Abstract
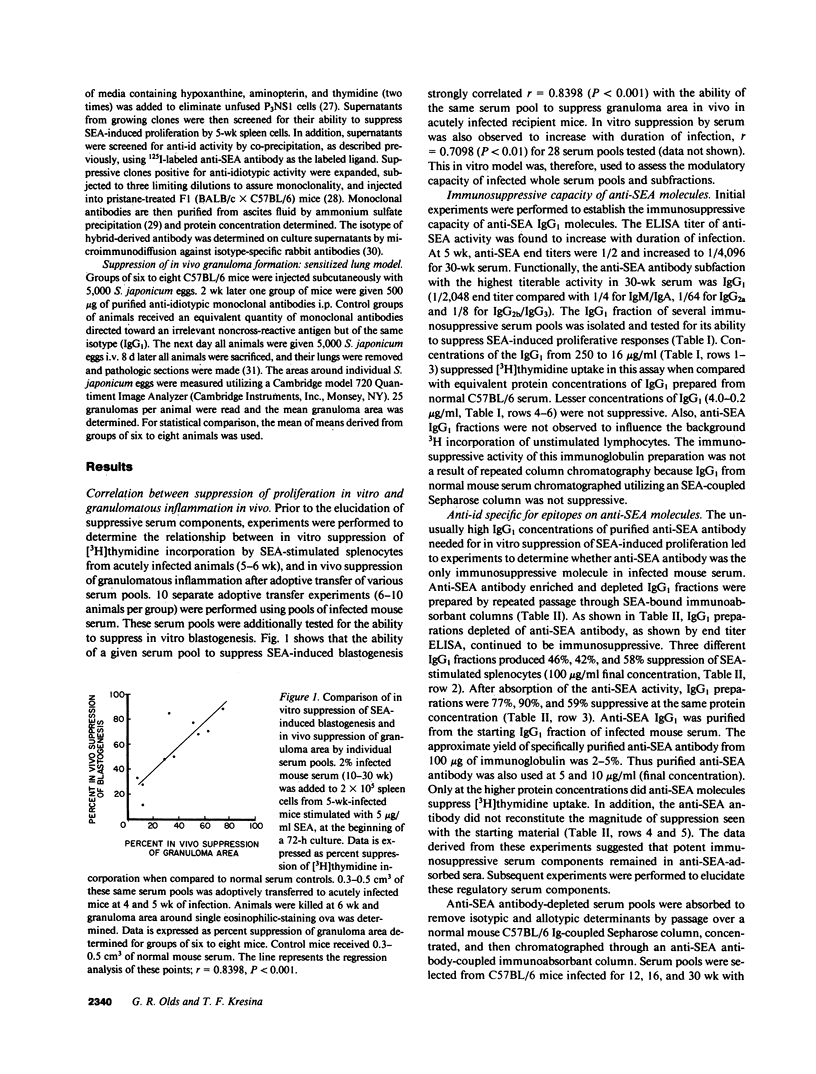
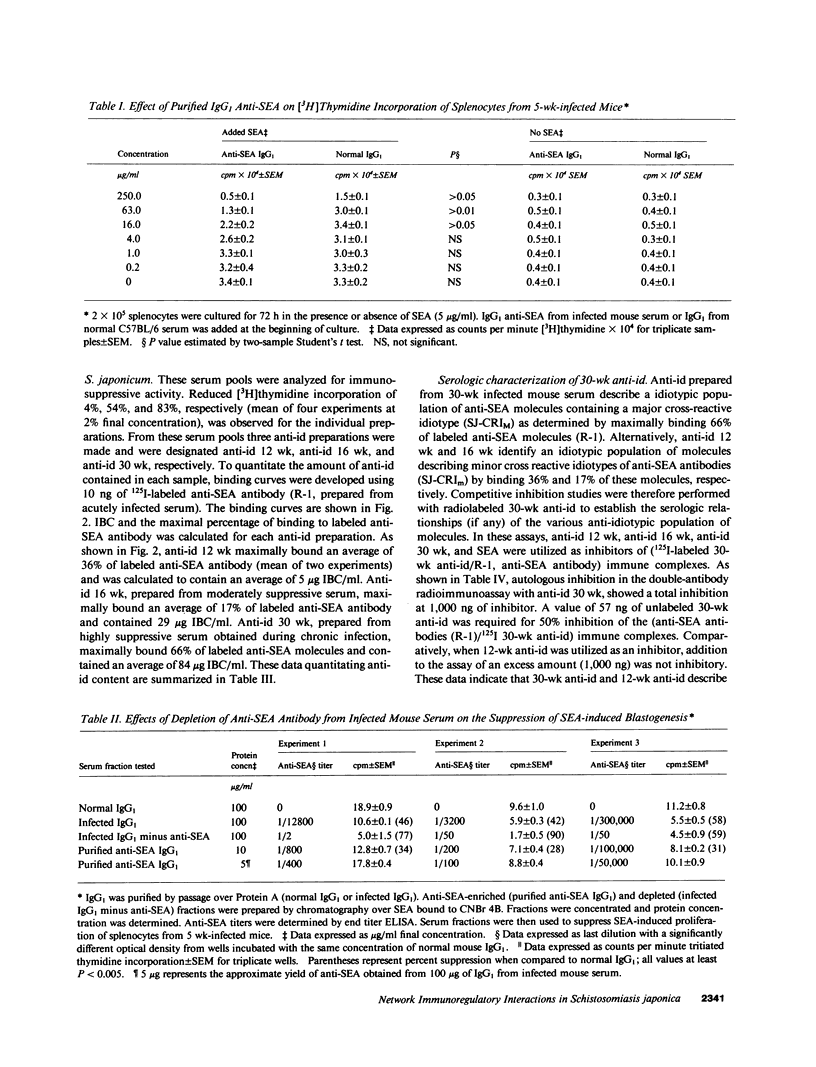
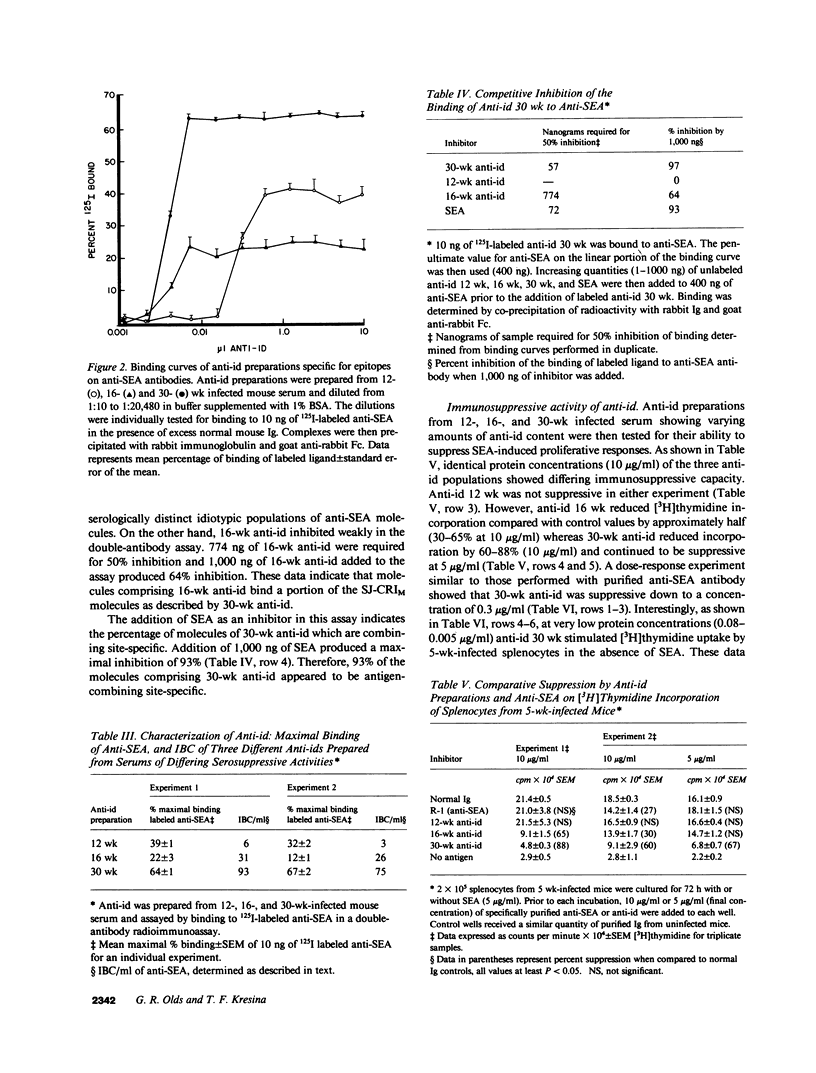
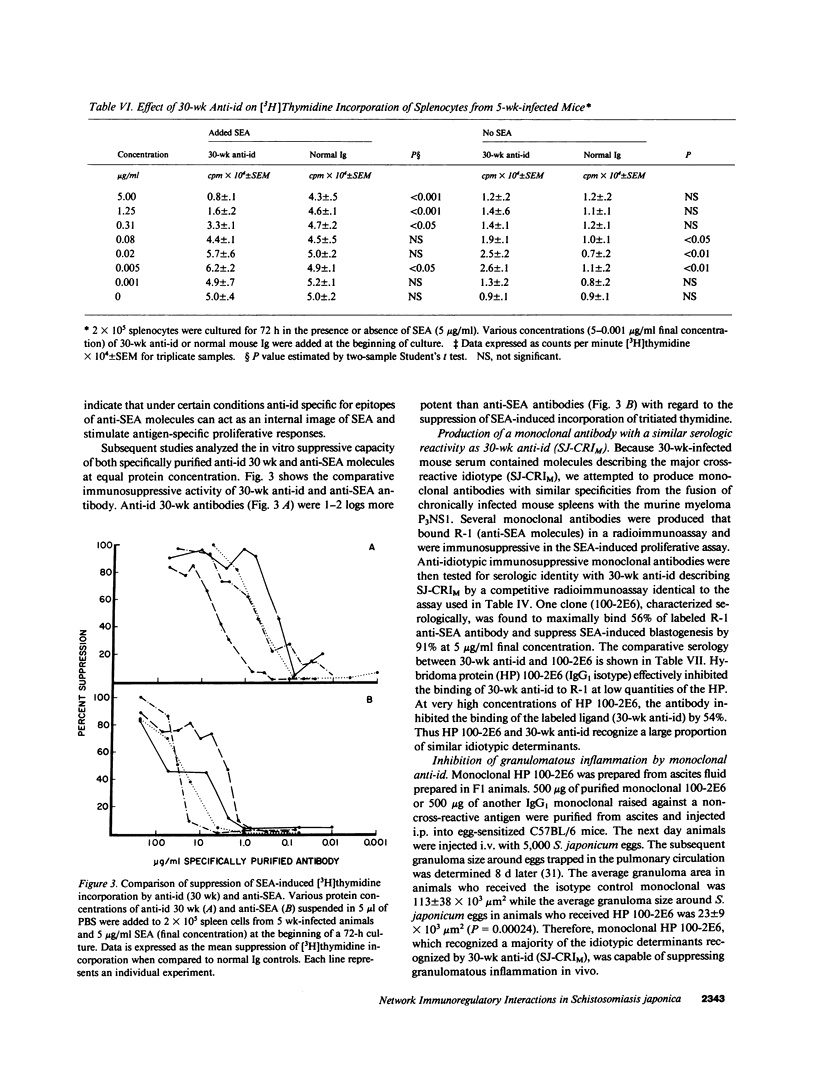
This study examined the role of naturally occurring anti-idiotypic antibody (anti-id), specific for epitopes on antibodies to schistosome egg antigens (SEA), in serosuppression during Schistosoma japonicum infection. Three anti-id preparations were obtained from pools of infected serum at 12, 16, and 30 wk of infection. Anti-id (12 wk) bound 36% of labeled anti-SEA antibodies, had an idiotype binding capacity (IBC) of 5 micrograms/ml, and did not suppress SEA-induced proliferation. Anti-id (16 wk) bound 17% of labeled anti-SEA antibodies, had 29 micrograms IBC/ml, and reduced 3H incorporation from 21.4 +/- 0.5 (10 micrograms/ml normal Ig) to 9.1 +/- 1.5 X 10(4) cpm (P less than 0.01). Anti-id (30 wk) bound 66% of labeled anti-SEA antibody, had 84 micrograms IBC/ml, and suppressed 3H incorporation by 88% (4.8 +/- 0.3 X 10(4) cpm, P less than 0.001). Analysis of the serologic reactivity of these three populations of anti-idiotypic antibodies revealed that anti-id (12 wk) described an idiotypic population of anti-SEA molecules containing a minor cross-reactive idiotype (SJ-CRIm). In contrast, anti-id (30 wk) described a serologically distinct, idiotypic population containing a major cross-reactive idiotype of anti-SEA molecules (SJ-CRIM). A monoclonal anti-id, which reacted with greater than 50% of the anti-SEA molecules describing SJ-CRIM, was profoundly suppressive in vitro and reduced granulomatous inflammation around parasite eggs in vivo from 113 X 10(3) micron2 to 23 X 10(3) micron2 (80% suppression, P less than 0.001). These observations suggest that immune network interactions modulate inflammation in chronic murine S. japonicum infection.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDRADE Z. A. WARREN KS: MILD PROLONGED SCHISTOSOMIASIS IN MICE: ALTERATIONS IN HOST RESPONSE WITH TIME AND THE DEVELOPMENT OF PORTAL FIBROSIS. Trans R Soc Trop Med Hyg. 1964 Jan;58:53–57. doi: 10.1016/0035-9203(64)90068-9. [DOI] [PubMed] [Google Scholar]
- Attallah A. M., Smith A. H., Murrell K. D., Fleischer T., Woody J., Vannier W. E., Scher I., Ahmed A., Sell K. W. Characterization of the immunosuppressive state during Schistosoma mansoni infection. J Immunol. 1979 Apr;122(4):1413–1420. [PubMed] [Google Scholar]
- Bona C., Kearney J. Anti-immunoglobulin antibodies. II. Expression of individual and cross-reactive idiotypes on syngeneic and homologous anti-idiotype antibodies. J Immunol. 1981 Aug;127(2):491–495. [PubMed] [Google Scholar]
- Boros D. L., Pelley R. P., Warren K. S. Spontaneous modulation of granulomatous hypersensitivity in schistosomiasis mansoni. J Immunol. 1975 May;114(5):1437–1441. [PubMed] [Google Scholar]
- Boros D. L., Pelley R. P., Warren K. S. Spontaneous modulation of granulomatous hypersensitivity in schistosomiasis mansoni. J Immunol. 1975 May;114(5):1437–1441. [PubMed] [Google Scholar]
- Boros D. L., Warren K. S. Delayed hypersensitivity-type granuloma formation and dermal reaction induced and elicited by a soluble factor isolated from Schistosoma mansoni eggs. J Exp Med. 1970 Sep 1;132(3):488–507. doi: 10.1084/jem.132.3.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boros D. L., Warren K. S. Delayed hypersensitivity-type granuloma formation and dermal reaction induced and elicited by a soluble factor isolated from Schistosoma mansoni eggs. J Exp Med. 1970 Sep 1;132(3):488–507. doi: 10.1084/jem.132.3.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briles D. E., Forman C., Hudak S., Claflin J. L. Anti-phosphorylcholine antibodies of the T15 idiotype are optimally protective against Streptococcus pneumoniae. J Exp Med. 1982 Oct 1;156(4):1177–1185. doi: 10.1084/jem.156.4.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. C., Rodkey L. S. Autoregulation of an antibody response via network-induced auto-anti-idiotype. J Exp Med. 1979 Jul 1;150(1):67–85. doi: 10.1084/jem.150.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheever A. W., Byram J. E., Hieny S., von Lichtenberg F., Lunde M. N., Sher A. Immunopathology of Schistosoma japonicum and S. mansoni infection in B cell depleted mice. Parasite Immunol. 1985 Jul;7(4):399–413. doi: 10.1111/j.1365-3024.1985.tb00086.x. [DOI] [PubMed] [Google Scholar]
- Cheever A. W., Duvall R. H., Hallack T. A., Jr Differences in hepatic fibrosis and granuloma size in several strains of mice infected with Schistosoma japonicum. Am J Trop Med Hyg. 1984 Jul;33(4):602–607. doi: 10.4269/ajtmh.1984.33.602. [DOI] [PubMed] [Google Scholar]
- Chensue S. W., Boros D. L., David C. S. Regulation of granulomatous inflammation in murine schistosomiasis. In vitro characterization of T lymphocyte subsets involved in the production and suppression of migration inhibition factor. J Exp Med. 1980 Jun 1;151(6):1398–1412. doi: 10.1084/jem.151.6.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chensue S. W., Boros D. L. Modulation of granulomatous hypersensitivity. I. Characterization of T lymphocytes involved in the adoptive suppression of granuloma formation in Schistosoma mansoni-infected mice. J Immunol. 1979 Sep;123(3):1409–1414. [PubMed] [Google Scholar]
- Chensue S. W., Wellhausen S. R., Boros D. L. Modulation of granulomatous hypersensitivity. II. Participation of Ly 1+ and Ly 2+ T lymphocytes in the suppression of granuloma formation and lymphokine production in Schistosoma mansoni-infected mice. J Immunol. 1981 Jul;127(1):363–367. [PubMed] [Google Scholar]
- Chien Y. H., Gascoigne N. R., Kavaler J., Lee N. E., Davis M. M. Somatic recombination in a murine T-cell receptor gene. Nature. 1984 May 24;309(5966):322–326. doi: 10.1038/309322a0. [DOI] [PubMed] [Google Scholar]
- Colizzi V., Giuntini M., Garzelli C., Campa M., Falcone G. Auto-anti-idiotypic antibodies inhibit T-cell-mediated hypersensitivity in BCG-infected mice. Cell Immunol. 1983 Aug;80(1):205–210. doi: 10.1016/0008-8749(83)90107-7. [DOI] [PubMed] [Google Scholar]
- Colley D. G. Adoptive suppression of granuloma formation. J Exp Med. 1976 Mar 1;143(3):696–700. doi: 10.1084/jem.143.3.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colley D. G., Freeman G. L., Jr Differences in adult Schistosoma mansoni worm burden requirements for the establishment of resistance to reinfection in inbred mice. II. C57BL/KsJ, SWR/J, SJL/J, BALB/cAnN, DBA/2N, A/J, B10.A(3R), and B10.A(5R) mice. Am J Trop Med Hyg. 1983 May;32(3):543–549. doi: 10.4269/ajtmh.1983.32.543. [DOI] [PubMed] [Google Scholar]
- Colley D. G., Lewis F. A., Todd C. W. Adoptive suppression of granuloma formation by T lymphocytes and by lymphoid cells sensitive to cyclophosphamide. Cell Immunol. 1979 Aug;46(1):192–200. doi: 10.1016/0008-8749(79)90258-2. [DOI] [PubMed] [Google Scholar]
- Cosenza H., Augustin A., Julius M. H. Induction and characterization of "autologous" anti-isiotypic antibodies. Eur J Immunol. 1977 May;7(5):273–278. doi: 10.1002/eji.1830070506. [DOI] [PubMed] [Google Scholar]
- Daniel T. M., Anderson P. A. The use of immunoabsorbents for the purification of mycobacterial antigens. J Lab Clin Med. 1977 Aug;90(2):354–360. [PubMed] [Google Scholar]
- Daniel T. M., DeMuth R. W. Immunochemical analyses of a major antigen of Mycobacterium szulgai. J Infect Dis. 1977 May;135(5):778–786. doi: 10.1093/infdis/135.5.778. [DOI] [PubMed] [Google Scholar]
- Daniel T. M., DeMuth R. W. Immunochemical analyses of a major antigen of Mycobacterium szulgai. J Infect Dis. 1977 May;135(5):778–786. doi: 10.1093/infdis/135.5.778. [DOI] [PubMed] [Google Scholar]
- Daniel T. M., Gonchoroff N. J., Katzmann J. A., Olds G. R. Specificity of Mycobacterium tuberculosis antigen 5 determined with mouse monoclonal antibodies. Infect Immun. 1984 Jul;45(1):52–55. doi: 10.1128/iai.45.1.52-55.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel T. M., Olds G. R. Demonstration of a shared epitope among mycobacterial antigens using a monoclonal antibody. Clin Exp Immunol. 1985 May;60(2):249–258. [PMC free article] [PubMed] [Google Scholar]
- Daus H., Hammer H. J., Rajki K., Mauch H. Influence of subclass-specific anti-idiotypic antibodies on the kinetics of the immune response to BCG. Immunology. 1984 Aug;52(4):697–702. [PMC free article] [PubMed] [Google Scholar]
- Doughty B. L., Phillips S. M. Delayed hypersensitivity granuloma formation and modulation around Schistosoma mansoni eggs in vitro. II. Regulatory T cell subsets. J Immunol. 1982 Jan;128(1):37–42. [PubMed] [Google Scholar]
- Eichmann K. Expression and function of idiotypes of lymphocytes. Adv Immunol. 1978;26:195–254. doi: 10.1016/s0065-2776(08)60231-x. [DOI] [PubMed] [Google Scholar]
- Eichmann K., Rajewsky K. Induction of T and B cell immunity by anti-idiotypic antibody. Eur J Immunol. 1975 Oct;5(10):661–666. doi: 10.1002/eji.1830051002. [DOI] [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay, Elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J Immunol. 1972 Jul;109(1):129–135. [PubMed] [Google Scholar]
- Fanning M. M., Peters P. A., Davis R. S., Kazura J. W., Mahmoud A. A. Immunopathology of murine infection with Schistosoma mansoni: relationship of genetic background to hepatosplenic disease and modulation. J Infect Dis. 1981 Aug;144(2):148–153. doi: 10.1093/infdis/144.2.148. [DOI] [PubMed] [Google Scholar]
- Garb K. S., Stavitsky A. B., Mahmoud A. A. Dynamics of antigen and mitogen-induced responses in murine schistosomiasis japonica: in vitro comparison between hepatic granulomas and splenic cells. J Immunol. 1981 Jul;127(1):115–120. [PubMed] [Google Scholar]
- Garb K. S., Stavitsky A. B., Olds G. R., Tracy J. W., Mahmoud A. A. Immune regulation in murine schistosomiasis japonica: inhibition of in vitro antigen- and mitogen-induced cellular responses by splenocyte culture supernatants and by purified fractions from serum of chronically infected mice. J Immunol. 1982 Dec;129(6):2752–2758. [PubMed] [Google Scholar]
- Gonyea L. M. Purification and iodination of antibody for use in an immunoradiometric assay for serum ferritin. Clin Chem. 1977 Feb;23(2 Pt 1):234–236. [PubMed] [Google Scholar]
- Hedrick S. M., Nielsen E. A., Kavaler J., Cohen D. I., Davis M. M. Sequence relationships between putative T-cell receptor polypeptides and immunoglobulins. Nature. 1984 Mar 8;308(5955):153–158. doi: 10.1038/308153a0. [DOI] [PubMed] [Google Scholar]
- Jerne N. K. Towards a network theory of the immune system. Ann Immunol (Paris) 1974 Jan;125C(1-2):373–389. [PubMed] [Google Scholar]
- Kennedy R. C., Dreesman G. R. Enhancement of the immune response to hepatitis B surface antigen. In vivo administration of antiidiotype induces anti-HBs that expresses a similar idiotype. J Exp Med. 1984 Mar 1;159(3):655–665. doi: 10.1084/jem.159.3.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kresina T. F., Nisonoff A. Passive transfer of the idiotypically suppressed state by serum from suppressed mice and transfer of suppression from mothers to offspring. J Exp Med. 1983 Jan 1;157(1):15–23. doi: 10.1084/jem.157.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuettner M. G., Wang A. L., Nisonoff A. Quantitative investigations of idiotypic antibodies. VI. Idiotypic specificity as a potential genetic marker for the variable regions of mouse immunoglobulin polypeptide chains. J Exp Med. 1972 Mar 1;135(3):579–595. doi: 10.1084/jem.135.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammie P. J., Linette G. P., Phillips S. M. Characterization of Schistosoma mansoni antigen-reactive T cell clones that form granulomas in vitro. J Immunol. 1985 Jun;134(6):4170–4175. [PubMed] [Google Scholar]
- McConahey P. J., Dixon F. J. A method of trace iodination of proteins for immunologic studies. Int Arch Allergy Appl Immunol. 1966;29(2):185–189. doi: 10.1159/000229699. [DOI] [PubMed] [Google Scholar]
- Mitchell G. F., Garcia E. G., Cruise K. M., Tiu W. U., Hocking R. E. Lung granulomatous hypersensitivity to eggs of Schistosoma japonicum in mice analysed by a radioisotopic assay and effects of hybridoma (idiotype) sensitization. Aust J Exp Biol Med Sci. 1982 Aug;60(Pt 4):401–416. doi: 10.1038/icb.1982.45. [DOI] [PubMed] [Google Scholar]
- Olds G. R., Griffin A., Kresina T. F. Dynamics of collagen accumulation and polymorphism in murine Schistosoma japonicum. Gastroenterology. 1985 Sep;89(3):617–624. doi: 10.1016/0016-5085(85)90459-7. [DOI] [PubMed] [Google Scholar]
- Olds G. R., Mahmoud A. A. Kinetics and mechanisms of pulmonary granuloma formation around Schistosoma japonicum eggs injected into mice. Cell Immunol. 1981 May 15;60(2):251–260. doi: 10.1016/0008-8749(81)90267-7. [DOI] [PubMed] [Google Scholar]
- Olds G. R., Olveda R., Tracy J. W., Mahmoud A. A. Adoptive transfer of modulation of granuloma formation and hepatosplenic disease in murine schistosomiasis japonica by serum from chronically infected animals. J Immunol. 1982 Mar;128(3):1391–1393. [PubMed] [Google Scholar]
- Powell M. R., Colley D. G. Demonstration of splenic auto-anti-idiotypic plaque-forming cells in mice infected with Schistosoma mansoni. J Immunol. 1985 Jun;134(6):4140–4145. [PubMed] [Google Scholar]
- Robertson M. Receptor gene rearrangement and ontogeny of T lymphocytes. 1984 Sep 27-Oct 3Nature. 311(5984):305–306. doi: 10.1038/311305a0. [DOI] [PubMed] [Google Scholar]
- Sacks D. L., Esser K. M., Sher A. Immunization of mice against African trypanosomiasis using anti-idiotypic antibodies. J Exp Med. 1982 Apr 1;155(4):1108–1119. doi: 10.1084/jem.155.4.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks D. L., Sher A. Evidence that anti-idiotype induced immunity to experimental African trypanosomiasis is genetically restricted and requires recognition of combining site-related idiotopes. J Immunol. 1983 Sep;131(3):1511–1515. [PubMed] [Google Scholar]
- Virgin H. W., 4th, Unanue E. R. Suppression of the immune response to Listeria monocytogenes. I. Immune complexes inhibit resistance. J Immunol. 1984 Jul;133(1):104–109. [PubMed] [Google Scholar]
- Von Lichtenberg F., Erickson D. G., Sadun E. H. Comparative histopathology of schistosome granulomas in the hamster. Am J Pathol. 1973 Aug;72(2):149–178. [PMC free article] [PubMed] [Google Scholar]
- Warren K. S., Berry E. G. Induction of hepatosplenic disease by single pairs of the Philippine, Formosan, Japanese, and Chinese strains of Schistosoma japonicum. J Infect Dis. 1972 Nov;126(5):482–491. doi: 10.1093/infdis/126.5.482. [DOI] [PubMed] [Google Scholar]
- Warren K. S., Boros D. L., Hang L. M., Mahmoud A. A. The Schistosoma japonicum egg granuloma. Am J Pathol. 1975 Aug;80(2):279–294. [PMC free article] [PubMed] [Google Scholar]
- Warren K. S., Domingo E. O. Granuloma formation around Schistosoma mansoni, S. HAEMATOBIUM, AND S. japonicum eggs. Size and rate of development, cellular composition, cross-sensitivity, and rate of egg destruction. Am J Trop Med Hyg. 1970 Mar;19(2):292–304. doi: 10.4269/ajtmh.1970.19.292. [DOI] [PubMed] [Google Scholar]
- Warren K. S. The relevance of schistosomiasis. N Engl J Med. 1980 Jul 24;303(4):203–206. doi: 10.1056/NEJM198007243030408. [DOI] [PubMed] [Google Scholar]


