla assessed in PAS-stained kidney sections was scored by counting the percentage of tubules that displayed tubular necrosis, cast formation, and tubular dilation as follows: 0, normal; 1, <10%; 2, 10%–25%; 3, 26%–50%; 4, 51%–75%; 5, >75%. Ten fields (×200 magnification) per kidney were used for counting. *P<0.05 compared with WT IRI (n=6). (E) The number of necrotic tubules was counted in PAS-stained kidney sections at 1 day after IRI. *P<0.05 compared with WT IRI 1 day (n=6).
PT-Specific KO of p53 Decreased Renal Histologic Damage after IRI
Ischemic kidneys from WT mice showed widespread necrosis, brush border blebbing, and sloughed cells in the proximal straight tubule, whereas these features were much less apparent in ischemic kidneys from p53 KO mice. The histologic changes after IRI were quantified by counting and scoring the percentage of tubules that displayed tubular necrosis, cast formation, and tubular dilation (Figure 2, C and D). The cumulative scores of histologic damage in the outer medulla at 1, 5, and 16 days as well as necrosis (Figure 2E) at 1 day were significantly lower in p53 KO kidneys compared with WT kidneys post-IRI, showing that gene ablation of p53 reduced tubular damage and cellular necrosis.
Renal Inflammation Was Reduced after IRI in p53 KO Mice
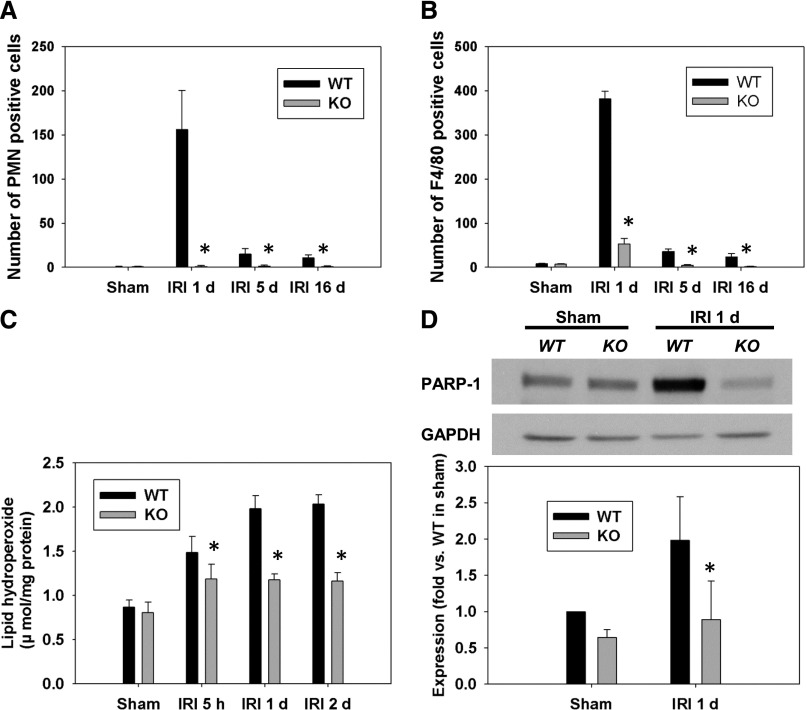
The infiltration of leukocytes in the outer medulla of WT and p53 KO mice at 1, 5, and 16 days post-IRI was assessed by immunostaining for neutrophils and macrophages. As shown in representative photographs (Supplemental Figure 2), WT mice exhibited increased infiltration of neutrophils and macrophages in the outer medulla, which was attenuated in p53 KO mice. The numbers of positively stained cells were counted in a blinded manner, and quantitative data indicate that the accumulation of neutrophils and macrophages was reduced in the outer medulla of p53 KO mice compared with WT mice at all time points after IRI (Figure 3, A and B).
Figure 3.
PT-specific KO of p53 decreased leukocyte infiltration, oxidative stress, and PARP-1 expression. The number of (A) neutrophils and (B) macrophages accumulated in the outer medulla of WT and p53 KO kidneys post-IRI was measured at 1, 5, and 16 days after IRI in high-magnification (×200) fields. *P<0.05 compared with WT IRI (n=4). (C) Lipid hydroperoxide levels in the WT and p53 KO mice after IRI. *P<0.05 compared with WT IRI (n=6). (D) Western blots showing increased expression of PARP1 24 h after IRI in WT kidneys. *P<0.05 compared with WT IRI (n=3). GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Attenuated Oxidative Stress after IRI in p53 KO Mice
Oxidative stress was assessed by lipid hydroperoxide levels in the kidney. Quantification of the whole-kidney lipid peroxide levels at 5 hours and 1 and 2 days post-IRI shows that its levels were significantly decreased in p53 KO mice compared with WT mice at all time points (Figure 3C).
PT-Specific Deletion of p53 Decreased PARP1 Expression after IRI
PARP1 can induce necrotic cell death after IRI. PARP1 expression was examined by Western blot analysis. PARP1 expression was significantly increased in WT kidneys after IRI, but its expression was downregulated in p53 KO mice (Figure 3D). This novel finding suggests that increased PARP1 function may be one of the mechanisms by which p53 activation regulates necrosis.
PT-Specific KO of p53 Decreased Apoptosis after IRI
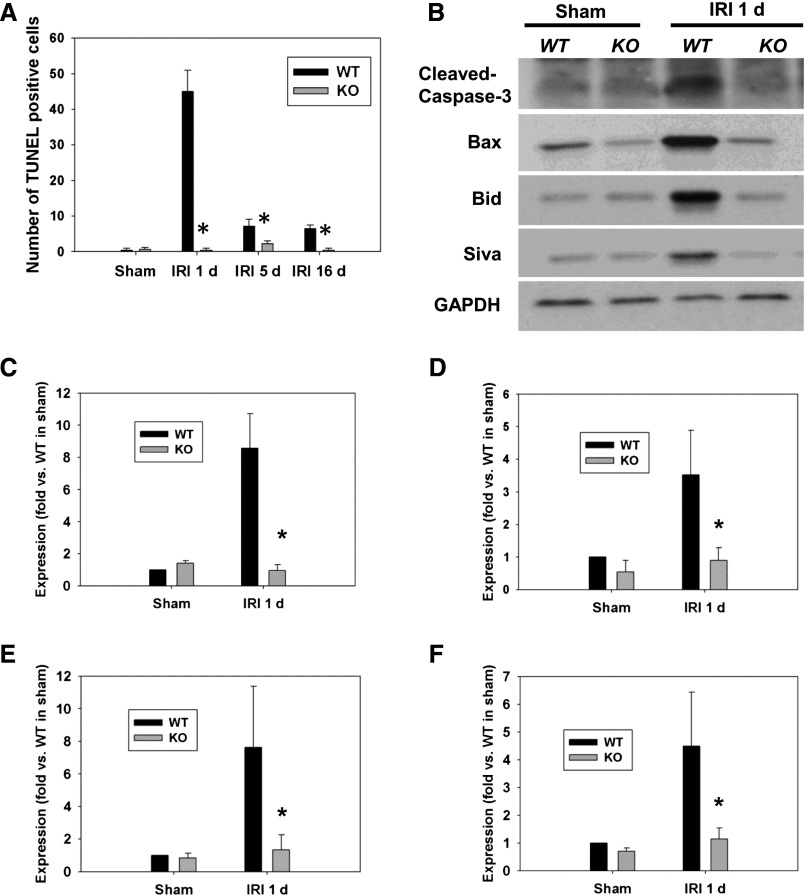
To determine whether there is a change of apoptotic levels in the outer medulla of WT mice compared with p53 KO mice, terminal deoxynucleotidyl transferase-mediated digoxigenin-deoxyuridine nick-end labeling (TUNEL) assay was performed together with Western blot analysis for proapoptotic proteins. The outer medulla of WT mice exhibited increased numbers of TUNEL-positive cells compared with that of p53 KO mice (Fig. 4A, Supplemental Figure 3). Cleaved Caspase-3 expression was increased in WT kidney 24 hours after IRI compared with p53 KO mice (Figure 4, B and C), further confirming increased apoptosis. Similarly, Western blot analysis showed enhanced activation of Bax in the kidney of WT mice compared with that of p53 KO mice 24 hours after IRI, whereas total Bax was not changed. The expression of proapoptotic proteins Bid and Siva was also increased in WT mice after IRI compared with p53 KO mice (Figure 4, B and D–F). These data suggest that specific deletion of p53 in the PT decreases apoptotic cell death after IRI by decreasing the levels of proapoptotic proteins.
Figure 4.
Apoptosis and pro-apoptotic molecule expression were reduced after IRI in p53 KO mice. (A) TUNEL assay to detect apoptotic cells was done using the In Situ Cell Death Detection Kit in kidneys derived from WT or p53 KO at 1, 5, and 16 days after IRI. The number of TUNEL-positive cells was measured in 10 randomly chosen high-magnification (×200) fields per kidney. *P<0.05 compared with WT IRI (n=5). Representative Western blot images showing increased expression of cleaved Caspase-3 (B, C), Bax (B, D), Bid (B, E), and Siva (B, F) in WT mice after 24 h of IRI. *P<0.05 compared with WT IRI (n=3–5). GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Loss of p53 Reduced Renal Fibrosis after IRI
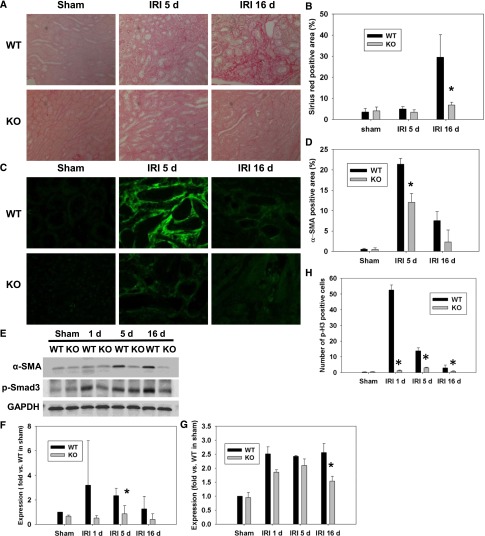
To investigate whether deletion of p53 reduces renal fibrosis, collagen deposition in the kidneys of WT and p53 KO mice was measured using Sirius red staining and α-SMA immunofluorescence staining. Sixteen days after IRI, WT mice showed a dramatic increase of Sirius red-positive area in the kidneys compared with p53 KO mice, indicating that deletion of p53 reduces renal fibrosis in the later stage of IRI (Figure 5, A and B). No change in the Sirius red staining was seen at 5 days postinjury. α-SMA expression, however, was decreased in p53 KO mice compared with WT mice at 5 days after IRI, which was shown by immunostaining and Western blot analysis (Figure 5, C–F). p-Smad3, a downstream signaling molecule of TGF-β, was also increased in WT mice but significantly reduced in p53 KO mice at 16 days post-IRI (Figure 5, E and G).
Figure 5.
Loss of p53 in PT reduced renal fibrosis and cell cycle arrest after IRI. (A) Collagen deposition detected by Sirius red staining in WT and p53 KO kidneys at 5 and 16 days after IRI. (B) The Sirius red-positive area was measured in four randomly chosen high-power (×200) fields per kidney using the National Institutes of Health ImageJ software. *P<0.05 compared with WT IRI (n=3). (C and D) Immunofluorescence staining of α-SMA in the outer medulla. The α-SMA–positive area was measured in four randomly chosen high-power (×200) fields per kidney. *P<0.05 compared with WT IRI (n=3). (E and F) Representative Western blot images and quantified data for expression of α-SMA in WT and p53 KO kidneys at 1, 5, and 16 days after IRI. *P<0.05 compared with WT IRI (n=3). (E and G) Representative Western blot films and quantified data for expression of p-Smad3 in WT and p53 KO kidneys at 1, 5, and 16 days after IRI. *P<0.05 compared with WT IRI (n=3). GAPDH served as a loading control. (H) Kidney sections were immunostained using p-H3 antibody. The number of p-H3–positive cells in WT and p53 KO kidneys at 1, 5, and 16 days after IRI was counted in 10 randomly selected high-power (×200) fields per kidney. *P<0.05 compared with WT IRI (n=4). GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Loss of p53 Reduced Cell Cycle Arrest after IRI
Cell cycle arrest at G2/M phase is associated with late kidney fibrosis in IRI. Histone H3 is phosphorylated during mitosis at Ser-10 in the G2/M phase.23 Phospho-H3 (p-H3) staining was performed to detect G2/M phase arrest. In WT mice, the number of p-H3–positive cells was significantly higher compared with the number of p-H3–positive cells in p53 KO mice at 1, 5, and 16 days after IRI (Fig. 5H, Supplemental Figure 4A). To confirm that the cells are truly arrested at the G2 phase and not in mitotic phase, we assessed the number of mitotic cells at the above time points. Mitotic entry is accompanied by the phosphorylation of several molecules, including mitotic protein monoclonal 2 (MPM-2), that may regulate the mitotic processes.24,25 Immunostaining using antiphospho-Ser/Thr-Pro and MPM-2 antibody (05–368; EMD Millipore, Bedford, MA) showed that the number of cells at M phase was increased in WT mice 1 day after IRI compared with in KO mice, but it only accounted for a small fraction of the cells arrested at G2/M phase, indicating that most p-H3–positive cells were arrested at G2 phase (Supplemental Figure 4, B and C). The number of cells at M phase was negligible at 5 and 16 days in both WT and p53 KO mice tissues.
Loss of p53 Reduced Necrotic Cell Death of PTC
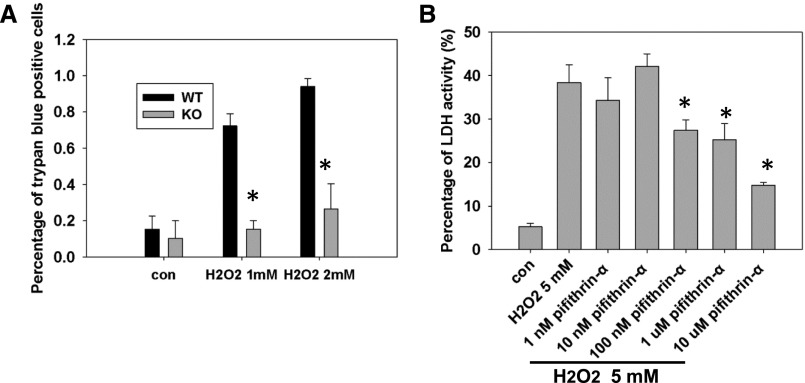
To further study the role of p53 in necrotic cell death, we used an in vitro H2O2-induced necrosis model. Necrosis was assayed using trypan blue staining and lactate dehydrogenase (LDH) release assay. After 1 hour of treatment of 1 or 2 mM H2O2, p53 KO primary PTCs had fewer trypan blue-positive cells compared with WT PTCs (Figure 6A). Pharmacological inhibition of p53 using pifithrin-α also reduced LDH release in LLC-PK1 cells after 5 mM H2O2 treatment (Figure 6B). These data indicate that deletion or inhibition of p53 reduces H2O2-induced necrosis.
Figure 6.
Loss of p53 reduced PTC necrosis. (A) Percentage of trypan blue-positive cells after H2O2 treatment in primary PTCs derived from p53 KO compared with WT mice kidneys. *P<0.05 (n=4). (B) Percentage of LDH activity after 5 mM H2O2 treatment in LLC-PK1 cells with or without different concentrations of the p53 inhibitor pifithrin-α. *P<0.05 (n=4). Con, control with PBS or DMSO (dimethyl sulfoxide) treatment.
Discussion
Several studies have previously investigated the role of p53 in IRI. Kelly et al.9 first showed that p53 expression is increased in the renal medulla after IRI and that pharmacological inhibition of p53 using pifithrin-α can reduce renal injury in rats. Molitoris et al.20 showed that systemic administration of p53-targeted small interfering RNA in mice attenuates ischemic and cisplatin-induced AKI. In a recent report, Yang et al.26 administered pifithrin-α in a single dose 3 and 14 days after unilateral IRI in mice and showed that it relieved epithelial cell cycle arrest and inhibited fibrogenesis. However, two recent reports showed that p53 inhibition is detrimental to renal function in IRI. Dagher et al.21 administered pifithrin-α daily, starting at the time of bilateral IRI in rats and continuing for 7 days, which ultimately increased renal fibrosis. To add to the paradox, Sutton et al.22 showed that global p53 deficiency or pharmacological inhibition of p53 exacerbates the injury by increasing and prolonging leukocyte infiltration into the renal parenchyma. These differing results suggest that the role of p53 in the pathophysiology of IRI is much more complicated and still incompletely understood.
In this study, we used KO mice with p53 gene specifically deleted in the PTC to study its effect on the kidney damage after IRI. This strategy not only specifically targets the PT, the major injury site of IRI,5 but also excludes the normal function of p53 in other cells within the kidney, like inflammatory cells recruited after IRI, in which absence of p53 may prolong inflammatory responses and increase renal injury.22 Our data show that specific deletion of p53 gene in the PT significantly improves kidney function, preserves renal histology, and reduces necrosis and apoptosis after IRI along with attenuated inflammatory response and late-term fibrogenesis. These findings support the concepts that p53 is a critical mediator of IRI and that its inhibition or its downstream signaling pathway prevents IRI and is a suitable therapeutic target.
A role for p53 in apoptosis is well established. p53 can upregulate the expression of several proapoptotic genes, including Bax, Fas/Apo-1, PERP, Siva, PUMA, and Noxa, under stress situations.16 Recently, Dong and colleagues,27 using two Bax-deficient mouse models, found that only conditional Bax deletion specifically from PTs could ameliorate IRI. Systemic deletion of Bax enhanced neutrophil infiltration without significant effect on kidney injury.27 Our data indicate that expression of Bax, Bid, and Siva are induced in ischemic kidneys but attenuated in p53 KO mice. The significance of Bid and Siva expression in IRI was previously reported. In a previous report, Dong and colleagues28 showed that Bid deficiency attenuated tubular disruption, tubular cell apoptosis, and caspase-3 activation during 48 hours of reperfusion. We previously reported that the expression of the proapoptotic p53 target Siva and its cognate receptor CD27 is highly upregulated and coexpressed in the ischemic rat and mouse renal tissues,29 indicating that Siva plays a critical role in apoptosis after IRI. It is likely that synergic functions of these proapoptotic molecules may lead to the execution of apoptosis after IRI.
Recently, Vaseva et al.30 showed that p53 may also be implicated in necrotic cell death through its interaction with cyclophilin D (CypD) in the inner membrane of mitochondria and subsequent opening of mitochondrial permeability transition pore (MPTP). Indeed, our data showed significantly reduced tubular cell necrosis in the outer medulla of p53 KO mice kidneys compared with WT after IRI (Figure 2). The role of CypD in necrotic cell death after IRI is established by us and other groups.31,32 Two key factors involved in CypD activation are redox stress and calcium mishandling. Our data indicate that p53 deletion can decrease oxidative stress, which could be a potential mechanism by which p53 inhibits necrosis in renal PTCs. Can p53 translocate to mitochondrial matrix and interact with CypD to open the MPTP after IRI? Our attempts to locate p53 to the mitochondria in various in vitro and in vivo models failed, suggesting that it may not be a prominent mechanism by which p53 induces necrosis in renal PTC (data not shown). However, our novel finding that p53 inhibition can downregulate PARP1 expression in renal PTC suggests that it could be an alternate mechanism by which p53 regulates necrosis after IRI. Activation of PARP1 is required for DNA repair, but excessive activation leads to necrotic cell death by depletion of intracellular ATP.33,34 We previously reported that pharmacological and genetic inhibition of PARP1 can prevent kidney dysfunction, oxidative stress, inflammation, and tubular necrosis but not apoptosis after ischemia/reperfusion7,35 and cisplatin nephrotoxicity.36 Furthermore, although bax/bak is generally regarded as an apoptosis inducer by regulating mitochondrial outer membrane permeabilization, recent evidence suggests that they may form the outer membrane channels of MPTP and contribute to necrotic cell death.37,38 Bax expression is attenuated after IRI as a function of p53 and thus, could play a role in attenuating necrosis.
It is well established that neutrophils, monocytes/macrophages, and T cells play major roles in the pathophysiology of renal ischemia-reperfusion injury in animal models and human AKI.39–42 Blocking the activation or trafficking of proinflammatory leukocytes into the kidney is shown to prevent renal function deterioration and histologic damage. Recently, Sutton et al.22 used bone marrow transplantation to produce chimeric mice lacking p53 in leukocytes and showed that p53 deletion prevents leukocyte apoptosis and increases their potential for cytokine secretion, thus worsening the pathophysiology of IRI. This study also showed that systemic deletion of p53 can worsen the injury.22 Our studies using mice lacking p53 in PT, however, showed that it can profoundly decrease the infiltration of leukocytes and thus, protect the kidneys from IRI. The apparent paradox in the data from the two studies could be because of the variations in the experimental protocol and the differing effects of inhibition of p53 in specific cell types within the kidney.
The role of cell cycle arrest and tubular apoptosis on the fibrogenic response of kidney tissue has recently been established in different injury models of CKD.26 Defects in progression of the cell cycle after injury, such as arrest at G1/G0 or G2/M phase, cause tubular cells to switch to a profibrotic phenotype with increased expression and release of TGF-β, thereby promoting fibrosis.26 p53 is a major player in cell cycle regulation. The effect of pharmacological inhibition of p53 using pifithrin-α in fibrosis development after IRI was previously examined by two groups21,26 (the opposing results are detailed above). Although the exact cause of these differing results is not clear, the different species and the timing of p53 inhibition in the two experiments may have contributed. Our data indicate that G2/M cell cycle arrest occurs after IRI in WT mice but not p53 KO mice, which was shown by reduced p-H3 staining, and may contribute to the attenuation of fibrosis. PT-specific deletion of p53 downregulated p21 expression, suggesting that it may titrate the level of p21, which allows the cells to progress through G2 into M phase. However, our data show that p53 deletion can prevent inflammation and both necrotic and apoptotic cell deaths. Although it is unclear if the severity of the injury after an ischemic episode directly affects the progression of CKD, it is likely that the attenuated inflammation and injury in the p53 KO mice may contribute to the decreased fibrosis. Additional studies are needed to answer this question.
In summary, our data show that p53 has profound effects on tubular cell necrosis and apoptosis after IRI. Absence of p53 in the PT significantly preserves renal function and markedly reduces kidney damage, renal oxidative stress, inflammation, and long-term fibrosis. Targeting proximal tubular cell death by modulating p53 or its downstream signaling pathways may provide a more efficient therapeutic strategy for IRI.
Concise Methods
Generation of PTC-Specific p53 KO Mice
Homozygous p53-floxed mice (C57BL/6J background) were obtained from The Jackson Laboratories (Bar Harbor, ME). The breeding strategy for transgenic mice that expressed Cre recombinase under the control of kidney-specific Pepck promoter (Pepck-Cre) was reported elsewhere.43 KO mice with the p53 gene specifically disrupted in renal proximal tubular epithelial cells (genotype p53fl/fl, Cre+/−) were developed by mating p53-floxed mice with Pepck-Cre transgenic mice. A routine PCR protocol was used for genotyping from tail DNA samples with the following primer pairs: Cre, 5′-CGGTGCTAACCAGCGTTTTC-3′ and 5′-TGGGCGGCATGGTGCAAGTT-3′ and p53, 5′-GGTTAAACCCAGCTTGACCA-3′ and 5′-GGAGGCAGAGACAGTTGGGAG-3′. Male littermates of the genotype p53fl/fl, Cre−/− were used as controls (WT). All animals were born at the expected Mendelian frequency and did not display any gross physical or behavioral abnormalities. Animal experiments were approved by the Institutional Animal Care and Use Committee of the University of Nebraska Medical Center.
Induction of IRI in Mice
IRI was induced in male mice as described previously.31,44 All animals were given free access to food and water. The mice were anesthetized by intraperitoneal administration of a cocktail containing ketamine (200 mg) and xylazine (16 mg) per 1 kg body wt. Ischemic injury was induced by bilateral renal pedicle clamping using microaneurysm clamps (Roboz Surgical Instrument, Gaithersburg, MD), and the core body temperature of mice was kept at 37°C. After 30 minutes of occlusion, the clamps were removed, and kidney reperfusion was verified visually. Sham-operated control animals underwent the same surgical procedure, except for the occlusion of the renal arteries. During the surgery, all animals were placed on a heating pad to maintain body temperature at 37°C. Blood samples were collected at the time of euthanasia or from the orbital sinus under isoflurane anesthesia at 0, 6, 24, and 48 hours post-IRI for measurement of serum creatinine and BUN. At the end of each time point (1, 5, or 16 days), renal tissue was collected, fixed in Bouins solution or snap frozen using liquid nitrogen, and stored at −80°C for future experiments.
Measurement of Serum Creatinine and BUN
Serum creatinine and BUN were measured to evaluate renal function using a Quantichrom assay kit (BioAssay Systems, Hayward, CA) according to the manufacturer’s protocol.
Morphologic Studies
WT and KO mice that underwent IRI were euthanized at 1 or 16 days. The kidneys then were processed at the University of Nebraska Medical Center histology core facility. Briefly, kidneys were fixed in formalin, embedded in paraffin, and cut into 5-μm sections. The tissue sections were then stained with Periodic acid–Schiff.
Immunofluorescence for Neutrophils
Formalin-fixed mouse kidney sections were processed for immunostaining as described previously.44 The slides were sequentially incubated with rabbit anti-mouse neutrophil antibody (Accurate, Westbury, NY) at a 1:100 dilution overnight at 4°C followed by FITC-conjugated goat anti-rabbit IgG (Vector Laboratories, Burlingame, CA) at a 1:200 dilution for 1 hour at room temperature. Neutrophil infiltration was quantified by counting the number of stained cells per field.
Immunohistochemistry for Macrophages and p-H3 Staining
Formalin-fixed mouse kidney sections were processed for immunostaining by sequential incubation with rabbit anti-F4/80 antibody (18705–1-AP; Proteintech, Chicago, IL) and anti–p-H3 antibody (sc-8656-R; Santa Cruz Biotechnology, Santa Cruz, CA) at a 1:100 dilution overnight at 4°C followed by horseradish peroxidase-conjugated goat anti-rabbit IgG (Vector Laboratories) at a 1:200 dilution for 1 hour at room temperature. The color development was induced by diaminobenzamide reagent (Vector Laboratories) according to the manufacturer’s instructions. Macrophage infiltration and p-H3–positive cells were quantified by counting the number of stained cells per field.
Lipid hydroperoxide assays were performed in the kidney extracts using kits (BioVision, Mountain View, CA) according to the manufacturer’s instructions.
Apoptosis Detection by TUNEL Staining
TUNEL staining of kidney tissue sections was carried out using the In Situ Cell Death Detection Kit, Fluorescein (Roche, Indianapolis, IN) according to the manufacturer’s protocol.
Collagen Deposition by Sirius Red
The rehydrated paraffin sections were stained with Sirius red solution (0.1% Direct Red 80 and 1.3% picric acid; Sigma-Aldrich, St. Louis, MO) and washed two times in acidified water (0.5% acetic acid; Sigma-Aldrich). Then, the sections were dehydrated and cleared before being observed under the microscope.45
α-SMA Immunofluorescence Staining
Formalin-fixed mouse kidney sections were processed for immunostaining as described previously.44 The slides were sequentially incubated with mouse anti–α-SMA antibody (A5228; Sigma-Aldrich) at a 1:500 dilution overnight at 4°C followed by FITC-conjugated horse anti-mouse IgG (Vector Laboratories) at a 1:200 dilution for 1 hour at room temperature. α-SMA deposition was quantified by measuring α-SMA–positive area per field.
Western Blot Analyses
Briefly, whole-renal tissue extracts (80 μg protein/lane) were separated on 10% SDS-PAGE gels and then transferred to Immobilon membranes (EMD Millipore). The membranes were incubated with anti-p53 (2524; Cell Signaling Technology, Beverly, MA), anti-p21 (sc-6246; Santa Cruz Biotechnology), antiactivated Bax (sc-23959; Santa Cruz Biotechnology), anticleaved Caspase-3 (9664; Cell Signaling Technology), anti-Bid (611866; BD Biosciences, San Jose, CA), anti-Siva (sc-48768; Santa Cruz Biotechnology), anti–α-SMA (A5228; Sigma-Aldrich), anti–p-Smad3 (ab51451; Abcam. Inc., Cambridge, MA), antiphospho-Ser/Thr-Pro, MPM-2 antibody (05–368; EMD Millipore), and anti-glyceraldehyde-3-phosphate dehydrogenase (sc-25778; Santa Cruz Biotechnology) antibodies overnight at 4°C. After washing, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies against the appropriate primary antibodies (1:5000; Vector Laboratories), exposed to Western Lighting Plus-ECL (NEL104001EA; PerkinElmer, Waltham, MA), and then developed with x-ray film. The area of each band was analyzed using the National Institutes of Health image software (ImageJ).
Proximal Tubular Cell Culture and In Vitro Experiment
Primary PT epithelial cells were isolated from p53 KO or WT male mice and cultured as described previously.7,46 The porcine-derived proximal tubular cell line LLCPK1 (ATCC, Rockville, MD) was cultured to 80%–90% confluent monolayer cultures as described previously.47 The cells were incubated with 1 or 2 mM H2O2 for 1 hour to induce necrosis.48 These concentrations and times were chosen for trypan blue staining as previously described.7,32 LDH release was measured enzymatically using a CytoTox 96 Non-Radioactive Cytotoxicity Assay Kit (Promega, Madison, WI).
Statistical Analyses
All data are expressed as means±SEMs. One-way ANOVA was used to compare the mean values of all groups. An unpaired t test was used to compare the means of two different groups. A P value<0.05 was considered statistically significant.
Disclosures
None.
Supplementary Material
Acknowledgments
The authors thank Dr. Volker H. Haase for providing the Pepck-Cre transgenic mouse.
This study was supported by a University of Nebraska Medical Center predoctoral fellowship (to Y.Y.) and National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-083291 (to B.J.P.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013121270/-/DCSupplemental.
References
- 1.Bonventre JV, Weinberg JM: Recent advances in the pathophysiology of ischemic acute renal failure. J Am Soc Nephrol 14: 2199–2210, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Liaño F, Pascual J, Madrid Acute Renal Failure Study Group : Epidemiology of acute renal failure: A prospective, multicenter, community-based study. Kidney Int 50: 811–818, 1996 [DOI] [PubMed] [Google Scholar]
- 3.Liaño F, Pascual J: Outcomes in acute renal failure. Semin Nephrol 18: 541–550, 1998 [PubMed] [Google Scholar]
- 4.Mason J, Torhorst J, Welsch J: Role of the medullary perfusion defect in the pathogenesis of ischemic renal failure. Kidney Int 26: 283–293, 1984 [DOI] [PubMed] [Google Scholar]
- 5.Venkatachalam MA, Bernard DB, Donohoe JF, Levinsky NG: Ischemic damage and repair in the rat proximal tubule: Differences among the S1, S2, and S3 segments. Kidney Int 14: 31–49, 1978 [DOI] [PubMed] [Google Scholar]
- 6.Yamamoto K, Wilson DR, Baumal R: Outer medullary circulatory defect in ischemic acute renal failure. Am J Pathol 116: 253–261, 1984 [PMC free article] [PubMed] [Google Scholar]
- 7.Devalaraja-Narashimha K, Padanilam BJ: PARP-1 inhibits glycolysis in ischemic kidneys. J Am Soc Nephrol 20: 95–103, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelly KJ, Plotkin Z, Dagher PC: Guanosine supplementation reduces apoptosis and protects renal function in the setting of ischemic injury. J Clin Invest 108: 1291–1298, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelly KJ, Plotkin Z, Vulgamott SL, Dagher PC: P53 mediates the apoptotic response to GTP depletion after renal ischemia-reperfusion: Protective role of a p53 inhibitor. J Am Soc Nephrol 14: 128–138, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Howard C, Tao S, Yang HC, Fogo AB, Woodgett JR, Harris RC, Rao R: Specific deletion of glycogen synthase kinase-3β in the renal proximal tubule protects against acute nephrotoxic injury in mice. Kidney Int 82: 1000–1009, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chien CT, Chang TC, Tsai CY, Shyue SK, Lai MK: Adenovirus-mediated bcl-2 gene transfer inhibits renal ischemia/reperfusion induced tubular oxidative stress and apoptosis. Am J Transplant 5: 1194–1203, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Youle RJ, Strasser A: The BCL-2 protein family: Opposing activities that mediate cell death. Nat Rev Mol Cell Biol 9: 47–59, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Faubel S, Ljubanovic D, Reznikov L, Somerset H, Dinarello CA, Edelstein CL: Caspase-1-deficient mice are protected against cisplatin-induced apoptosis and acute tubular necrosis. Kidney Int 66: 2202–2213, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Kunduzova OR, Escourrou G, Seguelas MH, Delagrange P, De La Farge F, Cambon C, Parini A: Prevention of apoptotic and necrotic cell death, caspase-3 activation, and renal dysfunction by melatonin after ischemia/reperfusion. FASEB J 17: 872–874, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Vousden KH, Lane DP: p53 in health and disease. Nat Rev Mol Cell Biol 8: 275–283, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Kruse JP, Gu W: Modes of p53 regulation. Cell 137: 609–622, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chipuk JE, Kuwana T, Bouchier-Hayes L, Droin NM, Newmeyer DD, Schuler M, Green DR: Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science 303: 1010–1014, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Zhou L, Fu P, Huang XR, Liu F, Lai KN, Lan HY: Activation of p53 promotes renal injury in acute aristolochic acid nephropathy. J Am Soc Nephrol 21: 31–41, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wei Q, Dong G, Yang T, Megyesi J, Price PM, Dong Z: Activation and involvement of p53 in cisplatin-induced nephrotoxicity. Am J Physiol Renal Physiol 293: F1282–F1291, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Molitoris BA, Dagher PC, Sandoval RM, Campos SB, Ashush H, Fridman E, Brafman A, Faerman A, Atkinson SJ, Thompson JD, Kalinski H, Skaliter R, Erlich S, Feinstein E: siRNA targeted to p53 attenuates ischemic and cisplatin-induced acute kidney injury. J Am Soc Nephrol 20: 1754–1764, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dagher PC, Mai EM, Hato T, Lee SY, Anderson MD, Karozos SC, Mang HE, Knipe NL, Plotkin Z, Sutton TA: The p53 inhibitor pifithrin-α can stimulate fibrosis in a rat model of ischemic acute kidney injury. Am J Physiol Renal Physiol 302: F284–F291, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sutton TA, Hato T, Mai E, Yoshimoto M, Kuehl S, Anderson M, Mang H, Plotkin Z, Chan RJ, Dagher PC: p53 is renoprotective after ischemic kidney injury by reducing inflammation. J Am Soc Nephrol 24: 113–124, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scrittori L, Hans F, Angelov D, Charra M, Prigent C, Dimitrov S: pEg2 aurora-A kinase, histone H3 phosphorylation, and chromosome assembly in Xenopus egg extract. J Biol Chem 276: 30002–30010, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Westendorf JM, Rao PN, Gerace L: Cloning of cDNAs for M-phase phosphoproteins recognized by the MPM2 monoclonal antibody and determination of the phosphorylated epitope. Proc Natl Acad Sci U S A 91: 714–718, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuang J, Ashorn CL, Gonzalez-Kuyvenhoven M, Penkala JE: cdc25 is one of the MPM-2 antigens involved in the activation of maturation-promoting factor. Mol Biol Cell 5: 135–145, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang L, Besschetnova TY, Brooks CR, Shah JV, Bonventre JV: Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat Med 16: 535–543, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wei Q, Dong G, Chen JK, Ramesh G, Dong Z: Bax and Bak have critical roles in ischemic acute kidney injury in global and proximal tubule-specific knockout mouse models. Kidney Int 84: 138–148, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei Q, Yin XM, Wang MH, Dong Z: Bid deficiency ameliorates ischemic renal failure and delays animal death in C57BL/6 mice. Am J Physiol Renal Physiol 290: F35–F42, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Singaravelu K, Padanilam BJ: p53 target Siva regulates apoptosis in ischemic kidneys. Am J Physiol Renal Physiol 300: F1130–F1141, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vaseva AV, Marchenko ND, Ji K, Tsirka SE, Holzmann S, Moll UM: p53 opens the mitochondrial permeability transition pore to trigger necrosis. Cell 149: 1536–1548, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Devalaraja-Narashimha K, Diener AM, Padanilam BJ: Cyclophilin D gene ablation protects mice from ischemic renal injury. Am J Physiol Renal Physiol 297: F749–F759, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Linkermann A, Bräsen JH, Darding M, Jin MK, Sanz AB, Heller JO, De Zen F, Weinlich R, Ortiz A, Walczak H, Weinberg JM, Green DR, Kunzendorf U, Krautwald S: Two independent pathways of regulated necrosis mediate ischemia-reperfusion injury. Proc Natl Acad Sci U S A 110: 12024–12029, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ha HC, Snyder SH: Poly(ADP-ribose) polymerase is a mediator of necrotic cell death by ATP depletion. Proc Natl Acad Sci U S A 96: 13978–13982, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moubarak RS, Yuste VJ, Artus C, Bouharrour A, Greer PA, Menissier-de Murcia J, Susin SA: Sequential activation of poly(ADP-ribose) polymerase 1, calpains, and Bax is essential in apoptosis-inducing factor-mediated programmed necrosis. Mol Cell Biol 27: 4844–4862, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martin DR, Lewington AJ, Hammerman MR, Padanilam BJ: Inhibition of poly(ADP-ribose) polymerase attenuates ischemic renal injury in rats. Am J Physiol Regul Integr Comp Physiol 279: R1834–R1840, 2000 [DOI] [PubMed] [Google Scholar]
- 36.Kim J, Long KE, Tang K, Padanilam BJ: Poly(ADP-ribose) polymerase 1 activation is required for cisplatin nephrotoxicity. Kidney Int 82: 193–203, 2012 [DOI] [PubMed] [Google Scholar]
- 37.Whelan RS, Konstantinidis K, Wei AC, Chen Y, Reyna DE, Jha S, Yang Y, Calvert JW, Lindsten T, Thompson CB, Crow MT, Gavathiotis E, Dorn GW, 2nd, O’Rourke B, Kitsis RN: Bax regulates primary necrosis through mitochondrial dynamics. Proc Natl Acad Sci U S A 109: 6566–6571, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karch J, Kwong JQ, Burr AR, Sargent MA, Elrod JW, Peixoto PM, Martinez-Caballero S, Osinska H, Cheng EH, Robbins J, Kinnally KW, Molkentin JD: Bax and Bak function as the outer membrane component of the mitochondrial permeability pore in regulating necrotic cell death in mice. Elife 2: e00772, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Awad AS, Ye H, Huang L, Li L, Foss FW, Jr., Macdonald TL, Lynch KR, Okusa MD: Selective sphingosine 1-phosphate 1 receptor activation reduces ischemia-reperfusion injury in mouse kidney. Am J Physiol Renal Physiol 290: F1516–F1524, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Awad AS, Rouse M, Huang L, Vergis AL, Reutershan J, Cathro HP, Linden J, Okusa MD: Compartmentalization of neutrophils in the kidney and lung following acute ischemic kidney injury. Kidney Int 75: 689–698, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kelly KJ, Williams WW, Jr., Colvin RB, Meehan SM, Springer TA, Gutierrez-Ramos JC, Bonventre JV: Intercellular adhesion molecule-1-deficient mice are protected against ischemic renal injury. J Clin Invest 97: 1056–1063, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Solez K, Morel-Maroger L, Sraer JD: The morphology of “acute tubular necrosis” in man: Analysis of 57 renal biopsies and a comparison with the glycerol model. Medicine (Baltimore) 58: 362–376, 1979 [PubMed] [Google Scholar]
- 43.Rankin EB, Tomaszewski JE, Haase VH: Renal cyst development in mice with conditional inactivation of the von Hippel-Lindau tumor suppressor. Cancer Res 66: 2576–2583, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zheng J, Devalaraja-Narashimha K, Singaravelu K, Padanilam BJ: Poly(ADP-ribose) polymerase-1 gene ablation protects mice from ischemic renal injury. Am J Physiol Renal Physiol 288: F387–F398, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Kim J, Padanilam BJ: Loss of poly(ADP-ribose) polymerase 1 attenuates renal fibrosis and inflammation during unilateral ureteral obstruction. Am J Physiol Renal Physiol 301: F450–F459, 2011 [DOI] [PubMed] [Google Scholar]
- 46.Hara-Chikuma M, Verkman AS: Aquaporin-1 facilitates epithelial cell migration in kidney proximal tubule. J Am Soc Nephrol 17: 39–45, 2006 [DOI] [PubMed] [Google Scholar]
- 47.Polosukhina D, Singaravelu K, Padanilam BJ: Activation of protein kinase C isozymes protects LLCPK1 cells from H2O2 induced necrotic cell death. Am J Nephrol 23: 380–389, 2003 [DOI] [PubMed] [Google Scholar]
- 48.Montero J, Dutta C, van Bodegom D, Weinstock D, Letai A: p53 regulates a non-apoptotic death induced by ROS. Cell Death Differ 20: 1465–1474, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.