Abstract
The aim of this work was to assess the accuracy of different extraction methods of phospholipids and to measure the effect that processing has on phospholipid composition. Four methods of extracting phospholipids from buttermilk powder were compared to optimize recovery of sphingomyelin. Using the optimal method, the phospholipid profile of four dairy products (raw milk, raw cream, homogenized and pasteurized milk, and buttermilk powder) was determined. A total lipid extraction by the Folch method followed by a solid-phase extraction using the Bitman method was the most efficient technique to recover milk sphingomyelin. Milk processing (churning, centrifuging, homogenization, spray-drying) affected the profile of milk phospholipids, leading to a loss of sphingomyelin and phosphatidylcholine after centrifugation for cream separation. We also observed a corresponding decrease in the saturation content of the raw cream phospholipids, and a loss of phosphatidylethanolamine after spray-drying to produce buttermilk powder.
Keywords: Phospholipids, lipid extraction, solid-phase extraction, milk processing, milk fat globule membrane, sphingomyelin
INTRODUCTION
Phospholipids account for about 1% of the total bovine milk lipids (1), and about 60% of these are found as part of the milk fat globule membrane (MFGM). Residual MFGM material is found in the skim milk phase, probably released during or after secretion of the lipid droplet (2). Upon processing, the MFGM is altered and loses some phospholipids, and the serum proteins tend to associate with the MFGM around the lipid core (3).
Milk can be processed by centrifugation to produce cream, homogenization, heat treatment, spray-drying to produce milk powder, and churning to produce butter. Buttermilk is a by-product of the butter-making process and is generally considered as a low-value product, however, it is rich in MFGM material (4). The surfactant properties of the buttermilk phospholipids could be of considerable importance for the nutritional and technological use of buttermilk and MFGM extracts, for example, in emulsions or for liposome formation.
Proteomics of the MFGM has been extensively studied whereas lipidomics still lacks some important information. Morin et al. (5) studied the effects of cream pasteurization and spray-drying of buttermilk on the phospholipid content of MFGM and buttermilk. Many studies have reported the phospholipid content of raw milk and buttermilk powder but, in general, there is a lack of data on the phospholipid composition of processed dairy products. In light of increasing knowledge about the health and nutritional benefits of the MFGM phospholipids, it is important to compare the efficiency of different methods of phospholipid extraction and determine the phospholipid profile of dairy products.
One important distinction of milk derived phospholipids from all other plant sources is that they contain sphingomyelin. Sphingomyelin has been associated with many health benefits. Its biological activity is due to its metabolites ceramide, sphingosine and sphingosine phosphate (6). MFGM phospholipids have been shown to have anti-carcinogenic properties, cholesterolemia lowering effects, and to be efficient in prevention of Alzheimer’s disease, gastric disease, stress and depression, and suppression of multiple sclerosis (6). Sphingomyelin has been implicated in microdomain formation in cell membranes, playing a role in regulation of several cellular processes (7).
Different methods of extraction of phospholipids from the same buttermilk powder were examined to determine the optimal recovery of sphingomyelin. The phospholipid profile, especially the sphingomyelin content and degree of saturation of the phospholipids present, from four processed dairy products (raw milk, raw cream, homogenized and pasteurized milk, and buttermilk powder) was also examined using the optimal extraction method as a function of four milk processing conditions: churning, homogenization, pasteurization, and spray-drying.
MATERIALS AND METHODS
Two sets of extractions were carried out: the purpose of the first set was to compare the methods of extraction of phospholipids from the same buttermilk powder, and the second set was to compare the phospholipid profile of the four dairy products using the same extraction method.
Samples and reagents
Bovine raw milk was collected from a bulk tank of milk from the Holstein and Jersey dairy herd at the Dairy Products Technology Center (California Polytechnic State University, San Luis Obispo, CA). Processed whole milk from Producers Dairy Foods, Inc. (Fresno, CA; pasteurized at 76°C for 15 seconds and homogenized at 14.2 MPa in the first stage and 10.1 MPa in the second) was bought at a local campus market. The first set of extractions was carried out on buttermilk powder purchased from Dairy America, Inc. (Fresno, CA). Buttermilk powder for the second set of extractions was provided by Land O’Lakes, Inc. (Arden Hills, MN). Deionized water was used unless specified otherwise. All solvents (chloroform, methanol, hexane, diethyl ether, petroleum ether, ethyl alcohol and ammonium hydroxide) were from Fisher Scientific (Pittsburgh, PA) and were of HPLC grade. Supelco Discovery® DSC-Silica gel-based solid-phase extraction (SPE) cartridges (bed weight 2 g, volume 12 mL) for the first set of extractions on buttermilk powder were purchased from Sigma-Aldrich (St. Louis, MO) and Strata silica SI-1 normal phase SPE cartridges (bed weight 2 g, volume 12 mL) for the second set of extractions on four dairy products from Phenomenex (Torrance, CA). Silica gel plates coated with glass (Fisher Scientific) were used for thin-layer chromatography (TLC).
Total lipid extraction: Folch method versus Mojonnier method
Total lipids from Dairy America buttermilk powder were extracted by the modified Mojonnier ether extraction method (8). To 5 g of powder (diluted in 5 mL of deionized water), 1.5 mL of NH4OH was added to neutralize acids and dissolve caseins. The first extraction was carried out with 10 mL of ethyl alcohol, 25 mL of ethyl ether and 25 mL of petroleum ether. The second extraction was with 5 mL of ethyl alcohol, 15 mL of ethyl ether and 15 mL of petroleum ether. The third extraction used 15 mL of ethyl ether and 15 mL of petroleum ether. The total lipids were recovered in hexane (100 mg•mL−1) after evaporation of the solvents at 65°C in a vacuum oven.
A total lipid extraction by the Folch (9) method was carried out on freeze-dried raw milk, freeze-dried processed milk, raw cream (from raw milk centrifuged at 3000 g for 5 minutes at 20°C in a benchtop Eppendorf centrifuge) and buttermilk powder from Dairy America (in the first set of experiments) and from Land O’Lakes (in the second set of experiments). The raw milk and raw cream phospholipids came from the same pool of milk whereas the processed milk and buttermilk powder were purchased from a different source.
The raw milk and processed milk samples contained 25% total fat on a dry matter basis, the raw cream 50% and the buttermilk powder 10%. After extraction first with chloroform:methanol (2:1, v/v) and then addition of a NaCl solution (8.76%) to purify the extract, the total lipids were dried with a roto-evaporator and recovered in hexane to a concentration of 100 mg.mL−1. Methanol was added first to improve the dissociation of lipid-protein interactions, and then chloroform was added to solubilize the lipids.
Comparison of solid-phase extraction methods: Bitman method versus Avalli method versus Vaghela method
It is necessary to remove the triglycerides and other neutral lipids prior to any quantitative analysis. Three methods of solid-phase extraction, the Bitman method (10), the Avalli method (11) and the Vaghela method (12), were carried out to separate the neutral lipids from the phospholipids. Only the Vaghela method was modified, as follows: a silica cartridge was used instead of an aminopropyl stationary phase, as Kaluzny et al. (13) showed a loss of acidic lipids using an aminopropyl-bonded phase. Each cartridge was loaded with 200 mg of lipids.
Briefly, the Bitman method involved conditioning the cartridge with chloroform and first, elution of the neutral lipids with 40 mL of hexane:ethyl ether (1:1, v/v), and then, elution of the phospholipids with a 20 mL wash of methanol followed by a 20 mL wash of chloroform:methanol:water (3:5:2, v/v/v). The Avalli method utilized hexane to condition the cartridge, 6 mL of hexane:diethyl ether (8:2, v/v) and 6 mL of hexane:diethyl ether (1:1, v/v) to elute the neutral lipids, and then, 8 mL of methanol and 4 mL of methanol plus 4 mL of chloroform:methanol:water (3:5:2, v/v/v). In the Vaghela method, the cartridge was conditioned with hexane. The neutral lipids were eluted first with 18 mL of chloroform:isopropyl alcohol (2:1, v/v), then the fatty acids with 18 mL of 2% (v/v) acetic acid in diethyl ether, and finally the phospholipids with 18 mL of methanol. After each SPE, the solvents were evaporated and the phospholipid extracts were dissolved in chloroform to a concentration of 10 mg.mL−1, transferred into amber glass vials capped with Teflon-lined caps and stored at −20°C until further analysis.
Phospholipid analysis
A TLC (chloroform:methanol:water, 65:25:4, v/v/v) was carried out to qualitatively identify the phospholipids present in each extract and check the presence of any residual traces of neutral lipids. Samples (20 µL) were spotted on the plates with a Hamilton gas-tight syringe (Fisher Scientific). Iodine vapor was applied overnight for the revelation of spots.
Electrospray ionization-tandem mass spectrometry (ESI-MS/MS) lipid profiling
The phospholipids extracts were taken to dryness under a gentle stream of nitrogen. A complete quantitative analysis of the phospholipid composition was analyzed by the Kansas Lipidomics Research Center (Manhattan, KS, USA). An automated ESI-MS/MS approach was used, and data acquisition, analysis and acyl group identification were carried out as described previously (14) with modifications. The dried samples were dissolved in 1 mL chloroform and an aliquot of 15 µL of extract in chloroform was used. Precise amounts of internal standards, obtained and quantified as previously described (15), were added in the following quantities (with some small variation in amounts in different batches of internal standards): 0.60 nmol di12:0-phosphatidylcholine (PC), 0.60 nmol di24:1-PC, 0.60 nmol 13:0-lysoPC, 0.60 nmol 19:0-lysoPC, 0.30 nmol di12:0-phosphatidylethanolamine (PE), 0.30 nmol di23:0-PE, 0.30 nmol 14:0-lysoPE, 0.30 nmol 18:0-lysoPE, 0.30 nmol 14:0-lysophosphatidylglycerol (lysoPG), 0.30 nmol 18:0-lysoPG, 0.30 nmol di14:0-phosphatidic acid (PA), 0.30 nmol di20:0(phytanoyl)-PA, 0.20 nmol di14:0-phosphatidylserine (PS), 0.20 nmol di20:0(phytanoyl)-PS, 0.23 nmol 16:0–18:0-phosphatidylinositol (PI), and 0.16 nmol di18:0-PI. The sample and internal standard mixture was combined with solvents, such that the ratio of chloroform/methanol/300mM ammonium acetate in water was 300/665/35, and the final volume was 1.2 mL.
Unfractionated lipid extracts were introduced by continuous infusion into the ESI source on a triple quadrupole MS/MS (4000QTrap, Applied Biosystems, Foster City, CA, USA). Samples were introduced using an autosampler (LC Mini PAL, CTC Analytics AG, Zwingen, Switzerland) fitted with the required injection loop for the acquisition time and presented to the ESI needle at 30 µL.min−1.
Lipid species were detected with the following scans: PC, sphingomyelin (SM), and lysoPC, [M + H]+ ions in positive ion mode with a precursor of 184.1; PE and lysoPE, [M + H]+ ions in positive ion mode with a neutral loss (NL) 141.0 (NL 141.0); PI, [M + NH4]+ in positive ion mode with NL 277.0; PS, [M + H]+ in positive ion mode with NL 185.0; and PA, [M + NH4]+ in positive ion mode with NL 115.0. SM was determined from the same mass spectrum as PC (precursors of m/z 184 in positive mode) (16, 17) and by comparison with PC internal standards using a molar response factor for SM (in comparison with PC) determined experimentally to be 0.39. The scan speed was 50 or 100 units per sec. The collision gas pressure was set at 2 (arbitrary units). The collision energies, with nitrogen in the collision cell, were +28 V for PE, +40 V for PC (and SM), +25 V for PI, PS and PA. Declustering potentials were +100 V. Entrance potentials were +15 V for PE, +14 V for PC (and SM), PI, PA, and PS. Exit potentials were +11 V for PE, +14 V for PC (and SM), PI, PA, PS. The mass analyzers were adjusted to a resolution of 0.7 atomic mass units full width at half height. For each spectrum, nine to 150 continuum scans were averaged in multiple channel analyzer mode. The source temperature (heated nebulizer) was 100°C, the interface heater was on, +5.5 kV or −4.5 kV were applied to the electrospray capillary, the curtain gas was set at 20 (arbitrary units), and the two ion source gases were set at 45 (arbitrary units).
The background of each spectrum was subtracted, the data were smoothed, and peak areas integrated using a custom script with Analyst software (Applied Biosystems).
Statistical analysis
The phospholipid composition and their fatty acid profile were evaluated for five replicates. Analysis of variance (ANOVA) was calculated using SPSS version 14.0 software (SPSS Inc., Chicago, IL, USA) and results were considered significantly different at P<0.05.
RESULTS
Comparison of methods to extract phospholipids from the same buttermilk powder
Phospholipid profile
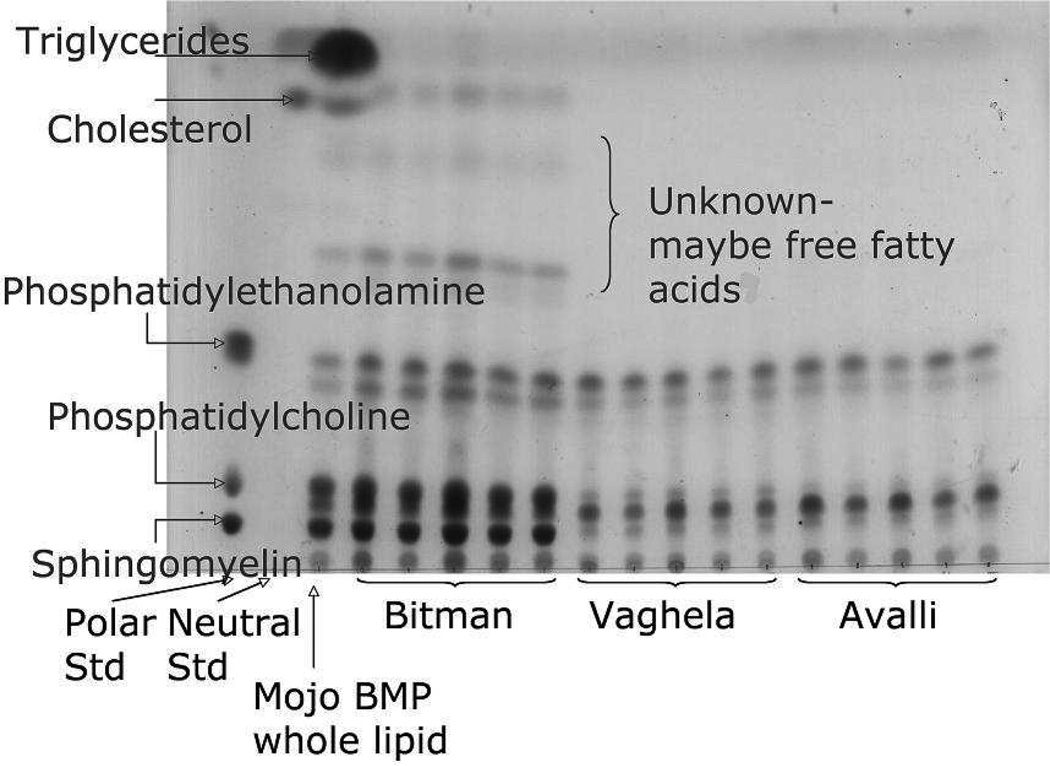
A purity check for each extracted phospholipid sample was carried out by TLC; the presence of triglycerides was not detectable in any of the three extracts (Figure 1). The phospholipid profile and fatty acid composition of the individual phospholipid species in buttermilk powder extracted following four methods of extraction were determined by ESI-MS/MS. The Mojonnier extraction followed by the Bitman SPE gave 33.6 nmol per mg lipid weight, the Mojonnier extraction followed by the Vaghela extraction 20.7 nmol per mg lipid weight, the Mojonnier extraction followed by the Avalli extraction 29.6 nmol per mg lipid weight, and the Folch extraction followed by the Bitman SPE 46.5 nmol per mg lipid weight.
Figure 1.
Thin-layer chromatography plate showing the migration of the phospholipids of buttermilk powder extracted by a total lipid extraction following the Mojonnier method, followed by an SPE using either the Bitman, Vaghela or Avalli method (Std: standard, BMP: buttermilk powder, Mojo: Mojonnier).
The Avalli and Vaghela methods yielded lower amounts of PC, ePC and SM (Table 1) compared to the Bitman method, but higher amounts of PE, ePE, PS and PI. The Bitman method gave low PE values (Table 1), likely due to a loss in PE recovery. LysoPE, LysoPC and PA were detected in all the extracted samples. At this stage it is not possible to say if they were originally present in the buttermilk powder resulting from the degradation of PE, PC and other phospholipids over processing and storage, or if the methods of extraction were responsible for this degradation. Christie et al. (18), using the Bligh and Dyer (19) total lipid extraction and Bitman SPE methods, reported the following concentrations of phospholipids in buttermilk: 29.5%w/v PC, 25.1%w/v SM and 38.1%w/v PE. Morin et al. (20), using the Mojonnier method, reported different concentrations of phospholipids in buttermilk: 33.2%w/w PC, 15.7%w/w SM, 33.9%w/w PE, 11.1%w/w PI and 6.2%w/w PS.
Table 1.
Phospholipid composition of buttermilk powder obtained following four methods of extraction.
| Samples Phospholipids |
Mojo/Vaghela mol% |
Mojo/Avalli mol% |
Mojo/Bitman mol% |
Folch/Bitman mol% |
|---|---|---|---|---|
| LysoPC | 0.1 ± 0.1 | 0.14 ± 0.03 | 0.6 ± 0.1 | 1.5 ± 0.1 |
| PC | 14.2 ± 1.5 | 9.7 ± 0.8 | 47.0 ± 0.7 | 35.3 ± 1.7 |
| SM | 2.0 ± 0.3 | 0.6 ± 0.2 | 22.6 ± 3.2 | 20.8 ± 0.4 |
| ePC | 1.8 ± 0.2 | 1.2 ± 0.1 | 7.0 ± 0.2 | 5.7 ± 0.2 |
| LysoPE | 0.5 ± 0.4 | 3.1 ± 0.6 | 0.2 ± 0.1 | 0.7 ± 0.1 |
| PE | 36.3 ± 4.7 | 34.5 ± 2.6 | 9.6 ± 1.6 | 8.8 ± 0.2 |
| PE-cer | 0.018±0.018 | 0.013±0.019 | 0.001±0.002 | 0.000±0.001 |
| ePE | 1.1 ± 0.4 | 1.2 ± 0.12 | 0.3 ± 0.1 | 0.24 ± 0.02 |
| PI | 35.9 ± 6.0 | 38.3 ± 2.2 | 10.0 ± 2.1 | 16.4 ± 2.8 |
| PS | 6.8 ± 2.0 | 9.3 ± 0.5 | 2.5 ± 0.4 | 10.0 ± 0.9 |
| PA | 1.3 ± 0.2 | 2.0 ± 0.1 | 0.3 ± 0.1 | 0.5 ± 0.2 |
Abbreviations. (LysoPC: lysophosphatidylcholine, PC: phosphatidylcholine; SM: sphingomyelin, ePC: ether phosphatidylcholine, LysoPE: lysophosphatidylethanolamine, PE: phosphatidylethanolamine, PE-cer: phosphoethanolamine ceramide, ePE: ether phosphatidylethanolamine, PI: phosphatidylinositol, PS: phosphatidylserine, PA: phosphatidic acid, Mojo: Mojonnier).
The low amount of lipids in the buttermilk powders (about 10% on dry basis), the high protein content and the presence of lipid-protein complexes make it hard to extract the lipids fully without altering them (21). The Mojonnier method involves a strong base, NH4OH, and heating of the samples to evaporate the solvents which both lead to hydrolysis or oxidation of phospholipids and their unsaturated fatty acids (22). Therefore cold extractions, such as the Folch method, are preferred. In addition to the processing conditions, such as spray-drying, the storage of the buttermilk powder may have influenced the degradation of some phospholipids into lyso-phospholipids. The Folch method, involving the use of an alcohol, allows the complete solubilization of the MFGM polar lipids (6). The alcohol denaturates the proteins and degrades the hydrogen bonds of the protein-lipid complexes.
Morin et al. (5) studied the effect of buttermilk processing on the phospholipid content; they used the Mojonnier method to extract the total lipids and analyzed the lipid content using a high-performance liquid chromatography (HPLC)-evaporative light scattering detector. They observed a similar decrease in the PE content of buttermilk and MFGM isolates due to spray-drying, suggesting that the formation of protein-phospholipid complexes during spray-drying renders some phospholipids non-extractable by the Mojonnier technique. The Folch method was also not successful in extracting all PE (Table 1).
Fatty acid distribution
ESI-MS/MS was employed to determine the structural information of a specific species. The method of extraction significantly affected the ratio of saturation:unsaturation of the fatty acids (Table 2). The polyunsaturation of a phospholipid means that the phospholipid contains either two unsaturated fatty acids or one saturated and one polyunsaturated. Slightly less saturated phospholipids were found using the Folch technique compared to the Mojonnier method, meaning that degradation may have occurred during the Mojonnier method when the solvents were evaporated by heat. The Vaghela and the Avalli methods were not efficient in the extraction of saturated fatty acids (Table 2), mainly due to poor extraction of SM (Table 1).
Table 2.
Degree of unsaturation of the phospholipids present in buttermilk powder obtained following four methods of extraction.
| Mojo/Vaghela mol% |
Mojo/Avalli mol% |
Mojo/Bitman mol% |
Folch/Bitman mol% |
||
|---|---|---|---|---|---|
| LysoPC | Saturated | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.23 ± 0.02 | 0.56 ± 0.05 |
| Monounsaturated | 0.03 ± 0.01 | 0.06 ± 0.01 | 0.24 ± 0.02 | 0.65 ± 0.06 | |
| Polyunsaturated | 0.03 ± 0.01 | 0.05 ± 0.01 | 0.11 ± 0.01 | 0.34 ± 0.03 | |
| PC | Saturated | 1.5 ± 0.1 | 0.8 ± 0.1 | 7.3 ± 0.5 | 5.7 ± 0.3 |
| Monounsaturated | 4.5 ± 0.5 | 2.3 ± 0.3 | 16.4 ± 1.0 | 12.4 ± 0.8 | |
| Polyunsaturated | 8.1 ± 0.8 | 6.6 ± 0.9 | 23.3 ± 1.2 | 17.3 ± 0.9 | |
| ePC | Saturated | 0.33 ± 0.05 | 0.20 ± 0.04 | 1.6 ± 0.1 | 1.3 ± 0.1 |
| Monounsaturated | 0.9 ± 0.1 | 0.5 ± 0.1 | 3.7 ± 0.2 | 3.1 ± 0.2 | |
| Polyunsaturated | 0.6 ± 0.1 | 0.5 ± 0.1 | 1.8 ± 0.1 | 1.3 ± 0.1 | |
| SM | Saturated | 1.7 ± 0.3 | 0.5 ± 0.2 | 21.2 ± 1.6 | 19.6 ± 1.4 |
| Monounsaturated | 0.29 ± 0.05 | 0.03 ± 0.01 | 1.4 ± 0.1 | 1.2 ± 0.1 | |
| Polyunsaturated | n.d. | n.d. | n.d. | n.d. | |
| LysoPE | Saturated | 0.010 ± 0.002 | 0.07 ± 0.02 | 0.003±0.001 | 0.03 ± 0.01 |
| Monounsaturated | 0.27 ± 0.04 | 1.2 ± 0.2 | 0.08 ± 0.01 | 0.41 ± 0.04 | |
| Polyunsaturated | 0.2 ± 0.1 | 1.8 ± 0.4 | 0.07 ± 0.01 | 0.22 ± 0.03 | |
| PE | Saturated | 0.23 ± 0.04 | 0.31 ± 0.05 | 0.04 ± 0.01 | 0.04 ± 0.01 |
| Monounsaturated | 6.1 ± 0.5 | 4.9 ± 0.5 | 1.4 ± 0.1 | 1.5 ± 0.1 | |
| Polyunsaturated | 30.0 ± 2.4 | 29.3 ± 2.0 | 8.2 ± 0.6 | 7.2 ± 0.2 | |
| PE-cer | Saturated | 0.018 ± 0.007 | 0.007±0.004 | 0.0006±0.0004 | 0.0002±0.0002 |
| Monounsaturated | 0.0003±0.0002 | 0.007±0.003 | 0.0001±0.0001 | n.d. | |
| Polyunsaturated | n.d. | n.d. | n.d. | n.d. | |
| ePE | Saturated | 0.02± 0.01 | 0.07 ± 0.03 | 0.004 ± 0.002 | 0.004 ± 0.002 |
| Monounsaturated | 0.33 ± 0.05 | 0.35 ± 0.05 | 0.10 ± 0.01 | 0.07 ± 0.01 | |
| Polyunsaturated | 0.7 ± 0.1 | 0.8 ± 0.1 | 0.18 ± 0.03 | 0.17 ± 0.04 | |
| PI | Saturated | 3.7 ± 0.4 | 4.2 ± 0.2 | 1.1 ± 0.1 | 1.7 ± 0.2 |
| Monounsaturated | 12.2 ± 1.2 | 11.5 ± 0.6 | 3.3 ± 0.2 | 5.1 ± 0.5 | |
| Polyunsaturated | 20.0± 2.6 | 22.6 ± 1.7 | 5.6 ± 0.4 | 9.6 ± 1.5 | |
| PS | Saturated | 0.003 ± 0.002 | 0.07 ± 0.02 | 0.002 ± 0.001 | 0.01 ± 0.01 |
| Monounsaturated | 3.1 ± 0.2 | 3.3 ± 0.4 | 0.9 ± 0.1 | 4.0 ± 0.3 | |
| Polyunsaturated | 3.7 ± 0.3 | 5.92 ± 0.05 | 1.5 ± 0.1 | 6.0 ± 0.2 | |
| PA | Saturated | 0.002 ± 0.001 | 0.018±0.003 | 0.003 ± 0.001 | 0.004 ± 0.002 |
| Monounsaturated | 0.08 ± 0.02 | 0.05 ± 0.01 | 0.025 ± 0.003 | 0.029 ± 0.007 | |
| Polyunsaturated | 1.2 ± 0.1 | 2.0 ± 0.1 | 0.31 ± 0.03 | 0.51 ± 0.05 | |
| PL | Saturated | 7.6 ± 0.9 | 6.3 ± 0.7 | 31.4 ± 2.3 | 28.9 ± 2.2 |
| Monounsaturated | 27.8 ± 2.6 | 24.2 ± 2.2 | 27.6 ± 1.7 | 28.4 ± 2.1 | |
| Polyunsaturated | 64.6 ± 6.4 | 69.5 ± 5.3 | 41.0 ± 2.6 | 42.7 ± 3.1 | |
| saturated: unsaturated | 0.1 | 0.1 | 0.5 | 0.4 | |
(PL: phospholipids; n.d.: not detected)
The Folch total lipid extraction method followed by the Bitman phospholipid SPE was chosen as it recovered more phospholipids (46.5nmol/mg lipid weight), more SM, a higher amount of saturated fatty acids and was more reproducible.
Comparison of the phospholipid composition of raw milk, raw cream, processed milk and buttermilk powder using the same extraction method
Phospholipid profile
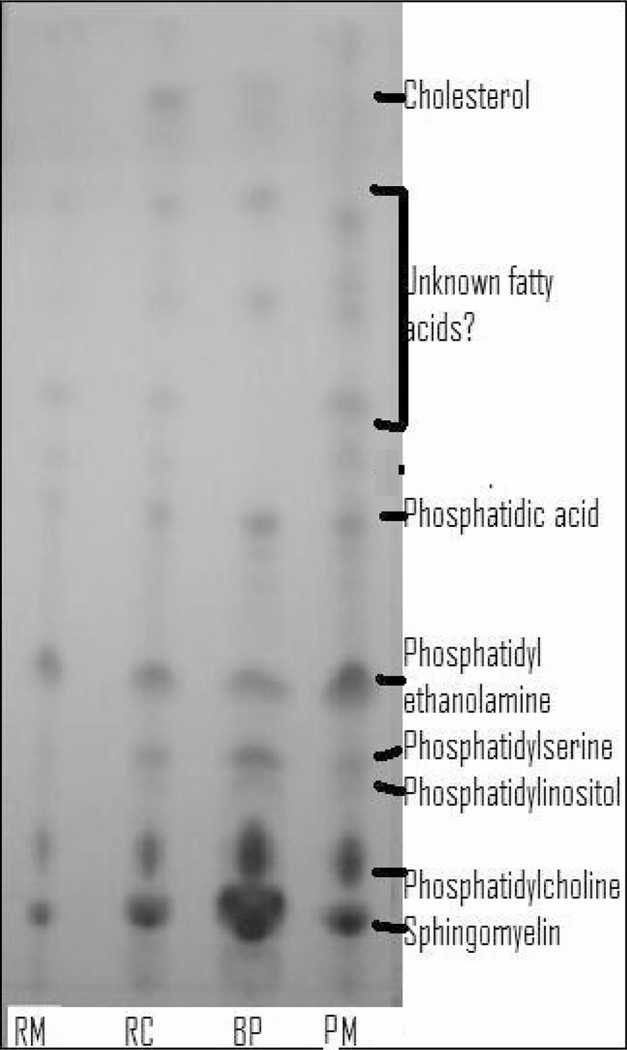
For a rapid and qualitative control of the efficiency of the solid-phase extraction in separating the phospholipids and the neutral lipids, a TLC was performed on mixtures of phospholipids from four products (Figure 2). The TLC shows that the solid-phase extraction did not totally eliminate all neutral lipids (Figure 2). Some cholesterol was still detected and other spots might correspond to free fatty acids. Although cholesterol is hydrophobic, it contains a polar 3β-hydroxyl group (23) and this could be the reason why cholesterol was found in the samples after solid-phase extraction. However, no further analysis was carried out as the main goal was to remove most of the triglycerides.
Figure 2.
Thin-layer chromatography plate showing the migration of the phospholipids of raw milk (RM), raw cream (RC), buttermilk powder (BP) and processed milk (PM) extracted by a total lipid Folch extraction followed by an SPE using the Bitman method.
The phospholipid profile and fatty acid composition of the individual phospholipid species in raw milk, raw cream, processed milk and buttermilk powder that were extracted following the Folch extraction method and the Bitman solid-phase extraction method are presented in Table 3. A statistical analysis of differences could not be carried out on LysoPC and PG due to unequal variance in the results. Results of buttermilk powder phospholipid profile extracted by the Folch/Bitman methods in the first set of experiments (Table 1) and in the second set of experiments (Table 3) differ, likely due to the buttermilk powders used in the two sets having derived from different sources of milk and processing conditions, especially spray-drying, as different protein aggregates were observed by confocal laser scanning microscopy (results not shown). In addition to the processing, the storage of the two powders may have affected the degradation of some phospholipids into lysophospholipids.
Table 3.
Phospholipid composition of raw milk, raw cream, processed milk and buttermilk powder. The phospholipids were extracted following the Folch method for total lipid extraction and the Bitman method for the solid-phase extraction.
| Samples Phospholipids |
Raw Milk mol% |
Raw cream mol% |
Processed milk mol% |
Buttermilk powder mol% |
|---|---|---|---|---|
| Total LysoPC | 1.0 ± 0.1 | 0.7 ± 0.1 | 1.08 ± 0.02 | 3.6 ± 0.2 |
| Total PC | 36.6b | 31.1a | 40.3c | 44.3d |
| Total SM | 21.8b | 17.7a | 24.1b | 23.9b |
| Total ePC | 5.4b | 4.4a | 5.2b | 6.8c |
| Total LysoPE | 0.5b | 0.4a | 0.5b | 0.4a |
| Total PE | 22.6c | 24.8d | 20.8b | 7.3a |
| Total PE-cer | 0.0087b | 0.0074ab | 0.0055ab | 0.0019a |
| Total ePE | 1.0c | 0.9bc | 0.9b | 0.3a |
| Total PI | 2.9b | 8.8d | 2.3a | 7.1c |
| Total PS | 1.7a | 6.7c | 1.3a | 4.0b |
| Total PG | 4.6 ± 0.8 | 3.6 ± 0.5 | 2.3 ± 0.1 | 0.51 ± 0.03 |
| Total PA | 1.8c | 0.8a | 1.2b | 1.8c |
Means within a row with different superscripts differ (P<0.05).
(PG: phosphatidylglycerol)
Before extraction, the raw milk was pumped and kept in a refrigerated holding tank at 4°C for few hours. This step may lead to hydrolysis of the neutral and polar lipids of the membrane (24), explaining the presence of lyso-PC, lyso-PE and PA in the raw milk phospholipid extracts. During processing of the buttermilk powder, the sheet-like MFGM material (25) and other components are subject to long holding time and drastic conditions such as spray-drying and high pasteurization temperatures. The buttermilk powder components are therefore likely to oxidize or denature. Buttermilk powder also contains more lysoPC than the other three dairy products (Table 3), meaning that PC is degraded during the churning process, the spray-drying or during the storage of the buttermilk powder, rather than during the Folch and Bitman extractions.
Deeth (26) used phospholipases to determine the asymmetrical distribution of the MFGM phospholipids. Centrifugation leads to a loss of phospholipids from the membrane surface (27). As SM and PC are polar lipids and mainly located in the outer leaflet of the bilayer (26), they are likely to be more easily released to the milk serum upon centrifugation. The lipidomics data confirmed this hypothesis since SM and PC are less abundant in raw cream than in raw milk. This has also been reported by Christie et al. (18) who observed a higher proportion of SM in skim-milk than in whole milk. The significantly lower amounts of SM and PC and higher amounts of PI and PS observed between the raw cream and raw milk phospholipids (Table 3) can also be explained by the difference in composition according to the size of the globules. Michalski et al. (28) found that small milk fat globules, separated by microfiltration, had different chemical and functional properties than large fat globules. This size separation could be advantageous for the quality of cheeses and the production of new dairy products. The raw cream, obtained after centrifugation of the raw milk, mainly consisted of the larger milk fat globules. After homogenization and pasteurization, a part of the MFGM rearranges at the surface of the globule with the absorbed whey proteins and caseins (29). The amount of the major phospholipids PC, PE and PI was significantly affected by these two processing steps (Table 3). Keenan (30) reported no alteration of the phospholipid ratios in MFGM and skim milk after homogenization. This leads to the conclusion that pasteurization may be responsible for the different phospholipid profile in raw milk and processed milk.
The amount of PE in raw milk, raw cream and processed milk phospholipids is within the range of the values reported in the literature (31), indicating that the Bitman method is not likely responsible for the low amount of PE found in buttermilk powders (Tables 1 and 3) but rather that the powders have a low PE content due to spray-drying as observed by Morin et al. (5). The relative composition of the buttermilk powder phospholipids has three times less PE than the other samples (Table 3). Another explanation is that this phospholipid is more hydrophobic than PC and SM and might be located on the layer surrounding the triglyceride core of the globule and on the inner leaflet of the bilayer, and thus partitions into the butter phase during butter manufacture. Keenan and Patton (24) reported that not all the membrane material goes into the buttermilk serum during churning; some MFGM material remains on the surface of the fat globules in the butter phase. This could indicate that PE is found in high quantity in the monolayer surrounding the lipid core.
The SM content was constant for raw milk, processed milk and buttermilk powder (Table 3). Only centrifugation significantly affected the relative amount of SM in the total phospholipid extract (Table 3). The amounts of PC and PE were significantly affected by processing (Table 3). PA and lyso-derivatives of PE and PC were found in all four samples. Keenan and Patton (24) reported the presence of lyso-derivatives of phospholipids in milk but these lyso-phospholipids, as well as PA, are also considered by others as artifacts due to degradation during extraction, or to phospholipase activity during handling and storage (18). Sánchez-Juanes et al. (31) did not detect the presence of LysoPE in raw whole milk but did in MFGM extracts. The values of raw milk and buttermilk powder phospholipids found in the literature are usually expressed as weight percentages and present a very wide variation. Pasteurized cream phospholipid data have been reported in the literature (32), however, only the main phospholipids, PC, PE, SM, PI and PS were reported (18, 31, 32). Sometimes PS and PI amounts are combined due to poor chromatographic separation, as seen in the study by Sánchez-Juanes et al. (31). These authors reported a higher amount of PE, PS and PI and a lower amount of PC and SM in MFGM than in whole milk. The raw cream phospholipids (Table 3) had the same profile as the MFGM phospholipids, as the raw cream phospholipids mainly contain MFGM phospholipids.
In the literature, the content of major phospholipids in raw milk varies greatly: 19.2–37.3% w/w PC, 19.8–42.0% w/w PE, 18.0–34.1% w/w SM, 0.6–11.8% w/w PI and 1.9–10.5% w/w PS (1, 6, 11, 32). Jensen and Clark (33) reported the phospholipid and sphingolipid composition of bovine milk as 34.5mol% PC, 31.8mol% PE, 25.2mol% SM, 3.1mol% PS, 4.7mol% PI, 3mol% plasmalogens, traces of lyso-PC, lyso-PE, ceramides and diphosphatidylglycerol. Hay and Morrison (34) reported the presence of ePE and ePC in buttermilk powder phospholipids, 4% of alkenyl ethers in PE and 1.3% in PC. These variations can be attributed to the differences in the method of extraction and analysis of the phospholipids, the handling of milk and animal factors such as breed, stage of lactation, diet and season of milking.
Fatty acid distribution
SM is a highly saturated phospholipid (Table 4). The main fatty acids in SM are C16:0, C22:0 and C24:0 for all four samples (Table 5). These fatty acids have also been reported in the literature as major dairy SM fatty acids (1, 35). PE-cer is mainly composed of the following fatty acids C16:0, C18:1 and C24:0 (results not shown). PC is the most saturated glycerophospholipid (Table 4). PE is the most polyunsaturated phospholipid and has very low degree of saturation (Table 4). Raw cream phospholipids were significantly less saturated than the phospholipids from raw milk, processed milk and buttermilk powder (Table 4) mainly due to a lower amount of SM in raw cream phospholipids (Table 3). The degree of monounsaturation was not significantly different across the range of processed dairy products. The values observed here differ from the values reported by Sánchez-Juanes et al. (31), possibly due to different techniques of extraction and analysis of phospholipids; the cold extraction method they used consisted of a total lipid extraction with a longer time that may have involved different degrees of recovery of certain classes of phospholipids (36). They did not use a solid-phase extraction to separate the phospholipids from neutral lipids and determine the classes of phospholipids by two-dimensional thin-layer chromatography (31). The analysis of phospholipid fatty acids, in their case, was carried out by gas chromatography-mass spectrometry.
Table 4.
Degree of unsaturation of the phospholipids present in raw milk, raw cream, processed milk and buttermilk powder obtained following the Folch method for total lipid extraction and the Bitman method for the solid-phase extraction
| Raw Milk mol% |
Raw cream mol% |
Processed milk mol% |
Buttermilk powder mol% |
||
|---|---|---|---|---|---|
| Total LysoPC | Saturated | 0.6 | 0.4 | 0.6 | 1.4 |
| Monounsaturated | 0.3 | 0.2 | 0.3 | 1.5 | |
| Polyunsaturated | 0.1 | 0.1 | 0.2 | 0.7 | |
| Total PC | Saturated | 11.7 | 7.8 | 9.8 | 10.2 |
| Monounsaturated | 11.5 | 9.6 | 13.1 | 11.6 | |
| Polyunsaturated | 13.3 | 13.5 | 17.2 | 22.3 | |
| Total ePC | Saturated | 1.3 | 1.0 | 1.2 | 1.5 |
| Monounsaturated | 3.4 | 2.8 | 3.2 | 4.0 | |
| Polyunsaturated | 0.7 | 0.7 | 0.7 | 1.3 | |
| Total SM | Saturated | 20.9 | 16.8 | 22.6 | 22.5 |
| Monounsaturated | 0.9 | 0.8 | 1.5 | 1.4 | |
| Polyunsaturated | - | - | - | - | |
| Total LysoPE | Saturated | 0.1 | 0.0 | 0.1 | 0.0 |
| Monounsaturated | 0.3 | 0.2 | 0.3 | 0.3 | |
| Polyunsaturated | 0.2 | 0.1 | 0.1 | 0.1 | |
| Total PE | Saturated | 0.1 | 0.1 | 0.1 | 0.0 |
| Monounsaturated | 4.5 | 4.6 | 4.0 | 1.1 | |
| Polyunsaturated | 18.1 | 20.1 | 16.7 | 6.2 | |
| Total PE-cer | Saturated | 0.006 | 0.005 | 0.004 | 0.001 |
| Monounsaturated | 0.003 | 0.002 | 0.001 | 0.000 | |
| Polyunsaturated | - | - | - | - | |
| Total ePE | Saturated | 0.0 | 0.1 | 0.0 | 0.0 |
| Monounsaturated | 0.3 | 0.3 | 0.3 | 0.1 | |
| Polyunsaturated | 0.6 | 0.6 | 0.6 | 0.2 | |
| Total PI | Saturated | 0.0 | 0.0 | 0.0 | 0.0 |
| Monounsaturated | 1.1 | 2.9 | 0.8 | 2.1 | |
| Polyunsaturated | 1.8 | 5.9 | 1.5 | 5.0 | |
| Total PS | Saturated | 0.0 | 0.0 | 0.0 | 0.0 |
| Monounsaturated | 0.7 | 2.4 | 0.5 | 1.3 | |
| Polyunsaturated | 1.0 | 4.3 | 0.9 | 2.6 | |
| Total PG | Saturated | 1.9 | 1.6 | 0.8 | 0.0 |
| Monounsaturated | 1.7 | 1.3 | 0.8 | 0.2 | |
| Polyunsaturated | 1.0 | 0.7 | 0.7 | 0.3 | |
| Total PA | Saturated | 0.0 | 0.0 | 0.0 | 0.0 |
| Monounsaturated | 0.1 | 0.1 | 0.1 | 0.1 | |
| Polyunsaturated | 1.6 | 0.7 | 1.0 | 1.7 | |
| Total PL | Saturated | 36.6b | 27.9a | 35.2b | 35.7b |
| Monounsaturated | 24.9±3.3 | 25.3±3.0 | 24.9±2.1 | 23.6±3.1 | |
| Polyunsaturated | 38.5a | 46.7c | 39.7ab | 40.5b | |
| saturated: unsaturated | 0.6b | 0.4a | 0.5b | 0.6b | |
Means within a row with different superscripts differ (P<0.05).
Table 5.
Fatty acid distribution of sphingomyelin present in the four dairy extracts obtained following the Folch method for total lipid extraction and the Bitman method for the solid-phase extraction
| Sample description | Raw Milk mol% |
Raw Cream mol% |
Processed Milk mol% |
Buttermilk Powder mol% |
|
|---|---|---|---|---|---|
| SM 16:1 | 0.29±0.01 | 0.24±0.02 | 0.38±0.02 | 0.35±0.02 | |
| SM 16:0 (or DSM 16:1) | 8.20±0.15 | 6.09±0.22 | 8.58±0.89 | 7.76±0.41 | |
| DSM 16:0 | n.d. | n.d. | 0.33±0.73 | n.d. | |
| SM 18:1 | 0.11±0.08 | 0.08±0.07 | 0.26±0.04 | 0.04±0.10 | |
| SM 18:0 (or DSM 18:1) | 0.69±0.09 | 0.69±0.30 | 1.44±0.47 | 0.72±0.67 | |
| DSM 18:0 | n.d. | n.d. | n.d. | n.d. | |
| SM 22:1 | n.d. | n.d. | n.d. | n.d. | |
| SM 22:0 or DSM 22:1) | 6.02±0.97 | 5.10±0.61 | 6.03±1.60 | 7.11±1.75 | |
| DSM 22:0 | 1.10±0.49 | 0.64±0.49 | 1.08±0.48 | 1.04±0.38 | |
| SM 24:1 | 0.53±0.05 | 0.52±0.09 | 0.86±0.06 | 1.02±0.13 | |
| SM 24:0 (or DSM 24:1) | 4.80±0.47 | 4.32±0.10 | 5.17±0.55 | 5.76±0.40 | |
| DSM 24:0 | 0.10±0.07 | n.d. | 0.01±0.02 | 0.09±0.06 | |
| Total SM | 21.85±1.08 | 17.68±0.85 | 24.14±2.06 | 23.89±2.17 | |
(DSM: dihydrosphingomyelin)
The ESI-MS/MS technique used in this study to determine the fatty acid profile of the phospholipids does not structurally differentiate between the two attached fatty acids. The results for SM, PE-cer and lysophospholipids give the chain length of the single fatty acid attached to the polar head group whereas the results for the other glycerophospholipids correspond to the summation of the individual chain lengths of the total acyl chains. The chain length of the fatty acids in SM (Table 5), PE-cer, lysoPE and lysoPC (results not shown) varied between C16 and C24. The added chain length of the acyl chains of the other glycerophospholipids in the four phospholipid mixtures was 26- to 44-carbons in length (results not shown). The MFGM phospholipids have longer chain fatty acids than the triglycerides of the lipid core (37). The milk phospholipid acyl chains has been reported to be 10- to 24-carbons in length with the major ones being C14:0, C16:0, C18:0, C18:1 and C18:2 (1, 31, 38). Sánchez et al. (31) reported the presence of medium-chain fatty acid (C10-C17), long-chain fatty acids (C18-C20) and very long-chain fatty acids (C>20) in phospholipids from MFGM extracts and whole milk; long-chain fatty acids were the most abundant in the glycerophospholipids whereas SM contained more of the very long-chain fatty acids. The presence of very long chain fatty acids (more than 20-carbons in length) in phospholipids and the high saturation content of SM fatty acids contribute to the structure of the milk fat globule membrane by maintaining its rigidity. The high degree of unsaturation of the glycerophospholipids (Table 4) may play an essential role in the fluidity of the MFGM (39). Again the differences observed in the literature arise from animal factors such as the diet and breed of cows and the methods of extraction and analysis of the phospholipids.
DISCUSSION
In this work, we found that for a more accurate estimation of the amount of lipids present in a sample, hydration of the sample prior to extraction is necessary (18). This was carried out in the Mojonnier extraction but it was not possible in the Folch extraction as it led to the formation of a stable suspension which was almost impossible to filter. The Folch method tends to give values on the low side (21).
Eder et al. (36) studied the effect of solvent and period of extraction on the recovery of extracted phospholipids from erythrocyte membranes. The recovery of PC and SM was not influenced by these two factors whereas PS, diacyl PE and plasmalogen PE and their fatty acid composition were greatly influenced by the solvent and the time chosen for the extraction. This could be related to the presence of PC and SM in the outer leaflet of the MFGM (26) making them readily extracted, and to the presence of PE and PS in the inner leaflet requiring more effective extraction conditions.
The extraction of phospholipids is a difficult task as they are usually associated with proteins or polysaccharides (18) and they are very sensitive to hydrolysis and oxidation. The cold extraction methods seem less oxidative of the polyunsaturated fatty acids (PUFA). The heating step in the Mojonnier technique may explain the partial degradation of the fatty acids of the phospholipids. The oxidative degradation of PUFA leads to undesirable oxidized flavors in dairy products (24). The analysis of phospholipids is often carried out by HPLC (40, 41, 42) or densitometric TLC (1) and the fatty acid composition is determined by gas-liquid chromatography of fatty acid methyl esters or butyl esters (1, 35) measuring fatty acids respectively from C:4 to C:22 with an improved detection of short-chain fatty acids by conversion into butyl esters (37). In this study, variation observed with the literature may arise from the use of a different technique of analysis, tandem mass spectrometry. ESI-MS/MS allows both the identification of phospholipid species and their fatty acid content requiring a smaller quantity of phospholipid extracts than TLC followed by gas chromatography or gas chromatography-mass spectrometry, for example (43).
Milk processes such as spray-drying lead to considerable oxidation of the phospholipids (18). They suggested that spray-dried buttermilk powders may not contain any phospholipids because of autoxidation during spray-drying processing. During churning, MFGM material binds to air cells leading to possible oxidation (24). The lower amount of PE in buttermilk powder due to spray-drying is also an important factor to consider when sourcing phospholipids to form vesicles or liposomes. Indeed, Waninge et al. (44) reported the impact of PE concentration on the phase behavior of phospholipid mixtures, shifting from a lamellar phase to a reversed hexagonal phase at high PE concentration.
The distribution of the fatty acids and the head group of the individual phospholipid species in the four dairy products under study determine the physical characteristics of the products and, in particular, the MFGM structure. The variability of the phospholipids present and their fatty acids content are a response of the MFGM to the surrounding environment. Saturated phospholipids such as SM are involved in lipid raft formation by association with cholesterol in biomembranes. PUFA play key roles such as providing essential fatty acids, eicosanoid precursors and membrane components. The degree of saturation and the length of the fatty acids of phospholipids play a key role in phase separation in membrane systems (45) and are thus involved in the fluidity/rigidity of the MFGM surrounding the liquid lipid core of the fat globule.
We can conclude that, after two major total lipid extractions and three common phospholipid solid-phase extractions were compared, the Folch cold extraction followed by the Bitman solid-phase extraction was the most efficient and reproducible method in the recovery of sphingomyelin, phosphatidylinositol and phosphatidylserine (compared to the Mojonnier/Bitman methods). Additionally, this method demonstrated more consistent results in the recovery of phosphatidylcholine, sphingomyelin and phosphatidylethanolamine (compared to the Mojonnier/Vaghela and Mojonnier/Avalli methods). The phospholipid profile of the four dairy products selected to exemplify different stages of milk processing was evaluated to study the effect of milk processing on their total phospholipid composition. We have focused on observed differences in sphingomyelin content as this component seems to be implicated in beneficial health aspects. The presence of long-chain and very long-chain fatty acids in all milk phospholipid extracts plays a role in the rigidity of the MFGM. Milk processing indeed affects the profile of milk phospholipids on the MFGM causing a redistribution between the membrane and the milk serum or to other fractions. We observed most significantly a loss of sphingomyelin by centrifugation and a loss of phosphatidylethanolamine after spray-drying of buttermilk. In some applications, sphingomyelin is an important factor to consider when sourcing dairy phospholipids as it is likely involved in valuable biological and structural functions. Sphingomyelin is also implicated in the formation of lipid rafts in cells, thus a loss of sphingomyelin (a saturated polar lipid) after centrifugation may induce changes in the fluidity of the MFGM and the structure of the lipid microdomains on the surface of the MFGM.
ACKNOWLEDGEMENTS
The authors thank Tammy Nelson, Diana Rios, Erin Doherty and Jeff Traughber for their help in the extraction of phospholipids from buttermilk powder used in the first set of experiments. The authors would like to also thank Karen Hein for her help in the statistical analysis. This work was funded by a University of Otago Postgraduate Scholarship, the California State University Agricultural Research Initiative, and the California Dairy Research Foundation. The Kansas Lipidomics Research Center was supported by NSF Grants MCB 0455318 and DBI 0521587, and NSF EPSCoR grant EPS-0236913 with matching support from the State of Kansas through Technology Enterprise Corporation and Kansas State University. The KLRC is also supported by K-INBRE (NIH Grant P20 RR16475 from the INBRE program of the National Center for Research Resources).
ABBREVIATIONS USED
- MFGM
milk fat globule membrane
- SPE
solid-phase extraction
- PC
phosphatidylcholine
- PE
phosphatidylethanolamine
- SM
sphingomyelin
- DSM
dihydrosphingomyelin
- PI
phosphatidylinositol
- PS
phosphatidylserine
- PA
phosphatidic acid
- PG
phosphatidylglycerol
- LysoPC
Lysophosphatidylcholine
- LysoPE
lysophosphatidylethanolamine
- LysoPG
lysophosphatidylglycerol
- ePC
ether phosphatidylcholine
- ePE
ether phosphatidylethanolamine
- PE-cer
phosphoethanolamine-ceramide
- PL
phospholipids
- n.d.
non-detected
- ESI-MS/MS
electrospray tandem mass spectrometry
- NL
neutral loss
- TLC
thin-layer chromatography
- Std
standard
- BMP
buttermilk powder
- Mojo
Mojonnier
- RM
raw milk
- RC
raw cream
- BP
buttermilk powder
- PM
processed milk
- PUFA
polyunsaturated fatty acid
- HPLC
high performance liquid chromatography
LITERATURE CITED
- 1.Bitman J, Wood DL. Changes in Milk-Fat Phospholipids During Lactation. Journal of Dairy Science. 1990;73:1208–1216. doi: 10.3168/jds.S0022-0302(90)78784-X. [DOI] [PubMed] [Google Scholar]
- 2.Keenan TW. Milk lipid globules and their surrounding membrane: A brief history and perspectives for future research. Journal of Mammary Gland Biology and Neoplasia. 2001;6:365–371. doi: 10.1023/a:1011383826719. [DOI] [PubMed] [Google Scholar]
- 3.Singh H. The milk fat globule membrane - A biophysical system for food applications. Current Opinion in Colloid & Interface Science. 2006;11:154–163. [Google Scholar]
- 4.Corredig M, Dalgleish DG. Buttermilk properties in emulsions with soybean oil as affected by fat globule membrane-derived proteins. Journal of Food Science. 1998;63:476–480. [Google Scholar]
- 5.Morin P, Jimenez-Flores R, Pouliot Y. Effect of processing on the composition and microstructure of buttermilk and its milk fat globule membranes. International Dairy Journal. 2007;17:1179–1187. [Google Scholar]
- 6.Rombaut R, Dewettinck K. Properties, analysis and purification of milk polar lipids. International Dairy Journal. 2006;16:1362–1373. [Google Scholar]
- 7.Simons K, Vaz WLC. Model systems, lipid rafts, and cell membranes. Annual Review of Biophysics and Biomolecular Structure. 2004;33:269–295. doi: 10.1146/annurev.biophys.32.110601.141803. [DOI] [PubMed] [Google Scholar]
- 8.CBIDF-ISO-AOAC. AOAC Official Method 989.05 Fat in Milk, Modified Mojonnier Ether Extraction Method. 1992 [Google Scholar]
- 9.Folch J, Lees M, Stanley GHS. A Simple Method for the Isolation and Purification of Total Lipides from Animal Tissues. Journal of Biological Chemistry. 1957;226:497–509. [PubMed] [Google Scholar]
- 10.Bitman J, Wood DL, Mehta NR, Hamosh P, Hamosh M. Comparison of the Phospholipid-Composition of Breast-Milk from Mothers of Term and Preterm Infants During Lactation. American Journal of Clinical Nutrition. 1984;40:1103–1119. doi: 10.1093/ajcn/40.5.1103. [DOI] [PubMed] [Google Scholar]
- 11.Avalli A, Contarini G. Determination of phospholipids in dairy products by SPE/HPLU/ELSD. Journal of Chromatography A. 2005;1071:185–190. doi: 10.1016/j.chroma.2005.01.072. [DOI] [PubMed] [Google Scholar]
- 12.Vaghela MN, Kilara A. A Rapid Method for Extraction of Total Lipids from Whey-Protein Concentrates and Separation of Lipid Classes with Solid-Phase Extraction. Journal of the American Oil Chemists Society. 1995;72:1117–1121. [Google Scholar]
- 13.Kaluzny MA, Duncan LA, Merritt MV, Epps DE. Rapid Separation of Lipid Classes in High-Yield and Purity using Bonded Phase Columns. Journal of Lipid Research. 1985;26:135–140. [PubMed] [Google Scholar]
- 14.Bartz R, Li WH, Venables B, Zehmer JK, Roth MR, Welti R, Anderson RGW, Liu PS, Chapman KD. Lipidomics reveals that adiposomes store ether lipids and mediate phospholipid traffic. Journal of Lipid Research. 2007;48:837–847. doi: 10.1194/jlr.M600413-JLR200. [DOI] [PubMed] [Google Scholar]
- 15.Welti R, Li M, Li W, Sang Y, Biesiada H, Zhou H-E, Rajashekar C, Williams T, Wang X. Profiling membrane lipids in plant stress response. J Biol Chem. 2002;277:31994–32002. doi: 10.1074/jbc.M205375200. [DOI] [PubMed] [Google Scholar]
- 16.Brugger B, Erben G, Sandhoff R, Wieland FT, Lehmann WD. Quantitative analysis of biological membrane lipids at the low picomole level by nano-electrospray ionization tandem mass spectrometry. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:2339–2344. doi: 10.1073/pnas.94.6.2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liebisch G, Lieser B, Rathenberg J, Drobnik W, Schmitz G. High-throughput quantification of phosphatidylcholine and sphingomyelin by electrospray ionization tandem mass spectrometry coupled with isotope correction algorithm. Biochimica Et Biophysica Acta-Molecular and Cell Biology of Lipids. 2004;1686:108–117. doi: 10.1016/j.bbalip.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 18.Christie WW, Noble RC, Davies G. Phospholipids in Milk and Dairy-Products. Journal of the Society of Dairy Technology. 1987;40:10–12. [Google Scholar]
- 19.Bligh EG, Dyer WJ. A Rapid Method of Total Lipid Extraction and Purification. Canadian Journal of Biochemistry and Physiology. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 20.Morin P, Britten M, Jimenez-Flores R, Poulio Y. Microfiltration of buttermilk and washed cream buttermilk for concentration of milk fat globule membrane components. Journal of Dairy Science. 2007;90:2132–2140. doi: 10.3168/jds.2006-832. [DOI] [PubMed] [Google Scholar]
- 21.Theodet C, Gandemer G. Comparison of 5 Methods for Lipid Extraction from Whey and Its by-Products. Lait. 1991;71:41–54. [Google Scholar]
- 22.Vaghela M, Kilara A. Lipid composition of whey protein concentrates manufactured commercially and in the laboratory. Journal of Dairy Science. 1996;79:1172–1183. [Google Scholar]
- 23.Yeagle PL. Cholesterol and the Cell-Membrane. Biochimica Et Biophysica Acta. 1985;822:267–287. doi: 10.1016/0304-4157(85)90011-5. [DOI] [PubMed] [Google Scholar]
- 24.Keenan TW, Patton S. The structure of milk: implications for sampling and storage. A. The milk lipid globule membrane. In: Jensen RG, editor. Handbook of milk composition. San Diego: Academic Press; 1995. pp. 5–50. [Google Scholar]
- 25.Corredig M, Dalgleish DG. Isolates from industrial buttermilk: Emulsifying properties of materials derived from the milk fat globule membrane. Journal of Agricultural and Food Chemistry. 1997;45:4595–4600. [Google Scholar]
- 26.Deeth HC. The role of phospholipids in the stability of milk fat globules. Australian Journal of Dairy Technology. 1997;52:44–46. [Google Scholar]
- 27.Anderson M, Brooker BE. Loss of Material During Isolation of Milk-Fat Globule Membrane. Journal of Dairy Science. 1975;58:1442–1448. [Google Scholar]
- 28.Michalski MC, Leconte N, Briard-Bion V, Fauquant J, Maubois JL, Goudédranche H. Microfiltration of raw whole milk to select fractions with different fat globule size distributions: Process optimization and analysis. Journal of Dairy Science. 2006;89:3778–3790. doi: 10.3168/jds.S0022-0302(06)72419-5. [DOI] [PubMed] [Google Scholar]
- 29.Michalski MC, Michel F, Geneste C. Appearance of submicronic particles in the milk fat globule size distribution upon mechanical treatments. Lait. 2002;82:193–208. [Google Scholar]
- 30.Keenan TW. Lipid Globules Retain Globule-Membrane Material after Homogenization. Journal of Dairy Science. 1983;66:196–203. [Google Scholar]
- 31.Sanchez-Juanes F, Alonso JM, Zancada L, Hueso P. Distribution and fatty acid content of phospholipids from bovine milk and bovine milk fat globule membranes. International Dairy Journal. 2009;19:273–278. [Google Scholar]
- 32.Rombaut R, Camp JV, Dewettinck K. Analysis of phospho- and sphingolipids in dairy products by a new HPLC method. Journal of Dairy Science. 2005;88:482–488. doi: 10.3168/jds.S0022-0302(05)72710-7. [DOI] [PubMed] [Google Scholar]
- 33.Jensen RG, Clark RM. Lipid Composition and Properties. In: Wong NP, editor. Fundamentals of Dairy Chemistry. 3rd ed. New York: Van Nostrand-Reinhold; 1988. pp. 171–214. [Google Scholar]
- 34.Hay JD, Morrison WR. Polar Lipids in Bovine Milk.3. Isomeric Cis and Trans Monoenoic and Dienoic Fatty Acids, and Alkyl and Alkenyl Ethers in Phosphatidylcholine and Phosphatidylethanolamine. Biochimica Et Biophysica Acta. 1971;248:71. [PubMed] [Google Scholar]
- 35.Fauquant C, Briard-Bion V, Leconte N, Guichardant M, Marie-Caroline Michalski MC. Membrane phospholipids and sterols in microfiltered milk fat globules. European Journal of Lipid Science and Technology. 2007;109:1167–1173. [Google Scholar]
- 36.Eder K, Reichlmayrlais AM, Kirchgessner M. Studies on the Extraction of Phospholipids from Erythrocyte-Membranes in the Rat. Clinica Chimica Acta. 1993;219:93–104. doi: 10.1016/0009-8981(93)90200-n. [DOI] [PubMed] [Google Scholar]
- 37.Jensen RG, Ferris AM, Lammikeefe CJ. Symposium - Milk-Fat Composition, Function, and Potential for Change - the Composition of Milk-Fat. Journal of Dairy Science. 1991;74:3228–3243. doi: 10.3168/jds.S0022-0302(91)78509-3. [DOI] [PubMed] [Google Scholar]
- 38.Fauquant C, Briard V, Leconte N, Michalski MC. Differently sized native milk fat globules separated by microfiltration: fatty acid composition of the milk fat globule membrane and triglyceride core. European Journal of Lipid Science and Technology. 2005;107:80–86. [Google Scholar]
- 39.Fong BY, Norris CS, MacGibbon AKH. Protein and lipid composition of bovine milk-fat-globule membrane. International Dairy Journal. 2007;17:275–288. [Google Scholar]
- 40.Christie WW. Rapid Separation and Quantification of Lipid Classes by High-Performance Liquid-Chromatography and Mass (Light-Scattering) Detection. Journal of Lipid Research. 1985;26:507–512. [PubMed] [Google Scholar]
- 41.Corredig M, Roesch RR, Dalgleish DG. Production of a novel ingredient from buttermilk. Journal of Dairy Science. 2003;86:2744–2750. doi: 10.3168/jds.S0022-0302(03)73870-3. [DOI] [PubMed] [Google Scholar]
- 42.Fagan P, Wijesundera C. Liquid chromatographic analysis of milk phospholipids with on-line pre-concentration. Journal of Chromatography A. 2004;1054:241–249. doi: 10.1016/j.chroma.2004.04.051. [DOI] [PubMed] [Google Scholar]
- 43.Pulfer M, Murphy RC. Electrospray mass spectrometry of phospholipids. Mass Spectrometry Reviews. 2003;22:332–364. doi: 10.1002/mas.10061. [DOI] [PubMed] [Google Scholar]
- 44.Waninge R, Nylander T, Paulsson M, Bergenstahl B. Phase equilibria of model milk membrane lipid systems. Chemistry and Physics of Lipids. 2003;125:59–68. doi: 10.1016/s0009-3084(03)00070-7. [DOI] [PubMed] [Google Scholar]
- 45.Mukherjee S, Maxfield FR. Membrane domains. Annual Review of Cell and Developmental Biology. 2004;20:839–866. doi: 10.1146/annurev.cellbio.20.010403.095451. [DOI] [PubMed] [Google Scholar]