Abstract
Aims
To investigate 25-hydroxycholecalciferol [25(OH)D] population pharmacokinetics in children and adolescents, to establish factors that influence 25(OH)D pharmacokinetics and to assess different vitamin D3 dosing schemes to reach sufficient 25(OH)D concentrations (>30 ng ml−1).
Methods
This monocentric prospective study included 91 young HIV-infected patients aged 3 to 24 years. Patients received a 100 000 IU vitamin D3 supplementation. A total of 171 25(OH)D concentrations were used to perform a population pharmacokinetic analysis.
Results
At baseline 28% of patients had 25(OH)D concentrations below 10 ng ml−1, 69% between 10 and 30 ng ml−1 and 3% above 30 ng ml−1. 25(OH)D pharmacokinetics were best described by a one compartment model with an additional production parameter reflecting the input from diet and sun exposure. The effects of skin phototype and bodyweight were significant on 25(OH)D production before any supplementation. The basal level was 27% lower in non-white skin phototype patients and was slightly decreased with bodyweight. No significant differences in 25(OH)D concentrations were related to antiretroviral drugs. To obtain concentrations between 30 and 80 ng ml−1, patients with baseline concentrations between 10 and 30 ng ml−1 should receive 100 000 IU per 3 months. However, vitamin D deficient patients (<10 ng ml−1) would need an intensive phase of 100 000 IU per 2 weeks (two times) followed 2 weeks later by a maintenance phase of 100 000 IU per 3 months.
Conclusions
Skin phototype and bodyweight had an influence on the basal production of 25(OH)D. According to 25(OH)D baseline concentrations, dosing schemes to reach sufficient concentrations are proposed.
Keywords: 25-hydroxycholecalciferol, children and adolescents, HIV-infected patients, population pharmacokinetics
What is already known about this subject
Vitamin D deficiency is associated with musculoskeletal disorders and has been recently related to a higher risk of mortality and HIV disease progression in adults.
A high prevalence of vitamin D deficiency in HIV-infected children and adolescents has been reported.
What this study adds
This is the first population pharmacokinetics analysis of 25-hydroxycholecalciferol (25(OH)D) in HIV-infected children and adolescents.
Skin phototype and bodyweight were shown to influence the 25(OH)D basal production.
Different vitamin D3 dosing regimens were assessed to reach sufficient 25(OH)D concentrations.
Introduction
High prevalences of vitamin D deficiency and insufficiency defined respectively as 25-hydroxycholecalciferol [25(OH)D, calcidiol] serum concentrations below 10 and between 10 and 30 ng ml−1 have been reported in the general population as well as in HIV-infected patients [1–3]. Vitamin D deficiency is associated with the risk of peripheral fractures, osteopenia or rickets [4–7]. Furthermore, many observational studies have reported that vitamin D insufficiency may be associated with non-skeletal disorders such as auto-immune diseases, cancers, infections or cardiovascular diseases [4,6]. Currently, a sufficient 25(OH)D concentration in adults is defined by most experts as a target of 30 ng ml−1 which corresponds to the minimum concentration that does not increase plasma concentrations of parathyroid hormone (PTH) [6,8,9]. 25(OH)D concentrations above this cutoff reduce the risk of peripheral fractures, osteopenia and rickets. Vitamin D toxicity is usually reported for serum concentrations above 150 ng ml−1. However an upper safety limit of 80 or 100 ng ml−1 has been previously considered [6,10,11]. Different factors such as a low sun exposure, insufficient dietary intake or skin phototype are known to impact the 25(OH)D serum concentrations. Regarding the HIV-infected patients, antiretroviral treatment could also have an influence by modifying vitamin D metabolism [3,12–14], especially with efavirenz (EFV) [1,15,16].
Children and adolescents have a particular risk regarding vitamin D deficiency and its impact on bone growth and calcium absorption. The prevalence of vitamin D deficiency between HIV-positive and HIV-negative children has been compared and no significant differences were pointed out [17–19]. However, very few studies have investigated the effect of antiretroviral therapies on vitamin D metabolism in HIV-infected children. The influence of EFV on vitamin D deficiency has been evaluated in adults. However it remains to be investigated further in children as few studies exist with contradictory results [17,20,21]. Moreover, even if several clinical trials have evaluated weekly, monthly or bi-monthly vitamin D3 supplementation schemes in HIV-infected adolescents, no dosing recommendations based on efficacy thresholds are currently available [20,22,23].
The aims of this study were i) to investigate the population pharmacokinetics of 25(OH)D in HIV-1-infected children and adolescents, ii) to investigate the factors that influence 25(OH)D pharmacokinetics in this population and iii) and to propose a dosing recommendation in order to reach the 25(OH)D target of 30–80 ng ml−1 for most patients.
Methods
Study protocol
This study was conducted prospectively between December 2010 and September 2011 in one medical centre in the Paris region (latitude: 48.50 N). All patients followed for HIV-1 infection were enrolled during ambulatory care visits to the Necker-Enfants Malades Hospital, Paris, France. This data collection has been used, in part, in a first analysis by Meyzer et al. to describe the prevalence of vitamin D insufficiency or deficiency in HIV-positive and HIV-negative (control group) children and young adults [21].
Age, bodyweight (BW), body mass index (BMI), skin pigmentation (scored from 0 to 6 by the Fitzpatrick skin phototype classification [24]), vitamin D supplementation in the past year, CD4 T cell count, HIV-1 viral load and antiretroviral drugs (ARV) were collected during consultation.
This observational study was approved by the CNIL (Commission Nationale de l'Informatique et des Libertés) in December 2010 and oral informed consent was obtained from parents and children according to French law.
As part of the usual follow-up and monitoring care, all the patients received at least one 100 000 IU vitamin D3 supplementation (Uvedose®, Crinex laboratories, France) during winter [25].
Analytical method
Serum 25(OH)D concentrations were measured on real-time (as part of routine blood testing) using the DiaSorin RIA method (Saluggia, Italy). The mean intra-assay coefficient of variation (CV) evaluated from the duplicate measurement of 201 serum samples was 4.1% (SD: 3.2%). Inter-assay CV evaluated from the daily measurement of serum pools used as an internal quality control ranged from 5.5% to 10%. The detection limit was 3 ng ml−1. The measurements were done for all patients in the same hospital laboratory. Vitamin D deficiency was defined as serum 25(OH)D concentrations < 10 ng ml−1 and vitamin D insufficiency as 25(OH)D concentrations between 10 and 30 ng ml−1.
Modelling strategy and data analysis
Different structural models for 25(OH)D pharmacokinetics were investigated: one or two compartments with linear elimination and first order or zero order absorption, with or without a lag time or a transit compartment for absorption. Because vitamin D3 supplementation was exclusively given by the oral route, clearance (CL) and volume of distribution (V) are apparent parameters, V/F and CL/F, where F is the unknown bioavailability fraction.
Data were analyzed using the non-linear mixed effect modelling software program Monolix version 4.1.4 (http://www.lixoft.eu) [26]. Parameters were estimated by computing the maximum likelihood estimator of the parameters without any approximation of the model (no linearization) using the stochastic approximation expectation maximization algorithm combined with a Markov Chain Monte Carlo (MCMC) procedure. The number of MCMC chains was fixed to five for all estimations. Several error models (proportional, additive or mixed) were investigated to describe the residual variability (ε). The between subject variabilities (η or BSVs) were assumed to be exponential. The likelihood ratio test (LRT) including the log-likelihood, the Akaike information criterion (AIC) and the Bayesian information criterion (BIC) were used to test different hypotheses regarding the final model, covariate effect(s) on pharmacokinetic parameter(s), residual variability model (proportional versus proportional plus additive error model), and structure of the variance-covariance matrix for the BSV parameters.
The main covariates of interest in the population were age, gender, skin phototype, seasons, BW, BMI and co-medications. The effect of each patient covariate was systematically tested via the LRT. Continuous covariates (COV), age, BW and BMI, were tested according to the following equation, using CL as an example,
where θCL is the typical value of clearance for a patient with the median covariate value and 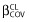 is the estimated influential factor for the continuous covariate. Binary covariates (CAT): skin phototype, gender, antiretroviral family or combined antiretroviral therapies were tested according to the following equation,
is the estimated influential factor for the continuous covariate. Binary covariates (CAT): skin phototype, gender, antiretroviral family or combined antiretroviral therapies were tested according to the following equation,
where 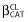 is the estimated influential factor for the binary covariate, CAT = 0 stands for the reference θCL value and CAT = 1 for the CL value in presence of the covariate.
is the estimated influential factor for the binary covariate, CAT = 0 stands for the reference θCL value and CAT = 1 for the CL value in presence of the covariate.
The effects of ARV were investigated as binary covariates, respectively, for protease inhibitors (PI), non-nucleoside reverse transcriptase inhibitors (NNRTI), EFV and tenofovir disoproxil fumarate (TDF). Combined therapies were also tested as binary covariates respectively for PI + TDF and NNRTI + TDF. The comparison of different ARV-induced effects was investigated as a categorical covariate with four different classes for PI, NNRTI, other antiretroviral association (PI + NNRTI or PI + NNRTI + integrase inhibitor (II) or PI + II) and no treatment.
Seasons were firstly tested as different groups, then non-significantly different groups were combined as long as the BIC value did not increase.
A covariate was finally retained if (i) its effect was biologically plausible, (ii) a reduction in AIC/BIC criteria was observed (LRT) and (iii) it produced a reduction in the variability of the pharmacokinetic parameter, assessed by the associated inter-subject variability.
Evaluation and validation
Graphical evaluation of the goodness-of-fit was mainly assessed by observed vs. predicted concentrations (PRED-DV) and weighted residuals vs. time and/or weighted residuals vs. PRED. The final population model was mainly appreciated by the normalized prediction distribution errors metrics [27] and the prediction-corrected visual predictive check [28]. Diagnostic graphics and distribution statistics were obtained using RfM (link on http://wfn.sourceforge.net) via the R program [29].
Dose simulation
Using the final model, dose simulations were performed in order to obtain 25(OH)D concentrations between 30 and 80 ng ml−1 during a 12 months follow-up. Based on 1000 simulations from the final model, different dosing schemes were evaluated in the whole population (e.g. monthly, bi-monthly or tri-monthly dosing schemes) and then the proportions of patients outside and within the therapeutic range after 1 year of treatment were compared. Different intensive and maintenance dosing schemes were also evaluated according to the patient 25(OH)D baseline concentrations, i.e. <10 ng ml−1 or between 10 and 30 ng ml−1.
Based on 400 simulations from the final model, the concentration−time profiles of these dosing schemes were finally evaluated for 12 months supplementation.
Results
Demographic data
Data from 91 vertically HIV-1-infected children and adolescents (47 boys, 44 girls) were collected for pharmacokinetic assessment. For boys, the median age and BW were 14 years (interquartile range (IQR) 10–17) and 47 kg (IQR 30–58), respectively. For girls, the median age was 15 years (IQR 11–17) and the median BW was 55 kg (IQR 41–61). A total of 29 patients had a white skin phototype (Fitzpatrick scale I to IV) and 62 had a non-white skin phototype (Fitzpatrick scale V and VI). With the exception of one HCV co-infected child, patients had no other co-morbidities or co-infections. A total of 171 25(OH)D concentrations were available for pharmacokinetic evaluation. Among the 91 patients, 74 had a second 25(OH)D concentration assessment and six of them were declared to be non-compliant. For the entire study, the median (min–max) number of samples per patient was 2 (1–3) and the mean follow-up time for patients who had a second 25(OH)D concentration assessment was 4 months (range 1–7, median 3.9).
At baseline 27 patients (30%) received tenofovir, 11 efavirenz (12%), 12 nevirapine or etravirine (13%), 73 were treated with a PI (80%), 12 received raltegravir (13%) and five patients (5%) received no ARV. The median 25(OH)D at baseline was 14 ng ml−1 (IQR, 8–17) for the total population, 28% of patients had concentrations below 10 ng ml−1, 69% between 10 and 30 ng ml−1 and 3% above 30 ng ml−1. At baseline, the median (IQR) total corrected calcium, serum phosphate and serum magnesium concentrations were, respectively, 2.3 (2.3–2.4) mmol l−1, 1.3 (1.2–1.5) mmol l−1 and 0.9 (0.9–1.0) mmol l−1. On average, 4 months after vitamin D3 supplementation, total corrected calcium, serum phosphate and serum magnesium concentrations were then, respectively, 2.4 (2.3–2.5) mmol l−1, 1.3 (1.1–1.4) mmol l−1 and 0.9 (0.9–1.0) mmol l−1. Table 1 summarizes the patients' baseline characteristics.
Table 1.
Baseline characteristics of the 91 patients
| Variables | 25(OH)D | 25(OH)D | All patients |
|---|---|---|---|
| <10 ng ml−1 | ≥10 ng ml−1 | ||
| n | 25 | 66 | 91 |
| Age, years | 17 (12–17) | 13 (10–17) | 14 (11–17) |
| Gender, n (%) | |||
| Boys | 13 (28) | 34 (72) | 47 (52) |
| Girls | 12 (27) | 32 (73) | 44 (48) |
| Ethnic origin, n (%) | |||
| White | 2 (7) | 27 (93) | 29 (32) |
| Other | 23 (37) | 39 (63) | 62 (68) |
| Bodyweight, kg | 52 (45–60) | 46 (29–59) | 50 (35–60) |
| BMI, kg m−2 | 21 (19–22) | 19 (17–23) | 20 (17–22) |
| HIV-1 viral load, log10 copies ml−1 | 1.6 (1.6–3.6) | 1.6 (1.6–2.4) | 1.6 (1.6–2.7) |
| CD4, cells mm−3 | 650 (462–832) | 754 (506–1073) | 703 (487–984) |
| Current ARV therapy duration, years | 1.6 (0.5–2.3) | 2 (1.3–2.3) | 2 (1.1–2.3) |
| Combined ARV drug, n (%) | |||
| PI + 2 NRTI | 16 (27) | 44 (73) | 60 (66) |
| NNRTI + 2 NRTI | 4 (31) | 9 (69) | 13 (14) |
| NNRTI + PI + II | 2 (25) | 6 (75) | 8 (9) |
| PI + II | 1 (33) | 2 (67) | 3 (3) |
| PI + NNRTI | 1 (50) | 1 (50) | 2 (2) |
| No treatment | 1 (20) | 4 (80) | 5 (5) |
| 25(OH)D, ng ml−1 | 7 (6–8) | 14 (12–18) | 12 (9–17) |
| Total corrected calcium, mmol l−1 | 2.3 (2.3–2.4) | 2.3 (2.3–2.4) | 2.3 (2.3–2.4) |
| Serum phosphate, mmol l−1 | 1.2 (1.2–1.4) | 1.3 (1.2–1.5) | 1.3 (1.2–1.5) |
| Serum magnesium, mmol l−1 | 0.9 (0.9–1.0) | 0.9 (0.9–1.0) | 0.9 (0.9–1.0) |
Data are described as median (IQR). 25(OH)D, 25-hydroxycholecalciferol (calcidiol); ARV, antiretroviral; NRTI, nucleoside reverse transcriptase inhibitor; NNRTI, non-nucleoside reverse transcriptase inhibitor; II, integrase inhibitor; PI, protease inhibitor.
Population pharmacokinetics
With regard to the long half-life of 25(OH)D, the absorption of vitamin D3 was considered to be very fast. The data were then best described by a one compartment model in which the absorption-formation and elimination 25(OH)D rate constants were equal. The basal level of 25(OH)D reflecting the production from the diet and sun exposure is described by the additional parameter, C0. The pharmacokinetic parameters were then the apparent volume of distribution (V/F) and elimination clearance (CL/F), where F is the unknown bioavailability, and C0. Between subject variability could be estimated for apparent CL and the basal level. A proportional model was used to describe the residual variability. The parameter estimates of this basic model were CL/F 0.9 l day−1 (BSV 0.47), V/F, 76.0 l, C0 12.3 ng ml−1 (BSV 0.43). In a second step, the skin phototype covariate was included in the model on the C0 parameter as a categorical covariate: ‘clear skin’ phototype (Fitzpatrick scale I to IV) vs. ‘dark skin’ phototype (V and VI). This improved the predictive performance of the model and significantly decreased the variability in this parameter from 0.43 to 0.41 and the BIC criteria from 1110 to 1104. In a third step, the C0 parameter was found to decrease with BW reducing the BIC criteria by 3 units and the corresponding variability from 0.41 to 0.39. No other covariate effect could be identified on CL/F or C0 parameters.
Table 2 summarizes the final population pharmacokinetic estimates. All parameters were estimated with relative standard errors lower than 39%. Finally, the C0 concentrations were shown to be 27% lower in non-white skin phototype patients and slightly decreased with BW.
Table 2.
Population pharmacokinetics parameters of 25(OH)D
| Parameter | Estimate (RSE %) |
|---|---|
| Structural model | |
| CL/F (l day−1) | 0.96 (13) |
| V/F (l) | 71.3 (3) |
| C0 (ng ml−1)† | 14.7 (8) |
| θNo white | 0.74 (9) |
| θBW | −0.29 (38) |
| Statistical model | |
| ω BSV CL/F | 0.46 (32) |
| ω BSV C0 | 0.39 (9) |
| σ proportional | 0.20 (11) |
C0 = C0*(θNo white)*(BW/median(BW))*∧θBW. For example, for a non-white patient weighing 40 kg, individual C0 is 14.7*.74*(40/50)∧ − 0.29 = 11.6 ng ml−1. RSE%, relative standard error (standard error of estimate/estimate*100); CL/F, apparent elimination clearance; V/F, apparent central volume of distribution; C0, basal 25(OH)D concentration; σ, residual variability estimates and BSV, between subject variability estimates; θNo white, influential factor on 25(OH)D C0 for a non-white skin phototype patient; θBW, influential factor on 25(OH)D C0 for bodyweight.
Evaluation and validation
The normalized prediction distribution error performed on the final model showed that the mean and variance were not significantly different from 0 (P = 0.8, Wilcoxon signed rank test) and 1 (P = 0.9, Fisher variance test) and their distribution was not different from a normal one (P = 0.7, Shapiro-Wilk test of normality). The prediction-corrected visual predictive check showed that the 10th, 50th and 90th percentiles of observed data were well included within the 90% CI of the 10th, 50th and 90th of simulated percentiles (Figure 1).
Figure 1.
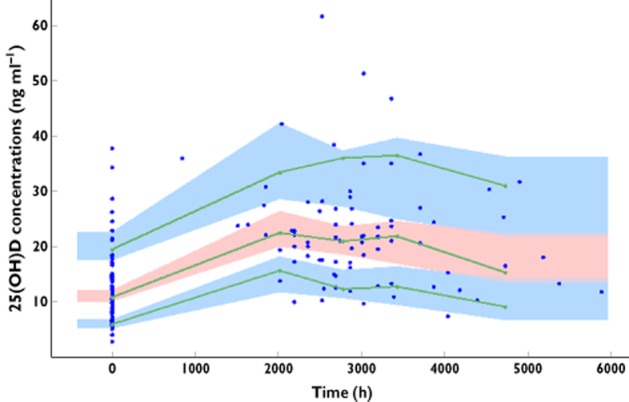
Prediction corrected visual predictive check for 25(OH)D concentrations vs. time. The lines show the 10th, 50th and 90th percentile of observed data. The areas represent the 90% confidence interval around the simulated percentiles
Doses simulation
Different dosing schemes were simulated to obtain 25(OH)D concentrations between 30 and 80 ng ml−1 for 1 year. Figure 2 shows the percentages of patients with 25(OH)D concentrations lower than 30 ng ml−1, between 30 and 80 ng ml−1 (target range) and higher than 80 ng ml−1 after 12 months of treatment. To avoid the risk of toxicity, the dose which produced less than 5% of patients above 80 ng ml−1 was retained.
Figure 2.
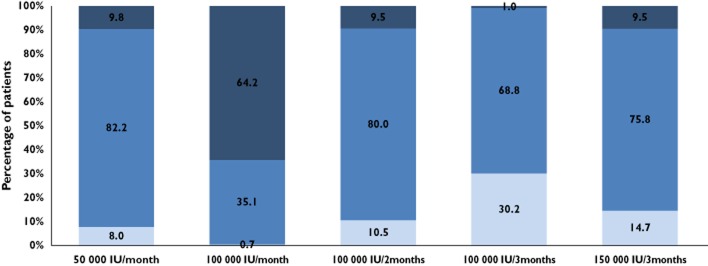
After 12 months of treatment, percentages of patients with 25(OH)D concentrations lower than 30 ng ml−1, between 30 and 80 ng ml−1 (target interval) and higher than 80 ng ml−1 (related to toxicity).  , <30 ng ml−1;
, <30 ng ml−1;  , 30–80 ng ml−1;
, 30–80 ng ml−1;  , >80 ng ml−1
, >80 ng ml−1
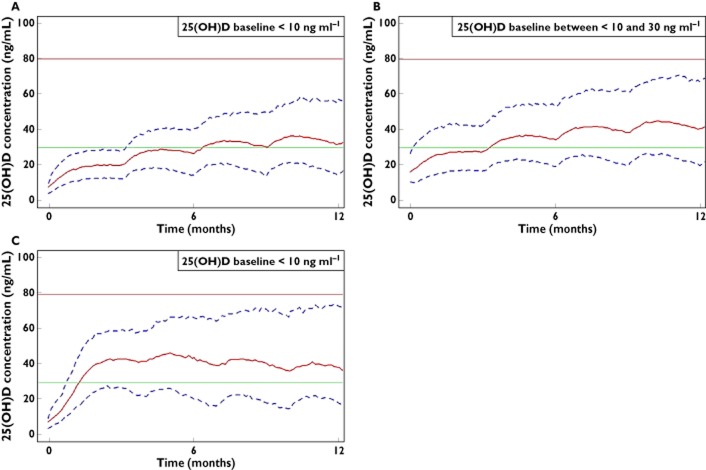
The concentration−time profiles obtained with a dose of 100 000 IU of vitamin D3 per 3 months according to different baselines, <10 ng ml−1 (Figure 3A) or between 10 and 30 ng ml−1 (Figure 3B) show that patients with vitamin D deficiency before supplementation have lower 25(OH)D concentrations after 12 months of treatment than patients with vitamin D insufficiency: median (90% CI) concentrations are 32 (15, 57) ng ml−1 and 41 (20, 67) ng ml−1, respectively. Furthermore, 6.4 months are necessary for half of the vitamin D deficient patients to reach the threshold of 30 ng ml−1, whereas patients having an insufficiency at baseline need only 3.4 months.
Figure 3.
Calculated 25(OH)D concentrations (solid line) and 90% CI (dashed lines) vs. time according to a 100 000 IU per 3 months dosing scheme in patients with baseline concentration <10 ng ml−1 (A), a 100 000 IU per 3 months dosing scheme in patients with baseline concentrations between 10 and 30 ng ml−1 (B) and an intensive (100 000 IU per 2 weeks, two times) and a maintenance dosing scheme (after 2 weeks, 100 000 IU per 3 months) in patients with baseline concentrations <10 ng ml−1 (C). The horizontal lines represent the 30 and 80 ng ml−1 25(OH)D targets for efficacy and toxicity, respectively
If children and adolescents have a deficient 25(OH)D status before beginning the treatment, a specific dosing scheme allowing to reach quickly sufficient 25(OH)D concentrations would be (i) an intensive phase 100 000 IU on day 1 and day 15 (intensive phase) and (ii) then beginning at day 30, a maintenance phase 100 000 IU every 3 months (Figure 3C). This dosing scheme results in higher 25(OH)D median (IQR) concentrations, 39 (18–73) ng ml−1, after 12 months of treatment and the time to observe half of the patients above 30 ng ml−1 is reduced to 1.3 months.
Discussion
The pharmacokinetics of 25(OH)D were satisfactorily described by a one compartment model with an additional basal level parameter representing the formation of 25(OH)D provided by food and sunlight exposure. This model has been previously published to describe the 25(OH)D pharmacokinetics in HIV-infected adults [30]. As shown in adult patients, the first order formation and elimination rate constant estimates were close, resulting in a flip-flop phenomenon. In order to interpret adequately the elimination slope, these two rate constants were defined as equal, which resulted in an improvement of both BIC criteria (decreased by 3 points) and relative standard errors of the estimates.
The skin phototype influenced the basal 25(OH)D production parameter, after the Fitzpatrick scale was reduced to two categories, ‘clear skin’ phototype and ‘dark skin’ phototype. The 25(OH)D production without any vitamin D3 supplementation was estimated to be 27% lower in non-white children and adolescents. A size effect including age, BMI, or BW was investigated on CL/F. The BW effect was also tested using an allometric scaling (exponents of ¾ and 1, for CL/F and V/F, respectively). Finally, BW was found to decrease significantly the basal production of 25(OH)D. Although a season effect was expected on the basal 25(OH)D production parameter, no significant relationship could be determined as most of 25(OH)D samples were collected during winter. Regarding the assessment of ARV, no significant differences were observed on pharmacokinetic parameters. However, the majority of patients had received an ARV combination including a PI + 2 nucleosides.
High prevalences of 25(OH)D insufficiency and deficiency were found among the 91 children and adolescents at inclusion: 28% of the patients had 25(OH)D concentrations below 10 ng ml−1, 69% between 10 and 30 ng ml−1, whereas only 3% had 25(OH)D concentrations above 30 ng ml−1. It should be noted that the role of dietary vitamin D intake could be lower in France than in Anglo-Saxon countries, as fortified milk or multivitamin intake are less common. A vitamin D3 dosing scheme allowing to reach and maintain the target of 30 ng ml−1 in most patients could ensure bone growth and limit some deleterious effects associated to the HIV disease. Based on the current study data and simulations from the final pharmacokinetic model, dosing schemes of 50 000 IU monthly or 100 000 IU bi-monthly should therefore be the most effective regimens. These results are in agreement with three previous studies which have evaluated equivalent vitamin D3 dosing regimens administered either weekly, monthly or bi-monthly in HIV-infected children and young adults [20,22,31]. The authors showed that these dosages resulted in a significant improvement of the vitamin D status. However, none of them reported the percentage of patients with 25(OH)D concentrations above 60 or 80 ng ml−1 and addressed long term toxicities issues. According to this study, the simulation of a bi-monthly dosing scheme of 100 000 IU showed that 9.5% of patients were above the toxic cutoff of 80 ng ml−1 at 12 months (1.2% over 100 ng ml−1). Therefore, to reduce the risk of toxicity the 100 000 IU per 3 months dosage was finally suggested. Regardless of the baseline value, this dosage will result, after 1 year of treatment, in more than 68% of patients having concentrations above 30 ng ml−1, without exceeding 80 ng ml−1 (<1%). These results are supported by a recent study which reported in 25 young patients receiving 100 000 IU per 3 months, that 80% of them reached sufficient 25(OH)D concentrations after 12 months of treatment [32].
Nevertheless, this dosing scheme could be insufficient for children and adolescents with vitamin D deficiency, as only 55% will reach the target of 30 ng ml−1 after 1 year of treatment. Thus, another dosing scheme was proposed which included an intensive phase of 100 000 IU per 2 weeks (two times) followed 2 weeks later by a maintenance phase (100 000 IU per 3 months).
These dosing propositions are in accordance with the vitamin D recommendations of the Institute of Medicine and the Endocrine Practice Guideline Committee in healthy children and adolescents [33].
The metabolism of vitamin D is known to be particularly complex as it depends on multiple factors. Whereas 25(OH)D is the major circulating form of vitamin D, it is also a biologically inactive form which needs a second hydroxylation in the kidneys to become active (1.25(OH)2D). This active form can inhibit its own synthesis through a negative feedback or by decreasing the secretion of PTH. Moreover other factors such as calcium or serum phosphate concentrations can modify the synthesis of 1,25(OH)2D [4]. However in this study only the 25(OH)D time course was evaluated. Thus it would be of great interest to develop a mechanistic model taking into account 1,25(OH)2D and PTH concentrations as well as other relevant factors to assess the impact of vitamin D3 supplementation.
This study reports 25(OH)D pharmacokinetics in HIV-infected children and adolescents. Dosing schemes were established to obtain sufficient 25(OH)D concentrations during the year and prevent toxic events. These conclusions should be prospectively confirmed.
Acknowledgments
We acknowledge Mrs Colas who performed administrative CNIL processes.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no support from any organization for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.
References
- 1.Dao CN, Patel P, Overton ET, Rhame F, Pals SL, Johnson C, Bush T, Brooks JT. Low vitamin D among HIV-infected adults: prevalence of and risk factors for low vitamin D Levels in a cohort of HIV-infected adults and comparison to prevalence among adults in the US general population. Clin Infect Dis Off Publ Infect Dis Soc Am. 2011;52:396–405. doi: 10.1093/cid/ciq158. [DOI] [PubMed] [Google Scholar]
- 2.Kim JH, Gandhi V, Psevdos G, Espinoza F, Park J, Sharp V. Evaluation of vitamin D levels among HIV-infected patients in New York City. 2011. AIDS Res Hum Retroviruses [Internet]. Available at http://www.ncbi.nlm.nih.gov/pubmed/21644847 (last accessed 27 November 2011) [DOI] [PubMed]
- 3.Mueller NJ, Fux CA, Ledergerber B, Elzi L, Schmid P, Dang T, Magenta L, Calmy A, Vergopoulos A, Bischoff-Ferrari HA. High prevalence of severe vitamin D deficiency in combined antiretroviral therapy-naive and successfully treated Swiss HIV patients. AIDS Lond Engl. 2010;24:1127–1134. doi: 10.1097/QAD.0b013e328337b161. [DOI] [PubMed] [Google Scholar]
- 4.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 5.Vieth R, Bischoff-Ferrari H, Boucher BJ, Dawson-Hughes B, Garland CF, Heaney RP, Holick MF, Hollis BW, Lamberg-Allardt C, McGrath JJ, Norman AW, Scragg R, Whiting SJ, Willett WC, Zittermann A. The urgent need to recommend an intake of vitamin D that is effective. Am J Clin Nutr. 2007;85:649–650. doi: 10.1093/ajcn/85.3.649. [DOI] [PubMed] [Google Scholar]
- 6.Souberbielle J-C, Body J-J, Lappe JM, Plebani M, Shoenfeld Y, Wang TJ, Bischoff-Ferrari HA, Cavalier E, Ebeling PR, Fardellone P, Gandini S, Gruson D, Guérin AP, Heickendorff L, Hollis BW, Ish-Shalom S, Jean G, von Landenberg P, Largura A, Olsson T, Pierrot-Deseilligny C, Pilz S, Tincani A, Valcour A, Zittermann A. Vitamin D and musculoskeletal health, cardiovascular disease, autoimmunity and cancer: recommendations for clinical practice. Autoimmun Rev. 2010;9:709–715. doi: 10.1016/j.autrev.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 7.Dawson-Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, Vieth R. Estimates of optimal vitamin D status. Osteoporos Int J Establ Result Coop Eur Found Osteoporos Natl Osteoporos Found USA. 2005;16:713–716. doi: 10.1007/s00198-005-1867-7. [DOI] [PubMed] [Google Scholar]
- 8.Rosen CJ. Clinical practice. Vitamin D insufficiency. N Engl J Med. 2011;364:248–254. doi: 10.1056/NEJMcp1009570. [DOI] [PubMed] [Google Scholar]
- 9.Holick MF. Vitamin D status: measurement, interpretation, and clinical application. Ann Epidemiol. 2009;19:73–78. doi: 10.1016/j.annepidem.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones G. Pharmacokinetics of vitamin D toxicity. Am J Clin Nutr. 2008;88:582S–586. doi: 10.1093/ajcn/88.2.582S. [DOI] [PubMed] [Google Scholar]
- 11.Shinchuk L, Holick MF. Vitamin D and rehabilitation: improving functional outcomes. Nutr Clin Pract. 2007;22:297–304. doi: 10.1177/0115426507022003297. [DOI] [PubMed] [Google Scholar]
- 12.Pasquet A, Viget N, Ajana F, de la Tribonniere X, Dubus S, Paccou J, Legroux-Gérot I, Melliez H, Cortet B, Yazdanpanah Y. Vitamin D deficiency in HIV-infected patients: associated with non-nucleoside reverse transcriptase inhibitor or efavirenz use? AIDS Lond Engl. 2011;25:873–874. doi: 10.1097/QAD.0b013e32834542fa. [DOI] [PubMed] [Google Scholar]
- 13.Lattuada E, Lanzafame M, Zoppini G, Concia E, Vento S. No influence of nevirapine on vitamin D deficiency in HIV-infected patients. AIDS Res Hum Retroviruses. 2009;25:849–850. doi: 10.1089/aid.2009.0063. [DOI] [PubMed] [Google Scholar]
- 14.Cozzolino M, Vidal M, Arcidiacono MV, Tebas P, Yarasheski KE, Dusso AS. HIV-protease inhibitors impair vitamin D bioactivation to 1,25-dihydroxyvitamin D. AIDS Lond Engl. 2003;17:513–520. doi: 10.1097/00002030-200303070-00006. [DOI] [PubMed] [Google Scholar]
- 15.Brown TT, McComsey GA. Association between initiation of antiretroviral therapy with efavirenz and decreases in 25-hydroxyvitamin D. Antivir Ther. 2010;15:425–429. doi: 10.3851/IMP1502. [DOI] [PubMed] [Google Scholar]
- 16.Fox J, Peters B, Prakash M, Arribas J, Hill A, Moecklinghoff C. Improvement in vitamin D deficiency following antiretroviral regime change: results from the MONET trial. AIDS Res Hum Retroviruses. 2011;27:29–34. doi: 10.1089/aid.2010.0081. [DOI] [PubMed] [Google Scholar]
- 17.Eckard AR, Judd SE, Ziegler TR, Camacho-Gonzalez AF, Fitzpatrick AM, Hadley GR, Grossmann RE, Seaton L, Seydafkan S, Mulligan MJ, Rimann N, Tangpricha V, McComsey GA. Risk factors for vitamin D deficiency and relationship with cardiac biomarkers, inflammation and immune restoration in HIV-infected youth. Antivir Ther. 2012;17:1069–1078. doi: 10.3851/IMP2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stephensen CB, Marquis GS, Kruzich LA, Douglas SD, Aldrovandi GM, Wilson CM. Vitamin D status in adolescents and young adults with HIV infection. Am J Clin Nutr. 2006;83:1135–1141. doi: 10.1093/ajcn/83.5.1135. [DOI] [PubMed] [Google Scholar]
- 19.Rutstein R, Downes A, Zemel B, Schall J, Stallings V. Vitamin D status in children and young adults with perinatally acquired HIV infection. Clin Nutr Edinb Scotl. 2011;30:624–628. doi: 10.1016/j.clnu.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 20.Havens PL, Mulligan K, Hazra R, Flynn P, Rutledge B, Van Loan MD, Lujan-Zilbermann J, Kapogiannis BG, Wilson CM, Stephensen CB. Serum 25-hydroxyvitamin D response to vitamin D3 supplementation 50 000 IU monthly in youth with HIV-1 infection. J Clin Endocrinol Metab. 2012;97:4004–4013. doi: 10.1210/jc.2012-2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meyzer C, Frange P, Chappuy H, Desse B, Veber F, Le Clésiau H, Friedlander G, Blanche S, Souberbielle J-C, Tréluyer J-M, Courbebaisse M. Vitamin D deficiency and insufficiency in HIV infected children and young adults. Pediatr Infect Dis J. 2013;32:1240–1244. doi: 10.1097/INF.0b013e3182a735ed. [DOI] [PubMed] [Google Scholar]
- 22.Kakalia S, Sochett EB, Stephens D, Assor E, Read SE, Bitnun A. Vitamin D supplementation and CD4 count in children infected with human immunodeficiency virus. J Pediatr. 2011;159:951–957. doi: 10.1016/j.jpeds.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 23.Arpadi SM, McMahon D, Abrams EJ, Bamji M, Purswani M, Engelson ES, Horlick M, Shane E. Effect of bimonthly supplementation with oral cholecalciferol on serum 25-hydroxyvitamin D concentrations in HIV-infected children and adolescents. Pediatrics. 2009;123:e121–126. doi: 10.1542/peds.2008-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fitzpatrick TBT. He validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124:869–871. doi: 10.1001/archderm.124.6.869. [DOI] [PubMed] [Google Scholar]
- 25.Vidailhet M, Mallet E, Bocquet A, Bresson J-L, Briend A, Chouraqui J-P, Darmaun D, Dupont C, Frelut M-L, Ghisolfi J, Girardet J-P, Goulet O, Hankard R, Rieu D, Simeoni U, Turck D. Vitamin D: still a topical matter in children and adolescents. A position paper by the Committee on Nutrition of the French Society of Paediatrics. Arch Pédiatrie Organe Off Sociéte Française Pédiatrie. 2012;19:316–328. doi: 10.1016/j.arcped.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 26.Kuhn E, Lavielle M. Maximum likelihood estimation in nonlinear mixed effects models. Comput Stat Data Anal. 2005;49:1020–1038. [Google Scholar]
- 27.Comets E, Brendel K, Mentré F. Computing normalised prediction distribution errors to evaluate nonlinear mixed-effect models: the NPDE add-on package for R. Comput Methods Programs Biomed. 2008;90:154–166. doi: 10.1016/j.cmpb.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 28.Bergstrand M, Hooker AC, Wallin JE, Karlsson MO. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J. 2011;13:143–151. doi: 10.1208/s12248-011-9255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Team R others. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Found Stat Comput; 2010. (01/19) [Google Scholar]
- 30.Foissac F, Tréluyer J-M, Souberbielle J-C, Rostane H, Urien S, Viard J-P. Vitamin D3 supplementation scheme in HIV-infected patients based upon pharmacokinetic modelling of 25-hydroxycholecalciferol. Br J Clin Pharmacol. 2013;75:1312–1320. doi: 10.1111/bcp.12006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arpadi SM, McMahon DJ, Abrams EJ, Bamji M, Purswani M, Engelson ES, Horlick M, Shane E. Effect of supplementation with cholecalciferol and calcium on 2-y bone mass accrual in HIV-infected children and adolescents: a randomized clinical trial. Am J Clin Nutr. 2012;95:678–685. doi: 10.3945/ajcn.111.024786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giacomet V, Vigano A, Manfredini V, Cerini C, Bedogni G, Mora S, Borelli M, Trabattoni D, Zuccotti GV. Cholecalciferol supplementation in HIV-infected youth with vitamin D insufficiency: effects on vitamin D status and T-cell phenotype: a randomized controlled trial. HIV Clin Trials. 2013;14:51–60. doi: 10.1310/hct1402-51. [DOI] [PubMed] [Google Scholar]
- 33.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]