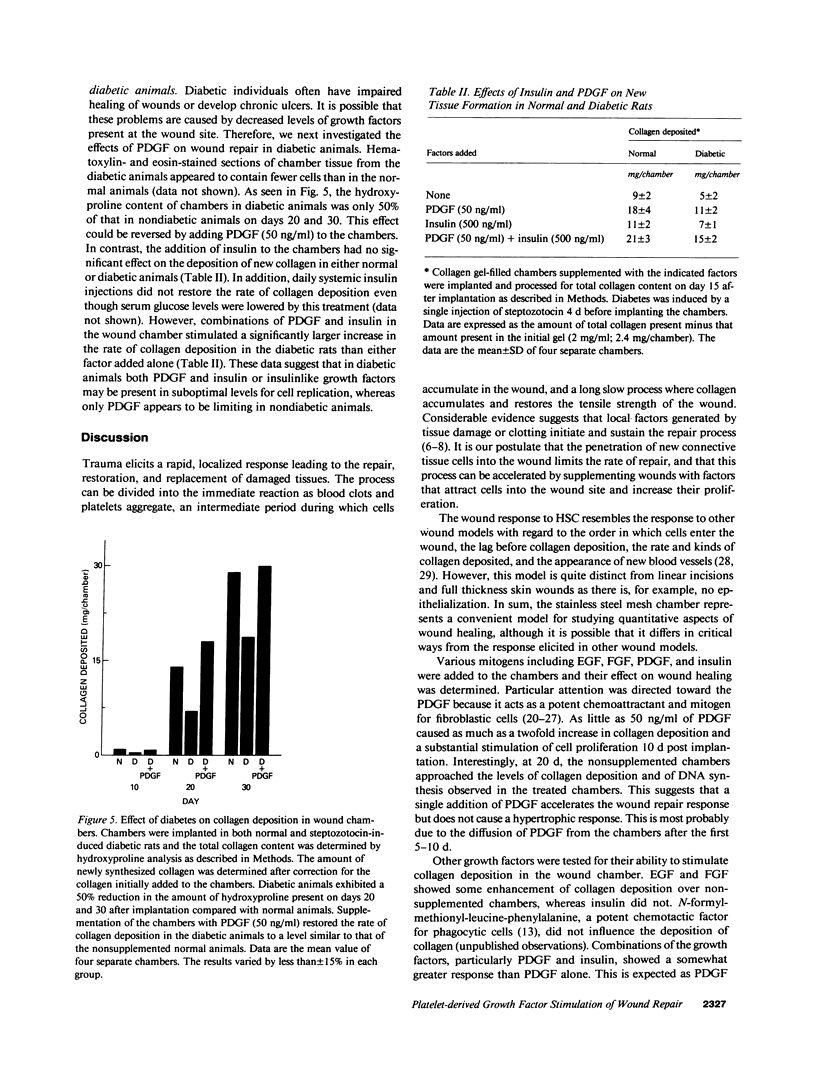
Abstract
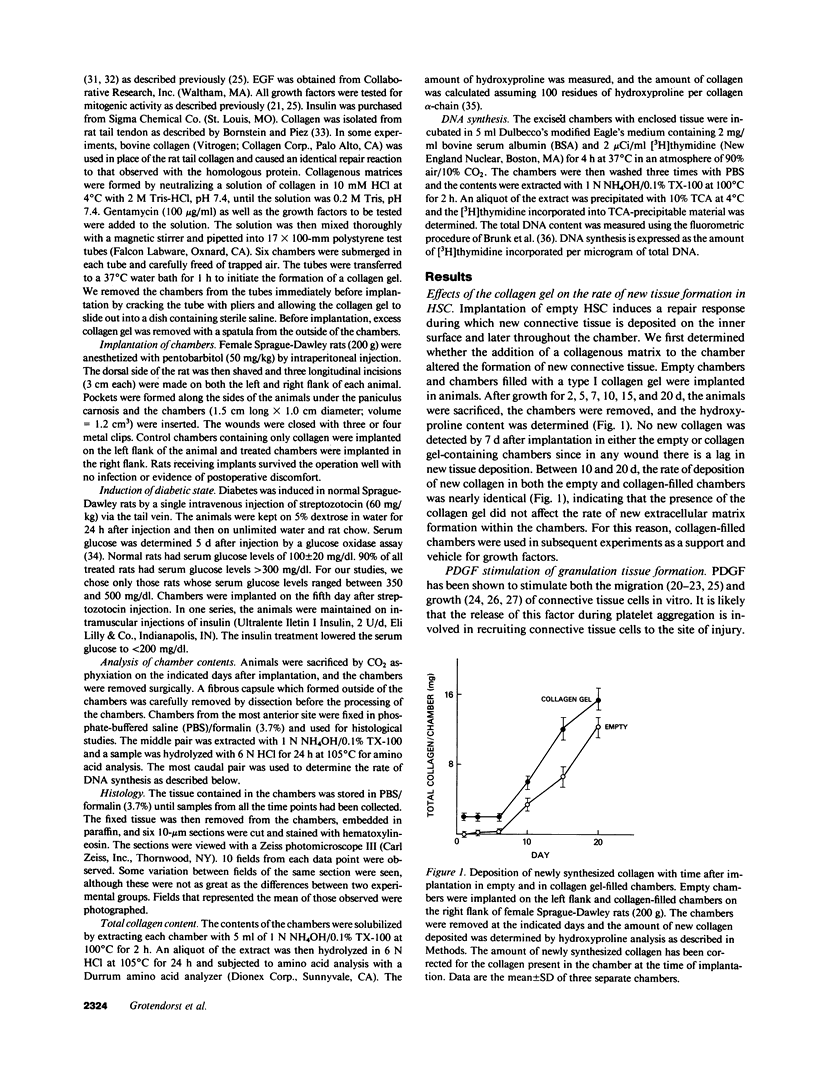
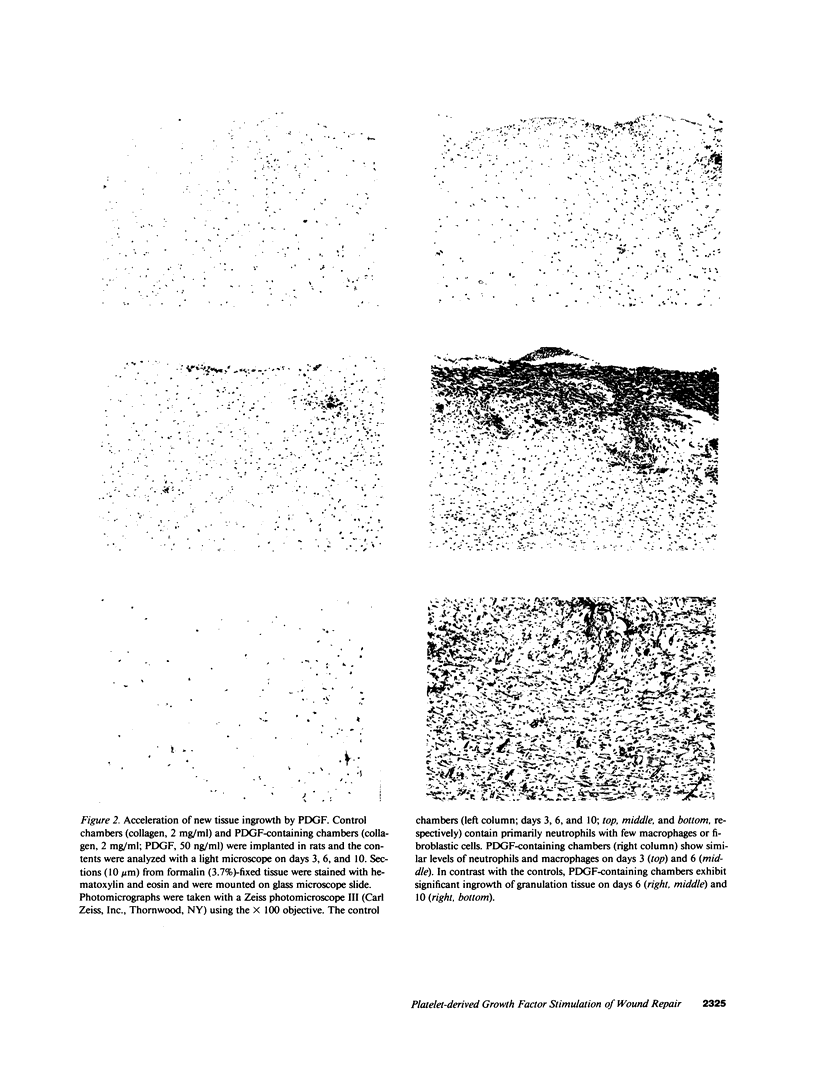
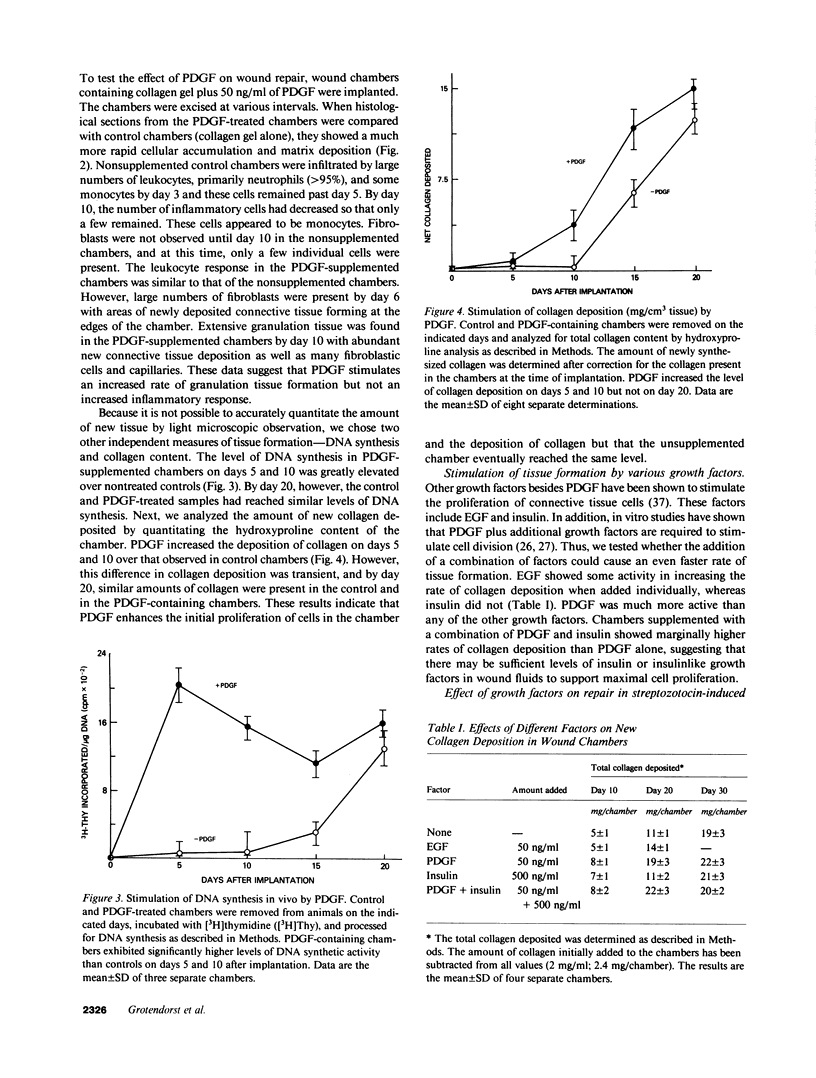
Subcutaneous implantation of Hunt-Schilling wound chambers in rats induces a wound repair response causing the chamber first to fill with fluid and subsequently with connective tissue. The presence of a type I collagen gel encouraged a more rapid dispersion of cells throughout the chamber but had no effect on the rate of new collagen deposition. Addition of platelet-derived growth factor (PDGF; 50 ng/chamber) to the collagen-filled chambers caused an earlier influx of connective tissue cells, a marked increase in DNA synthesis, and a greater collagen deposition in the chamber during the first 2 wk after implantation. After 3 wk, however, the levels of collagen were similar in PDGF-supplemented and control chambers. Diabetic animals exhibited a decreased rate of repair which was restored to normal by addition of PDGF to the wound chamber. Combinations of PDGF and insulin caused an even more rapid increase in collagen deposition. These results suggest that the levels of various growth factors, particularly PDGF, may be limiting at wound sites and that supplementation of wounds with these factors can accelerate the rate of new tissue formation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antoniades H. N., Scher C. D., Stiles C. D. Purification of human platelet-derived growth factor. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1809–1813. doi: 10.1073/pnas.76.4.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banda M. J., Knighton D. R., Hunt T. K., Werb Z. Isolation of a nonmitogenic angiogenesis factor from wound fluid. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7773–7777. doi: 10.1073/pnas.79.24.7773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein P., Piez K. A. The nature of the intramolecular cross-links in collagen. The separation and characterization of peptides from the cross-link region of rat skin collagen. Biochemistry. 1966 Nov;5(11):3460–3473. doi: 10.1021/bi00875a012. [DOI] [PubMed] [Google Scholar]
- Brunk C. F., Jones K. C., James T. W. Assay for nanogram quantities of DNA in cellular homogenates. Anal Biochem. 1979 Jan 15;92(2):497–500. doi: 10.1016/0003-2697(79)90690-0. [DOI] [PubMed] [Google Scholar]
- Deuel T. F., Huang J. S. Platelet-derived growth factor. Structure, function, and roles in normal and transformed cells. J Clin Invest. 1984 Sep;74(3):669–676. doi: 10.1172/JCI111482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuel T. F., Senior R. M., Chang D., Griffin G. L., Heinrikson R. L., Kaiser E. T. Platelet factor 4 is chemotactic for neutrophils and monocytes. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4584–4587. doi: 10.1073/pnas.78.7.4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuel T. F., Senior R. M., Huang J. S., Griffin G. L. Chemotaxis of monocytes and neutrophils to platelet-derived growth factor. J Clin Invest. 1982 Apr;69(4):1046–1049. doi: 10.1172/JCI110509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diegelmann R. F., Cohen I. K., Kaplan A. M. The role of macrophages in wound repair: a review. Plast Reconstr Surg. 1981 Jul;68(1):107–113. doi: 10.1097/00006534-198107000-00025. [DOI] [PubMed] [Google Scholar]
- Gauss-Müller V., Kleinman H. K., Martin G. R., Schiffmann E. Role of attachment factors and attractants in fibroblast chemotaxis. J Lab Clin Med. 1980 Dec;96(6):1071–1080. [PubMed] [Google Scholar]
- Grotendorst G. R. Alteration of the chemotactic response of NIH/3T3 cells to PDGF by growth factors, transformation, and tumor promoters. Cell. 1984 Feb;36(2):279–285. doi: 10.1016/0092-8674(84)90221-6. [DOI] [PubMed] [Google Scholar]
- Grotendorst G. R., Chang T., Seppä H. E., Kleinman H. K., Martin G. R. Platelet-derived growth factor is a chemoattractant for vascular smooth muscle cells. J Cell Physiol. 1982 Nov;113(2):261–266. doi: 10.1002/jcp.1041130213. [DOI] [PubMed] [Google Scholar]
- Grotendorst G. R., Seppä H. E., Kleinman H. K., Martin G. R. Attachment of smooth muscle cells to collagen and their migration toward platelet-derived growth factor. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3669–3672. doi: 10.1073/pnas.78.6.3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotendorst G. Can collagen metabolism be controlled? J Trauma. 1984 Sep;24(9 Suppl):S49–S54. [PubMed] [Google Scholar]
- Heldin C. H., Westermark B., Wasteson A. Platelet-derived growth factor. Isolation by a large-scale procedure and analysis of subunit composition. Biochem J. 1981 Mar 1;193(3):907–913. doi: 10.1042/bj1930907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes R. M., Hoopes J. E. Current concepts of wound healing. Clin Plast Surg. 1977 Apr;4(2):173–179. [PubMed] [Google Scholar]
- Hunt T. K., Twomey P., Zederfeldt B., Dunphy J. E. Respiratory gas tensions and pH in healing wounds. Am J Surg. 1967 Aug;114(2):302–307. doi: 10.1016/0002-9610(67)90388-1. [DOI] [PubMed] [Google Scholar]
- PIEZ K. A., WEISS E., LEWIS M. S. The separation and characterization of the alpha- and beta-components of calf skin collagen. J Biol Chem. 1960 Jul;235:1987–1991. [PubMed] [Google Scholar]
- Pledger W. J., Stiles C. D., Antoniades H. N., Scher C. D. Induction of DNA synthesis in BALB/c 3T3 cells by serum components: reevaluation of the commitment process. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4481–4485. doi: 10.1073/pnas.74.10.4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postlethwaite A. E., Keski-Oja J., Balian G., Kang A. H. Induction of fibroblast chemotaxis by fibronectin. Localization of the chemotactic region to a 140,000-molecular weight non-gelatin-binding fragment. J Exp Med. 1981 Feb 1;153(2):494–499. doi: 10.1084/jem.153.2.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postlethwaite A. E., Snyderman R., Kang A. H. The chemotactic attraction of human fibroblasts to a lymphocyte-derived factor. J Exp Med. 1976 Nov 2;144(5):1188–1203. doi: 10.1084/jem.144.5.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSS R., BENDITT E. P. Wound healing and collagen formation. I. Sequential changes in components of guinea pig skin wounds observed in the electron microscope. J Biophys Biochem Cytol. 1961 Dec;11:677–700. doi: 10.1083/jcb.11.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R. Wound healing. Sci Am. 1969 Jun;220(6):40–50. doi: 10.1038/scientificamerican0669-40. [DOI] [PubMed] [Google Scholar]
- SCHILLING J. A., JOEL W., SHURLEY H. M. Wound healing: a comparative study of the histochemical changes in granulation tissue contained in stainless steel wire mesh and polyvinyl sponge cylinders. Surgery. 1959 Oct;46:702–710. [PubMed] [Google Scholar]
- Schiffmann E., Corcoran B. A., Wahl S. M. N-formylmethionyl peptides as chemoattractants for leucocytes. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1059–1062. doi: 10.1073/pnas.72.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffmann E., Showell H. V., Corcoran B. A., Ward P. A., Smith E., Becker E. L. The isolation and partial characterization of neutrophil chemotactic factors from Escherichia coli. J Immunol. 1975 Jun;114(6):1831–1837. [PubMed] [Google Scholar]
- Senior R. M., Griffin G. L., Huang J. S., Walz D. A., Deuel T. F. Chemotactic activity of platelet alpha granule proteins for fibroblasts. J Cell Biol. 1983 Feb;96(2):382–385. doi: 10.1083/jcb.96.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seppä H., Grotendorst G., Seppä S., Schiffmann E., Martin G. R. Platelet-derived growth factor in chemotactic for fibroblasts. J Cell Biol. 1982 Feb;92(2):584–588. doi: 10.1083/jcb.92.2.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyderman R., Phillips J., Mergenhagen S. E. Polymorphonuclear leukocyte chemotactic activity in rabbit serum and Guinea pig serum treated with immune complexes: evidence for c5a as the major chemotactic factor. Infect Immun. 1970 Jun;1(6):521–525. doi: 10.1128/iai.1.6.521-525.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B., Shull J. H., Smith J. M., Ward J. M., Sodek J. Polypeptide transforming growth factors isolated from bovine sources and used for wound healing in vivo. Science. 1983 Mar 18;219(4590):1329–1331. doi: 10.1126/science.6572416. [DOI] [PubMed] [Google Scholar]
- Stiles C. D., Pledger W. J., Tucker R. W., Martin R. G., Scher C. D. Regulation of the Balb/c-3T3 cell cycle-effects of growth factors. J Supramol Struct. 1980;13(4):489–499. doi: 10.1002/jss.400130408. [DOI] [PubMed] [Google Scholar]
- Vogel A., Raines E., Kariya B., Rivest M. J., Ross R. Coordinate control of 3T3 cell proliferation by platelet-derived growth factor and plasma components. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2810–2814. doi: 10.1073/pnas.75.6.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward P. A., Lepow I. H., Newman L. J. Bacterial factors chemotactic for polymorphonuclear leukocytes. Am J Pathol. 1968 Apr;52(4):725–736. [PMC free article] [PubMed] [Google Scholar]
- Williams L. T., Antoniades H. N., Goetzl E. J. Platelet-derived growth factor stimulates mouse 3T3 cell mitogenesis and leukocyte chemotaxis through different structural determinants. J Clin Invest. 1983 Nov;72(5):1759–1763. doi: 10.1172/JCI111135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon J., Onodera T., Notkins A. L. Virus-induced diabetes mellitus: VIII. Passage of encephalomyocarditis virus and severity of diabetes in susceptible and resistant strains of mice. J Gen Virol. 1977 Nov;37(2):225–232. doi: 10.1099/0022-1317-37-2-225. [DOI] [PubMed] [Google Scholar]