Abstract
Calcium is an essential plant nutrient. It is required for various structural roles in the cell wall and membranes, it is a counter‐cation for inorganic and organic anions in the vacuole, and the cytosolic Ca2+ concentration ([Ca2+]cyt) is an obligate intracellular messenger coordinating responses to numerous developmental cues and environmental challenges. This article provides an overview of the nutritional requirements of different plants for Ca, and how this impacts on natural flora and the Ca content of crops. It also reviews recent work on (a) the mechanisms of Ca2+ transport across cellular membranes, (b) understanding the origins and specificity of [Ca2+]cyt signals and (c) characterizing the cellular [Ca2+]cyt‐sensors (such as calmodulin, calcineurin B‐like proteins and calcium‐dependent protein kinases) that allow plant cells to respond appropriately to [Ca2+]cyt signals.
Key words: Arabidopsis, ATPase, calcium (Ca2+), channel, cytosolic Ca2+, ecology, H+/Ca2+‐antiport (CAX), kinase, phylogeny, plasma membrane, root, vacuole
INTRODUCTION
Calcium is an essential plant nutrient. As the divalent cation (Ca2+), it is required for structural roles in the cell wall and membranes, as a counter‐cation for inorganic and organic anions in the vacuole, and as an intracellular messenger in the cytosol (Marschner, 1995). Calcium deficiency is rare in nature, but excessive Ca restricts plant communities on calcareous soils. Calcium is taken up by roots from the soil solution and delivered to the shoot via the xylem. It may traverse the root either through the cytoplasm of cells linked by plasmodesmata (the symplast) or through the spaces between cells (the apoplast). The relative contributions of the apoplastic and symplastic pathways to the delivery of Ca to the xylem are unknown (White, 2001). However, the movement of Ca through these pathways must be finely balanced to allow root cells to signal using cytosolic Ca2+ concentration ([Ca2+]cyt), control the rate of Ca delivery to the xylem, and prevent the accumulation of toxic cations in the shoot.
Calcium enters plant cells through Ca2+‐permeable ion channels in their plasma membranes (White, 2000). Since a high [Ca2+]cyt is cytotoxic, a submicromolar [Ca2+]cyt is maintained in unstimulated cells by Ca2+‐ATPases and H+/Ca2+‐antiporters (Sze et al., 2000; Hirschi, 2001). These enzymes remove cytosolic Ca2+ to either the apoplast or the lumen of intracellular organelles, such as the vacuole or endoplasmic reticulum (ER). The rapid influx of Ca2+ through cation channels in the plasma membrane, tonoplast and/or ER generates [Ca2+]cyt perturbations that initiate cellular responses to a diverse range of developmental cues and environmental challenges (White, 2000; Sanders et al., 2002). Proteins that change conformation or catalytic activity upon binding Ca2+, such as calmodulin (CaM), calcineurin B‐like proteins (CBLs) and Ca2+‐dependent protein kinases (CDPKs), allow the cellular perception and transduction of the [Ca2+]cyt signal. These proteins are termed ‘[Ca2+]cyt sensors’. It is speculated that cellular responses to specific biotic and abiotic stimuli are encoded by distinct [Ca2+]cyt perturbations and are transduced by particular [Ca2+]cyt sensors. Much current work on Ca in plants is dedicated to understanding the nature and specificity of [Ca2+]cyt signalling and response networks.
This article provides an overview of recent work on Ca in plants. First, it discusses the Ca requirements of different plant species, the mechanisms of Ca uptake and delivery to the xylem, and the impact of these on natural flora and the Ca content of crops. It then highlights the insights made possible by recent advances in electrophysiology, microscopy and plant molecular biology that have enabled researchers to characterize Ca2+ transporters in cellular membranes, begin to unravel the origins and specificity of [Ca2+]cyt signals, and identify the [Ca2+]cyt‐sensors that allow plant cells to respond to [Ca2+]cyt perturbations.
NUTRITION
The calcium requirements of plants
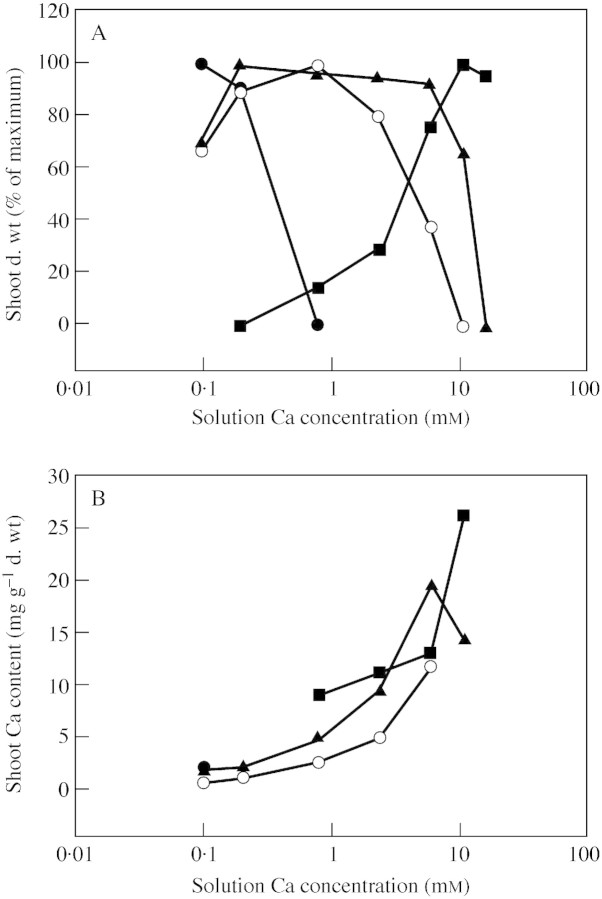
Plants growing with adequate Ca in their natural habitats have shoot Ca concentrations between 0·1 and 5 % d. wt (Marschner, 1995). These values reflect both Ca availability in the environment and the contrasting Ca requirements of different plant species. Calcium deficiency is rare in nature, but may occur on soils with low base saturation and/or high levels of acidic deposition (McLaughlin and Wimmer, 1999). By contrast, several costly Ca‐deficiency disorders occur in horticulture (Fig. 1; Shear, 1975). These generally arise when sufficient Ca is momentarily unavailable to developing tissues. Deficiency symptoms are observed (a) in young expanding leaves, such as in ‘tipburn’ of leafy vegetables, (b) in enclosed tissues, such as in ‘brown heart’ of leafy vegetables or ‘black heart’ of celery, or (c) in tissues fed principally by the phloem rather than the xylem, such as in ‘blossom end rot’ of watermelon, pepper and tomato fruit, ‘bitter pit’ of apples and ‘empty pod’ in peanut. They occur because Ca cannot be mobilized from older tissues and redistributed via the phloem. This forces the developing tissues to rely on the immediate supply of Ca in the xylem, which is dependent on transpiration. Transpiration is low in young leaves, in enclosed tissues and in fruit. Other physiological disorders, such as ‘cracking’ in tomato, cherry and apple fruit, occur in tissues lacking sufficient Ca upon hypo‐osmotic shock (following increased humidity or rainfall), presumably as a result of structural weaknesses in cell walls. When excessive Ca is present in the rhizosphere solution, plants may suffer Ca toxicity. This may prevent the germination of seeds and reduce plant growth rates (Fig. 2). In cultivated tomato, one symptom of excess calcium is the development of tiny yellowish flecks or ‘gold spot’ in the cell walls around the calyx and shoulders of the fruit (Fig. 1). These flecks are crystals of calcium oxalate and their abundance is increased by high humidity and high Ca fertilization (Bekreij et al., 1992).
Fig. 1. Calcium disorders in horticultural crops: (a) cracking in tomato fruit; (b) tipburn in lettuce; (c) calcium deficiency in celery; (d) blossom end rot in immature tomato fruit; (e) bitter pit in apples; (f) gold spot in tomato fruit with calcium oxalate crystals (inset). Photographs (A–E) are from the HRI collection and (F) is courtesy of Lim Ho (HRI‐Wellesbourne).
Fig. 2. The relationships between Ca concentration in the soil solution ([Ca2+]ext) and (A) shoot dry weight and (B) shoot Ca content of two calcifuge species Juncus squarrosus (filled circles) and Nardus stricta (filled triangles), the mineral‐tolerant Siegelingia decumbens (open circles), and the calcicole species Origanum vulgare (filled squares). Plants were grown for approx. 4 weeks after germination in a quartz sand to which was added a complete mineral solution containing various concentrations of CaCl2. Data are taken from Jefferies and Willis (1964), who observed (a) that Juncus squarrosus did not establish at [Ca2+]ext ≥ 0·8 mm and (b) that Origanum vulgare showed symptoms of Ca deficiency and was unable to survive at [Ca2+]ext less than about 0·8 mm.
Ecologists have classified plant species into calcifuges, which occur on acid soils with low Ca, and calcicoles, which occur on calcareous soils. The Ca concentrations in calcifuge and calcicole plants growing in their natural habitats differ markedly. However, it is the ability to tolerate excessive Al, Mn and Fe that largely determines the flora of acid soils, and an insensitivity to Fe‐ and P‐deficiencies that determines the flora of calcareous soils (Lee, 1999). Nevertheless, calcifuges generally grow well at low Ca2+ concentrations in the rhizosphere ([Ca2+]ext) and respond little to increased [Ca2+]ext, which may even inhibit growth (Fig. 2). Conversely, the mechanisms that enable calcicole plants to maintain low [Ca2+]cyt in their natural habitat are believed to restrict their growth at low [Ca2+]ext by inducing Ca‐deficiency (Fig. 2; Lee, 1999). This is consistent with the phenotype of plants overexpressing Ca2+‐transporters that remove Ca2+ from the cytoplasm to the vacuole which show Ca‐deficiency symptoms at low [Ca2+]ext (Hirschi, 2001). Hence, the optimal [Ca2+]ext for a plant in hydroponics often approximates the [Ca2+]ext of its natural habitat.
Distinct relationships between shoot Ca concentration ([Ca]shoot) and [Ca2+]ext have been attributed to contrasting Ca ‘physiotypes’ (Kinzel and Lechner, 1992). The ecology of different Ca physiotypes is thought to reflect their ability to utilize Ca as an osmoticum in primarily xerophytic, calcareous environments. One characteristic of calcicole plants, such as the Crassulaceae, Brassicaceae and Fabaceae, is a high soluble Ca concentration. In these plants, which are also termed ‘calciotrophs’ (Kinzel, 1982), Ca accumulation is stimulated greatly by increasing [Ca2+]ext. By contrast, calcifuges generally have a low soluble Ca concentration. They include the ‘potassium plants’, such as the Apiales and Asterales, which are characterized by high shoot K/Ca quotients, and the ‘oxalate plants’, which have high tissue oxalate concentrations. Plants that accumulate oxalate can be subdivided into species that contain soluble oxalate and those in which Ca‐oxalate is precipitated. Interestingly, the uptake of Ca does not appear to increase with increasing [Ca2+]ext in plants containing soluble oxalate, such as the Oxalidaceae (Kinzel and Lechner, 1992). In plants that precipitate Ca‐oxalate such as the Caryophyllales families Caryophyllaceae, Chenopodiaceae and Polygonaceae, there is a proportional increase in both Ca and oxalate concentration with increasing [Ca2+]ext (Libert and Franceschi, 1987; Kinzel and Lechner, 1992).
Calcium uptake and movement to the shoot
Calcium is acquired from the soil solution by the root system and translocated to the shoot via the xylem. The Ca flux to the xylem is high, and a rate of 40 nmol Ca h–1 g–1 f. wt root is not unreasonable in an actively growing plant (White, 1998). The delivery of Ca to the xylem is restricted to the extreme root tip and to regions in which lateral roots are being initiated (Clarkson, 1993; White, 2001). In these regions a contiguous, Casparian band between endodermal cells is absent or disrupted, and/or the endodermal cells surrounding the stele are unsuberized. The Casparian band restricts the apoplastic movement of solutes (Clarkson, 1984, 1993; White, 2001) and suberization prevents Ca2+ influx to endodermal cells (Moore et al., 2002). These observations suggest that Ca might reach the xylem solely via the apoplast in regions where the Casparian band is absent or disrupted, or circumvent the Casparian band by entering the cytoplasm of unsuberized endodermal cells when the Casparian band is present (Clarkson, 1984, 1993; White 2001). These are referred to as the apoplastic and symplastic pathways, respectively.
Each pathway of Ca movement across the root confers distinct advantages and disadvantages. The apoplastic pathway allows Ca to be delivered to the xylem without impacting on the use of [Ca2+]cyt for intracellular signalling (White, 1998). Intracellular signalling requires [Ca2+]cyt to be maintained at submicromolar levels in the resting cell and to increase rapidly in response to developmental cues or environmental challenges. Since the Ca2+ fluxes required for [Ca2+]cyt signalling are minute compared with those required for adequate nutrition, both these requirements for [Ca2+]cyt signalling might be compromised by high nutritional Ca2+ fluxes through root cells (White, 2001). However, the Ca flux to the xylem through the apoplastic pathway is influenced markedly by transpiration, which could lead to vagaries in the amount of Ca supplied to the shoot and the development of Ca disorders (Marschner, 1995; McLaughlin and Wimmer, 1999). Furthermore, the apoplastic pathway is relatively non‐selective between divalent cations (White, 2001; White et al., 2002b), and its presence could result in the accumulation of toxic solutes in the shoot. By contrast, the symplastic pathway allows the plant to control the rate and selectivity of Ca transport to the shoot (Clarkson, 1993; White, 2001). It is thought that Ca2+ enters the cytoplasm of endodermal cells through Ca2+‐permeable channels on the cortical side of the Casparian band, and that Ca2+ is pumped from the symplast by the plasma membrane Ca2+‐ATPases or Ca2+/H+‐antiporters of cells within the stele. By regulating the expression and activity of these transporters, Ca could be delivered selectively to the xylem at a rate consistent with the requirements of the shoot.
Several lines of evidence suggest that both apoplastic and symplastic pathways contribute to Ca delivery to the xylem. First, since Ca is delivered to the xylem in regions of the root where the Casparian band is fully developed and apoplastic Ca transport is restricted, some Ca might bypass the Casparian band through the cytoplasm of endodermal cells (Clarkson, 1984, 1993; White, 2001). Secondly, although Ca2+ channels and Ca2+‐ATPases are present and thermodynamically capable of catalysing Ca2+ influx and efflux across the plasma membrane of root endodermal cells, it has been calculated that there is insufficient ATP to power (Flowers and Yeo, 1992) and insufficient proteins to catalyse (White, 1998, 2001) the observed Ca2+ fluxes solely through the symplast. Thirdly, if Ca reached the xylem solely by a symplastic pathway, its accumulation in the shoot would be expected to show the hallmarks of protein‐catalysed transport, which it does not. For example, both Ca2+ channels in the plasma membrane of root cells and Ca2+‐ATPases discriminate between divalent cations, but there seems to be no discrimination between Ca2+, Ba2+ and Sr2+ in their transport to the shoot (White, 2001). Furthermore, there seems to be no competition, or interactions, between Ca2+, Ba2+ and Sr2+ during their transport to the shoot, and the accumulation of divalent cations in the shoot is often linearly related to their concentrations in the rhizosphere solution (White, 2001). Although the relative contributions of the apoplastic and symplastic pathways to the delivery of Ca to the xylem are unknown, it is likely that a functional separation of apoplastic Ca2+ fluxes (for transfer to the shoot) and symplastic Ca2+ fluxes (for cell signalling) would enable the root to fulfil the demand of the shoot for Ca without compromising intracellular [Ca2+]cyt signals.
The Ca concentration in xylem sap ([Ca]xylem) is influenced greatly by [Ca2+]ext, and [Ca]xylem between 300 µm and 16·5 mm has been reported (White et al., 1992; De Silva et al., 1998). The relative proportion of Ca2+ to total Ca also varies, with organic acids, such as malate and citrate, chelating Ca2+ in the xylem sap (Marschner, 1995). When abundant Ca is present in the xylem sap, there is a close relationship between Ca distribution to the shoot and transpiration. Within the leaf, Ca follows the apoplastic route of the transpiration stream and accumulates in either the mesophyll cells, trichomes or epidermal cells adjacent to guard cells, depending on the plant species (Karley et al., 2000). Both the [Ca2+]cyt in guard cells and the closing of stomata in detached epidermal strips are sensitive to apoplastic Ca2+ concentrations within the range of [Ca2+]xylem (McAinsh et al., 1995) and the ability of some calcicole species, such as Leontodon hispidus and Centaurea scabiosa, to tolerate high [Ca2+]ext may be related to their ability to accumulate Ca in their trichomes (De Silva et al., 1996, 1998).
The phylogeny of shoot calcium concentration
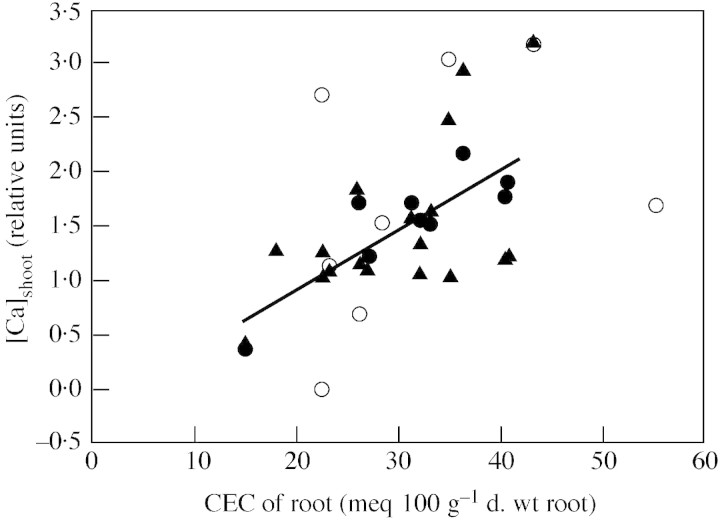
When grown under identical conditions, the [Ca]shoot of different plant species differs markedly (Figs 2 and 3 and Table 1; Broadley et al., 2003a). A large proportion of this variation can be attributed to the phylogenetic division between eudicots and monocots (Thompson et al., 1997; Broadley et al., 2003a). Eudicots generally have a higher [Ca]shoot than monocots. Within the eudicots, orders within both the rosid (Cucurbitales, Rosales, Malvales and Brassicales) and asterid (Apiales, Asterales, Lamiales and Solanales) clades have the highest [Ca]shoot. Within the monocots, [Ca]shoot is higher in the non‐commelinoid orders (e.g. Asparagales) than in the commelinoid orders (e.g. Poales). Phylogenetic differences in [Ca]shoot have not been resolved at lower taxonomic levels, but it is noteworthy that distinct Ca physiotypes (‘calciotrophes’, ‘potassium plants’ and ‘oxalate plants’) occur in particular plant families (Kinzel, 1982) and that traits such as the prevalence, shape and tissue distributions of Ca oxalate crystals, are used as taxonomic characters (e.g. Franceschi and Horner, 1980; Kuo‐Huang et al., 1994; Wu and Kuo‐Huang, 1997; Prychid and Rudall, 1999; Caddick et al., 2002).
Fig. 3. The relationships between root CEC, derived from a literature survey, and shoot calcium concentration, derived from both literature and experimental data, for angiosperm orders (Table 1). Circles represent shoot Ca content data from a literature survey. Filled circles and linear regression represent orders with n ≥ 3 species sampled. Triangles represent shoot Ca content estimated in a phylogenetically balanced experiment.
Table 1.
The mean relative shoot calcium concentration of angiosperm orders derived from both a literature survey and a phylogenetically balanced experiment, and their mean relative root cation exchange capacity (CEC)
| Literature | Literature | Experiment | ||||
| Order | Root CEC (meq 100 g–1 d. wt root) | No. spp. | Relative shoot [Ca] | No. spp. | Relative shoot [Ca] | No. spp. |
| Apiales | 40·67 | 3 | 1·90 | 5 | 1·21 | 4 |
| Aquifoliales | 27·28 | 1 | – | – | – | – |
| Asparagales | 27·08 | 6 | 1·22 | 4 | 1·10 | 13 |
| Asterales | 40·34 | 12 | 1·78 | 11 | 1·19 | 15 |
| Brassicales | 36·39 | 7 | 2·19 | 15 | 2·96 | 3 |
| Caryophyllales | 32·12 | 12 | 1·57 | 17 | 1·07 | 7 |
| Cucurbitales | 43·35 | 4 | 3·19 | 2 | 3·21 | 1 |
| Dipsacales | 35·01 | 1 | – | – | 1·04 | 2 |
| Ericales | 22·54 | 2 | –0·01 | 1 | 1·04 | 1 |
| Fabales | 33·03 | 23 | 1·53 | 78 | 1·66 | 9 |
| Gentianales | 32·21 | 1 | – | – | 1·36 | 4 |
| Geraniales | 28·41 | 4 | 1·53 | 2 | – | – |
| Lamiales | 25·96 | 2 | 1·72 | 5 | 1·84 | 7 |
| Laurales | –2·07 | 1 | – | – | – | – |
| Liliales | 24·06 | 2 | – | – | – | – |
| Malpighiales | 23·18 | 1 | 1·12 | 2 | 1·07 | 5 |
| Malvales | 34·91 | 1 | 3·04 | 2 | 2·50 | 3 |
| Myrtales | 26·21 | 1 | 0·70 | 1 | 1·13 | 6 |
| Poales | 14·94 | 50 | 0·37 | 48 | 0·43 | 18 |
| Ranunculales | 55·42 | 4 | 1·69 | 2 | – | – |
| Rosales | 22·54 | 4 | 2·73 | 1 | 1·25 | 3 |
| Sapidales | 17·98 | 6 | – | – | 1·29 | 4 |
| Solanales | 31·22 | 4 | 1·73 | 8 | 1·60 | 4 |
| Vitaceae | 15·67 | 2 | – | – | – | – |
Data for shoot Ca concentration were taken from Broadley et al. (2003a) and for root CEC from the comparative studies cited by Asher and Ozanne (1961), Heintz (1961), Crooke and Knight (1962), Fréjat et al. (1967), Snaydon and Bradshaw (1969) or Wacquant (1977).
All literature data sets were subjected to a residual maximum likelihood (REML) analysis, using a procedure described previously (Broadley et al., 2001). This procedure adjusted for differences in between‐study means to generate mean relative shoot Ca concentration (206 plant species in 18 orders from 244 studies) and mean relative root CEC (154 plant species from 93 studies).
Data are expressed as order means from n species sampled.
Although the Ca physiotype of a plant determines its ability to accumulate Ca in the shoot (Kinzel and Lechner, 1992; Broadley et al., 2003a), genotypic differences in the activities of Ca2+ transporters in root cell membranes (Hirschi, 2001; White et al., 2002a) and/or in the relative contributions of symplastic and apoplastic pathways to the delivery of Ca to the xylem (White, 2001) will contribute much to phylogenetic variation in [Ca]shoot. Historically, [Ca]shoot has been correlated with the cation exchange capacity (CEC) of plant roots (Fig. 3). The CEC is located in the root apoplast, and is attributed to the free carboxyl groups of galacturonic acids of cell wall pectins in the middle lamella (Haynes, 1980; Sattelmacher, 2001). If the pectin contents of shoot and root cell walls are similar, it is unsurprising that the phylogenetic variation in CEC in monocot roots parallels that in pectin content of shoot cell walls (Jarvis et al., 1988). The pectin contents of shoot cell walls are low in the Cyperaceae, Poaceae, Juncaceae and Restionaceae families (all assigned to the Poales) and intermediate in the commelinoid monocot families Commelinaceae (Commelinales), Bromeliaceae (unassigned to order) and Typhaceae (Poales) and in the non‐commelinoid Pandanaceae (Pandanales) family. The pectin content of shoot cell walls is similar to that of dicots in the commelinoid monocot families Musaceae (Zingiberales) and Sparganiaceae (Poales) and in the non‐commelinoid monocot families Velloziaceae (Pandanales), Alliaceae Amaryllidaceae, Asphodelaceae, Iridaceae, Orchidaceae (all Asparagales), Alismataceae, Araceae, Juncaginaceae, Potamogetonaceae, Zosteraceae (all Alismatales) and Liliaceae (Liliales).
In regions of the root where the Ca flux to the xylem is apoplastic, CEC may exert a direct effect on the transport of Ca2+ to the shoot. However, several reviews have asserted that there is no direct connection between the capacity of roots to bind cations in the free space and their active (energy‐dependent) accumulation (e.g. Haynes, 1980). Nevertheless, fixed negative charges, and charge screening, can influence both the absolute and relative concentrations of cations in the apoplast, especially at low ionic activities. Thus, root CEC could determine shoot cation content indirectly by affecting the rate and selectivity of cation uptake into the symplast in addition to cation transport through the apoplast (Asher and Ozanne, 1961; Wacquant, 1977). The effects of CEC on cation transport may have ecological implications. It has been suggested that, in solutions of low ionic strength, plants with higher root CEC compete for divalent cations more effectively, whereas plants with lower root CEC compete for monovalent cations more effectively (Smith and Wallace, 1956; Asher and Ozanne, 1961).
Quantifying the phylogenetic impact on [Ca]shoot has several uses. First, since [Ca]shoot correlates with ecological traits (Kinzel, 1982; Thompson et al., 1997; Grime, 2001), predictions of the responses of plant communities to environmental change can be improved using this information. Secondly, knowledge of phylogenetic variation in [Ca]shoot can be used to predict the movement of Ca (and chemically similar elements such as Sr, Mg and Ba; White, 2001; Broadley et al., 2003b) from soils to the shoots of plant species whose transfer coefficients are unknown and, thereby, improve nutrient and contaminant cycling models (Broadley et al., 2001). Thirdly, appreciation of phylogenetic influences on [Ca]shoot can improve the delivery of Ca to the human diet. Unsurprisingly, Ca‐deficiency disorders have been observed in populations whose dietary habits have changed from bean‐rich to rice‐rich sources of food (Graham et al., 2001). Phylogenetic information can help identify crops that are either predisposed to higher [Ca]shoot or offer genetic potential for breeding.
CALCIUM TRANSPORTERS IN CELLULAR MEMBRANES
Ca2+ efflux from the cytosol: Ca2+‐ATPases and H+/Ca2+‐antiporters
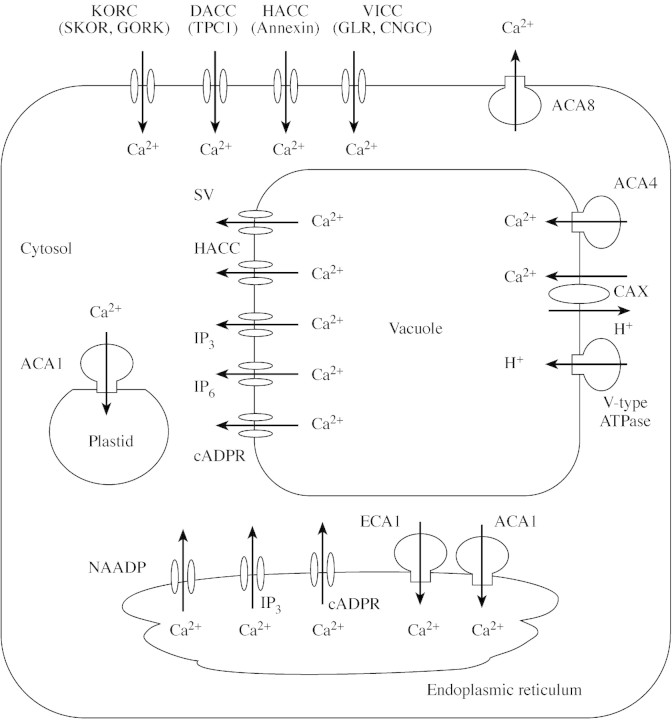
The removal of Ca2+ from the cytosol against its electrochemical gradient to either the apoplast or to intracellular organelles requires energized, ‘active’ transport. This is catalysed by Ca2+‐ATPases and H+/Ca2+‐antiporters (Fig. 4). By removing Ca2+ from the cytosol these enzymes perform several important functions (Sze et al., 2000; Hirschi, 2001): (1) they maintain a low [Ca2+]cyt in the resting (unstimulated) cell appropriate for cytoplasmic metabolism; (2) they restore [Ca2+]cyt to resting levels following a [Ca2+]cyt perturbation, thereby influencing the magnitude, kinetics and subcellular location of [Ca2+]cyt signals; (3) they replenish intracellular and extracellular Ca2+ stores for subsequent [Ca2+]cyt signals and permit the generation of local [Ca2+]cyt oscillations through their interplay with Ca2+ channels (Klüsener et al., 1995; Harper, 2001); (4) they provide Ca2+ in the ER for the secretory system to function (Blatt, 2000b; Ritchie et al., 2002); (5) they remove divalent cations, such as Mg2+, Mn2+, Ni2+ or Zn2+, from the cytosol, to support the specialized biochemistry of particular organelles and to prevent mineral toxicities (Hirschi, 2001; Wu et al., 2002). The relative importance of Ca2+‐ATPases and H+/Ca2+‐antiporters in each of these functions is unknown. Hirschi (2001) suggested that the Ca2+‐ATPases, which have high affinity (Km = 1–10 µm; Evans and Williams, 1998) but low capacity for Ca2+ transport, are responsible for maintaining [Ca2+]cyt homeostasis in the resting cell, whereas the H+/Ca2+‐antiporters, which have lower affinities (Km = 10–15 µm) but high capacities for Ca2+ transport, are likely to remove Ca2+ from the cytosol during [Ca2+]cyt signals and thereby modulate [Ca2+]cyt perturbations. Consistent with this hypothesis is the observation that an H+/Ca2+‐antiporter, but not the vacuolar Ca2+‐ATPase, resets [Ca2+]cyt in yeast following hypertonic shock (Denis and Cyert, 2002). However, the Arabidopsis de‐etiolated 3 (det3) mutant, which has reduced tonoplast H+‐ATPase and (presumably) H+/Ca2+‐antiporter activity, has a constitutively high [Ca2+]cyt (Allen et al., 2000), suggesting that H+/Ca2+‐antiporters contribute to [Ca2+]cyt homeostasis, and plants poisoned by the V‐type H+‐ATPase inhibitor bafilomycin show greater [Ca2+]cyt elevations in response to hypo‐osmotic shock (Takahashi et al., 1997).
Fig. 4. Cartoon illustrating the subcellular location of Ca2+ transporters in Arabidopsis thaliana based on Sze et al. (2000), Sanders et al. (2002) and White et al. (2002). In the plasma membrane there are hyperpolarization‐activated Ca2+ channels (HACC, possibly encoded by annexin genes), depolarization‐activated Ca2+ channels (DACC, one of which may be encoded by TPC1), Ca2+‐permeable outward rectifying K+ channels (KORC, encoded by SKOR and GORK), voltage‐insensitive cation channels (VICC, probably encoded by the CNGC and GLR genes) and Ca2+‐ATPases (one of which is ACA8). Biochemical and electrophysiological evidence indicates that IP3‐receptors, IP6‐receptors, cADPR‐activated (ryanodine)‐receptors and two types of voltage‐gated Ca2+ channels (the depolarization‐activated SV channels and the hyperpolarization‐activated Ca2+ channels) are present in the tonoplast together with Ca2+‐ATPases (including ACA4) and H+/Ca2+‐antiporters encoded by the CAX genes. There is also biochemical and electrophysiological evidence for the presence of NAADP‐receptors, IP3‐receptors, cADPR‐activated (ryanodine)‐receptors and depolarization‐activated Ca2+ channels in the endoplasmic reticulum (ER), together with Ca2+‐ATPases (including ECA1 and ACA2). The Ca2+‐ATPase ACA1 is located in the plastid inner membrane.
Plant Ca2+ ATPases belong to one of two major families (Evans and Williams, 1998; Geisler et al., 2000; Sze et al., 2000; Axelsen and Palmgren, 2001; Garciadeblas et al., 2001). The first family (the P‐type ATPase IIA family) lacks an N‐terminal autoregulatory domain. Four members of this family have been identified in the arabidopsis genome (termed AtECAs 1 to 4 by Axelsen and Palmgren, 2001). They are likely to be present in the plasma membrane, tonoplast and the ER/Golgi apparatus. In tomato, two transcripts of a type IIA Ca2+‐ATPase (LeLCA1) were observed in phosphate‐starved roots that correlated with two distinct protein isoforms (120 and 116 kDa) located in the plasma membrane and tonoplast of root cells, respectively (Navarro‐Avino et al., 1999). The second family of plant Ca2+ ATPases (the P‐type ATPase IIB family) is characterized by an autoinhibitory N‐terminal domain that contains a binding site for Ca‐CaM plus a serine‐residue phosphorylation site. Their catalytic activity can be modulated by [Ca2+]cyt either through activation upon binding CaM or by inhibition following phosphorylation by Ca2+‐dependent protein kinases (CDPK; Hwang et al., 2000). Since CaM binding‐sites are generally quite diverse, each type‐IIB Ca2+‐ATPase may have a different affinity for CaM or may bind a different CaM isoform. Ten members of the type‐IIB Ca2+‐ATPase family have been identified in the arabidopsis genome (termed AtACAs 1, 2 and 4 and AtACAs 7 to 13 by Axelsen and Palmgren, 2001). These reside on various cellular membranes including the plasma membrane (AtACA8), the tonoplast (AtACA4), ER (AtACA2) and the plastid inner membrane (AtACA1). Several explanations for the abundance of Ca2+‐ATPase isoforms, and also the presence of several isoforms on the same cellular membrane (such as AtECA1 and AtACA2 in the root ER) have been proposed (Geisler et al., 2000; Sze et al., 2000; Axelsen and Palmgren, 2001). Such explanations typically suggest that individual isoforms are functionally distinct and specialized to specific cellular processes requiring distinct spatial or temporal expression. They also imply a requirement for CaM‐independent and CaM‐dependent regulation of Ca2+‐ATPase activities in the modulation of [Ca2+]cyt perturbations during cell signalling. Multiple Ca2+‐ATPase genes are required to effect this because alternative splicing events are rare in plants. Intriguingly, the expression of many Ca2+‐ATPases is increased upon exposure to high salinity or high [Ca2+]ext, and some Ca2+‐ATPase genes are expressed only under stress conditions (Geisler et al., 2000; Garciadeblas et al., 2001). This may reflect a role in maintaining [Ca2+]cyt homeostasis or in reducing Na+ influx to the cytosol in saline environments.
The H+/Ca2+‐antiporters present in the plasma membrane and tonoplast have been characterized biochemically (Evans and Williams, 1998; Sanders et al., 2002). These have a lower affinity for Ca2+ than the Ca2+‐ATPases, and may also transport Mg2+. The stoichiometry of the dominant H+/Ca2+‐antiporter in the tonoplast is apparently 3H+/1Ca2+ (Blackford et al., 1990). Eleven genes encoding putative H+/Ca2+‐antiporters (AtCAX) have been identified in the genome of Arabidopsis thaliana (Hirschi, 2001; Mäser et al., 2001). The transporters AtCAX1, AtCAX2 and AtCAX4 are located at the tonoplast (Hirschi, 2001; N. Cheng et al., 2002, 2003). The AtCAX1 antiporter exhibits both a high affinity and high specificity for Ca2+. By contrast, the AtCAX2 transporter is a high‐affinity, high capacity H+/heavy metal cation antiporter. The cation specificity of other AtCAXs is unknown, but may reside in a specific stretch of nine amino acids termed the ‘Ca2+ domain’ (Shigaki et al., 2002). The increased transport capacity of truncated versions of AtCAX1, AtCAX3 and AtCAX4, and their relative inhibition by synthetic peptides corresponding to these deletions, suggest that they are subject to autoinhibition by their N‐termini in an isoform‐specific manner (N. Cheng et al., 2002; Pittman et al., 2002a, b). How, and whether, this regulatory mechanism operates in vivo has yet to be revealed.
The AtCAXs have homologues in other plant species and their physiological roles have been investigated using transgenic plants (Hirschi, 2001). Transgenic tobacco overexpressing AtCAX1 exhibits Ca‐deficiency disorders, such as tipburn, metal‐hypersensitivity and susceptibility to chilling, that can be reversed by increasing Ca supply. Since these plants have increased [Ca]shoot, Hirschi (2001) speculated that such phenotypes resulted from depleted [Ca2+]cyt, suggesting that the principal role of AtCAX1 was to maintain [Ca2+]cyt homeostasis by removing excess cytosolic Ca2+ to the vacuole, and noted that the expression of AtCAX1 and AtCAX3 (but not AtCAX2 or AtCAX4) was increased by raising Ca supply (Shigaki and Hirschi, 2000; Hirschi, 2001; N. Cheng et al., 2002).
Ca2+ influx to the cytosol: calcium channels
Calcium‐permeable channels have been found in all plant membranes (Fig. 4). They have been classified on the basis of their voltage‐dependence into depolarization‐activated (DACC), hyperpolarization‐activated (HACC) and voltage‐independent (VICC) cation channels (White, 2000; Miedema et al., 2001; Sanders et al., 2002). The presence of diverse classes of Ca2+‐permeable channels in a particular membrane is thought to enable physiological flexibility.
The principal roles of Ca2+‐permeable channels in the plasma membrane appear to be in cell signalling, but they may also contribute to nutritional Ca2+ fluxes in particular cell types (White, 1998, 2000; Miedema et al., 2001). Several types of DACCs have been observed in the plasma membrane of plant cells (White, 1998, 2000). Although each has distinct pharmacological and electrophysiological properties, all are permeable to both monovalent and divalent cations. They may therefore contribute to the uptake of essential or toxic cations in addition to Ca2+. Most DACCs activate significantly at voltages more positive than about –150 to –100 mV under physiological conditions (White, 1998). The dominant DACCs in protoplasts from arabidopsis tissues and carrot suspension cells appear to be controlled by cytoskeletal interactions and stabilized by the disruption of microtubules (Thion et al., 1996, 1998). It is argued that DACCs transduce general stress‐related signals since plasma membrane depolarization is common to many stimuli, occurs by many diverse mechanisms, and is likely to increase [Ca2+]cyt throughout the cell periphery (White, 1998, 2000). However, specific roles for DACCs acting in tandem with cytoskeletal rearrangements have been proposed in the acclimation of chilling‐resistant plants to low temperatures (Mazars et al., 1997; White, 1998; Xiong et al., 2002) and in the interactions of plants with microbes (White et al., 2002a). The outward‐rectifying K+ channels (KORCs) found in the plasma membrane of plant cells are also Ca2+‐permeable DACCs (White et al., 2002a). These channels activate significantly at voltages more positive than about –50 mV under most physiological conditions and catalyse a large K+ efflux simultaneously with a small Ca2+ influx (White, 1997; Gaymard et al., 1998; De Boer, 1999; Roberts and Snowman, 2000). The Ca2+ influx through KORCs might increase [Ca2+]cyt to coordinate ion transport, metabolism and gene expression. An elaborate model of how negative feedback through [Ca2+]cyt might control the loading of K+ into the root xylem by KORCs has been proposed by De Boer (1999).
Patch‐clamp electrophysiological techniques have identified HACCs in root cells (Kiegle et al., 2000a; Véry and Davies, 2000; Foreman et al., 2003), onion epidermal cells (Pickard and Ding, 1993), suspension‐cultured tomato cells (Blumwald et al., 1998), leaf mesophyll cells (Stoelzle et al., 2003) and stomatal guard cells (Hamilton et al., 2000, 2001; Pei et al., 2000; Murata et al., 2001; Perfus‐Barbeoch et al., 2002). These channels are permeable to many divalent cations including Ba2+, Ca2+, Mg2+, Mn2+, Cd2+ and Zn2+. They activate at voltages more negative than about –100 to –150 mV at physiological [Ca2+]cyt, but increasing [Ca2+]cyt shifts their activation potential to more positive voltages in root hairs (Véry and Davies, 2000) or more negative voltages in guard cells (Hamilton et al., 2000). At the apex of root hairs and pollen tubes, and in cells of the root elongation zone, the regulation of HACCs by [Ca2+]cyt may provide a positive feedback system to generate and maintain the elevated [Ca2+]cyt required for cell expansion (White, 1998, 2000; Véry and Davies, 2000; Miedema et al., 2001; Demidchik et al., 2002a). Mechanosensitive HACCs may orchestrate the changes in morphology induced by gravity, touch or flexure in a similar manner (Pickard and Ding, 1993; Bibikova et al., 1999). Reactive oxygen species also increase the activity of HACCs in root hairs (Foreman et al., 2003), guard cells (Pei et al., 2000; Murata et al., 2001; Klüsener et al., 2002; Köhler et al., 2003) and suspension‐cultured cells (Lecourieux et al., 2002) through the activity of NADPH oxidases. Oxidative bursts have been correlated with elevated [Ca2+]cyt, the initiation of [Ca2+]cyt waves, elongation growth, altered gene expression and the induction of antimicrobial activities. Elicitor‐activated HACCs, such as those activated by a heterotrimeric G‐protein/protein kinase cascade in suspension‐cultured tomato cells, are thought to raise [Ca2+]cyt to initiate cellular responses to pathogens (Blumwald et al., 1998). In guard cells, HACCs have a central role in closing stomata during water stress. Increasing ABA concentration shifts the activation potential of HACCs to more positive voltages, thereby promoting their opening, and the subsequent entry of Ca2+ not only depolarizes the plasma membrane but also initiates the [Ca2+]cyt‐dependent events, including [Ca2+]cyt‐dependent Ca2+ release (CICR) from intracellular stores, that lead to stomatal closure (Blatt, 2000a, b; White, 2000; Murata et al., 2001; Schroeder et al., 2001; Köhler and Blatt, 2002).
Many distinct VICCs are present in the plasma membrane of plant cells, which differ in cation selectivity, voltage‐dependence and pharmacology (White et al., 2002a; Demidchik et al., 2002b). Nevertheless, all VICCs appear to be permeable to both monovalent and divalent cations (Davenport and Tester, 2000; Demidchik and Tester, 2002; Demidchik et al., 2002a, b) and are likely to provide a weakly voltage‐dependent Ca2+ influx to cells under physiological ionic conditions (White and Davenport, 2002; Demidchik et al., 2002a). It has been suggested that Ca2+ influx through VICCs, which are open at physiological voltages and are generally insensitive to cytoplasmic modulators, is required to balance the perpetual Ca2+ efflux through Ca2+‐ATPases and H+/Ca2+‐antiporters to maintain [Ca2+]cyt homeostasis in an unstimulated plant cell (White and Davenport, 2002). This hypothesis is supported both by the pharmacology of Ca2+ influx to plant root cells and the linear dependence of their [Ca2+]cyt on cell membrane potential (Demidchik et al., 2002a). Furthermore, VICCs appear to be the only Ca2+‐permeable channels open at the resting potential of most plant cells.
There has been some recent speculation on the identity of genes encoding Ca2+‐permeable channels in the plasma membranes of plant cells (Demidchik et al., 2002b; Véry and Sentenac, 2002; White et al., 2002a). It has been suggested (a) that homologues of the arabidopsis AtTPC1 gene (Furuichi et al., 2001) encode DACCs regulated by [Ca2+]cyt, (b) that homologues of the arabidopsis AtSKOR (Gaymard et al., 1998) and AtGORK (Ache et al., 2000) genes encode KORCs, (c) that the annexin genes encode HACCs and (d) that genes from the cyclic‐nucleotide gated channel (CNGC) and glutamate receptor (GLR) families encode VICCs. Since CNGCs have a binding site for CaM within a C‐terminus binding site for cyclic nucleotide monophosphates, CNGC could impose a ‘coincidence hierarchy’ for [Ca2+]cyt signalling by integrating the response to two different signalling cascades acting simultaneously. The low‐affinity cation transporter in the plasma membrane of wheat root cells (TaLCT1) could also facilitate Ca2+ uptake into plant cells (Clemens et al., 1998), but it is not known whether this protein forms an ion channel.
Several Ca2+‐permeable channels co‐reside in the tonoplast (Allen and Sanders, 1997; White, 2000; Sanders et al., 2002). At least three types of pharmacologically distinct depolarization‐activated Ca2+‐permeable channels have been observed, of which the SV type is the most common. The SV channels are permeable to monovalent and divalent cations and catalyse a small Ca2+ influx to the cytoplasm under physiological conditions (Pottosin et al., 1997, 2001; White, 2000). They are regulated by many effectors, suggesting that they could be pivotal in effecting the coincidence control of [Ca2+]cyt signalling (Sanders et al., 1999, 2002; White, 2000). Increasing [Ca2+]cyt or decreasing vacuolar Ca2+ concentration ([Ca2+]vac) promotes their opening at physiological trans‐tonoplast voltages. Their response to [Ca2+]cyt is sensitized both by CaM and by cytosolic Mg2+. Cytoplasmic alkalinization or vacuolar acidification also promotes their opening under physiological conditions. The SV channels are regulated by [Ca2+]cyt‐dependent phosphorylation at two sites. Phos phorylation at one site is inhibitory, whereas phosphorylation at the other site activates the channel. It has been proposed that [Ca2+]cyt‐dependent regulation of SV channels might prevent an excessive rise in [Ca2+]cyt or modulate the kinetics of changes in [Ca2+]cyt (Sanders et al., 1999). The activity of SV channels is also reduced by 14‐3‐3 proteins (van den Wijngaard et al., 2001). The genes(s) encoding SV channels are unknown, but KCO1 has been implicated in their formation, since SV‐channel currents in mesophyll protoplasts from the arabidopsis kco1 mutant are smaller than those from wild‐type plants (Schonknecht et al., 2002). At least two types of pharmacologically distinct hyperpolarization‐activated, Ca2+‐permeable channels (HACC) have been reported in plant vacuoles (Allen and Sanders, 1997; White, 2000). These open at trans‐tonoplast voltages within the physiological range (–20 to –70 mV) and catalyse Ca2+ influx to the cytoplasm. Both tonoplast HACCs show spontaneous changes in their kinetic behaviour, which has been interpreted as resulting from interactions with (hypothetical) regulatory ligands. One type of tonoplast HACC is inhibited by a [Ca2+]cyt above 1 µm, whereas the other type is insensitive to [Ca2+]cyt. The opening of the second type of HACC is promoted by increasing [Ca2+]vac and/or vacuolar alkalinization.
Several highly selective Ca2+ channels activated by cytosolic second messengers (IP3, IP6 or cADPR) are also present in the tonoplast (Allen and Sanders, 1997; White, 2000; Sanders et al., 2002). All mediate Ca2+ influx to the cytoplasm. The IP3‐dependent Ca2+ channels are activated half‐maximally by IP3 concentrations as low as 200 nm. They open at physiological trans‐tonoplast voltages, and their opening is promoted by tonoplast hyperpolarization. They may have a role in turgor regulation in response to salt and hyper‐osmotic stresses (Allen and Sanders, 1997; Drøbak and Watkins, 2000; DeWald et al., 2001; Xiong et al., 2002), in nastic movements (Kim et al., 1996), in gravitropic movements of roots (Fasano et al., 2002) and pulvini (Perera et al., 1999), in stomatal closure (Staxén et al., 1999; Blatt, 2000a, b; Ng et al., 2001b; Schroeder et al., 2001; Klüsener et al., 2002) and in pollen tube elongation (Malhó et al., 1998; Rudd and Franklin‐Tong, 2001). They may also have roles in pollen tube self‐incompatibility (Malhó et al., 1998; Rudd and Franklin‐Tong, 2001) and in plant defence responses (Mithöfer et al., 1999; Sanders et al., 1999; Blume et al., 2000). The cADPR‐dependent Ca2+ channels also open at physiological trans‐tonoplast voltages, but are inhibited by [Ca2+]cyt greater than 600 nm (Leckie et al., 1998). The pharmacology of plant cADPR‐dependent Ca2+ channels resembles that of the cADPR‐activated, ryanodine‐receptor channels found in the endomembranes of animal cells (Allen and Sanders, 1997). They are activated half‐maximally by 20–40 nm cADPR, 20 nm ryanodine or millimolar concentrations of caffeine. They are inhibited by micromolar concentrations of ruthenium red and procaine. The cADPR‐dependent Ca2+ channels have been implicated in ABA‐signalling pathways leading to cold acclimation, desiccation tolerance (Wu et al., 1997) and stomatal closure (Leckie et al., 1998; Klüsener et al., 2002), in circadian [Ca2+]cyt rhythms and in the activation of plant defence responses (Durner et al., 1998; Klessig et al., 2000). Evidence for Ca2+ channels activated by sub‐micromolar concentrations of IP6 and having a role in stomatal closure is circumstantial but persuasive (Lemtiri‐Chlieh et al., 2000).
A variety of voltage‐dependent and ligand‐activated Ca2+ channels have also been revealed in the ER using biochemical or electrophysiological techniques (White, 2000). Depolarization‐activated Ca2+ channels were observed when ER vesicles from the touch‐sensitive tendrils of Bryonia dioica (BCC1) (Klüsener et al., 1995) or the root tips of Lepidium sativum (LCC1) (Klüsener and Weiler, 1999) were incorporated into planar lipid bilayers. Their activities were modulated by the Ca2+ gradient across the ER membrane and enhanced by cytoplasmic acidification. An interesting model for the generation of oscillations in [Ca2+]cyt through BCC1 has been proposed (Klüsener et al., 1995). At the resting membrane potential of the ER, which is assumed to be close to zero, and with submicromolar Ca2+ activities in the lumen of the ER, BCC1 is likely to be closed. However, if the lumenal Ca2+ activity is increased, for example by the activity of an ER Ca2+‐ATPase, BCC1 will open to release Ca2+ into the cytoplasm. This reduces the lumenal Ca2+ and the channel recloses. This cycle will generate transient local elevations of [Ca2+]cyt. The frequency of these elevations could be determined by modulation of BCC1, the Ca2+‐ATPase or the Ca2+ gradient across the ER. In addition to these voltage‐dependent Ca2+ channels, biochemical studies indicate the presence of Ca2+ channels activated by cADPR (Navazio et al., 2001), NAADP (nicotinic acid adenine dinucleotide phosphate) (Navazio et al., 2000) and (possibly) IP3 (Muir and Sanders, 1997). To date, no genes encoding ER Ca2+ channels have been identified in plants, and there appear to be no plant genes homologous to the IP3‐receptors, cADPR‐receptors, NAADP‐receptors or endomembrane voltage‐gated channels of animals.
A hyperpolarization‐activated Ca2+‐permeable channel has been recorded in excised patches from the nuclear envelope of red beet cells (Grygorczyk and Grygorczyk, 1998). This channel was unaffected by changes in [Ca2+]cyt, but when the [Ca2+] of the perinuclear space (the lumen of the nuclear envelope) exceeded about 1 µm the channel opened at physiological voltages. Based on this observation, a role for this channel in regulating Ca2+‐dependent nuclear processes was proposed (Grygorczyk and Grygorczyk, 1998). Interestingly, annexin‐like proteins with the potential to form Ca2+‐permeable cation channels have been located at the perinuclear membrane and in the nucleolus (Clark et al., 1998; Kovács et al., 1998; de Carvalho‐Niebel et al., 2002).
CYTOSOLIC CALCIUM SIGNALS
The evolution of [Ca2+]cyt signalling and the [Ca2+]cyt ‘signature’
The [Ca2+]cyt of plant cells increases in response to many developmental cues and environmental challenges (Table 2). This is considered essential for producing a physiological response. It is thought that elevating [Ca2+]cyt is a primitive, and universal, response to stress. Sanders et al. (1999) observed that the low solubility product of Ca2+ and phosphate would have necessitated a [Ca2+]cyt lower than the [Ca2+] of seawater to maintain energy metabolism. They presumed that this required the early evolution of mechanisms to remove Ca2+ from the cytoplasm, and noted that a homeostatically maintained submicromolar [Ca2+]cyt would have been ideal for the subsequent evolution of [Ca2+]cyt signalling systems, since it would confer sensitivity and speed to any signal. Sanders et al. (1999) also noted that the chemistry of Ca2+, which can coordinate six to eight uncharged oxygen atoms, had fortuitously made possible the evolution of proteins that change conformation upon binding Ca2+, allowing the cellular perception and transduction of a [Ca2+]cyt signal.
Table 2.
Examples of the developmental processes and responses to abiotic and biotic challenges initiated by a perturbation in cytosolic Ca2+ concentration ([Ca2+]cyt)
| Developmental process or environmental challenge | Characteristic [Ca2+]cyt perturbation | Stores releasing Ca2+ to cytosol | Selected references |
| Pollen tube elongation | Oscillation of high apical [Ca2+]cyt | Apoplast and internal | Malhó and Trewavas, 1996; Holdaway‐Clarke et al., 1997; Malhó et al., 1998; 2000; Messerli et al., 2000; Rudd and Franklin‐Tong, 2001 |
| Pollen tube self‐incompatibility response | Intracellular [Ca2+]cyt wave in shank | ApoplastInternal (IP3‐dependent) | Rudd and Franklin‐Tong, 2001; Straatman et al., 2001; Franklin‐Tong et al., 2002 |
| Cell polarity after fertilization | Intracellular [Ca2+]cyt wave from sperm fusion site leading to sustained [Ca2+]cyt elevation | Apoplast | Antoine et al., 2001 |
| Cell division | Elevated [Ca2+]cyt | Bush, 1995 | |
| Seed germination (giberellins) | Slow rise in [Ca2+]cyt | Bush, 1995; Anil and Sankara Rao, 2001 | |
| Apoptosis | Slow, sustained [Ca2+]cyt elevation | Levine et al., 1996 | |
| Red light | Elevated [Ca2+]cyt | Apoplast | Shacklock et al. 1992; Malhó et al., 1998 |
| Blue light | Brief spike in [Ca2+]cyt (seconds) | Apoplast | Malhó et al., 1998; Baum et al., 1999 |
| Circadian rhythms | Circadian [Ca2+]cyt oscillation | Johnson et al., 1995; Wood et al., 2001 | |
| Stomatal closure (ABA, sphingosine‐1‐phosphate) | (1) Elevated [Ca2+]cyt at cell periphery(2) Elevated [Ca2+]cyt around vacuole(3) Oscillations in [Ca2+]cyt | (1) Apoplast(2) Vacuole(3) Apoplast and internal | McAinsh et al., 1992; Allen et al., 1999, 2000; Blatt, 2000a, b; White, 2000; Anil and Sankara Rao, 2001; Evans et al.. 2001; Ng et al., 2001a, b; Schroeder et al., 2001; Klüsener et al., 2002 |
| CO2 | Elevated [Ca2+]cyt in guard cells | Apoplast | Webb et al., 1996 |
| Increasing apoplastic Ca2+ | Oscillations in [Ca2+]cyt of guard cells | Apoplast | McAinsh et al., 1995; Allen et al., 1999, 2000 |
| Auxin responses | (1) Slow, prolonged [Ca2+]cyt increase | Felle, 1988; Malhó et al., 1998; Ng et al., 2001b; Plieth, 2001; Plieth and Trewavas, 2002 | |
| (2) Oscillations in [Ca2+]cyt | |||
| Xylem K+ loading | Elevated [Ca2+]cyt | De Boer, 1999 | |
| Exocytosis | Elevated [Ca2+]cyt | Battey et al., 1999; Camacho and Malhó, 2003 | |
| Root cell elongation | Sustained [Ca2+]cyt elevation | Apoplast | Cramer and Jones, 1996; Demidchik et al., 2002a |
| Root hair elongation | Sustained high apical [Ca2+]cyt | Apoplast | Wymer et al., 1997; White, 1998; Bibikova et al., 1999 |
| Inhibition of cyclosis | Elevated [Ca2+]cyt | Ayling and Clarkson, 1996 | |
| Nodulation (nod factors) | Initial [Ca2+]cyt rise then oscillations in [Ca2+]cyt | Apoplast | Cárdenas et al., 2000; Wais et al., 2000; Walker et al., 2000; Lhuissier et al., 2001; Shaw and Long, 2003 |
| Senescence | Sustained [Ca2+]cyt elevation | Huang et al., 1997 | |
| UV‐B | Slow [Ca2+]cyt rise, elevated [Ca2+]cyt sustained for several minutes | Apoplast | Frohnmeyer et al., 1999 |
| Heat‐shock | Elevated [Ca2+]cyt sustained for 15–30 min | Apoplast and internal (IP3‐dependent) | Gong et al., 1998; Malhó et al., 1998 |
| Cold‐shock | (1) Single brief [Ca2+]cyt spike (seconds)(2) Oscillations in [Ca2+]cyt | (1) Apoplast | Knight et al., 1991; Malhó et al., 1998; White, 1998; Plieth et al., 1999; van der Luit, 1999; Allen et al., 2000; Kiegle et al., 2000b; Knight, 2000; Cessna et al., 2001; Plieth, 2001 |
| Slow cooling | Biphasic(1) Brief [Ca2+]cyt spike (seconds)(2) Slow [Ca2+]cyt elevation (minutes) | (1) Apoplast(2) Apoplast and internal (IP3‐dependent) | Knight et al., 1996; Plieth et al., 1999; Knight, 2000; Knight and Knight, 2000; Moore et al., 2002 |
| Oxidative stress (paraquat, superoxide, H2O2, ozone) | (1) Brief [Ca2+]cyt spike (2) Sustained [Ca2+]cyt elevation(3) Oscillations in [Ca2+]cyt | (1) Apoplast(2) Apoplast and internal (IP3‐dependent) | Price et al., 1994; Levine et al., 1996; McAinsh et al., 1996; Knight et al., 1998; Malhó et al., 1998; Clayton et al., 1999; Allen et al., 2000; Kawano and Muto, 2000; Knight, 2000; Klüsener et al., 2002; Lecourieux et al., 2002 |
| Anoxia | Biphasic(1) Slow spike (duration of minutes)(2) Sustained [Ca2+]cyt elevation (hours) | (1) Apoplast(2) Internal including mitochondria | Subbaiah et al., 1994, 1998; Sedbrook et al., 1996; Malhó et al., 1998; Plieth, 2001 |
| Drought/hyper‐osmotic stress (mannitol) | Biphasic(1) Slow spike (duration of minutes)(2) Sustained [Ca2+]cyt elevation (hours) | Apoplast and vacuole | Knight et al., 1997, 1998; Malhó et al., 1998; Kiegle et al., 2000b; Cessna et al., 2001; Pauly et al., 2001; Plieth, 2001 |
| Salinity (NaCl) | BiphasicTissue [Ca2+]cyt wave(1) Slow spike (duration of minutes)(2) Sustained [Ca2+]cyt elevation (hours)(3) Reduced [Ca2+]cyt (days) | Apoplast and vacuole (IP3‐dependent) | Knight et al., 1997; Kiegle et al., 2000b; Knight, 2000; DeWald et al., 2001; Pauly et al., 2001; Moore et al., 2002; Halperin et al., 2003 |
| Hypo‐osmotic stress | Biphasic(1) Small [Ca2+]cyt elevation(2) Large [Ca2+]cyt elevation | (1) Apoplast(2) Apoplast and internal (IP3‐dependent) | Takahashi et al., 1997; Malhó et al., 1998; Knight, 2000; Cessna and Low, 2001; Cessna et al., 2001; Pauly et al., 2001; Plieth, 2001 |
| Mechanical stimulation (motion, touch, wind) | Single brief [Ca2+]cyt spike (seconds)Tissue [Ca2+]cyt wave | Internal | Knight et al., 1991, 1992; Haley et al., 1995; Legue et al., 1997; Malhó et al., 1998; van der Luit, 1999; Plieth, 2001; Fasano et al., 2002 |
| Aluminium stress | Elevated [Ca2+]cyt | Zhang and Rengel, 1999 | |
| Pathogens (elicitors) | Biphasic(1) Slow spike (duration of minutes)(2) Sustained [Ca2+]cyt elevation (hours)(3) Oscillations in [Ca2+]cyt | (1) Apoplast(2) Apoplast and internal (IP3‐dependent) | Knight et al., 1991; Malhó et al., 1998; Mithöfer et al., 1999; Blume et al., 2000; Fellbrich et al., 2000; Grant et al., 2000; Cessna and Low 2001; Cessna et al. 2001; Rudd and Franklin‐Tong, 2001; Klüsener et al., 2002; Lecourieux et al., 2002 |
| (the relative magnitude of different phases varies with elicitor identity) | |||
The kinetics of the [Ca2+]cyt perturbations and the location of stores releasing Ca2+ into the cytosol associated with each developmental process or environmental challenge are indicated.
To effect an appropriate physiological response to a particular stimulus, it is thought that the [Ca2+]cyt perturbation (termed the [Ca2+]cyt ‘signature’) elicited by each developmental cue or environmental challenge is unique. This uniqueness is manifest in the sub‐cellular location and/or the kinetics or magnitude of the [Ca2+]cyt perturbation (McAinsh and Hetherington, 1998; Trewavas, 1999; Rudd and Franklin‐Tong, 2001). An increase in [Ca2+]cyt is effected by Ca2+ influx to the cytosol either from the apoplast, across the plasma membrane, or from intracellular stores such as the ER or vacuole. This Ca2+ influx is mediated by Ca2+‐permeable ion channels, and their type, cellular location and abundance will influence the spatial characteristics of [Ca2+]cyt perturbations. Since the diffusion of Ca2+ within the cytoplasm is low (Clapham, 1995), and the buffering of Ca2+ in the cytoplasm is high (0·1 to 1 mm; Malhó et al., 1998; Trewavas, 1999), the opening of a Ca2+ channel produces a local increase in [Ca2+]cyt that dissipates rapidly after the channel has closed. The subcellular localization of Ca2+ channels is therefore critical for the targeting of different cellular processes. In some circumstances, the proteins responding to the changes in [Ca2+]cyt must either be associated with the Ca2+ channel itself or tethered closely to the membrane. Thus, the opening of Ca2+ channels influences local biochemical processes.
To coordinate cellular responses, [Ca2+]cyt ‘waves’ are produced within the cytoplasm by the successive recruitment of receptive Ca2+ channels. Various authors have speculated how these waves might be initiated and propagated. Trewavas (1999) suggested that a local elevation of [Ca2+]cyt might generate soluble second messengers, such as IP3 or cADPR, that diffuse through the cytoplasm to activate a relay of spatially separated Ca2+‐channels. This might occur during the [Ca2+]cyt waves observed in the shank of poppy pollen tubes during the self‐incompatibility response (Straatman et al., 2001; Rudd and Franklin‐Tong, 2001) or in plant cells responding to salt stress (Drøbak and Watkins, 2000). Antoine et al. (2001) suggested that the [Ca2+]cyt wave that crosses the egg following fertilization was the result of the successive activation of (mechanosensitive) Ca2+ channels in the plasma membrane radiating from the site of sperm entry, and Franklin‐Tong et al. (2002) suggested that repetitive Ca2+ influx across the plasma membrane contributed to the [Ca2+]cyt waves that occurred in the shank of poppy pollen tubes during the self‐incompatibility response. The spatial changes in elevated [Ca2+]cyt that occur following the application of ABA to guard cells, first close to the plasma membrane and subsequently adjacent to the vacuole (McAinsh et al., 1992; Allen et al., 1999), are thought to reflect the sequential opening of hyperpolarization‐activated Ca2+ channels at the plasma membrane and then second‐messenger activated Ca2+ channels in the tonoplast (Grabov and Blatt, 1998; White, 2000; Schroeder et al., 2001). In addition to these subcellular [Ca2+]cyt waves, ‘waves’ of cells with high [Ca2+]cyt may also propagate through plant tissues. These can be induced in root tissues by mechanical stimulation (Legué et al., 1997; Fasano et al., 2002) or saline shock (Moore et al., 2002), in cotyledons by cold shock (Knight et al., 1993) and in leaves by chilling plant roots briefly (Campbell et al., 1996). Electrical action potentials, osmotic perturbations or chemical signals may trigger these waves.
Although an elevated [Ca2+]cyt is necessary for signal transduction, a prolonged increase in [Ca2+]cyt is lethal. Indeed, sustained high [Ca2+]cyt is implicated in apoptosis, both during normal development (e.g. in tissue patterning and xylogenesis) and in hypersensitive responses to pathogens (Levine et al., 1996). To effect other responses, [Ca2+]cyt perturbations must be either of low amplitude or transient. Transient increases in [Ca2+]cyt can be single (spike), double (biphasic) or multiple (oscillations). Unique [Ca2+]cyt spikes can be generated by delaying the [Ca2+]cyt rise, or by altering the rate of change of [Ca2+]cyt, the maximal [Ca2+]cyt reached or the duration [Ca2+]cyt is above a certain threshold. Oscillations can differ in their [Ca2+]cyt amplitudes, periodicity or duration (Evans et al., 2001). The production of [Ca2+]cyt waves allows specific spatiotemporal [Ca2+]cyt perturbations to be generated that may differ in their cellular location, rate and extent of propagation and/or their [Ca2+]cyt amplitude during propagation (Malhó et al., 1998). However, despite the seductive logic of a [Ca2+]cyt signature for each developmental cue or environmental challenge, plant tissues are comprised of populations of heterogeneous cells that may have contrasting abilities to generate [Ca2+]cyt signatures. For example, (a) within the root, the [Ca2+]cyt perturbations induced by mechanical perturbation (Legué et al., 1997), salinity, osmotic stress, cold shock or slow cooling (Kiegle et al., 2000b; Moore et al., 2002) differ markedly between cell types, and (b) shoot cells exhibit a biphasic [Ca2+]cyt perturbation during anoxia, whereas only a slow increase in [Ca2+]cyt is observed in root cells (Sedbrook et al., 1996; Plieth, 2001). Indeed, even cells of the same type, such as the two guard cells of a stomate, seldom generate an identical [Ca2+]cyt in response to a defined stimulus (Allen et al., 1999). This heterogeneity may arise from differences in cellular cytology, the distribution of cytoplasmic Ca2+ buffers and/or the activities of Ca2+ transporters.
The [Ca2+]cyt signature: temporal and spatial aspects
Several abiotic challenges result in an immediate, transient increase in [Ca2+]cyt that is restored to basal levels within minutes (Table 2). Such challenges include mechanical perturbation (Knight et al., 1991, 1992; Haley et al., 1995; Legué et al., 1997; Malhó et al., 1998; van der Luit, 1999; Plieth, 2001; Fasano et al., 2002) and rapid cooling for brief periods, termed ‘cold‐shock’ (Knight et al., 1991; Plieth et al., 1999; van der Luit, 1999; Kiegle et al., 2000b; Plieth, 2001). By contrast, sustained cooling below a threshold temperature results in a biphasic response in [Ca2+]cyt, in which an initial transient increase in [Ca2+]cyt is followed by a more prolonged, second transient elevation of [Ca2+]cyt (Plieth et al., 1999; Knight, 2000; Knight and Knight, 2000; Moore et al., 2002). Heat shock, acute salt (NaCl) stress, hyper‐osmotic (mannitol) stress, annoxia and exposure to oxidative stress also elicit an immediate, transient increase in [Ca2+]cyt in plant cells, which is followed by a more prolonged elevation of [Ca2+]cyt lasting many minutes or hours (Table 2; McAinsh et al., 1996; Sedbrook et al., 1996; Knight et al., 1997, 1998; Gong et al., 1998; Clayton et al., 1999; Kiegle et al., 2000b; Knight, 2000; DeWald et al., 2001; Plieth, 2001; Lecourieux et al., 2002; Moore et al., 2002). Biphasic [Ca2+]cyt perturbations have been observed in plant cells in response to diverse pathogens and elicitors, but the kinetics of these responses varies greatly (Lecourieux et al., 2002). Rudd and Franklin‐Tong (2001) speculated that the slow generation of a sustained [Ca2+]cyt elevation was a common feature of these responses, and the sustained increase in [Ca2+]cyt alone was subsequently correlated with the induction of defence responses (Cessna and Low, 2001; Lecourieux et al., 2002).
Several developmental cues and environmental challenges produce [Ca2+]cyt oscillations (Table 2). The duration, periodicity and amplitude of [Ca2+]cyt oscillations vary considerably, and their form is often dependent on the strength and combination of specific stimuli (Felle, 1988; McAinsh et al., 1995; Allen et al., 1999; Staxén et al., 1999; Evans et al., 2001). Periodicities may range from the circadian oscillations observed in various plant cells (Johnson et al., 1995; Wood et al., 2001), through the slow oscillations observed in coleoptile cells following the addition of auxin (periodicity ≈30 min; Felle, 1988) and in guard cells either spontaneously or in response to stimuli such as ABA, [Ca2+]ext, ozone, cold‐shock and elicitors (periodicity >3 min; Grabov and Blatt, 1998; Allen et al., 1999, 2000; Staxén et al., 1999; Evans et al., 2001; Ng et al., 2001a; Klüsener et al., 2002), to the rapid oscillations in [Ca2+]cyt observed at the tip of growing pollen tubes (periodicity 40–75 s; Holdaway‐Clarke et al., 1997; Malhó et al., 1998, 2000; Messerli et al., 2000; Rudd and Franklin‐Tong, 2001) and in root hairs during nodulation (periodicity 1–2 min; Wais et al., 2000; Walker et al., 2000; Shaw and Long, 2003). It is noteworthy that oscillations can be induced in guard cells by releasing caged Ca2+ into the cytosol (McAinsh et al., 1995) and oscillatory [Ca2+]cyt waves can be induced in pollen tubes by releasing caged Ca2+ or IP3 into the cytosol (Malhó and Trewavas, 1996; Malhó, 1998). Several hypotheses have been proposed for the generation of [Ca2+]cyt oscillations. These include models based on the successive emptying and refilling of finite Ca2+ stores (Klüsener et al., 1995; Harper, 2001), on the successive activation and deactivation of target Ca2+ channels through co‐incident signalling cascades (Sanders et al., 1999; White, 2000) or by the stretching and relaxation of a membrane (Holdaway‐Clarke et al., 1997).
Different stimuli generate contrasting spatial [Ca2+]cyt perturbations by mobilizing Ca2+ from different cellular stores and/or by activating Ca2+ channels in a restricted location (Table 2; Sanders et al., 1999). For example, Ca2+ influx from the apoplast is implicated in elevating [Ca2+]cyt during brief cold‐shock (White, 1998, 2000; Plieth et al., 1999; Kiegle et al., 2000b; Knight, 2000; Plieth, 2001) and oxidative stress (Knight et al., 1997; Clayton et al., 1999), but release from internal stores contributes significantly to the [Ca2+]cyt elevations in response to prolonged cooling, salinity, osmotic stresses or pathogens (Knight et al., 1996, 1997; Blume et al., 2000; Knight, 2000; Pauly et al., 2001; Lecourieux et al., 2002; Moore et al., 2002) and dominates the [Ca2+]cyt elevations in response to mechanical perturbations (Haley et al., 1995; Legué et al., 1997) and anoxia (Subbaiah et al., 1994, 1998). Van der Luit et al. (1999) demonstrated that separate nuclear [Ca2+] and [Ca2+]cyt perturbations were initiated in tobacco seedlings in response to wind and cold shock, respectively, and that each led to the expression of a different CaM isoform. Pauly et al. (2001) similarly proposed that different cytoplasmic and nuclear signals were involved in discriminating between hypo‐ and hyper‐osmotic shock.
Graded [Ca2+]cyt responses to stimuli
Perturbations in [Ca2+]cyt often show a graded response that is proportional to the strength of the stimulus. For example, the frequency and amplitude of oscillations in the [Ca2+]cyt of guard cells varies directly with the apoplastic ABA (Staxén et al., 1999) or sphingosine‐1‐phosphate (Ng et al., 2001a) concentration, with corresponding effects on the kinetics of stomatal closure. The form of the [Ca2+]cyt perturbations that occur during cooling are a complex function of the rate of cooling, the duration of cooling, and the magnitude of the temperature drop (Plieth et al., 1999). Plant cells respond to a brief cold‐shock with an immediate, transient increase in [Ca2+]cyt that is proportional to the rate of cooling, whereas they respond to sustained cooling below a threshold temperature with a prolonged biphasic [Ca2+]cyt perturbation (Table 2). The magnitude of the [Ca2+]cyt elevation induced in roots following their reorientation in a gravitational field is proportional to the angle of displacement up to a maximum of 135°, which corresponds to the optimal angle for amyloplasts to slide down the cell wall (Plieth and Trewavas, 2002), and the [Ca2+]cyt perturbation induced by mechanical stimulation is increased in proportion to the stress and/or the time an organ is in motion (Knight et al., 1992; Haley et al., 1995). Similarly, the magnitude of the initial [Ca2+]cyt elevation induced in tobacco suspension cells by hypo‐ or hyper‐osmotic shock depends upon the change in the osmolarity of the bathing medium (Takahashi et al., 1997; Pauly et al., 2001), the magnitude of the [Ca2+]cyt perturbation in response to elicitors increases proportionally to the elicitor concentration applied (Mithöfer et al., 1999; Blume et al., 2000; Lecourieux et al., 2002) and the magnitude of the [Ca2+]cyt elevation induced by oxidative stress increases in proportion to the concentrations of ozone, H2O2 or methyl viologen applied (Price et al., 1994; Levine et al., 1996; McAinsh et al., 1996; Clayton et al., 1999; Lecourieux et al., 2002).
Explicit [Ca2+]cyt perturbations produce defined physiological responses
Explicit [Ca2+]cyt signatures are responsible for the activation of specific genes in animal cells (Dolmetsch et al., 1997, 1998; Li et al., 1998). This is consistent with the hypothesis that explicit [Ca2+]cyt signatures produce defined physiological responses to specific developmental cues or environmental challenges. However, there have been few attempts to generate explicit [Ca2+]cyt perturbations artificially in plants. Although several experiments have demonstrated that elevating [Ca2+]cyt using ionophores induces the expression of Ca2+‐dependent genes and physiological responses to chilling and ABA (Monroy and Dhinsa, 1995; Sheen, 1996), the [Ca2+]cyt perturbations produced by ionophores are unlikely to have the same form as the [Ca2+]cyt perturbations elicited by these effectors. This suggested to Plieth (2001) that the form of the [Ca2+]cyt signature was inconsequential for these responses. Indeed, Plieth (2001) further argued that the notion of an explicit [Ca2+]cyt signature entirely responsible for a defined physiological response was fundamentally doubtful. He cited these observations: (a) both osmotic stress and salt stress induced similar [Ca2+]cyt perturbations, but result in different levels of expression of the p5cs gene (Knight et al., 1997), and (b) almost any [Ca2+]cyt perturbation could be elicited in plant cells with an appropriate manipulation of temperature (Plieth, 2001), to suggest that factors other than, or in addition to, [Ca2+]cyt were involved in producing an appropriate response to a particular challenge. Consistent with this suggestion, [Ca2+]cyt transients have recently been found to be necessary, but not sufficient, for the expression of ABA‐inducible genes in plant cells (Webb et al., 2001). Nevertheless, several biochemical and genetic consequences have been attributed to specific [Ca2+]cyt signatures.
An insight into the [Ca2+]cyt signatures required for specific cellular responses has been obtained by correlating biochemical or genetic responses with particular characteristics of [Ca2+]cyt perturbations common to diverse stimuli or by dissecting [Ca2+]cyt perturbations using pharmaceuticals. Thus, Cessna and Low (2001) used pharmaceuticals to alter the [Ca2+]cyt perturbations in response to hypo‐osmotic shock to demonstrate that only the Ca2+ flux from internal stores during the second transient increase in [Ca2+]cyt initiated an oxidative burst. They speculated that this was a consequence of either a local elevation of [Ca2+]cyt and/or a local distribution of the enzymes generating H2O2. Similarly, Lecourieux et al. (2002) demonstrated that only the second, sustained increase in [Ca2+]cyt elicited by cryptogein was required for a hypersensitive response and cell death. Blume et al. (2000) demonstrated that only a sustained [Ca2+]cyt elevation induced phytoallexin synthesis in response to elicitors, and Clayton et al. (1999) showed that the induction of glutathione‐S‐transferase (GST) gene expression by ozone relied only on the second, transient [Ca2+]cyt elevation. Finally, the changes in [Ca2+]cyt perturbations following combinations of oxidative stress and hyper‐osmotic stress correlated well with the expression of Ca2+‐regulated osmotic stress induced genes (p5cs and rab18), and the acquisition of osmotic stress tolerance (Knight et al., 1998).
A direct demonstration that an explicit [Ca2+]cyt perturbation was required to produce a defined physiological response was performed recently on arabidopsis guard cells (Allen et al., 2001). Artificially elevating [Ca2+]cytin guard cells through hyperpolarization (Grabov and Blatt, 1998; Allen et al., 2000, 2001) or H2O2 (Allen et al., 2000; Pei et al., 2000) can induce stomatal closure. However, although a single elevation of [Ca2+]cyt was sufficient for immediate stomatal closure, prolonged stomatal closure could only be induced if the initial increase in [Ca2+]cyt was followed by [Ca2+]cyt oscillations with a specific periodicity (Allen et al., 2001). Interestingly, appropriate [Ca2+]cyt oscillations are induced in guard cells by ABA and stomates of the ABA‐insensitive mutant gca2, which shows aberrant [Ca2+]cyt oscillations in response to ABA, can be induced to close when appropriate [Ca2+]cyt are produced artificially (Allen et al., 2001).
Habituation, ‘learning’ and ‘memory’
Trewavas (1999) has likened the [Ca2+]cyt signalling network to a basic cellular ‘memory’. He noted that, in common with a neural network, [Ca2+]cyt signals have the following properties: they may be (a) spatially structured, through the occurrence and location of cellular components; (b) subject to coincidence control, since certain elements are the targets of multiple [Ca2+]cyt cascades and can process or block specific signals arriving coincidentally; (c) synchronized, for example by membrane depolarization or a sudden increase in the concentration of a diffusable second messenger; (d) modified by exposure to specific challenges, through changes in the abundance of cellular components involved in [Ca2+]cyt signals; and (e) predisposed by previous challenges to generate an appropriate [Ca2+]cyt signal in response to a current challenge. He suggested that these prerequisites for cellular ‘learning’ allow plant cells to respond ‘intelligently’ to the challenges they experience through an innate phenotypic and physiological plasticity.
Previous sections have discussed the spatial distribution of Ca2+‐transporters within a plant cell and their modulation by components of diverse intracellular signalling cascades such as [Ca2+]cyt, cytoplasmic pH, reactive oxygen species, kinases, phosphatases, cNMPs, IP3 and cADPR. Such Ca2+ transporters could be a point of convergence, and integration, of many developmental and environmental signals. This phenomenon is termed ‘cross‐talk’ and might be a mechanism whereby plants develop cross‐tolerance to various biotic and abiotic stresses (Bowler and Fluhr, 2000; Knight, 2000). Similarly many targets of [Ca2+]cyt signals are also regulated by components of diverse signalling cascades (see below).
There is considerable evidence that [Ca2+]cyt signatures are modified by previous experience. A diminished [Ca2+]cyt elevation upon repetitive stimulation and/or a refractory period during which an increase in [Ca2+]cyt cannot be elicited by the same developmental cue or environmental challenge is commonly observed. The magnitude of the [Ca2+]cyt perturbation elicited by reorientation in a gravitational field (Plieth and Trewavas, 2002), touch (Legué et al., 1997) or wind‐induced motion (Knight et al., 1992) becomes progressively smaller upon repeated stimulation and a refractory period of several minutes is required before a full response is observed again. Following one brief pulse of phototropically active blue light, arabidopsis seedlings will not respond maximally again for several hours (Baum et al., 1999). A second exposure to an elicitor does not influence [Ca2+]cyt for several hours after its initial application (Blume et al., 2000), and plant cells challenged with H2O2 fail to respond to H2O2 again for several hours (Price et al., 1994). An anoxic treatment reduces the magnitude of the initial [Ca2+]cyt peak and delays the sustained elevation of [Ca2+]cyt in response to a second anoxic treatment (Sedbrook et al., 1996), and the magnitude of [Ca2+]cyt perturbations is diminished by continued chilling and rewarming (Plieth et al., 1999). These observations may indicate a desensitization of signalling cascades or a depletion of stores releasing Ca2+ to the cytosol. By contrast, the magnitude of the second (vacuolar) [Ca2+]cyt elevation observed during slow cooling was increased following a period of cold acclimation (Knight and Knight, 2000), and a pretreatment with mannitol increased the magnitude of the [Ca2+]cyt perturbations subsequently induced by hyper‐osmotic shock (Knight et al., 1998).
There is also evidence that the [Ca2+]cyt signatures elicited by one environmental challenge can be modified by prior exposure to a contrasting one. For example, the magnitude of the [Ca2+]cyt perturbations in response to oxidative stress was reduced by a prior exposure to hyper‐osmotic stress (Knight et al., 1997; Knight, 2000), and the magnitude of the [Ca2+]cyt perturbations in response to hyper‐osmotic stress was reduced by a prior exposure to oxidative stress (Knight et al., 1998; Knight, 2000). These observations may imply cross‐talk between the signalling cascades and/or Ca2+ channels and stores responding to these challenges. However, prior oxidative stress did not affect the [Ca2+]cyt transient observed upon cold shock or touch (Price et al., 1994; Knight et al., 1998), suggesting that contrasting signalling cascades and/or Ca2+ stores and channels are recruited by these challenges. Similarly, during the refractory period following heat shock, during which additional heat shocks fail to elevate [Ca2+]cyt, [Ca2+]cyt can be elevated by cold shock or mechanical perturbation (Gong et al., 1998), and during the refractory period following wind‐induced motion, cells can still raise [Ca2+]cyt in response to cold shock (Knight et al., 1992). The cross‐talk (and lack of cross‐talk) between diverse signalling cascades responding to each facet of the environment is thought to optimize a plant’s response to its surroundings.
RESPONDING TO CYTOSOLIC CALCIUM SIGNALS
To respond appropriately to a specific [Ca2+]cyt perturbation, a cell must activate a unique combination of Ca2+‐binding proteins. These [Ca2+]cyt sensors include CaMs, CaM‐like proteins, calcineurin B‐like (CBL) proteins and Ca2+‐dependent protein kinases (CDPKs). Many of these proteins bind Ca2+ using a helix–loop–helix structure termed the ‘EF hand’, which binds a single Ca2+ molecule with high affinity (Strynadka and James, 1989). Frequently, pairs of EF hands interact through antiparallel β‐sheets, which allows cooperativity in Ca2+ binding. When Ca2+ binds to [Ca2+]cyt sensors their structural and/or enzymatic properties change and their subsequent interactions with target proteins can alter solute transport and enzymatic activities, cytoskeletal orientation, protein phosphorylation cascades and gene expression. It is believed that these changes result in stress tolerance and/or a developmental switch. The form of the physiological response is determined not only by the [Ca2+]cyt perturbation itself, but also by the expression of the [Ca2+]cyt sensors, their affinities for both Ca2+ and target proteins, and the abundance and activity of the target proteins. Since different cell types, and probably even individuals of the same cell type, have contrasting transcript, protein and enzyme profiles, a similar [Ca2+]cyt perturbation will result in individual responses, which may contribute to phenotypic plasticity (Gilroy and Trewavas, 2001).
Calcium‐binding proteins: CaM and CaM‐related proteins
CaM is found in the apoplast and in the cytosol, ER and nucleus of plant cells. Within the cytosol, the estimated CaM concentration is 5 to 40 µm (Zielinski, 1998). Calmodulin has been implicated in Ca2+‐dependent responses to light, gravity, mechanical stress, phytohormones, pathogens, osmotic stress, salinity, heavy metals, xenobiotics, annoxia, oxidative stress, heat shock and chilling (Zielinski, 1998; Snedden and Fromm, 2001; Reddy, 2001; Rudd and Franklin‐Tong, 2001; Fasano et al., 2002). Calmodulin is a small (17 kDa), highly conserved, acidic protein with two globular domains each containing two EF hands connected by a flexible α‐helical spacer (Zielinski, 1998; Reddy, 2001; Snedden and Fromm, 2001; Luan et al., 2002). A Ca2+/CaM complex generally interacts with target proteins, although there are exceptions, such as the interaction of myosin‐like proteins with CaM alone (Reddy, 2001). The binding of Ca2+ to CaM, which has a Kd between 10–7 and 10–6 m, exposes a hydrophobic surface on each globular domain. This enables the Ca2+/CaM complex to wrap around its target protein and bind to its CaM‐binding site with an affinity in the nanomolar range through non‐specific van der Waals interactions. The co‐location of two EF hands in each globular domain allows Ca2+ to bind cooperatively, which ensures that CaM activation occurs over a narrow range of Ca2+ concentrations. The affinity of CaM for Ca2+ may be profoundly influenced by the presence of particular target proteins (Zielinski, 1998). Calmodulins bind to many different proteins implicated in diverse physiological processes including cation transport (including [Ca2+]cyt homeostasis), cytoskeletal rearrangements and cell division, phytohormone and phospholipid signalling, disease resistance (including the oxidative burst) and stress tolerance (Zielinski, 1998; Reddy, 2001; Snedden and Fromm, 2001; Luan et al., 2002; Reddy et al., 2002). Calmodulins can also regulate gene expression by binding to specific transcription factors (Szymanski et al., 1996; Reddy et al., 2000; Yang and Poovaiah, 2000; Bouché et al., 2002).
Small gene families encode CaM isoforms in plants. Many species possess several CaM genes encoding identical proteins as well as other genes encoding divergent isoforms (Zielinski, 1998; Snedden and Fromm, 2001). It is thought that the presence of genes encoding identical CaMs may reflect a need for diverse tissue‐specific, developmental or stress‐induced expression, and the presence of genes encoding different CaM isoforms may reflect a requirement for specific interactions with target proteins (Reddy, 2001; Snedden and Fromm, 2001). Indeed, it has been suggested that particular CaM isoforms transduce specific environmental or developmental signals. Consistent with this hypothesis, it has been shown that environmental challenges rapidly up‐regulate the expression of different CaM isoforms. For example, a subset of CaM genes is up‐regulated by touch in various plants (Braam et al. 1997; Verma and Upadhyaya, 1998; Zielinski, 1998), cold shock and wind stimuli lead to the expression of different CaM isoforms (van der Luit et al., 1999), and specific CaM genes are up‐regulated in response to wounding or pathogens (Heo et al., 1999; Yamakawa et al., 2001), auxin or salinity (Botella and Arteca, 1994). Many CaM isoforms show tissue‐specific and/or developmentally regulated expression (Gawienowski et al. 1993; Takezawa et al., 1995; Yang et al., 1998; Yamakawa et al., 2001; Duval et al., 2002), and CaM isoforms can differentially regulate target enzymes or have contrasting affinities for Ca2+ and CaM‐binding peptides (Liao et al., 1996; Liu et al., 1998; Reddy et al., 1999; Köhler and Neuhaus, 2000; Lee et al., 2000; Duval et al., 2002; Zielinski, 2002). Calmodulins can also be post‐translationally trimethylated, which influences both their stability and physiological activities (Zielinski, 1998). All these properties are consistent with [Ca2+]cyt signatures producing unique biochemical and physiological consequences in cells expressing specific CaM isoforms and target proteins. Interestingly, target proteins themselves may have CaM‐binding or non‐binding isoforms (Snedden and Fromm, 2001), which presumably allows for [Ca2+]cyt‐dependent and [Ca2+]cyt‐independent regulatory cascades.
Plants also possess ‘CAM‐like’ proteins. These have between one and six EF hands and a limited homology to CaM (defined arbitrarily as <75% identity with canonical CaM isoforms; Reddy, 2001; Snedden and Fromm, 2001; Luan et al., 2002; Zielinski, 2002). In arabidopsis, they include: CaBP‐22 (Ling and Zielinski, 1993), TCH2 and TCH3 (Braam et al., 1997), AtCP1 (Jang et al., 1998), centrins (Cordeiro et al., 1998), NADPH oxidases (AtrbohA–F; Torres et al., 1998), homologues of the rice ABA‐inducible EFA27 protein (Frandsen et al., 1996) and Ca2+‐binding protein phosphatases such as ABI1 and ABI2 (Leung et al., 1997). These proteins have been implicated in cellular responses to diverse environmental, developmental and pathological challenges.
Calcium‐binding proteins: calcineurin B‐like proteins
Calcineurin B‐like proteins (CBL) possess three EF hands (Luan et al., 2002). In arabidopsis there are at least ten AtCBL genes, including AtSOS3 (AtCBL4), which encodes a Ca2+‐sensor protein involved in salt tolerance (Liu and Zhu, 1998; Luan et al., 2002; Xiong et al., 2002). Many CBLs have a conserved myristoylation site in their N‐termini, which allows membrane association. It has been proposed that myristoylation of CBLs alters their cellular location and intracellular interactions (Luan et al., 2002). It is thought that particular CBLs transduce specific environmental or developmental signals. Consistent with this hypothesis is the induction of AtCBL1 expression by drought, salinity, cold and wounding (Kudla et al., 1999; Piao et al., 2001) and the accumulation of AtCBL1 and AtCBL2 transcripts in leaves upon illumination (Nozawa et al., 2001). A family of SNF1‐like serine/threonine protein kinases (AtCIPK1 through AtCIPK25) has been identified as Ca2+‐dependent targets for AtCBLs (Shi et al., 1999; Halfter et al., 2000; Albrecht et al., 2001; Y. Guo et al., 2001; Luan et al., 2002). The CIPKs interact with CBLs through a unique 24‐amino‐acid domain termed the ‘NAF domain’ (Albrecht et al., 2001) and require divalent‐cation cofactors for their activity (Luan et al., 2002). Both AtCIPK1 and AtCIPK2 require Mn2+ as a cofactor and AtSOS2 (AtCIPK24) requires Mg2+. Each CBL protein may interact with several CIPKs. For example, AtCBL1 interacts with numerous AtCIPKs (Shi et al., 1999) and AtSOS3 interacts with AtSOS2 (AtCIPK24) and at least seven other AtCIPKs (Halfter et al., 2000; Y. Guo et al., 2001). Conversely, certain AtCIPKs can interact with several AtCBLs (Shi et al., 1999; Kim et al., 2000; Albrecht et al., 2001; Y. Guo et al., 2001). Common interactions between AtCBLs and AtCIPKs may allow cross‐talk between signalling cascades, whereas preferential associations between CBLs and CIPKs are thought to underpin specific signalling cascades. In this context it is noteworthy that the expression of CIPKs (in addition to CBLs) can be tissue specific and/or regulated by environmental stresses such as cold, drought, salinity, wounding or nutrient starvation (Albrecht et al., 2001; Y. Guo et al., 2001; Kim et al., 2003).
Calcium‐binding proteins: calcium‐dependent protein kinases
The activity of many protein kinases can respond to [Ca2+]cyt signals directly. These can be placed in one of four classes: Ca2+‐dependent protein kinases (CDPKs), CDPK‐related proteins (CRKs), CaM‐dependent protein kinases (CaMKs) and chimeric Ca2+‐ and CaM‐dependent protein kinases (CCaMKs). McAinsh and Hetherington (1998) proposed an interesting model for generating specific physiological responses to [Ca2+]cyt perturbations through the action of Ca2+‐dependent and Ca2+‐independent protein kinases and phosphatases. In this model, specific [Ca2+]cyt perturbations influence the degree of phosphorylation of a target protein leading to contrasting or graded responses.
The CDPKs are ubiquitous in plants. There are at least 34 genes encoding CDPKs in the arabidopsis genome (Harmon et al., 2001; S. H. Cheng et al., 2002) and similar numbers in other plant species. They generally have four EF hands at their C‐terminus that bind Ca2+ to activate their serine/threonine kinase activity. They act as monomers and many may be autoinhibited by autophosphorylation of a pseudosubstrate domain (S. H. Cheng et al., 2002). Different CDPKs have contrasting affinities for Ca2+ (Lee et al., 1998) and the binding of Ca2+ to some CDPKs is modulated by lipids, interactions with 14‐3‐3 proteins or phosphorylation (Reddy, 2001; S. H. Cheng et al., 2002; Sanders et al., 2002). No CDPKs appear to be integral membrane proteins, but many are associated with the cytoskeleton, nucleus, plasma membrane and ER (Reddy, 2001; Sanders et al., 2002). At least 24 arabidopsis CDPKs can potentially undergo myristoylation and palmitoylation at their N‐termini, which may facilitate their association with membranes (S. H. Cheng et al., 2002; Xiong et al., 2002).
The CDPKs are capable of converting [Ca2+]cyt signals into biochemical and genetic consequences through the phosphorylation of diverse target proteins including membrane solute transporters (including the Ca2+‐ATPase, AtACA2), ion and water channels, NADPH oxidases, enzymes involved in carbon and nitrogen metabolism, cytoskeletal proteins, proteases and DNA‐binding proteins (Reddy, 2001; Rudd and Franklin‐Tong, 2001; S. H. Cheng et al., 2002; Sanders et al., 2002). They are implicated in pollen development, control of the cell cycle, phytohormone signal transduction, light‐regulated gene expression, gravitropism, thigmotropism, nodulation, cold acclimation, salinity tolerance, drought tolerance and responses to pathogens (Sheen, 1996; Saijo et al., 2000; Anil and Sankara Rao, 2001; Reddy, 2001; Romeis et al., 2001; S. H. Cheng et al., 2002; Xiong et al., 2002; Lee et al., 2003). It is thought that the possession of many CDPKs with contrasting Ca2+ affinities and target proteins allows plant cells to respond appropriately to specific [Ca2+]cyt perturbations (Anil and Sankara Rao, 2001; S. H. Cheng et al., 2002; Sanders et al., 2002). In various plant species specific CDPKs are induced by cold, drought, salinity, annoxia, mechanical stress, wounding and pathogen elicitors (Urao et al., 1994; Breviario et al., 1995; Monroy and Dhinsa, 1995; Botella et al., 1996; Tähtiharju et al., 1997; Yoon et al., 1999; Saijo et al., 2000; Anil and Sankara Rao, 2001; Romeis et al., 2001; Chico et al., 2002; Lee et al., 2003). In addition, the activities of CDPKs are affected by post‐translational modifications during development (Anil et al., 2000) or in response to environmental challenges such as wounding or pathogens (Romeis et al., 2001).
Other protein kinases responding to [Ca2+]cyt are less well characterized than the CDPKs. There are at least seven CRKs in the arabidopsis genome that are structurally similar to CDPKs, but have degenerate or truncated EF hands that may not be able to bind Ca2+ (Reddy, 2001). Orthologues of these are present in many plant species. Several CaMKs have also been cloned from arabidopsis and other plants (Zhang and Lu, 2003). Their kinase activity is activated by CaM‐dependent autophosphorylation and their catalytic activity is also modulated by CaM. They are expressed highly in rapidly growing cells and tissues of the root and flower (Zhang and Lu, 2003). Chimeric CCaMKs are expressed in the anthers of several plant species, including lily and tobacco (Liu et al., 1998), but none have been identified in arabidopsis (Zhang and Lu, 2003). The CCaMKs possess a CaM‐binding domain and three EF hands. They require only Ca2+ for autophosphorylation, but Ca2+ and CaM for substrate phosphorylation. Liu et al. (1998) demonstrated that different CaM isoforms have contrasting effects on substrate phosphorylation by lily and tobacco CCaMKs, suggesting that these kinases could respond to specific environmental or developmental challenges.
Calcium‐binding proteins: proteins without EF hands
Several proteins lacking EF hands are also capable of binding Ca2+ (Reddy, 2001; Anil and Sankara Rao, 2001). For example, the activity of phospholipase D (PLD), which cleaves membrane phospholipids into a soluble head group and phosphatidic acid, is regulated by [Ca2+]cyt through a Ca2+/phospholipid binding‐site termed the ‘C2 domain’ (Wang, 2001). Phospholipase D activity is implicated in cellular responses to ethylene and ABA, α‐amylase synthesis in aleurone cells, stomatal closure, pathogen responses, leaf senescence and drought tolerance (Ritchie et al., 2002). Plants possess several PLD isoforms that differ in their affinity for Ca2+ and their modulation by phosphoinositides, free fatty acids and lysolipids (Wang, 2001). These biochemical modulators of PLD activity are the substrates or products of phospholipase C, which generates IP3 and diacylglycerol, phospholipase A2 and diacylglycerol kinase, both of which are regulated by CaM. It has therefore been suggested that [Ca2+]cyt signalling cascades might coordinate the activities of these diverse enzymes to effect specific responses to contrasting environmental or developmental stimuli (Wang, 2001).
In annexins, Ca2+ is bound by the ‘endonexin fold’, which contains a characteristic GXGT‐{38}‐(D/E) motif (Delmer and Potikha, 1997). This often appears only in the first quarter of plant annexins, although animal annexins generally possess four such motifs. The binding of Ca2+ to annexins enables them to associate with membranes to form cation‐channels (Hofmann et al., 2000; White et al., 2002a). In plants, annexins are encoded by small gene families. Seven annexin genes (AnnAt1‐7) have been identified in arabidopsis (Clark et al., 2001; White et al., 2002a). Most annexin genes are expressed throughout the plant, but annexins are especially abundant in highly secretory cell types, where they are located at the cell periphery and may have a role in membrane fusion, membrane trafficking and/or secretion (Clark et al., 2001). The increased expression of certain annexins upon specific developmental or environmental challenges has linked them with Ca2+ signals during root nodulation, pathogen attack, ABA responses, fruit ripening and cold acclimation (White et al., 2002a).
Other Ca2+‐binding proteins include calreticulin, calsequestrin, calnexin and BiP, which sequester Ca2+ in the ER. These proteins are implicated in Ca2+ homeostasis, protein folding and post‐translational modifications (Crofts and Denecke, 1998; Michalak et al., 1998). The expression of calreticulin is increased in reproductive tissues. Calreticulin possesses two Ca2+‐binding domains. The first is a proline‐rich, low‐capacity/high‐affinity Ca2+‐binding domain (Kd =1 µm; 1 mol Ca2+ mol protein–1) that contains a highly conserved stretch of amino acids (KPEDWD). The second is a high‐capacity/low‐affinity Ca2+‐binding domain (Kd = 2 mm; 25 mol Ca2+ mol protein–1) comprising acidic amino acid residues (generally glutamate and aspartate). Both calreticulin and BiP, and a Ca2+‐binding protein (PCP) expressed in Brassica pistils and implicated in pollen–pistil interactions (Furuyama and Dzelzkalns, 1999) also possess high‐capacity/low‐affinity Ca2+‐binding domains. Calnexin possesses a proline‐rich low‐capacity/high‐affinity Ca2+‐binding domain.
Calcium and CaM‐binding transcription factors
Changes in [Ca2+]cyt may influence nuclear Ca2+ activities, but cell nuclei are also capable of initiating autonomous Ca2+ signals (van der Luit et al., 1999; Pauly et al., 2001). Perturbations in nuclear Ca2+ activities are thought to regulate DNA transcription, the cell cycle during mitosis and meiosis, DNA replication and DNA fragmentation during programmed cell death (Snedden and Fromm, 2001). Signal transduction through Ca2+/CaM has been inferred in several instances (Yang and Poovaiah, 2000; Rudd and Franklin‐Tong, 2001; Snedden and Fromm, 2001; Townley and Knight, 2002). It is possible for Ca2+/CaM to interact with target proteins in the cytosol, which subsequently transduce a signal to the nucleus, or with target proteins in the nucleus itself (Snedden and Fromm, 2001). Several CaM isoforms have been located in the nuclei of plant cells. In animals, [Ca2+]cyt signals appear to direct the translocation of CaM into the nucleus and environmental signals have been shown to alter the distribution of petunia CaM53 (Rodríguez‐Concepción et al., 1999) and rice OsCaM61 (Dong et al., 2002) between the plasma membrane and the nucleus by influencing their prenylation. Transcription factors are among the target proteins for Ca2+/CaM in plant cell nuclei (Szymanski et al., 1996; Reddy et al., 2000; Yang and Poovaiah, 2000; Bouché et al., 2002; Reddy et al., 2002). Such interactions have been suggested in the transduction of phytohormone signals, phytochrome‐ responses and acclimation to low temperatures. Interestingly, the expression of some CaM isoforms might be autoregulated through interactions between Ca2+/CaM and transcription factors (Szymanski et al., 1996). It has been suggested that this could supply stoichiometric levels of CaM isoforms for the activation of target proteins and, thereby, provide a mechanism for their long‐term or subsequent activation. A CaM‐like protein has also been implicated in the suppression of post‐transcriptional gene silencing in plants (Anandalakshmi et al., 2000). In addition to Ca2+/CaM signal transduction cascades, CaM‐independent CDPKs have also been implicated in the regulation of nuclear gene expression (Patharkar and Cushman, 2000) and a nuclear transcription factor with two EF hands (AtSUB1) has been implicated in transcriptional responses to blue and far‐red light (H. Guo et al., 2001). The arabidopsis genome contains two homologues of AtSUB1: AtSUL1 and AtSUL2.
PERSPECTIVES
It has long been known that Ca is an essential element for plants, and that plant species differ in both the amounts of Ca they require and their tolerance of Ca in the rhizosphere. These differences between plant species not only influence the natural flora of calcareous soils but also have consequences for breeding crops to improve the delivery of Ca to the human diet. Recent research in this area has concentrated on elucidating the physiology and biochemistry underpinning contrasting Ca physiotypes and in developing a phylogenetic framework to describe the variation in [Ca]shoot within the angiosperms. This work will be used to inform the modelling of responses of plant communities to environmental change, of nutrient and contaminant partitioning within an ecosystem, and of the delivery of Ca to the food chain.
More recently, research on Ca in plants has been focused at the cellular level. This is presumably a consequence of the realization, some two decades ago, that [Ca2+]cyt was an obligate intracellular messenger coordinating the cellular responses to numerous developmental cues and environmental challenges. Since then, biochemists have characterized the Ca2+‐ATPases and H+/Ca2+‐antiporters required to maintain submicromolar [Ca2+]cyt in a resting cell, and electrophysiologists have characterized the Ca2+‐permeable channels that raise [Ca2+]cyt to initiate [Ca2+]cyt signals. Genes encoding these transport proteins have been identified, and their spatial and temporal expression patterns, subcellular locations, functional properties and physiological roles are being studied using a variety of biochemical, physiological, molecular biological and genetic techniques. In parallel, the origin and specificity of [Ca2+]cyt signals are being addressed. In particular, the hypothesis that each developmental cue or environmental challenge produces a unique [Ca2+]cyt signature to elicit specific cellular responses is being tested using cell‐specific targeting of transgenic aequorin (Knight et al., 1991) and cameleon (Allen et al., 1999) Ca2+‐reporters in combination with single‐cell transcript profiling (Karrer et al., 1995; Brandt et al., 2002). Researchers are also identifying the cellular [Ca2+]cyt‐sensors that allow plant cells to respond appropriately to [Ca2+]cyt signals, and elucidating their biochemical properties and interactions with target proteins. The involvement of specific [Ca2+]cyt‐sensors in particular physiological responses is indicated by their diverse tissue expression patterns and by their contrasting changes in expression in response to developmental or environmental stimuli. This hypothesis will be tested through the analysis of transgenic plants mis‐expressing [Ca2+]cyt‐sensors.
Undoubtedly, the application of functional genomics will provide further insights to the biology of Ca in plants. For example, oligonucleotide microarrays might be used to identify the genetic consequences of acclimation to low or high [Ca2+]ext. This will provide a unique insight into the physiology of Ca accumulation. Genetic responses to changes in [Ca2+]ext might include the differential expression of transport proteins, of enzymes in the biochemical pathways containing compounds that chelate Ca, or of transcriptional cascades that lead to the modification of plant anatomy and/or morphology. These responses could not be predicted a priori. In parallel, genes that impact on Ca accumulation in the shoot might be identified through the resolution of quantitative trait loci (QTL) detected using mapping populations that show variation in their ability to accumulate Ca in the shoot. These are available for several plant species. Information from both microarray and QTL analyses could be used to formulate hypotheses on the impact of specific genes on [Ca]shoot, which might be tested by investigating the phenotype of transgenic plants mis‐expressing candidate genes. Such approaches could ultimately lead to the development of crops that accumulate less of contaminants, such as radiostrontium, or deliver more Ca to the food chain. The challenge of the immediate future will be to integrate the massive data sets arising from plant ‘omic’ technologies into a cohesive conceptual framework for Ca in plants that embraces both its roles in biochemistry and cell signalling.
ACKNOWLEDGEMENTS
We thank Graham Seymour (HRI) for comments on cell wall composition, Lim Ho (HRI) for his photographs of horticultural disorders, Marc Knight (Oxford) for the overview of [Ca2+]cyt signals he presented at ‘Calcium 2002’ at HRI‐Wellesbourne, and Tim Flowers (Sussex) for his editorial encouragement. The research in our laboratories is supported by the Biotechnology and Biological Sciences Research Council (UK) and by the Department for Environment, Food and Rural Affairs (UK).
Supplementary Material
Received: 7 January 2003; Returned for revision: 26 March 2003; Accepted: 6 June 2003 Published electronically: 21 August 2003
References
- AcheP, Becker D, Ivashikina N, Dietrich P, Roelfsema MRG, Hedrich R.2000. GORK, a delayed outward rectifier expressed in guard cells of Arabidopsis thaliana, is a K+‐selective, K+‐sensing ion channel. FEBS Letters 486: 93–98. [DOI] [PubMed] [Google Scholar]
- AlbrechtV, Ritz O, Linder S, Harter K, Kudla J.2001. The NAF domain defines a novel protein–protein interaction module conserved in Ca2+‐regulated kinases. EMBO Journal 20: 1051–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AllenGJ, Sanders D.1997. Vacuolar ion channels in higher plants. Advances in Botanical Research 25: 217–252. [Google Scholar]
- AllenGJ, Chu SP, Harrington CL, Schumacher K, Hoffmann T, Tang YY, Grill E, Schroeder JI.2001. A defined range of guard cell calcium oscillation parameters encodes stomatal movements. Nature 411: 1053–1057. [DOI] [PubMed] [Google Scholar]
- AllenGJ, Chu SP, Schumacher K, Shimazaki CT, Vafeados D, Kemper A, Hawke SD, Tallman G, Tsien RY, Harper JF et al.2000. Alteration of stimulus specific guard cell calcium oscillations and stomatal closing in Arabidopsis det3 mutant. Science 289: 2338–2342. [DOI] [PubMed] [Google Scholar]
- AllenGJ, Kwak JM, Chu SP, Llopis J, Tsien RY, Harper JF, Schroeder JI.1999. Cameleon calcium indicator reports cytoplasmic calcium dynamics in Arabidopsis guard cells. Plant Journal 19: 735–747. [DOI] [PubMed] [Google Scholar]
- AnandalakshmiR, Marathe R, Ge X, Herr JM, Mau C, Mallory A, Pruss G, Bowman L, Vance VB.2000. A calmodulin‐related protein that suppresses posttranscriptional gene silencing in plants. Science 290: 142–144. [DOI] [PubMed] [Google Scholar]
- AnilVS, Harmon AC, Sankara Rao K.2000. Spatio‐temporal accumulation and activity of calcium‐dependent protein kinases during embryogenesis, seed development, and germination in sandalwood. Plant Physiology 122: 1035–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AnilVS, Sankara Rao K.2001. Calcium‐mediated signal transduction in plants: a perspective on the role of Ca2+ and CDPKs during early plant development. Journal of Plant Physiology 158: 1237–1256. [Google Scholar]
- AntoineAF, Dumas C, Faure J‐E, Feijó JA, Rougier M.2001. Egg activation in flowering plants. Sexual Plant Reproduction 14: 21–26. [Google Scholar]
- AsherCJ, Ozanne PG.1961. The cation exchange capacity of plant roots and its relationship to the uptake of insoluble nutrients. Australian Journal of Agricultural Research 12: 755–766. [Google Scholar]
- AxelsenKB, Palmgren MG.2001. Inventory of the superfamily of P‐type ion pumps in Arabidopsis. Plant Physiology 126: 696–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AylingSM, Clarkson DT.1996. The cytoplasmic streaming response of tomato root hairs to auxin: the role of calcium. Australian Journal of Plant Physiology 23: 699–708. [Google Scholar]
- BatteyNH, James NC, Greenland AJ, Brownlee C.1999. Exocytosis and endocytosis. Plant Cell 11: 643–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BaumG, Long JC, Jenkins GI, Trewavas AJ.1999. Stimulation of the blue light phototropic receptor NPH1 causes a transient increase in cytosolic Ca2+ Proceedings of the National Academy of Sciences of the USA 96: 13554–13559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BekreijC, Janse J, Vangoor BJ, Vandoesburg JDJ.1992. The incidence of calcium‐oxalate crystals in fruit walls of tomato Lycopersicon esculentum Mill. as affected by humidity, phosphate and calcium supply. Journal of Horticultural Science 67: 45–50. [Google Scholar]
- BibikovaTN, Blancaflor EB, Gilroy S.1999. Microtubules regulate tip growth and orientation in root hairs of Arabidopsis thaliana Plant Journal 17: 657–665. [DOI] [PubMed] [Google Scholar]
- BlackfordS, Rea PA, Sanders D.1990. Voltage sensitivity of H+/Ca2+ antiport in higher plant tonoplast suggests a role in vacuolar calcium accumulation. Journal of Biological Chemistry 265: 9617–9620. [PubMed] [Google Scholar]
- BlattMR.2000a. Cellular signaling and volume control in stomatal movements in plants. Annual Review of Cell and Developmental Biology 16: 221–241. [DOI] [PubMed] [Google Scholar]
- BlattMR.2000b. Ca2+ signalling and control of guard‐cell volume in stomatal movements. Current Opinion in Plant Biology 3: 196–204. [PubMed] [Google Scholar]
- BlumeB, Nürnberger T, Nass N, Scheel D.2000. Receptor‐mediated increase in cytoplasmic free calcium required for activation of pathogen defense in parsley. Plant Cell 12: 1425–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BlumwaldE, Aharon GS, Lam BC‐H.1998. Early signal transduction pathways in plant‐pathogen interactions. Trends in Plant Science 3: 342–346. [Google Scholar]
- BotellaJR, Arteca RN.1994. Differential expression of two calmodulin genes in response to physical and chemical stimuli. Plant Molecular Biology 24: 757–766. [DOI] [PubMed] [Google Scholar]
- BotellaJR, Arteca JM, Somodevilla M, Arteca RN.1996. Calcium‐dependent protein kinase gene expression in response to physical and chemical stimuli in mungbean (Vigna radiata). Plant Molecular Biology 30: 1129–1137. [DOI] [PubMed] [Google Scholar]
- BouchéN, Scharlat A, Snedden W, Bouchez D, Fromm H.2002. A novel family of calmodulin‐binding transcription activators in multicellular organisms. Journal of Biological Chemistry 277: 21851–21861. [DOI] [PubMed] [Google Scholar]
- BowlerC, Fluhr R.2000. The role of calcium and activated oxygens as signals for controlling cross‐tolerance. Trends in Plant Science 5: 241–246. [DOI] [PubMed] [Google Scholar]
- BraamJ, Sistrunk ML, Polisensky DH, Xu W, Purugganan MM, Antosiewicz DM, Campbell P, Johnson KA.1997. Plant responses to environmental stress: regulation and functions of the Arabidopsis TCH genes. Planta 203: S35–S41. [DOI] [PubMed] [Google Scholar]
- BrandtS, Kloska S, Altmann T, Kehr J.2002. Using array hybridization to monitor gene expression at the single cell level. Journal of Experimental Botany 53: 2315–2323. [DOI] [PubMed] [Google Scholar]
- BreviarioD, Morello L, Giani S.1995. Molecular cloning of two novel rice cDNA sequences encoding putative calcium‐dependent protein kinases. Plant Molecular Biology 27: 953–967. [DOI] [PubMed] [Google Scholar]
- BroadleyMR, Bowen HC, Cotterill HL, Hammond JP, Meacham MC, Mead A, White PJ.2003a. Variation in the shoot calcium content of angiosperms. Journal of Experimental Botany 54: 1431–1446. [DOI] [PubMed] [Google Scholar]
- BroadleyMR, Bowen HC, Cotterill HL, Hammond JP, Meacham MC, Mead A, White PJ.2003b. Quantifying the phylogenetic variation in the shoot mineral content of angiosperms. Journal of Experimental Botany 54: in press. [DOI] [PubMed] [Google Scholar]
- BroadleyMR, Willey NJ, Wilkins JC, Baker AJM, Mead A, White PJ.2001. Phylogenetic variation in heavy metal accumulation in angiosperms. New Phytologist 152: 9–27. [DOI] [PubMed] [Google Scholar]
- BushDS.1995. Calcium regulation in plant cells and its role in signaling. Annual Review of Plant Physiology and Plant Molecular Biology 46: 95–122. [Google Scholar]
- CaddickLR, Rudall PJ, Wilkin P, Hedderson TAJ, Chase MW.2002. Phylogenetics of Dioscoreales based on combined analyses of morphological and molecular data. Botanical Journal of the Linnean Society 138: 123–144. [Google Scholar]
- CamachoL, Malhó R.2003. Endo/exocytosis in the pollen tube apex is differentially regulated by Ca2+ and GTPases. Journal of Experimental Botany 54: 83–92. [DOI] [PubMed] [Google Scholar]
- CampbellAK, Trewavas AJ, Knight MR.1996. Calcium imaging shows differential sensitivity to cooling and communication in luminous transgenic plants. Cell Calcium 19: 211–218. [DOI] [PubMed] [Google Scholar]
- CárdenasL, Holdaway‐Clarke TL, Sánchez F, Quinto C, Feijó JA, Kunkel JG, Hepler PK.2000. Ion changes in legume root hairs responding to Nod factors. Plant Physiology 123: 443–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CessnaSG, Low PS.2001. Activation of the oxidative burst in aequorin‐transformed Nicotiana tabacum cells is mediated by protein kinase‐ and anion channel‐dependent release of Ca2+ from internal stores. Planta 214: 126–134. [DOI] [PubMed] [Google Scholar]
- CessnaSG, Messerli MA, Robinson KR, Low PS.2001. Measurement of stress‐induced Ca2+ pulses in single aequorin‐transformed tobacco cells. Cell Calcium 30: 151–156. [DOI] [PubMed] [Google Scholar]
- ChengN, Pitman JK, Shigaki T, Hirschi KD.2002. Characterization of CAX4, an Arabidopsis H+/cation antiporter. Plant Physiology 128: 1245–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ChengN‐H, Pitman JK, Barkla BJ, Shigaki T, Hirschi KD.2003. The Arabidopsis cax1 mutant exhibits impaired ion homeostasis, development, and hormonal responses and reveals interplay among vacuolar transporters. Plant Cell 15: 347–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ChengS‐H, Willmann MR, Chen H‐C, Sheen J.2002. Calcium signaling through protein kinases. The Arabidopsis calcium‐dependent protein kinase gene family. Plant Physiology 129: 469–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ChicoJM, Raíces M, Téllez‐Iñón MT, Ulloa RM.2002. A calcium‐dependent protein kinase is systemically induced upon wounding in tomato plants. Plant Physiology 128: 256–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ClaphamDE.1995. Calcium signaling. Cell 80: 259–268. [DOI] [PubMed] [Google Scholar]
- ClarkGB, Dauwalder M, Roux SJ.1998. Immunological and biochemical evidence for nuclear localization of annexin in peas. Plant Physiology and Biochemistry 36: 621–627. [DOI] [PubMed] [Google Scholar]
- ClarkGB, Sessions A, Eastburn DJ, Roux SJ.2001. Differential expression of members of the annexin multigene family in Arabidopsis. Plant Physiology 126: 1072–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ClarksonDT.1984. Calcium transport between tissues and its distribution in the plant. Plant, Cell and Environment 7: 449–456. [Google Scholar]
- ClarksonDT.1993. Roots and the delivery of solutes to the xylem. Philosophical Transactions of the Royal Society of London Series B 341: 5–17. [Google Scholar]
- ClaytonH, Knight MR, Knight H, McAinsh MR, Hetherington AM.1999. Dissection of the ozone‐induced calcium signature. Plant Journal 17: 575–579. [DOI] [PubMed] [Google Scholar]
- ClemensS, Antiosiewicz DM, Ward JM, Schachtman DP, Schroeder JI.1998. The plant cDNA LCT1 mediates the uptake of calcium and cadmium in yeast. Proceedings of the National Academy of Sciences of the USA 95: 12043–12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CordeiroMCR, Piqueras R, de Oliveira DE, Castresana C.1998. Characterization of early induced genes in Arabidopsis thaliana responding to bacterial inoculation: identification of centrin and of a novel protein with two regions related to kinase domains. FEBS Letters 434: 387–393. [DOI] [PubMed] [Google Scholar]
- CramerGR, Jones RL.1996. Osmotic stress and abscisic acid reduce cytosolic calcium activities in roots of Arabidopsis thaliana Plant, Cell and Environment 19: 1291–1298. [Google Scholar]
- CroftsAJ, Denecke J.1998. Calreticulin and calnexin in plants. Trends in Plant Science 3: 396–399. [Google Scholar]
- CrookeWM, Knight AH.1962. An evaluation of published data on the mineral composition of plants in the light of the cation‐exchange capacities of their roots. Soil Science 93: 365–373. [Google Scholar]
- DavenportRJ, Tester M.2000. A weakly voltage‐dependent nonselective cation channel mediates toxic sodium influx in wheat. Plant Physiology 122: 823–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De BoerAH.1999. Potassium translocation into the root xylem. Plant Biology 1: 36–45. [Google Scholar]
- de Carvalho‐NiebelF, Timmers ACJ, Chabaud M, Defaux‐Petras A, Barker DG.2002. The Nod factor‐elicited annexin MtAnn1 is preferentially localised at the nuclear periphery in symbiotically activated root tissues of Medicago truncatula Plant Journal 32: 343–352. [DOI] [PubMed] [Google Scholar]
- DelmerDP, Potikha TS.1997. Structure and functions of annexins in plants. Cellular and Molecular Life Sciences 53: 546–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DemidchikVV, Tester MA.2002. Sodium fluxes through non‐selective cation channels in the plasma membrane of protoplasts from Arabidopsis roots. Plant Physiology 128: 379–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DemidchikV, Bowen HC, Maathuis FJM, Shabala SN, Tester MA, White PJ, Davies JM.2002a.Arabidopsis thaliana root non‐selective cation channels mediate calcium uptake and are involved in growth. Plant Journal 32: 799–808. [DOI] [PubMed] [Google Scholar]
- DemidchikVV, Davenport RJ, Tester MA.2002b. Non‐selective cation channels in plants. Annual Review of Plant Biology 53: 67–107. [DOI] [PubMed] [Google Scholar]
- DenisV, Cyert MS.2002. Internal Ca2+ release in yeast is triggered by hypertonic shock and mediated by a TRP channel homologue. Journal of Cell Biology 156: 29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSilvaDLR, Hetherington AM, Mansfield TA.1996. Where does all the calcium go? Evidence of an important regulatory role for trichomes in two calcicoles. Plant, Cell and Environment 19: 880–886. [Google Scholar]
- DeSilvaDLR, Hetherington AM, Mansfield TA.1998. The regulation of apoplastic calcium in relation to intracellular signalling in stomatal guard cells. Zeitschrift fur Pflanzenernährung und Bodenkunde 161: 533–539. [Google Scholar]
- DeWaldDB, Torabinejad J, Jones CA, Shope JC, Cangelosi AR, Thompson JE, Prestwich GD, Hama H.2001. Rapid accumulation of phosphatidylinositol 4,5‐bisphosphate and inositol 1,4,5‐trisphosphate correlates with calcium mobilization in salt‐stressed Arabidopsis. Plant Physiology 126: 759–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DolmetschRE, Lewis RS, Goodnow CC, Healy JI.1997. Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature 386: 855–858. [DOI] [PubMed] [Google Scholar]
- DolmetschRE, Xu K, Lewis RS.1998. Calcium oscillations increase the efficiency and specificity of gene expression. Nature 392: 933–936. [DOI] [PubMed] [Google Scholar]
- DongA, Xin H, Yu Y, Sun C, Cao K, Shen W‐H.2002. The subcellular localization of an unusual rice calmodulin isoform, OsCaM61, depends on its prenylation state. Plant Molecular Biology 48: 203–210. [DOI] [PubMed] [Google Scholar]
- DrøbakBK, Watkins PAC.2000. Inositol(1,4,5)trisphosphate production in plant cells: an early response to salinity and hyperosmotic stress. FEBS Letters 481: 240–244. [DOI] [PubMed] [Google Scholar]
- DurnerJ, Wendehenne D, Klessig DF.1998. Defense gene induction in tobacco by nitric oxide, cyclic GMP, and cyclic ADP‐ribose. Proceedings of the National Academy of Sciences of the USA 95: 10328–10333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuvalFD, Renard M, Jaquinod M, Biou V, Montrichard F, Macherel D.2002. Differential expression and functional analysis of three calmodulin isoforms in germinating pea (Pisum sativum L.) seeds. Plant Journal 32: 481–493. [DOI] [PubMed] [Google Scholar]
- EvansDE, Williams LE.1998. P‐type calcium ATPases in higher plants – biochemical, molecular and functional properties. Biochimica et Biophysica Acta 1376: 1–25. [DOI] [PubMed] [Google Scholar]
- EvansNH, McAinsh MR, Hetherington AM.2001. Calcium oscillations in higher plants. Current Opinion in Plant Biology 4: 415–420. [DOI] [PubMed] [Google Scholar]
- FasanoJM, Massa GD, Gilroy S.2002. Ionic signaling in plant responses to gravity and touch. Journal of Plant Growth Regulation 21: 71–88. [DOI] [PubMed] [Google Scholar]
- FellbrichG, Blume B, Brunner F, Hirt H, Kroj T, Ligterink W, Romanski A, Nürnberger T.2000.Phytophthora parasitica elicitor‐induced reactions in cells of Petroselinum crispum Plant and Cell Physiology 41: 692–701. [DOI] [PubMed] [Google Scholar]
- FelleH.1988. Auxin causes oscillations of cytosolic free calcium and pH in Zea mays coleoptiles. Planta 174: 495–499. [DOI] [PubMed] [Google Scholar]
- FlowersTJ, Yeo AR.1992.Solute transport in plants. London: Blackie Academic and Professional. [Google Scholar]
- ForemanJ, Demidchik V, Bothwell JHF, Mylona P, Miedema H, Torres MA, Linstead P, Costa S, Brownlee C, Jones JDGet al.2003. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 422: 442–446. [DOI] [PubMed] [Google Scholar]
- FranceschiVR, Horner HT.1980. Calcium oxalate crystals in plants. Botanical Reviews 46: 361–427. [Google Scholar]
- FrandsenG, Müller‐Uri F, Nielsen M, Mundy J, Skriver K.1996. Novel plant Ca2+‐binding protein expressed in response to abscisic acid and osmotic stress. Journal of Biological Chemistry 271: 343–348. [DOI] [PubMed] [Google Scholar]
- Franklin‐TongVE, Holdaway‐Clarke TL, Straatman KR, Kunkel JG, Hepler PK.2002. Involvement of extracellular calcium influx in the self‐incompatibility response of Papaver rhoeas Plant Journal 29: 333–345. [DOI] [PubMed] [Google Scholar]
- FréjatA, Anstett A, Lemaire F.1967. Capacité d’échange de cations des systèmes radiculaires et des sols, et leurs relations avec l’alimentation minérale. Annales Agronomique 18: 31–64. [Google Scholar]
- FrohnmeyerH, Loyall L, Blatt MR, Grabov A.1999. Millisecond UV‐B irradiation evokes prolonged elevation of cytosolic‐free Ca2+ and stimulates gene expression in transgenic parsley cell cultures. Plant Journal 20: 109–117. [DOI] [PubMed] [Google Scholar]
- FuruichiT, Cunningham KW, Muto S.2001. A putative two pore channel AtTPC1 mediates Ca2+ flux in Arabidopsis leaf cells. Plant and Cell Physiology 42: 900–905. [DOI] [PubMed] [Google Scholar]
- FuruyamaT, Dzelzkalns VA.1999. A novel calcium‐binding protein is expressed in Brassica pistils and anthers late in flower development. Plant Molecular Biology 39: 729–737. [DOI] [PubMed] [Google Scholar]
- GarciadeblasB, Benito B, Rodríguez‐Navarro A.2001. Plant cells express several stress calcium ATPases but apparently no sodium ATPase. Plant and Soil 235: 181–192. [Google Scholar]
- GawienowskiMC, Szymanski D, Perera IY, Zielinski RE.1993. Calmodulin isoforms in Arabidopsis encoded by multiple divergent mRNAs. Plant Molecular Biology 22: 215–225. [DOI] [PubMed] [Google Scholar]
- GaymardF, Pilot G, Lacombe B, Bouchez D, Bruneau D, Boucherez J, Michaux‐Ferrière N, Thibaud J‐B, Sentenac H.1998. Identification and disruption of a plant shaker‐like outward channel involved in K+ release into the xylem sap. Cell 94: 647–655. [DOI] [PubMed] [Google Scholar]
- GeislerM, Axelsen KB, Harper JF, Palmgren MG.2000. Molecular aspects of higher plant P‐type Ca2+‐ATPases. Biochimica et Biophysica Acta 1465: 52–78. [DOI] [PubMed] [Google Scholar]
- GilroyS, Trewavas A.2001. Signal processing and transduction in plant cells: the end of the beginning? Nature Reviews Molecular Cell Biology 2: 307–314. [DOI] [PubMed] [Google Scholar]
- GongM, van der Luit AH, Knight MR, Trewavas AJ.1998. Heat‐shock‐induced changes in intracellular Ca2+ level in tobacco seedlings in relation to thermotolerance. Plant Physiology 116: 429–437. [Google Scholar]
- GrabovA, Blatt MR.1998. Membrane voltage initiates Ca2+ waves and potentiates Ca2+ increases with abscisic acid in stomatal guard cells. Proceedings of the National Academy of Sciences of the USA 95: 4778–4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GrahamRD, Welch RM, Bouis HE.2001. Addressing micronutrient malnutrition through enhancing the nutritional quality of staple foods: principles, perspectives and knowledge gaps. Advances in Agronomy 70: 77–142. [Google Scholar]
- GrantM, Brown I, Adams S, Knight M, Ainslie A, Mansfield J.2000. The RPM1 plant disease resistance gene facilitates a rapid and sustained increase in cytosolic calcium that is necessary for the oxidative burst and hypersensitive cell death. Plant Journal 23: 441–450. [DOI] [PubMed] [Google Scholar]
- GrimeJP.2001Plant strategies, vegetation processes, and ecosystem properties, 2nd edn. Chichester, UK: Wiley. [Google Scholar]
- GrygorczykC, Grygorczyk R.1998. A Ca2+‐ and voltage‐dependent cation channel in the nuclear envelope of red beet. Biochimica et Biophysica Acta 1375: 117–130. [DOI] [PubMed] [Google Scholar]
- GuoH, Mockler T, Duong H, Lin C.2001. SUB1, an Arabidopsis Ca2+‐binding protein involved in cryptochrome and phytochrome coaction. Science 291: 487–490. [DOI] [PubMed] [Google Scholar]
- GuoY, Halfter U, Ishitani M, Zhu J‐K.2001. Molecular characterization of functional domains in the protein kinase SOS2 that is required for plant salt tolerance. Plant Cell 13: 1383–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HaleyA, Russell AJ, Wood N, Allan AC, Knight M, Campbell AK, Trewavas AJ.1995. Effects of mechanical signaling on plant cell cytosolic calcium. Proceedings of the National Academy of Sciences of the USA 92: 4124–4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HalfterU, Ishitani M, Zhu J‐K.2000. The Arabidopsis SOS2 protein kinase physically interacts with and is activated by the calcium‐binding protein SOS3. Proceedings of the National Academy of Sciences of the USA 97: 3735–3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HalperinSJ, Gilroy S, Lynch JP.2003. Sodium chloride reduces growth and cytosolic calcium, but does not affect cytosolic pH, in root hairs of Arabidopsis thaliana L. Journal of Experimental Botany 54: 1269–1280. [DOI] [PubMed] [Google Scholar]
- HamiltonDWA, Hills A, Blatt MR.2001. Extracellular Ba2+ and voltage interact to gate Ca2+ channels at the plasma membrane of stomatal guard cells. FEBS Letters 491: 99–103. [DOI] [PubMed] [Google Scholar]
- HamiltonDWA, Hills A, Köhler B, Blatt MR.2000. Ca2+ channels at the plasma membrane of stomatal guard cells are activated by hyperpolarization and abscisic acid. Proceedings of the National Academy of Sciences of the USA 97: 4967–4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HarmonAC, Gribskov M, Gubrium E, Harper JF.2001. The CDPK superfamily of protein kinases. New Phytologist 151: 175–183. [DOI] [PubMed] [Google Scholar]
- HarperJF.2001. Dissecting calcium oscillators in plant cells. Trends in Plant Science 6: 395–397. [DOI] [PubMed] [Google Scholar]
- HaynesRJ.1980. Ion exchange properties of roots and ionic interactions within the root apoplasm: their role in ion accumulation by plants. Botanical Review 46: 75–99. [Google Scholar]
- HeintzeSG.1961. Studies on cation‐exchange capacities of roots. Plant and Soil 13: 365–383. [Google Scholar]
- HeoWD, Lee SH, Kim MC, Kim JC, Chung WS, Chun HJ, Lee KJ, Park CY, Park HC, Choi JY, Cho MJ.1999. Involvement of specific calmodulin isoforms in salicylic acid‐independent activation of plant disease resistance responses. Proceedings of the National Academy of Sciences of the USA 96: 766–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HirschiK.2001. Vacuolar H+/Ca2+ transport: who’s directing the traffic? Trends in Plant Science 6: 100–104. [DOI] [PubMed] [Google Scholar]
- HofmannA, Proust J, Dorowski A, Schantz R, Huber R 2000. Annexin 24 from Capsicum annuum X‐ray structure and biochemical characterization. Journal of Biological Chemistry 275: 8072–8082. [DOI] [PubMed] [Google Scholar]
- Holdaway‐ClarkeTL, Feijó JA, Hackett GR, Kunkel JG, Hepler PK.1997. Pollen tube growth and the intracellular cytosolic calcium gradient oscillate in phase while extracellular calcium influx is delayed. Plant Cell 9: 1999–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HuangF‐Y, Philosoph‐Hadas S, Meir S, Callaham DA, Sabato R, Zelcer A, Hepler PK.1997. Increases in cytosolic Ca2+ in parsley mesophyll cells correlate with leaf senescence. Plant Physiology 115: 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HwangI, Sze H, Harper JF.2000. A calcium‐dependent protein kinase can inhibit a calmodulin‐stimulated Ca2+ pump (ACA2) located in the endoplasmic reticulum of Arabidopsis Proceedings of the National Academy of Sciences of the USA 97: 6224–6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JangHJ, Pih KT, Kang SG, Lim JH, Jin JB, Piao HL, Hwang I.1998. Molecular cloning of a novel Ca2+‐binding protein that is induced by NaCl stress. Plant Membrane Biology 37: 839–847. [DOI] [PubMed] [Google Scholar]
- JarvisMC, Forsyth W, Duncan HJ.1988. A survey of the pectic content of nonlignified monocot cell walls. Plant Physiology 88: 309–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JefferiesRL, Willis AJ.1964. Studies on the calcicole‐calcifuge habit. II. The influence of calcium on the growth and establishment of four species in soil and sand cultures. Journal of Ecology 52: 691–707. [Google Scholar]
- JohnsonCH, Knight MR, Kondo T, Masson P, Sedbrook J, Haley A, Trewavas A.1995. Circadian oscillations of cytosolic and chloroplastic free calcium in plants. Science 269: 1863–1865. [DOI] [PubMed] [Google Scholar]
- KarleyAJ, Leigh RA, Sanders D.2000. Where do all the ions go? The cellular basis of differential ion accumulation in leaf cells. Trends in Plant Science 5: 465–470. [DOI] [PubMed] [Google Scholar]
- KarrerEE, Lincoln JE, Hogenhout S, Bennett AB, Bostock RM, Martineau B, Lucas WJ, Gilchrist DG, Alexander D.1995.In situ isolation of mRNA from individual plant cells: creation of cell‐specific cDNA libraries. Proceedings of the National Academy of Sciences of the USA 92: 3814–3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KawanoT, Muto S.2000. Mechanism of peroxidase actions for salicylic acid‐induced generation of active oxygen species and an increase in cytosolic calcium in tobacco cell suspension culture. Journal of Experimental Botany 51: 685–693. [PubMed] [Google Scholar]
- KiegleE, Gilliham M, Haseloff J, Tester M.2000a. Hyperpolarisation‐activated calcium currents found only in cells from the elongation zone of Arabidopsis thaliana roots. Plant Journal 21: 225–229. [DOI] [PubMed] [Google Scholar]
- KiegleE, Moore CA, Haseloff J, Tester MA, Knight MR.2000b. Cell‐type‐specific calcium responses to drought, salt and cold in the Arabidopsis root. Plant Journal 23: 267–278. [DOI] [PubMed] [Google Scholar]
- KimHY, Coté GG, Crain RC.1996. Inositol 1,4,5‐trisphosphate may mediate closure of K+ channels by light and darkness in Samanea saman motor cells. Planta 198: 279–287. [DOI] [PubMed] [Google Scholar]
- KimK‐N, Cheong YH, Grant JJ, Pandey GK, Luan S.2003.CIPK3, a calcium sensor‐associated protein kinase that regulates abscisic acid and cold signal transduction in Arabidopsis. Plant Cell 18: 411–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KimK‐N, Cheong YH, Gupta R, Luan S.2000. Interaction specificity of Arabidopsis calcineurin B‐like calcium sensors and their target kinases. Plant Physiology 124: 1844–1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KinzelH.1982.Pflanzenökologie und Mineralstoffwechsel. Stuttgart, Germany: Ulmer. [Google Scholar]
- KinzelH, Lechner I.1992. The specific mineral metabolism of selected plant species and its ecological implications. Botanica Acta 105: 355–361. [Google Scholar]
- KlessigDF, Durner J, Noad R, Navarre DA, Wendehenne D, Kumar D, Zhou JM, Shah J, Zhang S, Kachroo Pet al.2000. Nitric oxide and salicylic acid signaling in plant defense. Proceedings of the National Academy of Sciences of the USA 97: 8849–8855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KlüsenerB, Weiler EW.1999. A calcium‐selective channel from root‐tip endomembranes of garden cress. Plant Physiology 119: 1399–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KlüsenerB, Boheim G, LißH, Engelberth J, Weiler EW.1995. Gadolinium‐sensitive, voltage‐dependent calcium release channels in the endoplasmic reticulum of a higher plant mechanoreceptor organ. EMBO Journal 14: 2708–2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KlüsenerB, Young JJ, Murata Y, Allen GJ, Mori IC, Hugouvieux V, Schroeder JI.2002. Convergence of calcium signaling pathways of pathogenic elicitors and abscisic acid in Arabidopsis guard cells. Plant Physiology 130: 2152–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KnightH.2000. Calcium signaling during abiotic stress in plants. International Review of Cytology 195: 269–324. [DOI] [PubMed] [Google Scholar]
- KnightH, Knight MR.2000. Imaging spatial and cellular characteristics of low temperature calcium signature after cold acclimation in Arabidopsis Journal of Experimental Botany 51: 1679–1686. [DOI] [PubMed] [Google Scholar]
- KnightH, Brandt S, Knight MR.1998. A history of stress alters drought calcium signalling pathways in Arabidopsis Plant Journal 16: 681–687. [DOI] [PubMed] [Google Scholar]
- KnightH, Trewavas AJ, Knight MR.1996. Cold calcium signaling in Arabidopsis involves two cellular pools and a change in calcium signature after acclimation. Plant Cell 8: 489–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KnightH, Trewavas AJ, Knight MR.1997. Calcium signalling in Arabidopsis thaliana responding to drought and salinity. Plant Journal 12: 1067–1078. [DOI] [PubMed] [Google Scholar]
- KnightMR, Campbell AK, Smith SM, Trewavas AJ.1991. Transgenic plant aequorin reports the effects of touch and cold‐shock and elicitors on cytoplasmic calcium. Nature 352: 524–526. [DOI] [PubMed] [Google Scholar]
- KnightMR, Read ND, Campbell AK, Trewavas AJ.1993. Imaging calcium dynamics in living plants using semisynthetic recombinant aequorins. Journal of Cell Biology 121: 83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KnightMR, Smith SM, Trewavas AJ.1992. Wind‐induced plant motion immediately increases cytosolic calcium. Proceedings of the National Academy of Sciences of the USA 89: 4967–4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KöhlerB, Blatt MR.2002. Protein phosphorylation activates the guard cell Ca2+ channel and is a prerequisite for gating by abscisic acid. Plant Journal 32: 185–194. [DOI] [PubMed] [Google Scholar]
- KöhlerB, Hills A, Blatt MR.2003. Control of guard cell ion channels by hydrogen peroxide and abscisic acid indicates their action through alternate signaling pathways. Plant Physiology 131: 385–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KöhlerC, Neuhaus D.2000. Characterization of calmodulin binding to cyclic nucleotide‐gated ion channels from Arabidopsis thaliana FEBS Letters 471: 133–136. [DOI] [PubMed] [Google Scholar]
- KovácsI, Ayaydin F, Oberschall A, Ipacs I, Bottka S, Pongor S, Dudits D, Tóth EC.1998. Immunolocalization of a novel annexin‐like protein encoded by a stress and abscisic acid responsive gene in alfalfa. Plant Journal 15: 185–187. [DOI] [PubMed] [Google Scholar]
- KudlaJ, Xu Q, Harter K, Gruissem W, Luan S.1999. Genes for calcineurin B‐like proteins in Arabidopsis are differentially regulated by stress signals. Proceedings of the National Academy of Sciences of the USA 96: 4718–4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo‐HuangL‐L, Sheeu C‐R, Yang Y‐P, Chiang S‐HT.1994. Calcium oxalate crystals in some aquatic angiosperms of Taiwan. Botanical Bulletin of Academia Sinica 35: 179–188. [Google Scholar]
- LeckieCP, McAinsh MR, Allen GJ, Sanders D, Hetherington AM.1998. Abscisic acid‐induced stomatal closure mediated by cyclic ADP‐ribose. Proceedings of the National Academy of Sciences of the USA 95: 15837–15842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LecourieuxD, Mazars C, Pauly N, Ranjeva R, Pugin A.2002. Analysis and effects of cytosolic free calcium increases in response to elicitors in Nicotiana plumbaginifolia cells. Plant Cell 14: 2627–2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeeJA.1999. The calcicole‐calcifuge problem revisited. Advances in Botanical Research 29: 1–30. [Google Scholar]
- LeeJY, Yoo BC, Harmon AC.1998. Kinetic and calcium‐binding properties of three calcium‐dependent protein kinase isoenzymes from soybean. Biochemistry 37: 6801–6809. [DOI] [PubMed] [Google Scholar]
- LeeSH, Johnson JD, Walsh MP, Van Lierop JE, Sutherland C, Xu A, Snedden WA, Kosk‐Kosicka D, Fromm H, Narayanan N, Cho MJ.2000. Differential regulation of Ca2+/calmodulin‐dependent enzymes by plant calmodulin isoforms and free Ca2+ concentration. Biochemical Journal 350: 299–306. [PMC free article] [PubMed] [Google Scholar]
- LeeSS, Cho HS, Yoon GM, Ahn J‐W, Kim H‐H, Pai H‐S.2003. Interaction of NtCDPK1 calcium‐dependent protein kinase with NtRpn3 regulatory subunit of the 26S proteasome in Nicotiana tabacum Plant Journal 33: 825–840. [DOI] [PubMed] [Google Scholar]
- LeguéV, Blancaflor E, Wymer C, Perbal G, Fantin D, Gilroy S.1997. Cytoplasmic free Ca2+ in Arabidopsis roots changes in response to touch but not gravity. Plant Physiology 114: 789–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemtiri‐ChliehF, MacRobbie EAC, Brearley CA.2000. Inositol hexakisphosphate is a physiological signal regulating the K+‐inward rectifying conductance in guard cells. Proceedings of the National Academy of Sciences of the USA 97: 8687–8692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeungJ, Merlot S, Giraudat J.1997. The arabidopsis ABSCISIC ACID‐INSENSITIVE2 (ABI2) and ABI1 genes encode homologous protein phosphatases 2C involved in abscisic acid signal transduction. Plant Cell 9: 759–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LevineA, Pennell RI, Alvarez ME, Palmer R, Lamb C.1996. Calcium‐mediated apoptosis in a plant hypersensitive disease resistance response. Current Biology 6: 427–437. [DOI] [PubMed] [Google Scholar]
- LhuissierFGP, De Ruijter NCA, Sieberer BJ, Esseling JJ, Emons AMC.2001. Time course of cell biological events evoked in legume root hairs by Rhizobium Nod factors: state of the art. Annals of Botany 87: 289–302. [Google Scholar]
- LiW‐H, Llopsis J, Whitney M, Zlokarnik G, Tsien RY.1998. Cell‐permeant caged InsP3 ester shows that Ca2+ spike frequency can optimize gene expression. Nature 392: 936–941. [DOI] [PubMed] [Google Scholar]
- LiaoB, Gawienowski MC, Zielinski RE.1996. Differential stimulation of NAD kinase and binding of peptide substrates by wild‐type and mutant plant calmodulin isoforms. Archives of Biochemistry and Biphysics 327: 53–60. [DOI] [PubMed] [Google Scholar]
- LibertB, Franceschi VR.1987. Oxalate in crop plants. Journal of Agriculture and Food Chemistry 35: 926–938. [Google Scholar]
- LingV, Zielinski RE.1993. Isolation of an Arabidopsis cDNA sequence encoding a 22 kDa calcium‐binding protein (CaBP‐22) related to calmodulin. Plant Molecular Biology 22: 207–214. [DOI] [PubMed] [Google Scholar]
- LiuJ, Zhu J‐K.1998. A calcium sensor homolog required for plant salt tolerance. Science 280: 1943–1945. [DOI] [PubMed] [Google Scholar]
- LiuZ, Xia M, Poovaiah BW.1998. Chimeric calcium/calmodulin‐dependent protein kinase in tobacco: differential regulation by calmodulin isoforms. Plant Molecular Biology 38: 889–897. [DOI] [PubMed] [Google Scholar]
- LuanS, Kudla J, Rodríguez‐Concepción M, Yalovsky S, Gruissem W 2002. Calmodulins and calcineurin B‐like proteins: calcium sensors for specific signal response coupling in plants. Plant Cell 14: S389–S400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAinshMR, Hetherington AM.1998. Encoding specificity in Ca2+ signalling systems. Trends in Plant Science 3: 32–36. [Google Scholar]
- McAinshMR, Brownlee C, Hetherington AM.1992. Visualizing changes in cytosolic‐free Ca2+ during the response of stomatal guard cells to abscisic acid. Plant Cell 4: 1113–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAinshMR, Clayton H, Mansfield TA, Hetherington AM.1996. Changes in stomatal behavior and guard cell cytosolic free calcium in response to oxidative stress. Plant Physiology 111: 1031–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAinshMR, Webb AAR, Taylor JE, Hetherington AM.1995. Stimulus‐induced oscillations in guard cell cytosolic free calcium. Plant Cell 7: 1207–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlinSB, Wimmer R.1999. Calcium physiology and terrestrial ecosystem processes. New Phytologist 142: 373–417. [Google Scholar]
- MalhóR.1998. Role of 1,4,5‐inositol triphosphate‐induced Ca2+ release in pollen tube orientation. Sexual Plant Reproduction 11: 231–235. [Google Scholar]
- MalhóR, Trewavas AJ.1996. Localized apical increases of cytosolic free calcium control pollen tube orientation. Plant Cell 8: 1935–1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MalhóR, Camacho L, Moutinho A.2000. Signalling pathways in pollen tube growth and reorientation. Annals of Botany 85: 59–68. [Google Scholar]
- MalhóR, Moutinho A, van der Luit A, Trewavas AJ.1998. Spatial characteristics of calcium signalling: the calcium wave as a basic unit in plant cell calcium signalling. Philosophical Transactions of the Royal Society of London Series B 353: 1463–1473. [Google Scholar]
- MarschnerH.1995.Mineral nutrition of higher plants, 2nd edn. London: Academic Press. [Google Scholar]
- MäserP, Thomine S, Schroeder JI, Ward JM, Hirschi K, Sze H, Talke IN, Amtmann A, Maathuis FJM, Sanders Det al.2001. Phylogenetic relationships within cation transporter families of Arabidopsis. Plant Physiology 126: 1646–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MazarsC, Thion L, Thuleau P, Graziana G, Knight MR, Moreau M, Ranjeva R.1997. Organization of cytoskeleton controls the changes in cytosolic calcium of cold‐shocked Nicotiana plumbaginifolia protoplasts. Cell Calcium 22: 413–420. [DOI] [PubMed] [Google Scholar]
- MesserliMA, Créton R, Jaffe LF, Robinson KR.2000. Periodic increases in elongation rate precede increases in cytosolic Ca2+ during pollen tube growth. Developmental Biology 222: 84–98. [DOI] [PubMed] [Google Scholar]
- MichalakM, Mariani P, Opas M.1998. Calreticulin, a multifunctional Ca2+ binding chaperone of the endoplasmic reticulum. Biochemistry and Cell Biology 76: 779–785. [DOI] [PubMed] [Google Scholar]
- MiedemaH, Bothwell JHF, Brownlee C, Davies JM.2001. Calcium uptake by plant cells – channels and pumps acting in concert. Trends in Plant Science 6: 514–519. [DOI] [PubMed] [Google Scholar]
- MithöferA, Ebel J, Bhagwat AA, Boller T, Neuhaus‐Url G.1999. Transgenic aequorin monitors cytosolic calcium transients in soybean cells challenged with β‐glucan or chitin elicitors. Planta 207: 566–574. [Google Scholar]
- MonroyAF, Dhinsa RS.1995. Low‐temperature signal transduction: induction of cold acclimation‐specific genes of alfalfa by calcium at 25 °C. Plant Cell 7: 321–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MooreCA, Bowen HC, Scrase‐Field S, Knight MR, White PJ.2002. The deposition of suberin lamellae determines the magnitude of cytosolic Ca2+ elevations in root endodermal cells subjected to cooling. Plant Journal 30: 457–466. [DOI] [PubMed] [Google Scholar]
- MuirSR, Sanders D.1997. Inositol 1,4,5‐trisphosphate‐sensitive Ca2+ release across nonvacuolar membranes in cauliflower. Plant Physiology 114: 1511–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MurataY, Pei ZM, Mori IC, Schroeder J.2001. Abscisic acid activation of plasma membrane Ca2+ channels in guard cells requires cytosolic NADPH and is differentially disrupted upstream and downstream of reactive oxygen species production in abi1‐1 and abi2‐1 protein phosphatase 2C mutants. Plant Cell 13: 2513–2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro‐AvinoJP, Hentzen AE, Bennett AB.1999. Alternative transcription initiation sites generate two LCA1 Ca2+‐ATPase mRNA transcripts in tomato roots. Plant Molecular Biology 40: 133–140. [DOI] [PubMed] [Google Scholar]
- NavazioL, Bewell MA, Siddiqua A, Dickinson GD, Galione A, Sanders D.2000. Calcium release from the endoplasmic reticulum of higher plants elicited by the NADP metabolite nicotinic acid adenine dinucleotide phosphate. Proceedings of the National Academy of Sciences of the USA 97: 8693–8698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NavazioL, Mariani P, Sanders D.2001. Mobilization of Ca2+ by cyclic ADP‐ribose from the endoplasmic reticulum of cauliflower florets. Plant Physiology 125: 2129–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NgCK‐Y, Carr K, McAinsh MR, Powell B, Hetherington AM.2001a. Drought‐induced guard cell signal transduction involves sphingosine‐1‐phosphate. Nature 410: 596–598 [DOI] [PubMed] [Google Scholar]
- NgCK‐Y, McAinsh MR, Gray JE, Hunt L, Leckie CP, Mills L, Hetherington AM.2001b. Calcium‐based signalling systems in guard cells. New Phytologist 151: 109–120. [DOI] [PubMed] [Google Scholar]
- NozawaA, Koizumi, Sano H.2001. An Arabidopsis SNF1‐related protein kinase, AtSR1, interacts with a calcium‐binding protein, AtCBL2, of which transcripts respond to light. Plant and Cell Physiology 42: 976–981. [DOI] [PubMed] [Google Scholar]
- PatharkarOR, Cushman JC.2000. A stress‐induced calcium‐dependent protein kinase from Mesembryanthemum crystallinum phosphoryl ates a two‐component pseudo‐response regulator. Plant Journal 24: 679–691. [DOI] [PubMed] [Google Scholar]
- PaulyN, Knight MR, Thuleau P, Graziana A, Muto S, Ranjeva R, Mazars C.2001. The nucleus together with the cytosol generates patterns of specific cellular calcium signatures in tobacco suspension culture cells. Cell Calcium 30: 413–421. [DOI] [PubMed] [Google Scholar]
- PeiZ‐M, Murata Y, Benning G, Thomine S, Klüsener B, Allen GJ, Grill E, Schroeder JI.2000. Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 406: 731–734. [DOI] [PubMed] [Google Scholar]
- PereraIY, Heilmann, Boss WF.1999. Transient and sustained increases in inositol 1,4,5‐trisphosphate precede the differential growth response in gravistimulated maize pulvini. Proceedings of the National Academy of Sciences of the USA 96: 5838–5843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfus‐BarbeochL, Leonhardt N, Vavasseur A, Forestier C.2002. Heavy metal toxicity: cadmium permeates through calcium channels and disturbs the plant water status. Plant Journal 32: 539–548. [DOI] [PubMed] [Google Scholar]
- PiaoHL, Lim JH, Kim SJ, Cheong G‐W, Hwang I.2001. Constitutive over‐expression of AtGSK1 induces NaCl stress responses in the absence of NaCl stress and results in enhanced NaCl tolerance in Arabidopsis Plant Journal 27: 305–314. [DOI] [PubMed] [Google Scholar]
- PickardBG, Ding JP.1993. The mechanosensory calcium‐selective ion channel: key component of a plasmalemmal control centre? Australian Journal of Plant Physiology 20: 439–459. [DOI] [PubMed] [Google Scholar]
- PittmanJK, Shigaki T, Cheng N‐H, Hirschi KD.2002a. Mechanism of N‐terminal autoinhibition in the Arabidopsis Ca2+/H+ antiporter CAX1. Journal of Biological Chemistry 277: 26452–26459. [DOI] [PubMed] [Google Scholar]
- PittmanJK, Sreevidya CS, Shigaki T, Ueoka‐Nakanishi H, Hirschi KD.2002b. Distinct N‐terminal regulatory domains of Ca2+/H+ antiporters. Plant Physiology 130: 1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PliethC.2001. Plant calcium signaling and monitoring: pros and cons and recent experimental approaches. Protoplasma 218: 1–23. [DOI] [PubMed] [Google Scholar]
- PliethC, Trewavas AJ.2002. Reorientation of seedlings in the earth’s gravitational field induces cytosolic calcium transients. Plant Physiology 129: 786–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PliethC, Hansen U‐P, Knight H, Knight MR.1999. Temperature sensing by plants: the primary characteristics of signal perception and calcium response. Plant Journal 18: 491–497. [DOI] [PubMed] [Google Scholar]
- PottosinII, Dobrovinskaya OR, Muñiz J.2001. Conduction of monovalent and divalent cations in the slow vacuolar channel. Journal of Membrane Biology 181: 55–65. [DOI] [PubMed] [Google Scholar]
- PottosinII, Tikhonova LI, Hedrich R, Schönknecht G.1997. Slowly activating vacuolar channels cannot mediate Ca2+‐induced Ca2+‐release. Plant Journal 12: 1387–1398. [Google Scholar]
- PriceAH, Taylor A, Ripley SJ, Griffiths A, Trewavas AJ, Knight MR.1994. Oxidative signals in tobacco increase cytosolic calcium. Plant Cell 6: 1301–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PrychidCJ, Rudall PJ.1999. Calcium oxalate crystals in monocotyledons: a review of their structure and systematics. Annals of Botany 84: 725–739. [Google Scholar]
- ReddyASN.2001. Calcium: silver bullet in signaling. Plant Science 160: 381–404. [DOI] [PubMed] [Google Scholar]
- ReddyASN, Reddy VS, Golovkin M.2000. A calmodulin binding protein from Arabidopsis is induced by ethylene and contains a DNA‐binding motif. Biochemical and Biophysical Research Communications 279: 762–769. [DOI] [PubMed] [Google Scholar]
- ReddyVS, Ali GS, Reddy ASN.2002. Genes encoding calmodulin‐binding proteins in the Arabidopsis genome. Journal of Biological Chemistry 277: 9840–9852. [DOI] [PubMed] [Google Scholar]
- ReddyVS, Safadi F, Zielinski RE, Reddy ASN.1999. Interaction of a kinesin‐like protein with calmodulin isoforms from Arabidopsis Journal of Biological Chemistry 274: 31727–31733. [DOI] [PubMed] [Google Scholar]
- RitchieSM, Swanson SJ, Gilroy S.2002. From common signalling components to cell specific responses: insights from the cereal aleurone. Physiologia Plantarum 115: 342–351. [DOI] [PubMed] [Google Scholar]
- RobertsSK, Snowman BN.2000. The effects of ABA on channel‐mediated K+ transport across higher plant roots. Journal of Experimental Botany 51: 1585–1594. [DOI] [PubMed] [Google Scholar]
- Rodríguez‐ConcepciónM, Yalovsky S, Zik M, Fromm H, Gruissem W.1999. The prenylation status of a novel plant calmodulin directs plasma membrane or nuclear localization of the protein. EMBO Journal 18: 1996–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RomeisT, Ludwig AA, Martin R, Jones JDG.2001. Calcium‐dependent protein kinases play an essential role in a plant defence response. EMBO Journal 20: 5556–5567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RuddJJ, Franklin‐Tong VE.2001. Unravelling response‐specificity in Ca2+ signalling pathways in plant cells. New Phytologist 151: 7–33. [DOI] [PubMed] [Google Scholar]
- SaijoY, Hata S, Kyozuka J, Shimamoto K, Izui K.2000. Over‐expression of a single Ca2+‐dependent protein kinase confers both cold and salt/drought tolerance on rice plants. Plant Journal 23: 319–327. [DOI] [PubMed] [Google Scholar]
- SandersD, Brownlee C, Harper JF.1999. Communicating with calcium. Plant Cell 11: 691–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SandersD, Pelloux J, Brownlee C, Harper JF.2002. Calcium at the crossroads of signaling. Plant Cell 14: S401–S417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SattelmacherB.2001. The apoplast and its significance for plant mineral nutrition. New Phytologist 149: 167–192. [DOI] [PubMed] [Google Scholar]
- SchonknechtG, Spoormaker P, Steinmeyer R, Bruggeman L, Ache P, Dutta R, Reintanz B, Godde M, Hedrich R, Palme K.2002. KCO1 is a component of the slow‐vacuolar SV ion channel. FEBS Letters 511: 28–32. [DOI] [PubMed] [Google Scholar]
- SchroederJI, Allen GJ, Hugouvieux V, Kwak JM, Waner D.2001. Guard cell signal transduction. Annual Review of Plant Physiology and Plant Molecular Biology 52: 627–658. [DOI] [PubMed] [Google Scholar]
- SedbrookJC, Kronebusch PJ, Borisy GG, Trewavas AJ, Masson PH.1996. Transgenic AEQUORIN reveals organ‐specific cytosolic Ca2+ responses to anoxia in Arabidopsis thaliana seedlings. Plant Physiology 111: 243–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ShacklockPS, Read ND, Trewavas AJ.1992. Cytosolic free calcium mediates red light‐induced photomorphogenesis. Nature 358: 753–755. [Google Scholar]
- ShawSL, Long SR.2003. Nod factor elicits two separable calcium responses in Medicago truncatula root hair cells. Plant Physiology 131: 976–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ShearCB.1975. Calcium‐related disorders of fruits and vegetables. HortScience 10: 361–365. [Google Scholar]
- SheenJ.1996. Ca2+‐dependent protein kinases and stress signal transduction in plants. Science 274: 1900–1902. [DOI] [PubMed] [Google Scholar]
- ShiJ, Kim K‐N, Ritz O, Albrecht V, Gupta R, Harter K, Luan S, Kudla J.1999. Novel protein kinases associated with calcineurin B‐like calcium sensors in Arabidopsis. Plant Cell 11: 2393–2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ShigakiT, Hirschi K.2000. Characterisation of CAX‐like genes in plants: implications for functional diversity. Gene 257: 291–298. [DOI] [PubMed] [Google Scholar]
- ShigakiT, Sreevidya C, Hirschi KD.2002. Analysis of the Ca2+ domain in the Arabidopsis H+/Ca2+ antiporters CAX1 and CAX3. Plant Molecular Biology 50: 475–483. [DOI] [PubMed] [Google Scholar]
- SmithRL, Wallace A.1956. Cation‐exchange capacity of roots and its relation to calcium and potassium content of plants. Soil Science 81: 97–109. [Google Scholar]
- SnaydonRW, Bradshaw AD.1969. Differences between natural populations of Trifolium repens L. in response to mineral nutrients. Journal of Applied Ecology 6: 185–202. [Google Scholar]
- SneddenWA, Fromm H.2001. Calmodulin as a versatile calcium signal transducer in plants. New Phytologist 151: 35–66. [DOI] [PubMed] [Google Scholar]
- StaxénI, Pical C, Montgomery LT, Gray JE, Hetherington AM, McAinsh MR.1999. Abscisic acid induces oscillations in guard‐cell cytosolic free calcium that involve phosphoinositide‐specific phospholipase C. Proceedings of the National Academy of Sciences of the USA 96: 1779–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- StoelzleS, Kagawa T, Wada M, Hedrich R, Dietrich P.2003. Blue light activates calcium‐permeable channels in Arabidopsis mesophyll cells via the phototropin signaling pathway. Proceedings of the National Academy of Sciences of the USA 100: 1456–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- StraatmanKR, Dove SK, Holdaway‐Clarke T, Hepler PK, Kunkel JG, Franklin‐Tong VE.2001. Calcium signalling in pollen of Papaver rhoeas undergoing the self‐incompatibility SI response. Sexual Plant Reproduction 14: 105–110. [Google Scholar]
- StrynadkaNCJ, James MNG.1989. Crystal structures of the helix–loop–helix calcium‐binding proteins. Annual Review of Biochemistry 58: 951–998. [DOI] [PubMed] [Google Scholar]
- SubbaiahCC, Bush DS, Sachs MM.1994. Elevation of cytosolic calcium precedes anoxic gene expression in maize suspension‐cultured cells. Plant Cell 6: 1747–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SubbaiahCC, Bush DS, Sachs MM.1998. Mitochondrial contribution to the anoxic Ca2+ signal in maize suspension‐cultured cells. Plant Physiology 118: 759–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SzeH, Liang F, Hwang I, Curran AC, Harper JF.2000. Diversity and regulation of plant Ca2+ pumps: insights from expression in yeast. Annual Review of Plant Physiology and Plant Molecular Biology 51: 433–462. [DOI] [PubMed] [Google Scholar]
- SzymanskiDB, Liao B, Zielinski RE.1996. Calmodulin isoforms differentially enhance the binding of cauliflower nuclear proteins and recombinant TGA3 to a region derived from the Arabidopsis Cam‐3 promoter. Plant Cell 8: 1069–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TähtiharjuS, Sangwan V, Monroy AF, Dhindsa RS, Borg M.1997. The induction of kin genes in cold‐acclimating Arabidopsis thaliana Evidence of a role for calcium. Planta 203: 442–447. [DOI] [PubMed] [Google Scholar]
- TakahashiK, Isobe M, Knight MR, Trewavas AJ, Muto S.1997. Hypoosmotic shock induces increases in cytosolic Ca2+ in tobacco suspension‐culture cells. Plant Physiology 113: 587–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TakezawaD, Liu ZH, An G, Poovaiah BW.1995. Calmodulin gene family in potato: developmental and touch‐induced expression of the mRNA encoding a novel isoform. Plant Molecular Biology 27: 693–703. [DOI] [PubMed] [Google Scholar]
- ThionL, Mazars C, Nacry P, Bouchez D, Moreau M, Ranjeva R, Thuleau P.1998. Plasma membrane depolarization‐activated calcium channels, stimulated by microtubule‐depolymerizing drugs in wild‐type Arabidopsis thaliana protoplasts, display constitutively large activities and a longer half‐life in ton2 mutant cells affected in the organization of cortical microtubules. Plant Journal 13: 603–610. [DOI] [PubMed] [Google Scholar]
- ThionL, Mazars C, Thuleau P, Graziana A, Rossignol M, Moreau M, Ranjeva R.1996. Activation of plasma‐membrane voltage‐dependent calcium‐permeable channels by disruption of microtubules in carrot cells. FEBS Letters 393: 13–18. [DOI] [PubMed] [Google Scholar]
- ThompsonK, Parkinson JA, Band SR, Spencer RE.1997. A comparative study of leaf nutrient concentrations in a regional herbaceous flora. New Phytologist 136: 679–689. [DOI] [PubMed] [Google Scholar]
- TorresMA, Onouchi H, Hamada S, Machida C, Hammond‐Kosack KE, Jones JDG.1998. Six Arabidopsis thaliana homologues of the human respiratory burst oxidase (gp91phox). Plant Journal 14: 365–370. [DOI] [PubMed] [Google Scholar]
- TownleyHE, Knight MR.2002. Calmodulin as a potential negative regulator of Arabidopsis COR gene expression. Plant Physiology 128: 1169–1172. [DOI] [PubMed] [Google Scholar]
- TrewavasA.1999. Le calcium, c’est la vie: calcium makes waves. Plant Physiology 120: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UraoT, Katagiri T, Mizoguchi T, Yamaguchi‐Shinozaki K, Hayashida N, Shinozaki K.1994. Two genes that encode Ca2+‐dependent protein kinases are induced by drought and high‐salt stresses in Arabidopsis thaliana Molecular and General Genetics 244: 331–340. [DOI] [PubMed] [Google Scholar]
- van den WijngaardPWJ, Bunney TD, Roobeek I, Schonknecht G, de Boer AH.2001. Slow vacuolar channels from barley mesophyll cells are regulated by 14‐3‐3 proteins. FEBS Letters 488: 100–104. [DOI] [PubMed] [Google Scholar]
- van der LuitAH, Olivari C, Haley A, Knight MR, Trewavas AJ.1999. Distinct calcium signaling pathways regulate calmodulin gene expression in tobacco. Plant Physiology 121: 705–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VermaPK, Upadhyaya KC 1998. A multiplex RT‐PCR assay for analysis of relative transcript levels of different members of multigene families: application to Arabidopsis calmodulin gene family. Biochemistry and Molecular Biology International 46: 699–706. [DOI] [PubMed] [Google Scholar]
- VéryA‐A, Davies JM.2000. Hyperpolarization‐activated calcium channels at the tip of Arabidopsis root hairs. Proceedings of the National Academy of Sciences of the USA 97: 9801–9806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VéryA.‐A, Sentenac H.2002. Cation channels in the Arabidopsis plasma membrane. Trends in Plant Science 7: 168–175. [DOI] [PubMed] [Google Scholar]
- WacquantJ.‐P.1977. Physicochemical selectivity for cations and CEC of grass roots. Plant and Soil 47: 257–261. [Google Scholar]
- WaisRJ, Galera C, Oldroyd G, Catoira R, Penmetsa RV, Cook D, Gough C, Dénarie J, Long SR.2000. Genetic analysis of calcium spiking responses in nodulation mutants of Medicago truncatula Proceedings of the National Academy of Sciences of the USA 97: 13407–13412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WalkerSA, Viprey V, Downie JA.2000. Dissection of nodulation signaling using pea mutants defective for calcium spiking induced by Nod factors and chitin oligomers. Proceedings of the National Academy of Sciences of the USA 97: 13413–13418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WangX.2001. Plant phospholipases. Annual Review of Plant Physiology and Plant Molecular Biology 52: 211–231. [DOI] [PubMed] [Google Scholar]
- WebbAAR, Larman MG, Montgomery LT, Taylor JE, Hetherington AM.2001. The role of calcium in ABA‐induced gene expression and stomatal movements. Plant Journal 26: 351–362. [DOI] [PubMed] [Google Scholar]
- WebbAAR, McAinsh MR, Mansfield TA, Hetherington AM.1996. Carbon dioxide induces increases in guard cell cytosolic free calcium. Plant Journal 9: 297–304. [Google Scholar]
- WhitePJ.1997. Cation channels in the plasma membrane of rye roots. Journal of Experimental Botany 48: 499–514. [DOI] [PubMed] [Google Scholar]
- WhitePJ.1998. Calcium channels in the plasma membrane of root cells. Annals of Botany 81: 173–183. [Google Scholar]
- WhitePJ.2000. Calcium channels in higher plants. Biochimica et Biophysica Acta 1465: 171–189. [DOI] [PubMed] [Google Scholar]
- WhitePJ.2001. The pathways of calcium movement to the xylem. Journal of Experimental Botany 52: 891–899. [DOI] [PubMed] [Google Scholar]
- WhitePJ, Davenport RJ.2002. The voltage‐independent cation channel in the plasma membrane of wheat roots is permeable to divalent cations and may be involved in cytosolic Ca2+ homeostasis. Plant Physiology 130: 1386–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WhitePJ, Banfield J, Diaz M.1992. Unidirectional Ca2+ fluxes in roots of rye (Secale cereale L.). A comparison of excised roots with roots of intact plants. Journal of Experimental Botany 43: 1061–1074. [Google Scholar]
- WhitePJ, Bowen HC, Demidchik V, Nichols C, Davies JM.2002a. Genes for calcium‐permeable channels in the plasma membrane of plant root cells. Biochimica et Biophysica Acta 1564: 299–309. [DOI] [PubMed] [Google Scholar]
- WhitePJ, Whiting SN, Baker AJM, Broadley MR.2002b. Does zinc move apoplastically to the xylem in roots of Thlaspi caerulescens? New Phytologist 153: 199–211. [Google Scholar]
- WoodNT, Haley A, Viry‐Moussaïd, Johnson CH, van der Luit AH, Trewavas AJ.2001. The calcium rhythms of different cell types oscillate with different circadian phases. Plant Physiology 125: 787–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WuCC, Kuo‐Huang LL.1997. Calcium crystals in the leaves of some species of Moraceae. Botanical Bulletin of Academia Sinica 38: 97–104. [Google Scholar]
- WuY, Kuzma J, Maréchal E, Graeff R, Lee HC, Foster R, Chua NH.1997. Abscisic acid signaling through plant cyclic ADP‐ribose in plants. Science 278: 2126–2130. [DOI] [PubMed] [Google Scholar]
- WuZ, Liang F, Hong B, Young JC, Sussman MR, Harper JF, Sze H.2002. An endoplasmic reticulum‐bound Ca2+/Mn2+ pump, ECA1, supports plant growth and confers tolerance to Mn2+ stress. Plant Physiology 130: 128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WymerCL, Bibikova TN, Gilroy S.1997. Cytoplasmic free calcium distributions during the development of root hairs of Arabidopsis thaliana Plant Journal 12: 427–439. [DOI] [PubMed] [Google Scholar]
- XiongL, Schumaker KS, Zhu J‐K.2002. Cell signaling during cold, drought, and salt stress. Plant Cell 14: S165–S183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YamakawaH, Mitsuhara I, Ito N, Seo S, Kamada H, Ohashi Y.2001. Transcriptionally and post‐transcriptionally regulated response of 13 calmodulin genes to tobacco mosaic virus‐induced cell death and wounding in tobacco plants. European Journal of Biochemistry 268: 3916–3929. [DOI] [PubMed] [Google Scholar]
- YangT, Poovaiah BW 2000. An early ethylene up‐regulated gene encoding a calmodulin‐binding protein involved in plant senescence and death. Journal of Biological Chemistry 275: 38467–38473. [DOI] [PubMed] [Google Scholar]
- YangT, Lev‐Yadun S, Feldman M, Fromm H.1998. Developmentally regulated organ‐, tissue‐, and cell‐specific expression of calmodulin genes in common wheat. Plant Molecular Biology 37: 109–120. [DOI] [PubMed] [Google Scholar]
- YoonGM, Cho HS, Ha HJ, Liu JR, Lee HP.1999. Characterization of NtCDPK1, a calcium‐dependent protein kinase gene in Nicotiana tabacum, and the activity of its encoded protein. Plant Molecular Biology 39: 991–1001. [DOI] [PubMed] [Google Scholar]
- ZhangL, Lu Y‐T.2003. Calmodulin‐binding protein kinases in plants. Trends in Plant Science 8: 123–127. [DOI] [PubMed] [Google Scholar]
- ZhangW‐H, Rengel Z.1999. Aluminium induces an increase in cytoplasmic calcium in intact wheat root apical cells. Australian Journal of Plant Physiology 26: 401–409. [Google Scholar]
- ZielinskiRE.1998. Calmodulin and calmodulin‐binding proteins in plants. Annual Review of Plant Physiology and Plant Molecular Biology 49: 697–725. [DOI] [PubMed] [Google Scholar]
- ZielinskiRE.2002. Characterization of three new members of the Arabidopsis thaliana calmodulin gene family: conserved and highly diverged members of the gene family functionally complement a yeast calmodulin null. Planta 214: 446–455. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.