Abstract
Calcium ions function as intracellular second messengers in regulating a plethora of cellular processes from acclimative stress responses to survival and programmed cell death. The generation of specificity in Ca2+ signals is dependent on influx and efflux from the extracellular milieu, cytosol and intracellular organelles. One aspect of plant Ca2+ signalling that is currently attracting a great deal of interest is how ‘Ca2+‐signatures’, specific spatio‐temporal changes in cytosolic‐free Ca2+, encode the necessary information to bring about this range of physiological responses. Here, current information is reviewed on how Ca2+‐signatures are generated in plant cells and how stimulus‐specific information can be encoded in the form of Ca2+‐signatures.
Key words: Calcium, waves, oscillations, hot‐spots, cytosolic free calcium ([Ca2+]cyt), guard cells, ion channels
INTRODUCTION
The Ca2+ ion is now firmly established as a ubiquitous signalling molecule in plants. Numerous plant signal transduction pathways have been shown to use Ca2+ as an integral signalling component (Sanders et al., 1999, 2002). The universality of the Ca2+ ion in signalling highlights the importance of understanding how specificity can be encoded in elevations in the cytosolic concentration of this ion (Sanders et al., 1999, 2002; Berridge et al., 2000). Perhaps the best example of this in plants is the response of stomata to the plant hormones abscisic acid (ABA) and auxin, which bring about the diametrically opposite effects of stomatal closure and opening, respectively, via changes in guard cell turgor mediated in both cases through increases in the concentration of cytosolic‐free Ca2+ ([Ca2+]cyt) (McAinsh et al., 1990; Irving et al., 1992). One plausible explanation is that each stimulus generates a unique increase in [Ca2+]cyt. The spatial and temporal components of this increase in [Ca2+]cyt, or ‘Ca2+‐signature’ as it is sometimes called, then dictate the outcome of the final response (McAinsh et al., 1997; McAinsh and Hetherington, 1998; Ng et al., 2001b). It has been suggested that the key to generating stimulus‐specific Ca2+‐signatures lies in the ability to access differentially the cellular machinery controlling Ca2+ influx and release from intracellular stores (McAinsh et al., 1997; Blatt, 2000; Evans et al., 2001; Ng et al., 2001b; Schroeder et al., 2001).
Ca2+‐mobilizing signalling intermediates that have been implicated in mediating elevations in [Ca2+]cyt in plants include, inositol‐1,4,5‐trisphosphate (InsP3), inositol hexakisphosphate (InsP6), nicotinic acid adenine dinucleotide phosphate (NAADP), phosphatidylinositol 3‐ and 4‐phosphate (PI3P and PI4P), cyclic adenosine 5′‐diphosphoribose (cADPR), hydrogen peroxide (H2O2) and sphingosine‐1‐phosphate (S1P) (Gilroy et al., 1990; McAinsh et al., 1996; Leckie et al., 1998; Staxén et al., 1999; Lemtiri‐Chlieh et al., 2000; Navazio et al., 2000; Pei et al., 2000; Ng et al., 2001a; Jung et al., 2002). At the cellular level, it is possible that this complexity may contribute to the spatio‐temporal variations in the Ca2+‐signatures, needed for specifying stimulus‐specific responses. Furthermore, the locale within the cell of such elevations in [Ca2+]cyt, whether the appropriate response elements are present in a given region of the cell to decode the information encrypted in the Ca2+ signal, may also be important (Trewavas and Malhó, 1997; McAinsh and Hetherington, 1998; Malhó et al., 1998; Evans et al., 2001; Ng et al., 2001b). It is not the intention here to provide an exhaustive review of Ca2+ signalling in plants; for this information, the reader is referred to Sanders et al. (1999, 2002). Instead, the purpose of this review is to draw attention to the different types of Ca2+ elevations that occur in plants, which can take the form of hot‐spots, puffs, sparks, oscillations and waves, and the importance of stimulus‐specific Ca2+‐signatures for encoding information necessary for eliciting the appropriate physiological responses in plant cells. The various forms of Ca2+ elevations are discussed below, with examples given in the figures: hot‐spots (Fig. 2), puffs and sparks (Figs 1 and 3), oscillations (Fig. 5) and waves (Fig. 1).
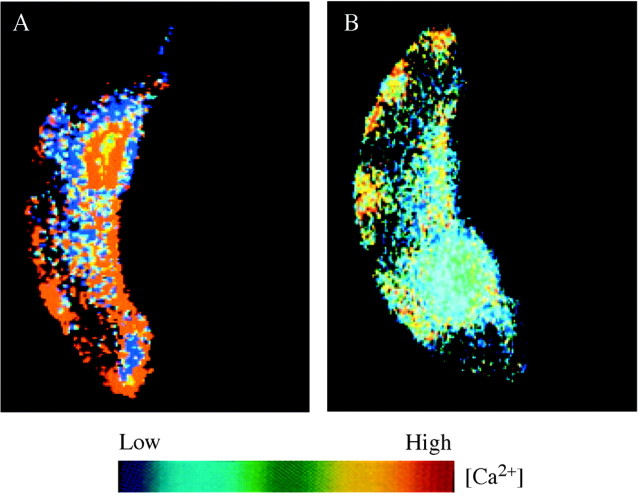
Fig. 2. Spatial heterogeneities in guard cell [Ca2+]cyt in response to 100 nM ABA (A) and 1 mm [Ca2+]ext (B). [Ca2+]cyt levels are colour‐coded; blue indicates low [Ca2+]cyt, red indicates high [Ca2+]cyt. These data suggest that plant cells have the capacity to encode specificity in the Ca2+ signal in the form of localized increases in [Ca2+]cyt. Reproduced, with permission, from McAinsh et al. (1992, 1995).
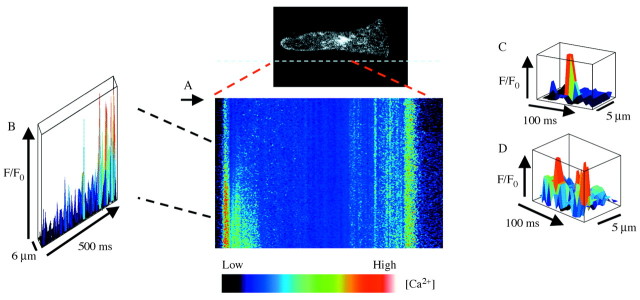
Fig. 1. Elemental Ca2+ elevations during Ca2+ wave propagation in a Fucus rhizoid cell. (A), Single‐line confocal scans of Ca2+ Green fluorescence along the longitudinal axis of the cell during the initation of Ca2+ waves are displayed sequentially to show the relative change in fluorescence following hypo‐osmotic treatment (to 50 % sea water). (B), Three‐dimensional plot shows a non‐uniform increase in Ca2+ during the onset of the Ca2+ wave in the rhizoid apex. (C), Elemental Ca2+ elevations in the perinuclear region. (D), Elemental Ca2+ events appear to arise repetitively at the same location in perinuclear region. Reproduced, with persmission, from Goddard et al. (2000).
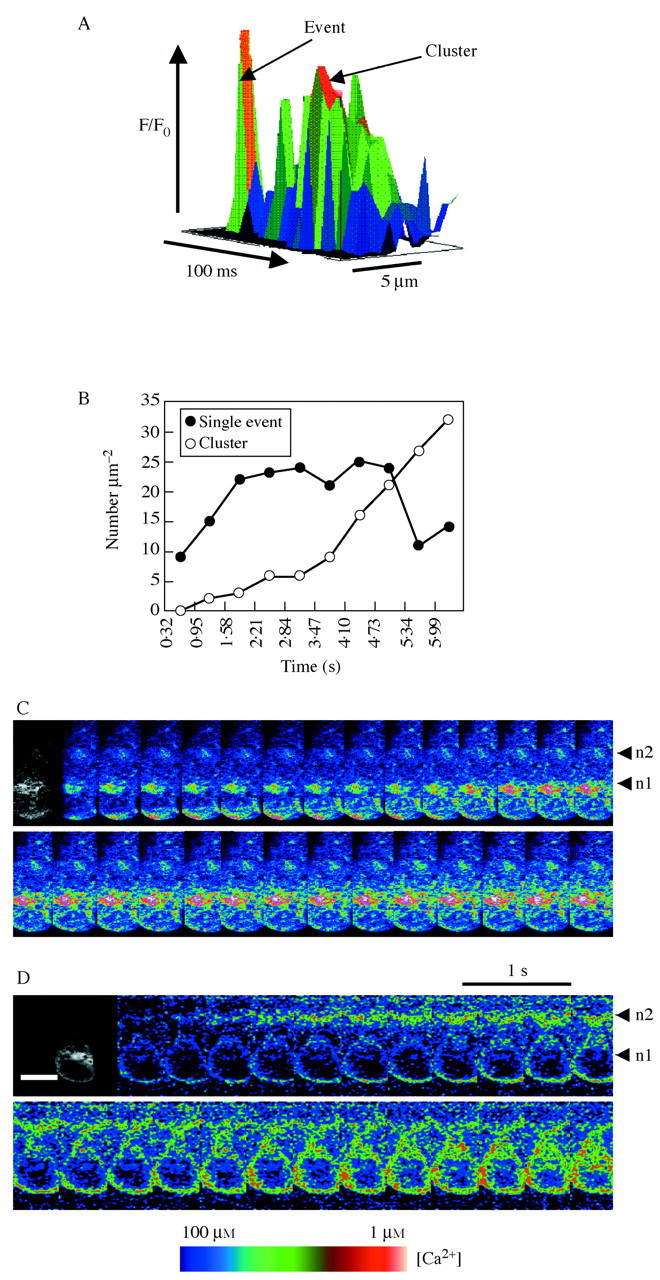
Fig. 3. (Opposite) Elemental Ca2+ elevations during Ca2+ wave propagation in a Fucus rhizoid cell and patterns of Ca2+ elevations following hypo‐osmotic treatment in dividing cells. (A), Elemental Ca2+ events in the perinuclear region either occur individually or appeared to cluster into more prolonged elevations. (B), Number of discrete elevations at the rhizoid apex increased initially during the first 1·5 s of wave propagation and then declined with the appearance of more prolonged elevations. (C), Ca2+ Green to Teax Red ratio images during the onset of a hypo‐osmotically induced Ca2+ waves (100 % sea water to 50 % sea water) shows an initial elevation of Ca2+ in the rhizoid apex which declines before the onset of Ca2+ elevation arising in the apical nucleus region. (D), A minority of cells shows a variation of the pattern shown in part C where the Ca2+ elevations were observed to arise in the subapical nucleus simultaneous with the apical Ca2+ elevation. Reproduced, with permission, from Goddard et al. (2000).
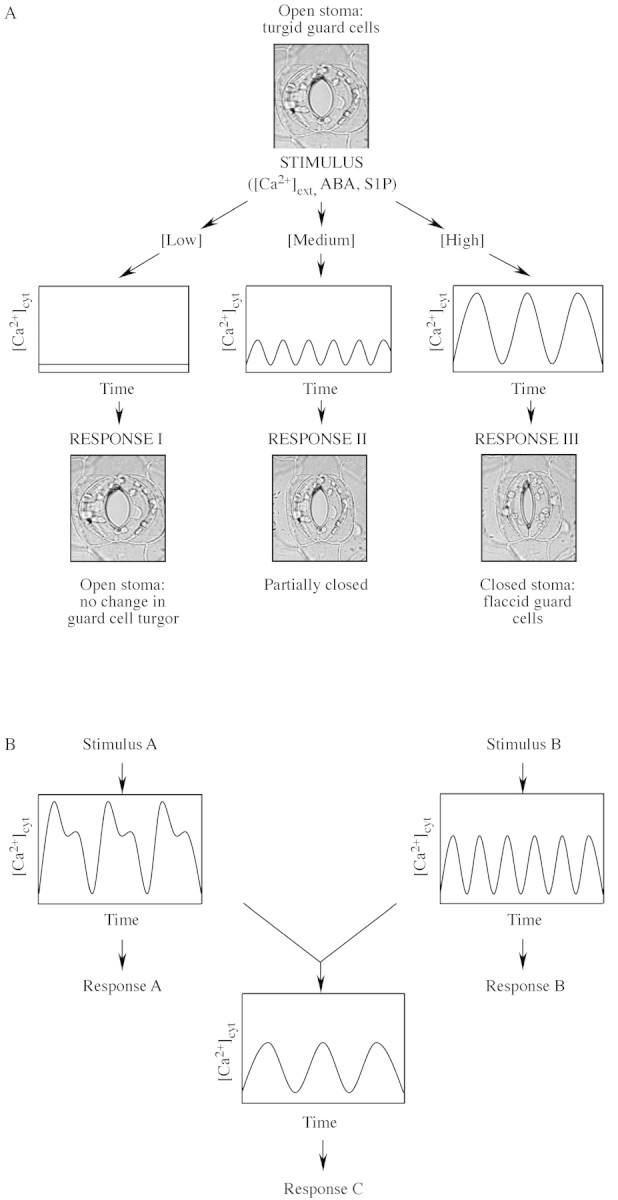
Fig. 5. Encoding signalling information in plant Ca2+ signatures. (A), In guard cells the strength of the stimulus has been correlated directly with the pattern of [Ca2+]cyt oscillations (i.e. the period, frequency and amplitude) which, in turn, dictates the resultant steady‐state stomatal aperture. (B), Guard cells are able to integrate signalling information from a number of stimuli that induce oscillations in [Ca2+]cyt applied simultaneously to generate a novel Ca2+ signature when formulating the final stomatal aperture. Reproduced, with permission, from Evans et al. (2001).
HOT‐SPOTTING: ELEMENTAL EVENTS
Studies using animal cells have shown that elevations in [Ca2+]cyt induced by agonists, e.g. InsP3 and cADPR, through their respective Ca2+ release‐channels, InsP3R and ryanodine receptors (RyR), respectively, involve a hierarchically distinguishable series of sub‐threshold events (Berridge et al., 2000; Bootman et al., 2001). Low levels of stimulation result in single channel events, leading to Ca2+ elevations known as ‘quarks’ and ‘blips’. The Ca2+ release associated with higher levels of stimulations is termed ‘puffs’ and ‘sparks’. Quarks, blips, puffs and sparks constitute what is collectively termed ‘elemental events’ and form the fundamental building blocks for global Ca2+ signalling, e.g. Ca2+ waves. In plants, the best examples of elemental events in Ca2+ signalling were obtained using Fucus serratus embryos by Brownlee and co‐workers (Goddard et al., 2000; Fig. 1). They showed that hypo‐osmotic shock induced unitary Ca2+ elevations in discrete domains within the cytosol of Fucus embryos. These discrete elevations in [Ca2+]cyt are in the range 200–300 nm and last between 15 and 30 ms (Fig. 1C). Interestingly, these unitary increases in [Ca2+]cyt occur repetitively and are spatially separated by regions with fewer events (Fig. 1D). Unitary elevations in Ca2+ of similar magnitudes, amplitudes and spatial dimensions were also induced by UV‐photolysis of caged‐InsP3, and suggest a role for InsP3 in the generation of osmotically induced Ca2+ signals, although direct evidence for this is lacking (Goddard et al., 2000). The observation of these unitary increases in [Ca2+]cyt in Fucus provides the first evidence in support of elemental events as fundamental building blocks in plant Ca2+ signalling.
In stomatal guard cells, stimulus‐induced elevations in [Ca2+]cyt also show marked spatial heterogeneities (Fig. 2). McAinsh et al. (1992) observed that ABA‐induced elevations in [Ca2+]cyt were unevenly distributed and appeared as ‘hot‐spots’ and Ca2+‐quiescent regions. Spatial heterogeneity in guard cell [Ca2+]cyt has also been reported by Gilroy et al. (1991) and McAinsh et al. (1995). It is possible that the spatial heterogeneities in [Ca2+]cyt elevations could result from (a) differential accessibility of the primary stimulus to only a subset of the signalling machinery, or (b) the non‐uniform distribution of the intracellular signalling machinery. These observations suggest the potential for encoding specificity in the form of localized increases in [Ca2+]cyt. It is tempting to suggest that these localized Ca2+ ‘hot‐spots’ observed in guard cells represent elemental events (puffs and sparks). However, due to the spatial and temporal resolution used in these studies, it is likely that these localized elevations in [Ca2+]cyt represent longer transients in [Ca2+]cyt, as opposed to the unitary Ca2+ elevations observed in Fucus in response to osmotic stress and UV‐photolysis of caged‐InsP3.
TRIGGERING WAVES
Ca2+ waves represent a form of global increase in [Ca2+]cyt that are triggered by the clustering of unitary Ca2+ elevations leading to Ca2+‐induced Ca2+‐release (CICR). Ca2+ waves have been extensively studied in animal cells (Berridge et al., 2000; Bootman et al., 2001). It is now well established that Ca2+ signalling in plants can also take the form of a propagating Ca2+ wave from studies using Fucus (Taylor et al., 1996). Goddard et al. (2000) showed an increase in the number of unitary Ca2+ elevations following exposure to hypo‐osmotic stress, leading subsequently to clustering and more prolonged elevations (Fig. 3A and B). Interestingly, they observed that clustering and prolonged elevations preceded the formation of Ca2+ waves. This suggests that the clustering of unitary Ca2+ elevations acts as the trigger for the generation of a Ca2+ wave (Fig. 3C). In this respect, the unitary Ca2+ elevations in Fucus are reminiscent of Ca2+ quarks/blips reported in animal cells and suggest that the clustering of such elemental events may be representative of Ca2+ puffs and sparks.
Importantly, the authors showed that the spatio‐temporal changes in [Ca2+]cyt can vary with the strength of the hypo‐osmotic shock treatment, suggesting that variations in the signature of the Ca2+ wave can determine downstream physiological responses (Goddard et al., 2000). In rhizoid cells that had undergone nuclear division but not partition wall formation, Ca2+ waves were observed to propagate from two nuclear regions, with most cells showing an initial transient Ca2+ elevation in the rhizoid apex, followed by Ca2+ elevations in the apical nuclear region (n1) that subsequently spread to the sub‐apical nucleus (n2) (Fig. 3C). However, in 20 % of the cells, Ca2+ elevations were observed to occur in the region of the sub‐apical nucleus (n2) either before or simultaneously with Ca2+ elevations in the rhizoid apex followed by Ca2+ elevations in the apical nucleus (n1) (Fig. 3D). Additionally, the authors showed that these Ca2+ elevations were highly correlated with the distribution of endoplasmic reticulum (ER) and that these specific patterns of Ca2+ wave generation may encode the necessary information for differential regulation of cell volume changes and the rate of cell division (Goddard et al., 2000).
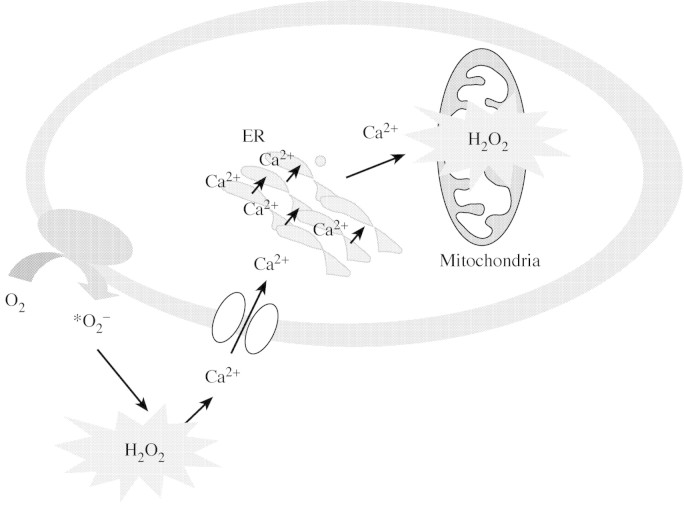
Recently, Coelho et al. (2002) showed that the generation of reactive oxygen species (ROS) are tightly linked to the generation of Ca2+ waves in Fucus and suggested the following sequence of events may occur during osmotic stress (Fig. 4). (a) Sensing of osmotic changes by an unidentified osmosensor results in extracellular production of ROS. (b) This initial ROS burst then activates the influx of Ca2+ through Ca2+‐permeable channels on the plasma membrane. Interestingly, H2O2 has also been reported to activate plasma membrane Ca2+‐permeable channels in guard cells (Pei et al., 2000). (c) Ca2+ influx then leads to elevations in [Ca2+]cyt, triggering CICR from the ER via InsP3‐sensitive release mechanisms. (d) This InsP3‐ dependent release of Ca2+ from the ER results in the formation of a Ca2+ wave followed by mitochondrial uptake of Ca2+ and subsequent mitochondrial production of ROS. Although the significance of the subsequent production of ROS by the mitochondria remains to be established, this study nevertheless highlights the importance of peripheral ROS production and Ca2+ influx through plasma membrane Ca2+‐permeable channels in the generation of Ca2+ waves during acclimative responses to osmotic stress in Fucus.
Fig. 4. Proposed signalling pathway during hyperosmotic stress in Fucus embryos. Osmotic change is sensed by an unidentified sensor leading to the production of extracellular ROS production (which may be important for strengthening the cell wall). The H2O2 formed also diffuses into the cell, leading to localized peripheral intracellular increase. The extracellular H2O2 also increases Ca2+‐channel activity. This leads to Ca2+‐induced Ca2+ release from intracellular stores (ER) resulting in Ca2+ wave propagation and mitochondrial ROS production. Reproduced, with permission, from Coelho et al. (2002).
OSCILLATIONS: SIMPLY UP AND DOWN?
Since the initial discovery of external Ca2+ ([Ca2+]ext)‐induced oscillations in [Ca2+]cyt in guard cells (McAinsh et al., 1995), stimulus‐induced oscillations in [Ca2+]cyt have also been observed in other cell types, including pollen tubes (Holdaway‐Clarke et al., 1997), roots (Kiegle et al., 2000) and root hairs (Ehrhardt et al., 1996). Oscillations in [Ca2+]cyt result from the dynamic balance of fluxes of Ca2+ into and out of the cytosol and include release and uptake from intracellular stores (vacuole and ER) and nucleus as well as influx and efflux across the plasma membrane. These fluxes are mediated through the regulated activities of Ca2+ channels and Ca2+‐pumps located on the plasma and endo‐membranes (Bunney et al., 1999; Sanders et al., 1999, 2002; Pauly et al., 2000; Harper, 2001; Miedema et al., 2001). Oscillations in [Ca2+]cyt allow for information to be encoded in both the amplitude and frequency (Fig. 5) (Berridge et al., 1988; Fewtrell, 1993; McAinsh et al., 1997; McAinsh and Hetherington, 1998; Evans et al., 2001; Ng et al., 2001b). Of the various cell types that have been used to study stimulus‐induced oscillations in [Ca2+]cyt, the guard cell has emerged as the most intensively used system.
Studies in the Hetherington and McAinsh laboratories using manganese quenching and imaging have shown that [Ca2+]ext‐induced oscillations in [Ca2+]cyt in guard cells of Commelina communis result from both Ca2+ influx through the plasma membrane and release from intracellular stores (McAinsh et al., 1995). Later studies from the Blatt and Schroeder laboratories using Vicia faba and Arabidopsis thaliana guard cells demonstrated that Ca2+ influx through the plasma membrane is gated by hyperpolarization‐dependent Ca2+ channels (Grabov et al., 1998, 1999; Hamilton et al., 2000; Pei et al., 2000). Interestingly, Blatt and co‐workers showed that the influx of Ca2+ through the plasma membrane Ca2+ channels are coupled to oscillations in plasma membrane potentials, and that ABA can regulate this influx of Ca2+ by increasing the probability of channel opening and by shifting the voltage sensitivity of these channels to more depolarizing potentials. Together these results suggest that ABA‐induced oscillations in [Ca2+]cyt is the result of influx of Ca2+ through the plasma membrane due in part to channel gating by oscillations in membrane potentials and greater probabilities of Ca2+ channel opening (Grabov et al., 1998, 1999; Hamilton et al., 2000). More recently, the Schroeder laboratory showed that ABA induced H2O2 production, and subsequent H2O2‐activation of Ca2+ influx through hyperpolarization‐activated guard cell plasma membrane Ca2+‐permeable channels can contribute to increases in [Ca2+]cyt (Pei et al., 2000).
In addition to the role of plasma membrane Ca2+ influx, a variety of second messenger systems exist for the release of Ca2+ from intracellular stores, for the generation of increases in [Ca2+]cyt. One of the earliest indications of an intracellular route for Ca2+ release in guard cells was reported by the Trewavas laboratory (Gilroy et al., 1990). They showed that UV‐photolysis of caged InsP3 microinjected into the cytosol of guard cells elevated [Ca2+]cyt and stimulated stomatal closure. This artificial elevation of cytosolic InsP3 could also reversibly inactivate inward‐rectifying K+ channels (IK,in), whilst at the same time activating an inward current that depolarizes the plasma membrane (Blatt et al., 1990). Many elements of an InsP3‐mediated signalling pathway have been identified in plants, and Lee et al. (1996) have shown that ABA induced the rapid turnover of phosphoinositides in guard cell protoplasts. Staxén et al. (1999) have also demonstrated the involvement of a phosphoinositide‐specific phospholipase C (PI‐PLC)‐InsP3 Ca2+‐mobilizing pathway in the regulation of guard cell turgor by ABA but not [Ca2+]ext. Their data suggest that ABA‐induced oscillations in [Ca2+]cyt have the potential to encode information on the strength of the initial ABA stimulus and for maintaining steady‐state stomatal apertures (Staxén et al., 1999). Hunt et al. (2003) have subsequently confirmed the role of PI‐PLC in the control of stomatal aperture by ABA. Another inositol phosphate that has been implicated in ABA‐mediated changes in guard cells is InsP6 (Lemtiri‐Chlieh et al., 2000). ABA has been shown to induce a rapid production of InsP6 and that loading of InsP6 into the cytosol of guard cell protoplasts inhibits IK,in in a Ca2+‐dependent manner. It will be of interest to determine the exact nature of the Ca2+‐dependence of InsP6‐mediated inhibition of IK,in and the modulation of [Ca2+]cyt by InsP6.
More recently, Jung et al. (2002) used a pharmacological approach to demonstrate that PI3P and PI4P are components of the ABA signal transduction pathway mediating stomatal closure, and that inhibitors of PI3‐kinase and PI4‐kinase (wortmanin and LY294002) inhibited ABA‐induced oscillations in [Ca2+]cyt. Together, these data suggest that inositol phosphates are important regulatory components in guard cell ABA signalling. Other Ca2+‐mobilizing second messengers known to function in regulation of guard cell turgor include cADPR and the recently identified sphingolipid metabolite, S1P. Leckie et al. (1998) observed that most cells microinjected with cADPR showed a sustained increase in [Ca2+]cyt lasting in excess of 10 min while oscillations were observed in some cells. The increase in [Ca2+]cyt elicited by cADPR ranged from 0·05 to 0·4 µm. In the case of S1P, the increase in [Ca2+]cyt take the form of oscillations that have a period and amplitude that is characteristic of the concentration of S1P used. The differential pattern of [Ca2+]cyt induced by different concentrations of S1P can be correlated with the rate of stomatal closure and is further evidence in support of oscillations in the control of guard cell turgor (Ng and Hetherington, 2001; Ng et al., 2001a).
The removal of Ca2+ from the cytosol is also an important factor in the generation of oscillations in [Ca2+]cyt causing the downward phase of the oscillation. The importance of cytosolic Ca2+ removal is highlighted by Allen et al. (2000) who showed that the V‐type ATPase activity is required for returning [Ca2+]cyt to resting levels. The authors showed in the det3 (de‐etiolated 3) mutant of arabidopsis (which exhibits decreased endomembrane energization due to a 60 % decline in the expression of the C‐subunit of the V‐type ATPase) that a correlation exists between stomatal closure and [Ca2+]ext‐induced [Ca2+]cyt oscillations. Guard cells of det3 mutants do not close in response to [Ca2+]ext and do not exhibit oscillations in [Ca2+]cyt compared with wild‐type guard cells. Interestingly, [Ca2+]cyt oscillations induced by cold treatment and ABA were not affected in guard cells of the det3 mutants and suggest a scenario where multiple pathways of cytosolic Ca2+ removal exist and that interact differentially to bring about the complexities observed in stimulus‐induced oscillations in [Ca2+]cyt.
The mechanism by which stimulus‐specific information may be encoded in [Ca2+]cyt oscillations has been studied in the Schroeder laboratory. In an elegant study, Allen et al. (2001) showed that the information encrypted in elevations in [Ca2+]cyt can be separated into two components: (1) ‘Ca2+‐reactive’ and (2) ‘Ca2+‐programmed’. These two distinct components encode information relating to short‐term and long‐term acclimative responses, respectively. The ‘Ca2+‐reactive’ component of the elevation in [Ca2+]cyt occurs rapidly and encodes information for the initiation of stomatal closure. This rapid reactive component usually takes the form of the initial transient elevation in [Ca2+]cyt. On the other hand, the ‘Ca2+‐programmed’ component encodes information related to the maintenance of steady‐state stomatal apertures and is usually represented by a series of oscillations. The number of transients (a complete cycle consisting of an upward and downward phase) determines the final steady‐state aperture: the greater the number of transients, the smaller the steady‐state stomatal aperture (Allen et al., 2001). Taken together, these data make a compelling case for the encryption of important physiological information in the form of [Ca2+]cyt oscillations.
CONCLUSIONS AND FUTURE PROSPECTS
The potential for encoding specificity in the Ca2+ signal has been demonstrated by the ability of guard cells to generate stimulus‐specific temporal changes in [Ca2+]cyt in the form of oscillations. Additional information can also be encoded in the form of complex patterns of [Ca2+]cyt oscillations generated in response to two or more stimuli (Hetherington et al., 1998; McAinsh et al., 2000; Ng et al., 2001b). The ability to modulate the pattern of the Ca2+‐signature suggests the presence of a complex cellular machinery capable of integrating stimuli perception into a meaningful physiological response. While [Ca2+]cyt oscillations play an important role in turgor regulation in stomatal guard cells, stimulus‐specific patterns of Ca2+ wave formation and propagation appear to be important in specifying the information necessary for differential regulation of cell volume changes and the rate of cell division. The ability for encryption of stimulus‐specific information immediately raises the question of how the cell is able to decode the information into a meaningful physiological response. It has been suggested that a variety of Ca2+‐binding proteins, Ca2+‐dependent protein kinases and protein phosphatases may provide the diversity needed for decoding the encrypted Ca2+ signal (Luan et al., 1993; Berkowitz et al., 2000; Guo et al, 2002). Webb and Hetherington (1997) have suggested that signalling components may be organized in the form of a ‘cassette’ and function as focal points for signal convergence and integration. Trewavas and Malhó (1997) also suggested a similar concept of a ‘transducon’, large protein complexes where signalling components may be spatially organized within the cellular milieu. The hypotheses that signalling components are organized into spatially distinct cassettes/transducons was lent credence by the recent observation of Guo et al. (2002) who showed the physical interaction of ScaBP5, a Ca2+‐binding protein with PKS3, its interacting protein kinase. PKS3 in turn interacts with ABI2 and ABI1. This suggests that ScaBP5, PKS3 and ABI2 and/or ABI1 are spatially organized, perhaps in a cassette/transducon, for efficient transduction of the ABA signal. Insights into how stimulus‐specific signals are encrypted and the underlying mechanism(s) for decoding the Ca2+ signal into meaningful physiological responses are likely to be gained through the systematic identification of genes, their products and the interactions between the various signalling components.
ACKNOWLEDGEMENTS
We are grateful to the Biotechnology and Biological Sciences Research Council, UK (M.R.M.), The Royal Society, UK (M.R.M.) and University College Dublin (C.K.‐Y.N.) for research funding.
Supplementary Material
Received: 8 May 2003; Returned for revision: 2 June 2003; Accepted: 15 June 2003 Published electronically: 21 August 2003
References
- AllenGJ, Chu SP, Harrington CL, Schumacher K, Hoffmann T, Tang YY, Grill E, Schroeder JI.2001. A defined range of guard cell calcium oscillation parameters encodes stomatal movements. Nature 411: 1053–1057. [DOI] [PubMed] [Google Scholar]
- AllenGJ, Chu SP, Schumacher K, Shimazaki CT, Vafeados D, Kemper A, Hawke SD, Tallman G, Tsien RY, Harper JFet al.2000. Alteration of stimulus‐specific guard cell calcium oscillations and stomatal closing in Arabidopsis det3 mutants. Science 289: 2338–2342. [DOI] [PubMed] [Google Scholar]
- BerkowitzG, Zhang X, Mercier R, Leng Q, Lawton M.2000. Co‐expression of calcium‐dependent protein kinase with the inward rectified guard cell K+ channel KAT1 alters current parameters in Xenopus laevis oocytes. Plant and Cell Physiology 41: 785–790. [DOI] [PubMed] [Google Scholar]
- BerridgeMJ, Cobbold PH, Cuthbertson KSR.1988. Spatial and temporal aspects of cell signalling. Philosophical Transactions of the Royal Society of London Series B 320: 325–343. [DOI] [PubMed] [Google Scholar]
- BerridgeMJ, Lipp P, Bootman MD.2000. The versatility and universality of calcium signalling. Nature Reviews – Molecular Cell Biology 1: 11–21. [DOI] [PubMed] [Google Scholar]
- BlattMR.2000. Cellular signalling and volume control in stomatal movements in plants. Annual Review of Cell and Developmental Biology 16: 221–241. [DOI] [PubMed] [Google Scholar]
- BlattMR, Thiel G, Trentham DR.1990. Reversible inactivation of K+ channels in Vicia stomatal guard cells following the photolysis of caged inositol 1,4,5‐triphosphate. Nature 346: 766–769. [DOI] [PubMed] [Google Scholar]
- BootmanMD, Lipp P, Berridge MJ.2001. The organisation and functions of local Ca2+ signals. Journal of Cell Science 114: 2213–2222. [DOI] [PubMed] [Google Scholar]
- BunneyTD, Shaw PJ, Watkins PAC, Taylor JP, Beven AF, Wells B, Calder GM, Drøbak BK.1999. ATP‐dependent regulation of nuclear Ca2+ levels in plant cells. FEBS Letters 476: 145–149. [DOI] [PubMed] [Google Scholar]
- CoelhoSM, Taylor AR, Ryan KP, Sousa‐Pinto I, Brown MT, Brownlee C.2002. Spatiotemporal patterning of reactive oxygen production and Ca2+ wave propagation in Fucus rhizoid cells. Plant Cell 14: 2369–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EhrhardtDW, Wais R, Long SR.1996. Calcium spiking in plant root hairs responding to rhizobium nodulation signals. Cell 85: 673–681. [DOI] [PubMed] [Google Scholar]
- EvansNH, McAinsh MR, Hetherington AM.2001. Calcium oscillations in higher plants. Current Opinion in Plant Biology 4: 415–420. [DOI] [PubMed] [Google Scholar]
- FewtrellC.1993. Ca2+ oscillations in nonexcitable cells. Annual Review of Physiology 55: 427–454. [DOI] [PubMed] [Google Scholar]
- GilroyS, Read ND, Trewavas AJ.1990. Elevation in cytoplasmic calcium by caged calcium or caged inositol trisphosphate initiates stomatal closure. Nature 346: 769–771. [DOI] [PubMed] [Google Scholar]
- GilroyS, Fricker MD, Read ND, Trewovas AJ. 1991. Role of calcium in signal transduction of Commelina guard cells. Plant Cell 3: 333–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GoddardH, Manison NFH, Tomos D, Brownlee C.2000. Elemental propagation of calcium signals in response‐specific patterns determined by environmental stimulus strength. Proceedings of the National Academy of Sciences of the USA 97: 1932–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GrabovA, Blatt MR.1998. Membrane voltage initiates Ca2+ waves and potentiates Ca2+ increases with abscisic acid in stomatal guard cells. Proceedings of the National Academy of Sciences of the USA 95: 4778–4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GrabovA, Blatt MR.1999. A steep dependence of inward‐rectifying potassium channels on cytosolic free calcium concentration increase evoked by hyperpolarisation in guard cells. Plant Physiology 119: 277–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GuoY, Xiong L, Song CP, Gong D, Halfter U, Zhu JK.2002. A calcium sensor and its interacting protein kinase are global regulators of abscisic acid signalling in Arabidopsis Developmental Cell 3: 233–244. [DOI] [PubMed] [Google Scholar]
- HamiltonDWA, Hills A, Köhler B, Blatt MR.2000. Ca2+ channels at the plasma membrane of stomatal guard cells are activated by hyerpolarisation and abscisic acid. Proceedings of the National Academy of Sciences of the USA 97: 4967–4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HarperJF.2001. Dissecting calcium oscillators in plant cells. Trends in Plant Science 6: 395–397. [DOI] [PubMed] [Google Scholar]
- HetheringtonAM, Gray JE, Leckie CP, McAinsh MR, Ng C, Pical C, Priestley AJ, Staxén I, Webb AAR.1998. The control of specificity in guard cell signal transduction. Philosophical Transactions of the Royal Society of London Series B 353: 1489–1494. [Google Scholar]
- Holdaway‐ClarkeTL, Feijo JA, Hackett GR, Kunkel JG, Hepler PK.1997. Pollen tube growth and the intracellular cytosolic calcium gradient oscillate in phase while extracellular calcium influx is delayed. Plant Cell 9: 1999–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HuntL, Mills, LN, Pical, C, Leckie, CP, Aitken, FL, Kopka J, Mueller‐Roeber B, McAinsh MR, Hetherington AM, Gray JE.2003. Phospholipase C is required for the control of stomatal aperture by ABA. Plant Journal 34: 47–55. [DOI] [PubMed] [Google Scholar]
- IrvingHR, Gehring CA, Parish RW. 1992. Changes in cytostolic pH and calcium guard cells precede stomatal movements. Proceedings of the National Academy of Sciences of the USA 89: 1790–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JungJY, Kim YW, Kwak JM, Hwang JU, Young Jm Schroeder JI, Hwang I, Lee Y.2002. Phosphatidylinositol 3‐ and 4‐phosphate are required for normal stomatal movements. Plant Cell 14: 2399–2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KiegleE, Moore CA, Haseloff J, Tester MA, Knight MR.2000. Cell‐type‐specific calcium responses to drought, salt and cold in the Arabidopsis root. Plant Journal 23: 267–278. [DOI] [PubMed] [Google Scholar]
- LeckieCP, McAinsh MR, Allen GJ, Sanders D, Hetherington AM.1998. Abscisic acid‐induced stomatal closure mediated by cyclic ADP‐ribose. Proceedings of the National Academy of Sciences of the USA 95: 15837–15842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeeYS, Choi YB, Suh S, Lee J, Assmann SM, Joe CO, Kelleher JF, Crain RC.1996. Abscisic acid‐induced phosphoinositide turnover in guard cell protoplasts of Vicia faba Plant Physiology 110: 987–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemtiri‐ChliehF, MacRobbie EAC, Brearley CA.2000. Inositol hexakisphosphate is a physiological signal regulating K+‐inward rectifying conductances in guard cells. Proceedings of the National Academy of Sciences of the USA 97: 8687–8692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LuanS, Li W, Rusnak F, Assmann SM, Schreiber SL.1993. Immunosuppresants implicate protein phosphatase regulation of K+ channels in guard cells. Proceedings of the National Academy of Sciences of the USA 90: 2202–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MalhóR, Moutinho A, van der Luit A, Trewavas AJ.1998. Spatial characteristics of calcium signalling: the calcium wave as a basic unit of plant cell calcium signalling. Philosophical Transactions of the Royal Society of London Series B 353: 1463–1473. [Google Scholar]
- McAinshMR, Hetherington AM.1998. Encoding specificity in Ca2+ signalling systems. Trends in Plant Science 3: 32–36. [Google Scholar]
- McAinshMR, Brownlee C, Hetherington AM.1990. Abscisic acid‐induced elevation of guard cell cytosolic Ca2+ precedes stomatal closure. Nature 343: 186–188. [Google Scholar]
- McAinshMR, Brownlee C, Hetherington AM.1992. Visualising changes in cytosolic‐free Ca2+ during the response of stomatal guard cells to abscisic acid. Plant Cell 4: 1113–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAinshMR, Brownlee C, Hetherington AM.1997. Calcium ions as second messengers in guard cell signal transduction. Physiologia Plantarum 100: 16–29. [Google Scholar]
- McAinshMR, Clayton H, Mansfield, TA, Hetherington AM.1996. Changes in stomatal behaviour and guard cell cytosolic free calcium in response to oxidative stress. Plant Physiology 111: 1031–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAinshMR, Gray JE, Hetherington AM, Leckie CP, Ng C.2000. Calcium signalling in stomatal guard cells. Biochemical Society Transactions 28: 476–481. [PubMed] [Google Scholar]
- McAinshMR, Webb AAR, Taylor JE, Hetherington AM.1995. Stimulus‐induced oscillations in guard cell cytosolic‐free calcium. Plant Cell 7: 1207–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MiedemaH, Bothwell JHF, Brownlee C, Davies JM.2001. Calcium uptake by plant cells – channels and pumps acting in concert. Trends in Plant Science 6: 514–519. [DOI] [PubMed] [Google Scholar]
- NavazioL, Bewell MA, Siddiqua A, Dickson GD, Galione A, Sanders D.2000. Calcium release from the endoplasmic reticulum of higher plants elicited by the NADP metabolite nicotinic acid adenine dinucleotide phosphate. Proceedings of the National Academy of Sciences of the USA 97: 8693–8698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NgCKY, Hetherington AM.2001. Sphingolipid‐mediated signalling in plants. Annals of Botany 88: 957–965. [Google Scholar]
- NgCKY, Carr K, McAinsh MR, Powell B, Hetherington AM.2001a. Drought‐induced guard cell signal transduction involves sphingosine‐1‐phosphate. Nature 410: 596–599. [DOI] [PubMed] [Google Scholar]
- NgCKY, McAinsh MR, Gray JE, Hunt L, Leckie CP, Mills L, Hetherington AM.2001b. Calcium‐based signalling systems in guard cells. New Phytologist 151: 109–120. [DOI] [PubMed] [Google Scholar]
- PaulyN, Knight MR, Thuleau P, van der Luit A, Moreau M, Trewavas AJ, Ranjeva R, Mazars C.2000. Cell signalling: control of free calcium in plant cells. Nature 405: 754–755. [DOI] [PubMed] [Google Scholar]
- PeiZM, Murata Y, Benning G, Thomine S, Klûsener B, Allen GJ, Grill E, Schroeder JI.2000. Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 406: 731–734. [DOI] [PubMed] [Google Scholar]
- SandersD, Brownlee C, Harper JF.1999. Communicating with calcium. Plant Cell 11: 691–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SandersD, Pelloux J, Brownlee C, Harper JF.2002. Calcium at the crossroads of signalling. Plant Cell (Supp 1): S401–S417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SchroederJI, Allen GJ, Hugovieux V, Kwak JM, Waner D.2001. Guard cell signal transduction. Annual Review of Plant Physiology and Plant Molecular Biology 52: 627–658. [DOI] [PubMed] [Google Scholar]
- StaxénI, Pical C, Montgomery LT, Gray JE, Hetherington AM.1999. Abscisic acid induces oscillations in guard‐cell cytosolic free calcium that involve phosphoinositide‐specific phospholipase C. Proceedings of the National Academy of Sciences of the USA 96: 1779–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TaylorAR, Manison NFH, Fernandez C, Wood JW, Brownlee C.1996. Spatial organization of calcium signalling involved in cell volume control in the Fucus rhizoid. Plant Cell 8: 1935–1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TrewavasAJ, Malhó R.1997. Signal perception and transduction: the origin of the phenotype. Plant Cell 9: 1181–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WebbAAR, Hetherington AM.1997. Convergence of the ABA, CO2 and extracellular calcium signal transduction pathways in stomatal guard cells. Plant Physiology 114: 1557–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.