Abstract
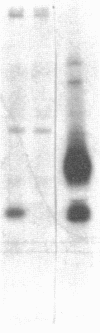
We have recently shown that human monocytes and U937 cells possess two molecular classes of Fc gamma receptor. One, a 72,000-mol-wt sialoglycoprotein, has high affinity for certain subclasses of human and murine monomeric IgG. The other is a 40,000-mol-wt protein (p40) with low affinity for monomeric IgG but with the capacity to bind IgG aggregates or IgG-coated particles. In the present study, a 40,000-mol-wt single chain protein, apparently identical to p40 from U937 cells, was isolated from surface-radioiodinated human platelets by affinity purification using a murine IgG2b monoclonal antibody to p40. This 40,000-mol-wt protein was not seen when control IgG2b or unrelated murine monoclonal antibodies were employed in place of anti-p40. The same 40,000-mol-wt protein was also recovered from an IgG-Sepharose affinity adsorbent, but not from ovalbumin-or myoglobin-Sepharose. The 72,000-mol-wt Fc gamma receptor of monocytes was not identified on platelets. Monoclonal anti-p40 and Fab fragments derived from this antibody blocked platelet aggregation by heat-aggregated human IgG, whereas a control murine IgG2b protein had little or no inhibitory effect at 500-1,000-fold higher concentrations. A murine IgG1 monoclonal antibody, reactive with an unrelated platelet-specific membrane antigen, did not inhibit platelet responses to aggregated IgG. Anti-p40 did not affect platelet aggregation by thrombin, collagen, or fibrinogen plus ADP. Although anti-p40 did not directly aggregate platelets in the concentrations employed, cross-linking with F(ab')2 goat anti-murine Ig induced apyrase-sensitive aggregation of anti-p40-treated platelets. This indicates that p40 possesses transmembrane linkage for platelet activation and secretion. These observations strongly suggest that this newly recognized 40,000-mol-wt platelet membrane protein serves as an Fc gamma receptor.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. L., Abraham G. N. Characterization of the Fc receptor for IgG on a human macrophage cell line, U937. J Immunol. 1980 Dec;125(6):2735–2741. [PubMed] [Google Scholar]
- Anderson C. L. Isolation of the receptor for IgG from a human monocyte cell line (U937) and from human peripheral blood monocytes. J Exp Med. 1982 Dec 1;156(6):1794–1806. doi: 10.1084/jem.156.6.1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson C. L., Spence J. M., Edwards T. S., Nusbacher J. Characterization of a polyvalent antibody directed against the IgG Fc receptor of human mononuclear phagocytes. J Immunol. 1985 Jan;134(1):465–470. [PubMed] [Google Scholar]
- Breckenridge R. T., Rosenfeld S. I., Graff K. S., Leddy J. P. Hereditary C5 deficiency in man. III. Studies of hemostasis and platelet responses to zymosan. J Immunol. 1977 Jan;118(1):12–16. [PubMed] [Google Scholar]
- Cheng C. M., Hawiger J. Affinity isolation and characterization of immunoglobulin G Fc fragment-binding glycoprotein from human blood platelets. J Biol Chem. 1979 Apr 10;254(7):2165–2167. [PubMed] [Google Scholar]
- Ginsberg M. H., Henson P. M. Enhancement of platelet response to immune complexes and IgG aggregates by lipid A-rich bacterial lipopolysaccharides. J Exp Med. 1978 Jan 1;147(1):207–217. doi: 10.1084/jem.147.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenstein J. L., Solomon A., Abraham G. N. Monoclonal antibodies reactive with idiotypic and variable-region specific determinants on human immunoglobulins. Immunology. 1984 Jan;51(1):17–25. [PMC free article] [PubMed] [Google Scholar]
- Henson P. M., Spiegelberg H. L. Release of serotonin from human platelets induced by aggregated immunoglobulins of different classes and subclasses. J Clin Invest. 1973 May;52(5):1282–1288. doi: 10.1172/JCI107296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heusser C. H., Anderson C. L., Grey H. M. Receptors for IgG: subclass specificity of receptors on different mouse cell types and the definition of two distinct receptors on a macrophage cell line. J Exp Med. 1977 May 1;145(5):1316–1327. doi: 10.1084/jem.145.5.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isersky C., Taurog J. D., Poy G., Metzger H. Triggering of cultured neoplastic mast cells by antibodies to the receptor for IgE. J Immunol. 1978 Aug;121(2):549–558. [PubMed] [Google Scholar]
- Ishizaka T., Ishizaka K. Triggering of histamine release from rat mast cells by divalent antibodies against IgE-receptors. J Immunol. 1978 Mar;120(3):800–805. [PubMed] [Google Scholar]
- Israels E. D., Nisli G., Paraskevas F., Israels L. G. Platelet Fc receptor as a mechanism for Ag-Ab complex-induced platelet injury. Thromb Diath Haemorrh. 1973 May 10;29(2):434–444. [PubMed] [Google Scholar]
- Jones D. H., Looney R. J., Anderson C. L. Two distinct classes of IgG Fc receptors on a human monocyte line (U937) defined by differences in binding of murine IgG subclasses at low ionic strength. J Immunol. 1985 Nov;135(5):3348–3353. [PubMed] [Google Scholar]
- Kahn C. R., Baird K. L., Jarrett D. B., Flier J. S. Direct demonstration that receptor crosslinking or aggregation is important in insulin action. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4209–4213. doi: 10.1073/pnas.75.9.4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karas S. P., Rosse W. F., Kurlander R. J. Characterization of the IgG-Fc receptor on human platelets. Blood. 1982 Dec;60(6):1277–1282. [PubMed] [Google Scholar]
- Kulczycki A., Jr Role of immunoglobulin E and immunoglobulin E receptors in bronchial asthma. J Allergy Clin Immunol. 1981 Jul;68(1):5–14. doi: 10.1016/0091-6749(81)90116-0. [DOI] [PubMed] [Google Scholar]
- Kunicki T. J., Russell N., Nurden A. T., Aster R. H., Caen J. P. Further studies of the human platelet receptor for quinine- and quinine-dependent antibodies. J Immunol. 1981 Feb;126(2):398–402. [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Moore A., Ross G. D., Nachman R. L. Interaction of platelet membrane receptors with von Willebrand factor, ristocetin, and the Fc region of immunoglobulin G. J Clin Invest. 1978 Nov;62(5):1053–1060. doi: 10.1172/JCI109210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller-Eckhardt C., Lüscher E. F. Immune reactions of human blood platelets. I. A comparative study on the effects on platelets of heterologous antiplatet antiserum, antigen-antibody complexes, aggregated gammaglobulin and thrombin. Thromb Diath Haemorrh. 1968 Nov 15;20(1):155–167. [PubMed] [Google Scholar]
- Perez-Montfort R., Kinet J. P., Metzger H. A previously unrecognized subunit of the receptor for immunoglobulin E. Biochemistry. 1983 Dec 6;22(25):5722–5728. doi: 10.1021/bi00294a007. [DOI] [PubMed] [Google Scholar]
- Pfueller S. L., Lüscher E. F. The effects of aggregated immunoglobulins on human blood platelets in relation to their complement-fixing abilities. I. Studies of immunoglobulins of different types. J Immunol. 1972 Sep;109(3):517–525. [PubMed] [Google Scholar]
- Schechter Y., Hernaez L., Schlessinger J., Cuatrecasas P. Local aggregation of hormone-receptor complexes is required for activation by epidermal growth factor. Nature. 1979 Apr 26;278(5707):835–838. doi: 10.1038/278835a0. [DOI] [PubMed] [Google Scholar]
- Unkeless J. C., Fleit H., Mellman I. S. Structural Aspects and Heterogeneity of Immunoglobulin Fc Receptors. Adv Immunol. 1981;31:247–270. doi: 10.1016/s0065-2776(08)60922-0. [DOI] [PubMed] [Google Scholar]
- Young J. D., Unkeless J. C., Kaback H. R., Cohn Z. A. Macrophage membrane potential changes associated with gamma 2b/gamma 1 Fc receptor-ligand binding. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1357–1361. doi: 10.1073/pnas.80.5.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman T. S., Kolb W. P. Human platelet-initiated formation and uptake of the C5-9 complex of human complement. J Clin Invest. 1976 Jan;57(1):203–211. doi: 10.1172/JCI108261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman T. S., Spiegelberg H. L. Pneumococcus-induced serotonin release from human platelets. Identification of the participating plasma/serum factor as immunoglobulin. J Clin Invest. 1975 Oct;56(4):828–834. doi: 10.1172/JCI108161. [DOI] [PMC free article] [PubMed] [Google Scholar]