Abstract
This paper describes the isolation of wheat mutants in the hard red spring Scarlet resulting in reduced sensitivity to the plant hormone abscisic acid (ABA) during seed germination. ABA induces seed dormancy during embryo maturation and inhibits the germination of mature seeds. Wheat sensitivity to ABA gradually decreases with dry after-ripening. Scarlet grain normally fails to germinate when fully dormant, shows ABA sensitive germination when partially after-ripened, and becomes ABA insensitive when after-ripened for 8–12 months. Scarlet ABA-insensitive (ScABI) mutants were isolated based on the ability to germinate on 5 µM ABA after only 3 weeks of after-ripening, a condition under which Scarlet would fail to germinate. Six independent seed-specific mutants were recovered. ScABI 1, ScABI2, ScABI3 and ScABI4 are able to germinate more efficiently than Scarlet at up to 25 µM ABA. The two strongest ABA insensitive lines, ScABI3 and ScABI4, both proved to be partly dominant suggesting that they result from gain-of-function mutations. The ScABI1, ScABI2, ScABI3, ScABI4, and ScABI5 mutants after-ripen more rapidly than Scarlet. Thus, ABA insensi-tivity is associated with decreased grain dormancy in Scarlet wheat. This suggests that ABA sensitivity is an important factor controlling grain dormancy in wheat, a trait that impacts seedling emergence and pre-harvest sprouting resistance.
Keywords: Wheat, Abscisic acid, Dormancy, Pre-harvest sprouting
Introduction
The control of seed dormancy and germination is very important in cereal crops where excessive dormancy leads to problems with uneven stand establishment, and insufficient seed dormancy can result in pre-harvest sprouting (PHS). PHS occurs in cereals including wheat, barley, millet, and sorghum when cool rainy conditions occur prior to harvest (reviewed by Gubler et al. 2005) and results in economic losses to farmers when sprouted grain is rated as feed. While environmental conditions induce sprouting, there are clearly genetic differences in susceptibility (Flintham 2000). Susceptibility to PHS is associated with lack of seed dormancy, whereas resistance is associated with higher seed dormancy. Seed dormancy describes the state in which mature seeds are unable to germinate under favorable conditions (reviewed by Finkelstein et al. 2008). Dormant seeds acquire the ability to germinate through dormancy breaking treatments including dry after-ripening, a period of dry storage, or cold stratification, a period of moist chilling. Dormancy and PHS resistance are associated with differences in grain coat color, hormone accumulation or responsiveness, and spike architecture (reviewed by Schramm et al. 2010). This study examines the contribution of abscisic acid (ABA) response to control of wheat grain dormancy in a hard red spring wheat, Scarlet.
Bread wheat, Triticum aestivum L., is an allohex-aploid crop plant that is a staple for human nutrition. Allohexaploid wheat has a haploid chromosome number of 21 (AABBDD, 2n = 42, x = 7) such that three related diploid progenitors contributed seven chromosomes each through two polyploidization events (Jiang and Gill 1994; Galili et al. 2000; Dubcovsky and Dvorak 2007). Hybridization of the A genome donor T. urartu Tumanian ex Gandilyan with the B genome donor, a close relative of Aegilops speltoides, resulted in tetraploid durum wheat (AABB, 2n = 28, x = 7). Hybridization of durum wheat with the D genome donor Ae. tauschii resulted in modern hexaploid bread wheat approximately 8,000 years ago. Because the three wheat progenitors were close relatives, the gene-coding regions of the A, B, and D genomes are approximately 95 % identical at the nucleotide level. Many wheat genes are present as three homologous copies on the A, B, and D genome referred to as homoeologues. Thus, selection for mutants in wheat is likely to give rise either to single dominant mutations or to recessive mutations either in rare single copy genes or in genes that evolved tissue-specific gene expression.
Wheat cultivars with a red testa color resulting from the accumulation of catechin and proanthocyanidin in the testa or seed coat tend to have greater dormancy and PHS resistance than cultivars that have a white testa resulting from recessive mutations in all three copies of the Red-1 locus (R-A1, R-B1, and R-D1) (Miyamoto and Everson 1958; McCallum and Walker 1990). The R-1 gene encodes TaMyb10, an R2R3 type Myb domain transcription factor that is a homologue of Arabidopsis thaliana TT2 (Himi et al. 2011). The line Chinese Spring contains a single dominant R-D1 gene for red testa color. An induced r-D1 mutation in Chinese Spring reduced, but did not completely eliminate grain dormancy (Warner et al. 2000). Moreover, there are red cultivars with low seed dormancy and white cultivars with high seed dormancy (Morris et al. 1989; Torada and Amano 2002). Thus, red testa color contributes to, but is not the sole source of seed dormancy. Differences in ABA sensitivity or accumulation appear to account for some variation in wheat (Walker-Simmons 1987; McKibbin et al. 2002; Schramm et al. 2010) and barley (Chono et al. 2006) grain dormancy. In addition, red testa color is associated with higher apparent ABA sensitivity (Himi et al. 2002), although the effect may be dependent on the level of dormancy of the grain (Warner et al. 2000).
Seed dormancy can result from two mechanisms, seed coat imposed dormancy and embryo dormancy (reviewed by Bewley 1997). In seed coat imposed dormancy tissues covering the embryo can inhibit germination by inhibiting gas exchange or water uptake, by acting as a physical barrier to radicle emergence, or though diffusible inhibitors. Coat imposed dormancy can be relieved by cutting or removing the seed coat. In embryo dormancy, the embryo fails to grow even when surrounding tissues are removed. Wheat exhibits both types of dormancy (reviewed by Bewley and Black 1994; Schramm et al. 2010). Cutting wheat seeds in half generally improves the germination capacity of wheat grains. The embryo half of cut grains can be assayed for ABA sensitivity independent of coat imposed dormancy (Walker-Simmons 1987; Schramm et al. 2010).
ABA is a sesquiterpenoid plant hormone that induces seed dormancy and desiccation tolerance during plant embryo maturation (reviewed by Holdsworth et al. 2008). ABA maintains dormancy in mature seed, whereas the hormone gibberellic acid (GA) breaks dormancy and stimulates germination (Karssen and Lac¸ka 1986). The current understanding of the role of ABA in inducing and maintaining seed dormancy results, to a large extent, from studies of mutants with altered ABA accumulation or response. Loss of function mutations in ABA biosynthetic enzymes or in positive regulators of ABA signaling result in reduced seed dormancy in Arabidopsis and tomato, and vivipary in maize (Koornneef et al. 1982; McCarty et al. 1991; Groot and Karssen 1992; Schwartz et al. 1997). Loss of function mutations in the ABA catabolic enzyme, ABA 8′-hydroxylase, or in negative regulators of ABA signaling result in increased seed dormancy in Arabidopsis (Kushiro et al. 2004; Okamoto et al. 2006). Loss-of-function mutations in the RCAR/PYR/PYL family of ABA receptors result in ABA insensitive seed germination in Arabidopsis (Ma et al. 2009; Park et al. 2009). The ABA receptors, members of the START/Bet v onefold superfamily (reviewed by Klinger et al. 2010), bind and inhibit the activity of a family of protein phosphatase type 2Cs (PP2Cs) in an ABA-dependent manner (Nishimura et al. 2010).
The effects of ABA signaling mutations can be pleiotropic because in addition to inducing seed dormancy and desiccation tolerance in seeds, ABA also induces vegetative responses to environmental stresses including drought, cold, and salt tolerance [reviewed by Zhang et al. (2006), reviewed by Tuteja (2007)]. ABA induces stomatal closure in response to drought stress to conserve water, and also induces osmotic protectants such as proline and dehydrin protein accumulation. For example, gain-of-function mutations in PP2Cs result in decreased seed dormancy, ABA insensitive seed germination, a vegetative wilty phenotype, and early flowering (Koornneef et al. 1984; Leung et al. 1997).
The purpose of this work was to examine the role of ABA in seed dormancy of wheat, a member of the Triticae tribe. Seed dormancy in barley (Hordeum vulgare), another member of the Triticae tribe, is associated with high level expression of the noid dioxygenase), and low level expression of the ABA catabolic gene HvABA8’OH-1 suggesting that seed dormancy is also regulated by ABA accumulation in these cereals (Millar et al. 2006). This evidence indicates that ABA accumulation regulates seed dormancy in the Triticae.
If ABA regulates wheat grain dormancy, then we would expect that mutants selected for increased ABA sensitivity would result in higher seed dormancy, whereas mutations selected for decreased ABA sensitivity would result in decreased grain dormancy. We previously found that although Warm mutants (Wheat ABA responsive mutants) selected for increased ABA sensitivity result in increased seed dormancy, this phenotype is dependent on the degree of after-ripening (Schramm et al. 2010). Both normal and Warm wheat grain lose ABA sensitivity as a result of after-ripening. However, Warm lines after-ripen more slowly than wild-type.
In order to examine the role of ABA in inducing wheat seed dormancy, this study has isolated ABA insensitive mutants in the hard red spring Scarlet. These mutations result in reduced seed dormancy and a decreased requirement for after-ripening prior to seed germination. While the effect of these mutations is mostly seed-specific, some mutations do have secondary phenotypes.
Materials and methods
Germination assays
The term seed is used in reference to the wheat caryopsis or grain. Because of variation in the degree of dormancy between seed lots, germination assays were always performed using seeds from parents grown side-by-side that had reached a suitable degree of after-ripening to maximize the difference between wild-type and mutant ABA sensitivity (determined experimentally, ranging from 2 weeks to 4 months, depending on the experiment). Germination assays were performed using either whole or cut half-grains plated on a 9-cm petri dish lined with a single germination disc (Anchor steel blue germination blotter (SDB3.375), Anchor Paper Co., St. Paul MN) moistened with an aqueous solution of 6 mL of 5 mM MES (pH 5.5) buffer (2-[N-morpholino] ethane sulfonic acid, Sigma, St. Louis, MO) containing varying concentrations of optically pure (+)-ABA (PBI58, gift from S. Abrams). MES buffer without ABA was used as a negative control. (+)-ABA was maintained as a 0.1 M stock in methanol at −20 °C in the dark. The plates were sealed with Parafilm to prevent evaporation, wrapped with foil, and incubated at 30 °C in the dark (Walker-Simmons 1987). Germination was scored based on radicle emergence every 24 h for 5 days. Germination was expressed either as a percentage germinated or as a weighted germination index (GI) calculated over 5 days of scoring as (5 × gday 1 + 4 × gday 2… + 1 × gday 5)/(5 × n) where g is the number of germinated seeds and n is the total number of viable seeds (Walker-Simmons 1987), except for the ScABI3 F3 germination, which used a GI calculation over 6 days of germination scoring. Using this formula, the maximum possible GI is 1.0, and the speed of germination as well as number of germinated seeds are represented by a single value.
Plant material and mutagenesis
Wild type seed for the hard red spring cultivar ‘Scarlet’ were obtained from Kidwell et al. (1999). Five thousand Scarlet grains were mutagenized with 0.3 % ethyl methanesulfonate (EMS; Sigma Chemicals, St. Louis, MO) as previously described (Okubara et al. 2009). Briefly, grains were presoaked in 50 mM potassium phosphate buffer (pH 7.0) for 5 h, then mutagenized in 200 mL 0.3 % (v/v) EMS in 50 mM phosphate buffer (pH 7.0) with shaking for 16 h. EMS was then neutralized with an equal volume of 10 % (w/v) sodium thiosulfate and washed 10 times in water. Mutagenized grain (M1) was grown under standard growth conditions in the greenhouse. The primary spike from each M1 plant was harvested. A total of four grains each from 600 independent M1 spikes were plated on 5 µM ABA such that a total of 2,400 M2 grains were screened for the ability to germinate on ABA after 3 weeks of dry after-ripening at room temperature. Original isolation numbers for mutants were generated based on experiment number, and are renamed as follows: 1B = ScABI1, 1C = ScABI2, 2A = ScABI3, 2C = ScABI4, 2D = ScABI5, 2E = ScABI6 (Scarlet ABA Insensitive).
Growth conditions
Plants were grown in the greenhouse with a photoperiod of 16 h at a light intensity of 400 µmol m−2 s−1, with a daytime temperature of 21–24 °C, and a nighttime temperature of 15–18 °C. During winter months, supplemental light was provided with high pressure sodium lamps. Seeds or seedlings were planted into 3 L pots containing Sunshine LC1 potting soil mixture (Sun Gro Horticulture). Pots were watered to saturation every 2 days. A nutrient solution (Peters Professional 20-10-20 Peat-Lite Special) was supplied once a week. For germination experiments, spikes were harvested at physiological maturity and seeds were hand threshed to avoid scarification of the seed coat. Seeds were subject to dry after-ripening at room temperature and thereafter stored at −20 °C to maintain dormancy unless otherwise indicated.
M6 ScABI plants used to determine vegetative sensitivity to ABA in the leaf transpiration assay, final plant height, and length of time required to flower were grown in a growth room with a 16 h photoperiod (22 °C day, 15 °C night) and light intensity of 400 µmol m−2 s−1 with a 1:1 ratio of metal halide: sodium lamps. Plant height was measured from the base of the plant to the top of the spike on the tallest tiller excluding awns.
Leaf transpiration experiment
Vegetative transpiration and sensitivity to ABA was examined using a detached leaf transpiration assay adapted from Raskin and Ladyman (1988). Modifications included different ABA concentrations as determined by pilot experiments to be appropriate for wheat treated with (+)-ABA instead of barley treated with (±)-ABA. Uppermost fully expanded leaves were detached and placed cut end down in a 15 mL tube filled with 8 mL of treatment solution (either MES (pH 5.5) with methanol as a negative control, or MES with various concentrations of ABA in methanol). The leaf transpiration experiment was conducted in the same growth room used to grow the leaf donor plants in order to avoid the need to acclimate the leaves to a new environment. Tubes containing treatment solutions were weighed before the experiment to determine the starting weight. Parafilm was wrapped around the tube opening to hold the leaf upright in the treatment solution, and to prevent evaporation. After 24 h, leaves were scanned using a flatbed scanner and exposed transpiring leaf area determined by analyzing images with ImageJ (version 1.37) (Abramoff et al. 2004). After leaves were removed, tubes were capped and re-weighed so that the amount of solution transpired could be calculated. Transpiration rates were calculated by dividing the volume of treatment solution transpired in 24 h by the leaf area.
Statistical analysis
Data were analyzed using an analysis of variance, and tests of differences between each mutant and wild type were conducted using the Dunnett’s multiple comparison adjustment using Minitab software (version 16).
Results
Identification of ABA insensitive mutants in hard red spring Scarlet
The hard red spring Scarlet was chosen as a background for isolation of EMS-induced ABA insensitive mutations because it is quite dormant requiring up to 9 months to fully after-ripen (Schramm et al. 2010). A total of 2,400 M2 seeds derived from 600 independent EMS-mutagenized M1 plants were after-ripened for 3 weeks after harvest and then screened for the ability to germinate rapidly on 5 µM ABA in order to identify ABA-insensitive mutants in the Scarlet background (referred to here as Scarlet ABA Insensitive mutants (ScABI)). Eleven putative mutants were isolated in the M2, and then advanced by single plant descent to the M4 for retesting. In the M4 generation, putative mutant seeds were tested for germination intact (whole) and as cut half-grains, with and without ABA after 1 month of after-ripening. Six mutants each derived from independent M1 plants showed increased germination compared to Scarlet wild type (Table 1) either in the absence of ABA (ScABI5 and ScABI6), or both in the absence and presence of 5 µM ABA (ScABI1, ScABI2, ScABI3, and ScABI4) suggesting that these mutants are both less dormant and less sensitive to ABA than wild-type Scarlet. The ABA insensitive phenotype was reproducible in experiments performed on progeny of ABA insensitive plants in the M5 and M6 generation using seed that had after-ripened for 3 and 2 months respectively (Table 1, Online Resource 1). All six mutants (ScABI1-ScABI6) show an increase in germination compared to Scarlet on ABA as cut or whole seeds.
Table 1.
Germination percentages for six ScABI mutants and Scarlet wild type
| Genotype | M4a |
M5b | M6c |
||||
|---|---|---|---|---|---|---|---|
| 5 µM ABA |
MES, no ABA |
5 µM ABA | 5 µM ABA |
||||
| Cut | Whole | Cut | Whole | Whole | Cut | Whole | |
| ScABI1 | 30.0 | 25.0 | 40.0 | 40.0 | 68.3 | 85.0d | 77.5d |
| ScABI2 | 23.3 | 16.7 | 73.3 | 26.7 | 77.8 | 52.0 | 4.0 |
| ScABI3 | 40.0 | 50.0 | 100.0 | 80.0 | 99.3 | 97.1 | 94.3 |
| ScABI4 | 55.0 | 15.0 | 100.0 | 100.0 | 99.3 | 92.9 | 95.3 |
| ScABI5 | 20.0 | 10.0 | 60.0 | 30.0 | 60.0 | 57.5 | 72.5 |
| ScABI6 | 20.0 | 10.0 | 50.0 | 40.0 | 55.0 | 91.4 | 57.1 |
| Scarlet | 16.7 | 6.7 | 25.0 | 10.0 | 38.0 | 16.7 | 13.3 |
M4 seed was tested after 1 month of after-ripening. Ten to 30 seeds were tested per genotype and treatment combination. Germination percentages after 120 h are shown for all experiments except cut seeds on MES without ABA, for which 48 h is shown
M5 seed was tested after 3 months of after-ripening. Ninety to 285 seeds were tested per genotype. The 72 h time point is shown
M6 seed was tested after 2 months of after-ripening. Twenty-five to 85 seeds were tested per genotype. The 72 h time point is shown for cut seeds, and the 120 h time point is shown for whole seeds
ScABI1 was tested in a separate experiment in the M6 generation. Corresponding Scarlet germination was 30 % for cut seeds and 40 % for whole seeds
Two Scarlet ABA insensitive mutants, ScABI5 and ScABI6, were associated with secondary phenotypes that persisted over the five generations of selection. Both have altered seed shape and size compared to Scarlet wild type. ScABI5 seeds are brown, and significantly longer and narrower than Scarlet seeds (Online Resource 2a, c, d). ScABI6 seeds show no change in color, but appear to be short and rounder in shape (Online Resource 2b, c, d).
Characterization of mutant ABA dose–response during seed germination
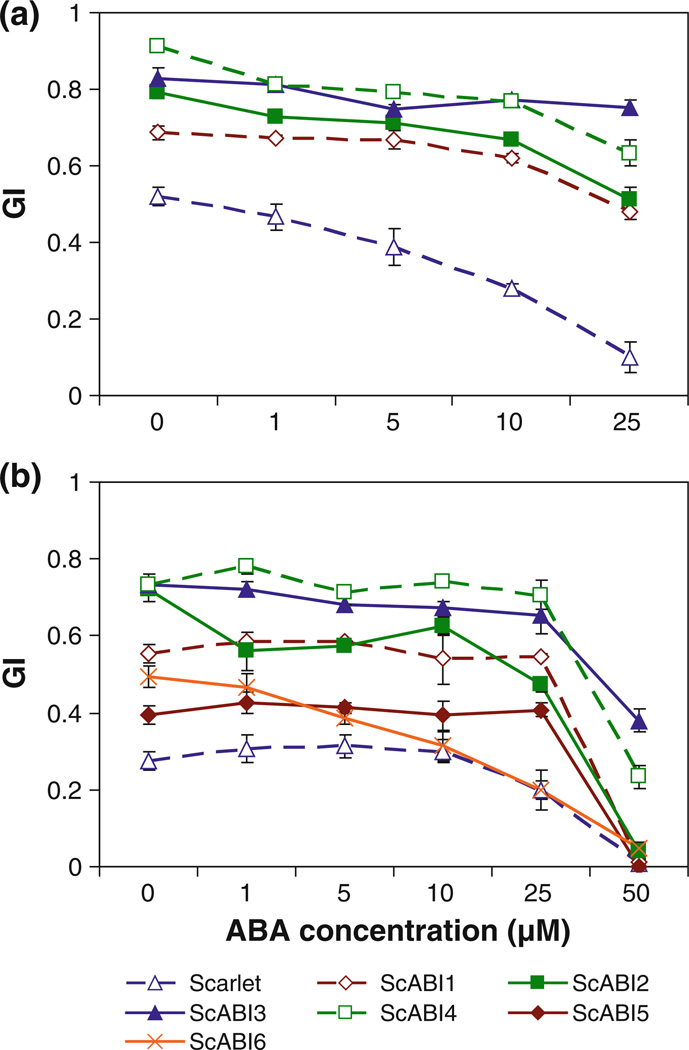
The ABA sensitivity of seed germination of ScABI mutants was examined in two dose–response experiments performed on the M5 and M6 generations. In the first experiment, intact M5 seeds were after-ripened for 3 months and then plated on increasing concentrations of ABA. ScABI1, ScABI2, ScABI3, and ScABI4 showed increased germination compared to Scarlet both in the absence of ABA and at all ABA concentrations tested (Fig. 1a). The results of the second experiment performed on intact M6 seeds (Fig. 1b) after-ripened for 2 months were consistent with the M5 experiment. ScABI3 and ScABI4 appear to be the most ABA insensitive since they germinate most efficiently at all ABA concentrations examined. Even at the highest ABA concentration tested in the M5 experiment, 25 µM, ScABI3 and ScABI4 showed germination indices (GI) of 0.75 and 0.63, respectively, compared to a GI of 0.1 for Scarlet wild type (Fig. 1a). ScABI6 appears to have more efficient germination than Scarlet wild type in the absence of ABA (GI of 0.49 vs. 0.27) and at low concentrations of ABA, but is similar to the wild type at concentrations at or above 10 µM ABA (Fig. 1b). ScABI5 appears to be less sensitive to ABA than Scarlet wild type at all concentrations examined except 50 µM, but is not as ABA insensitive as ScABI1, ScABI2, ScABI3, and ScABI4. All six mutations appear to result in reduced dormancy based on the fact that they show higher germination efficiency than Scarlet in the absence of ABA in both experiments.
Fig. 1.
ABA dose response in germination of (a) M5 and (b) M6 whole grain of ScABI mutants and Scarlet wild type. M5 grain was tested after 3 months of after-ripening, and M6 grain after 2 months of after-ripening. Germination index (GI) values shown are an average of three replicates of 10 seeds each counted over 5 days. Note that the x-axis is not drawn to scale. Error bars represent standard errors of three replicates
Segregation analysis of ABA insensitive mutants
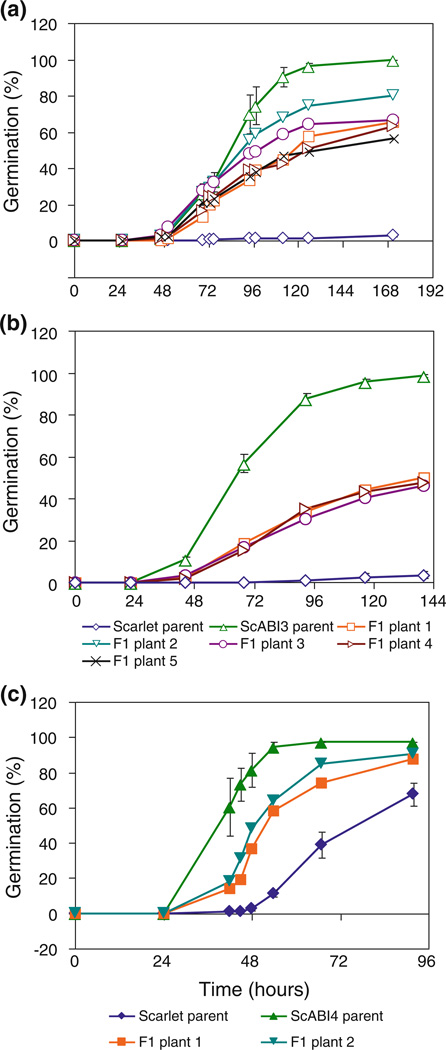
Segregation analysis was performed on ScABI3 and ScABI4, the mutants showing the strongest ABA-insensitive germination phenotype. F2 populations were derived from five independent ScABI3 × Scarlet F1 plants, and from two independent ScABI4 × Scarlet F1 plants. F1 plants were grown side-by-side with the Scarlet wild-type and mutant parents so that the parental seed germination could be used as an indicator of the likely germination capacity of the +/+ and −/− individuals segregating in the F2 populations that were harvested and after-ripened at the same time.
For ScABI3, the percent germination of intact parental and F2 seeds after-ripened for 6 weeks was determined on 2 µM ABA (Table 2). The germination of F2 populations derived from independent F1 plants varied, but all were intermediate between the parental types (Fig. 2a). The 112 h time point was chosen for Chi-square analysis of genetic segregation because the two parents showed the highest degree of difference at this time point when the ScABI3 parents showed 90.7 % germination, and the Scarlet parent showed 1.3% germination. Based on the parental germination, we would expect the F2 seeds to show 68.3 % germination if dominant (¾ of the seed are −/− or +/− giving 90.7 % germination, and ¼ of the seed are +/+ giving 1.3 % germination), and 23.7 % germination if recessive. When all F2 families were combined, the percent germination was 52.0 %. Chi-square analysis indicates that this is neither a dominant nor recessive trait (Online Resource 3a). For the sake of Chi-square analysis, if we assume that heterozygous seeds have a percent germination mid-way between the parents, then it appears that the mutation shows partial dominance (χ2 = 6.522). However, this hypothesis based on the notion that germination of heterozygotes is intermediate between the parents does not fully explain the germination of every independent F2 population examined, or the F2 data as a whole (Online Resource 3a). When F2 populations derived from independent F1 plants are examined separately, some F2 populations appear to fit the dominant model whereas others fit the semi-dominant model (Online Resource 3a).
Table 2.
Germination percentage and Chi-square analysis of F2 segregation data for two ScABI mutants with accompanying parental germination
| Genotype | Generation | n | Germination (%)a | χ2b | P value |
|---|---|---|---|---|---|
| ScABI3/Scarlet | F2 | 450 | 52.0 | 6.522 | 0.843 |
| ScABI3 parent | M6 | 150 | 90.7 | ||
| Scarlet parent | 150 | 1.3 | |||
| ScABI4/Scarlet | F2 | 254 | 42.9 | 0.039 | 0.011 |
| ScABI4 parent | M7 | 71 | 81.7 | ||
| Scarlet parent | 105 | 2.9 |
ScABI3 segregation analysis and parental germination testing was performed on intact seeds after-ripened for 6 weeks and treated with 2 µM ABA. ScABI4 segregation analysis and parental germination testing was performed on half seeds after-ripened for 3 weeks and treated with 5 µM ABA. Germination percentages after 112 h of imbibition are shown for ScABI3, and germination percentages after 48 h of imbibition are shown for ScABI4
Chi-square values calculated based on a hypothesis of incomplete dominance. Calculations were based on parental percentages using the midparent value to estimate germination of the heterozygous state
Fig. 2.
Kinetics of germination during segregation analysis for two ScABI mutants. Average percent germination of: a F2 populations derived from five F1 plants compared to parental ScABI3 and Scarlet seed derived from five independent plants; b F3 populations grouped as families derived from separate F1 plants compared to ScABI3 and Scarlet parental controls derived from 25 independent plants; and c ScABI4 F2 populations derived from two F1 plants compared to ScABI4 and Scarlet parents derived from two and four plants, respectively. Error bars represent standard error. Germination conditions for F2 data as described in Table 2. Intact F3 seeds were incubated on 2 µM ABA after 8 weeks after-ripening
Next we examined the germination phenotype of F3 progeny derived from 107 F2 plants randomly advanced from the F2 segregation analysis. F3 seeds were tested for germination on 2 µM ABA after 8 weeks of after-ripening. Mean germination of F3 seeds was again intermediate between the parental lines tested under the same conditions (Fig. 2b). F3 average germination after 6 days imbibition on ABA was 48.1 %, whereas ScABI3 parental lines averaged 98.6 % germination, and Scarlet lines averaged 3.6 % germination.
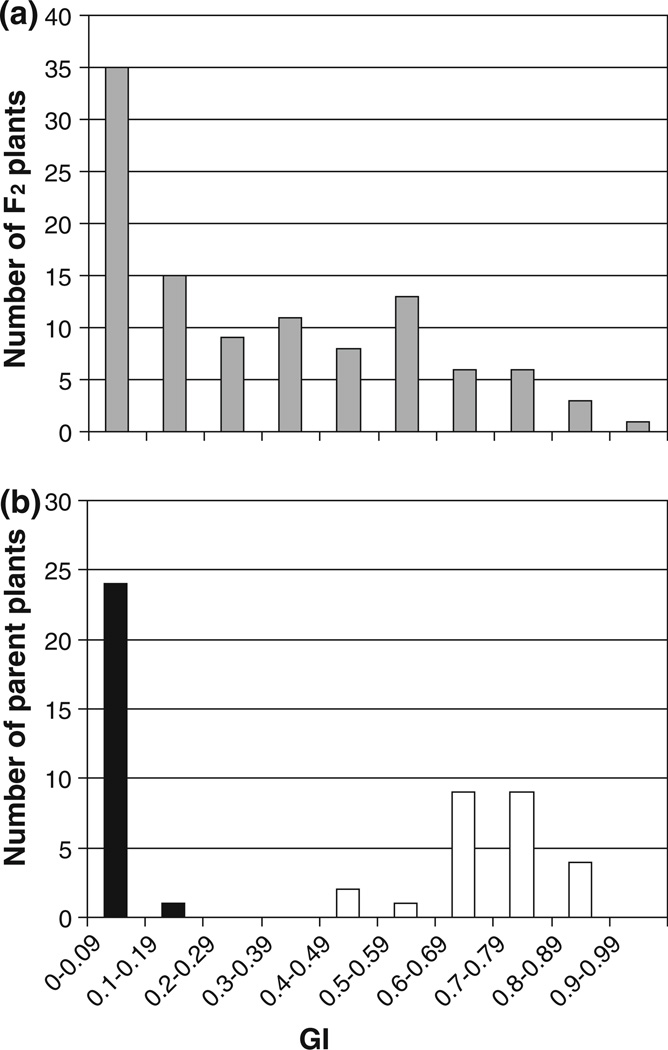
In order to visualize the range of phenotypes of the progeny of F2 plants compared to parental pheno-types, a histogram of germination data was constructed (Fig. 3). While the final percent germination was intermediate to parental germination, it appeared that the speed of germination also varied among F2 plants, and differed from the mutant parent. To integrate speed with extent of germination, ScABI3 F3 germination data was converted to GI over the 6 days of germination scored. The mean GI for the ScABI3 parents examined was 0.70, whereas Scarlet parent mean GI was only 0.01 (Fig. 3b). The mean of the F2 plants was 0.29. If the mutation is partially dominant, the distribution of F2 plants should be made up of three distributions corresponding to homozygous wild type segregants, homozygous mutant segregants, and heterozygotes (Online Resource 3b). There is a clear peak corresponding to GI of wild type segre-gants, but no clear peak representing the homozygous mutant or heterozygous F2 plants (Fig. 3a). While the GI of the wild type parent has a very narrow distribution, the GI of mutant parents has a fairly wide distribution of GIs peaking near the mean (Fig. 3b). Thus, it seems likely that the reason that there are no distinct peaks for F2 heterozygous and homozygous mutant plants because these two categories have wide distributions that overlap at the tails (Online Resource 3b).
Fig. 3.
Germination index (GI) of a F3 progeny of 107 individual F2 plants derived from a cross between ScABI3 and Scarlet and b accompanying parental seed germination. In b, Scarlet wild type seed germination is represented by black bars, and ScABI3 mutant seed germination by white bars. Seeds were incubated for 6 days on 2 µM ABA after 8 weeks of after-ripening
Next we examined whether the F3 germination phenotype was consistent with that of the F2 parents. It was expected that the 37 F2 plants grown from F2 seeds that did not germinate after 7 days of incubation would give rise mainly to wild type progeny. These gave an average F3 GI of 0.06, indicating that most of these plants were wild type segregants. On the other hand, 53 F2 plants grown from seeds that germinated by 112 h of incubation were expected to mostly give rise to mutant and heterozygous progeny. These F3 families had a mean GI of 0.44 and included only six plants that appeared to be homozygous wild type (average GI of 0.03) under conditions resulting in GIs of 0.70 for ScABI3 and 0.01 for Scarlet.
For the ScABI4 segregation analysis, the percent germination of cut seeds was determined for parental and F2 seeds after-ripened for 3 weeks on 5 µM ABA (Table 2; Fig. 2c). The germination of F2 populations derived from two independent F1 plants showed some variation, but both were intermediate between the parental types (Fig. 2c). The time point chosen for segregation analysis had an impact on the conclusion reached by Chi-square analysis, with time points after 48 h deviating farther from the hypothesis of incomplete dominance (Online Resource 3c). The 48 h time point is the most representative of the phenotypic difference because that is the last time point where wild type Scarlet germinates at a very low rate (Fig. 2c). At 48 h, the ScABI4 parents showed on average 81.7 % germination, the wild type 2.9 % germination, and the F2 seeds showed 42.9 % germination (Table 2). Based on the parental germination, we would expect the F2 to show 62.0 % germination if dominant (¾ of the seed are −/− or +/− giving 81.7 % germination, and ¼ of the seed are +/+ giving 2.9 % germination), and 22.6 % germination if recessive. The F2 germination percentage, 42.9 %, is intermediate between the two percentages suggesting that the mutation is partially dominant (χ2 = 0.039). This was consistent over F2 populations from four F1 plants derived from two independent crosses (Online Resource 3c, d).
Next, we examined the ABA sensitivity of F3 progeny derived from 13 F2 plants that germinated within 48 h of incubation on ABA in the F2 segregation analysis experiment. Among these, four appeared to be derived from homozygous −/− F2 plants (80–100 % germination), eight appeared to be derived from heterozygous F2 plants, and one appeared to be derived from a homozygous wild-type plant (0 % germination) when whole seeds after-ripened for 4 months were incubated on 5 µM ABA for 48 h, conditions under which ScABI4 and Scarlet showed 92.1 and 5 % average germination, respectively. Thus, it appears that most of the seeds that germinated on ABA in the F2 segregation analysis are either homozygous or heterozygous for the ScABI4 mutation. Thus, ScABI4 is likely a partly dominant mutation.
ABA-insensitive mutants after-ripen more rapidly than wild type Scarlet
In characterizing these mutants, we found that a difference between wild-type and mutant can be seen between 1 and 4 months after harvest. However, the magnitude of this difference can vary depending on the dormancy of the seed lot, the length of time seeds are after-ripened, whether or not the seed was cut, and the length of time the seeds were incubated on ABA media (Table 1; Fig. 2; Online Resource 1 and 3).
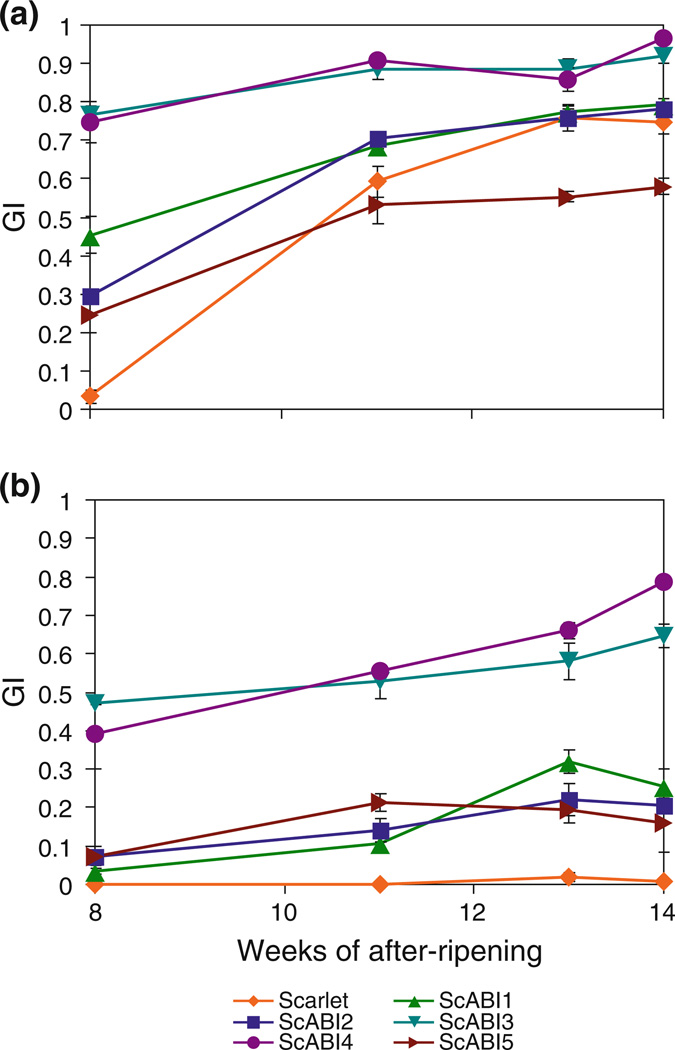
In order to better understand the effect of the ScABI mutants on seed dormancy and the requirement for after-ripening, we compared the germination of intact ScABI1, ScABI2, ScABI3, ScABI4, and ScABI5 mutant seed to wild-type Scarlet in the presence and absence of 5 µM ABA over an after-ripening time course (Fig. 4). The time course was initiated after 8 weeks of after-ripening when wild type Scarlet still failed to germinate in the absence of ABA. At 8 weeks after-ripening, all of the ScABI mutants showed higher germination efficiency than Scarlet in the absence of ABA; the GIs of the mutants ranged from 0.25 to 0.77 compared to Scarlet with a GI of 0.03 (Fig. 4a). However, by 13 weeks of after-ripening, Scarlet germinated as efficiently as ScABI1 and ScABI2, and more efficiently than ScABI5. Thus, while these mutants initially after-ripen more rapidly than Scarlet, Scarlet eventually reaches similar levels of seed germination. Scarlet showed little or no germination (maximum GI of 0.02) on 5 µM ABA throughout the after-ripening time course (Fig. 4b). All of the mutants germinated more efficiently than Scarlet on ABA. ScABI3 and ScABI4 after-ripen more rapidly and are more ABA insensitive than the other lines.
Fig. 4.
Germination of ScABI mutants and Scarlet wild type in the a absence and b presence of 5 µM ABA over a 6 week after-ripening time course. Intact seeds stored at room temperature were tested starting at 8 weeks of after-ripening. Germination index (GI) values shown are an average of three replicates of 10 seeds each counted over 5 days. Error bars represent standard errors of three replicates
ScABI3 and ScABI4 show little to no dormancy at 1 month of after-ripening (M4 retest, Table 1). Therefore, an experiment was undertaken to determine if these ScABI mutants lack dormancy as freshly harvested grain or at time points prior to 1 month of after-ripening. Seed of both mutants tested just 1 week after harvest displayed marked dormancy. ScABI3 showed 8 % germination after 5 days of incubation, whereas Scarlet showed 0 % germination. ScABI4 and Scarlet wild type showed 0 % germination after 1 week of imbibition. An independent ScABI4 seed lot that had after-ripened for 19 days showed 47.5 % germination after 5 days of incubation, whereas Scarlet showed 7.5 % germination. Given that ScABI3 and ScABI4 show 80–100 % germination with 4–8 weeks of after-ripening (Table 1), it appears that these two mutants are initially dormant but after-ripen more rapidly than Scarlet (Fig. 4). These data indicate that the ScABI3 and ScABI4 mutants are associated with rapid after-ripening but do not completely eliminate seed dormancy in the hard red spring Scarlet.
Characterization of vegetative phenotypes, plant height, flowering time, and leaf transpiration
In Arabidopsis, ABA insensitive mutants can be either seed specific or show both seed and vegetative phenotypes. To characterize the vegetative phenotypes of the ScABI mutants, we have examined the effects of these mutations on plant height, flowering time, and transpiration.
To determine whether ScABI mutants had changes in final plant height compared to Scarlet wild type, experiments were conducted on M6 plants to compare the height of ScABI mutants to wild type. Only ScABI1, ScABI2, and ScABI4 were significantly shorter than Scarlet wild type (Table 3). To determine if the reduced height co-segregated with the germination phenotype for ScABI4, F3 and parent plants from the segregation analysis experiment were measured at maturity. Reduced height did not appear to co-segregate with ABA insensitive germination based on six lines classified as ScABI4 homozygotes (data not shown).
Table 3.
Final plant height
| Genotype | Height (cm)a | Ratiob | P valuec |
|---|---|---|---|
| ScABI1 | 76.68 | 0.92 | <0.05 |
| ScABI2 | 67.95 | 0.82 | <0.001 |
| ScABI3 | 78.61 | 0.95 | NS |
| ScABI4 | 65.35 | 0.79 | <0.001 |
| ScABI5 | 84.75 | 1.02 | NS |
| ScABI6 | 79.66 | 0.96 | NS |
| Scarlet | 83.14 | – | – |
NS not significant
Mean plant height of 11–15 M6 plants per genotype grown in the greenhouse
Plant height of mutant relative to Scarlet wild type
P values refer to significance determined by analysis of variance using Dunnett’s multiple comparison adjustment
To determine whether ScABI mutants are associated with a change in time required to transition to flowering, a greenhouse experiment was conducted using M6 plants. In this experiment, ScABI1, ScABI2, and ScABI3 took significantly longer to head than Scarlet wild type (Table 4), whereas ScABI4, ScABI5, and ScABI6 were not significantly different from Scarlet wild type.
Table 4.
Time to transition to flowering
| Genotype | Days to headinga | P valueb |
|---|---|---|
| ScABI1 | 49.00 | <0.001 |
| ScABI2 | 48.58 | <0.001 |
| ScABI3 | 48.50 | <0.001 |
| ScABI4 | 46.25 | NS |
| ScABI5 | 45.33 | NS |
| ScABI6 | 46.00 | NS |
| Scarlet | 45.07 | – |
NS not significant
Mean number of days to first head emergence for 11–15 M6 plants per genotype grown in the greenhouse
P values refer to significance determined by analysis of variance using Dunnett’s multiple comparison adjustment
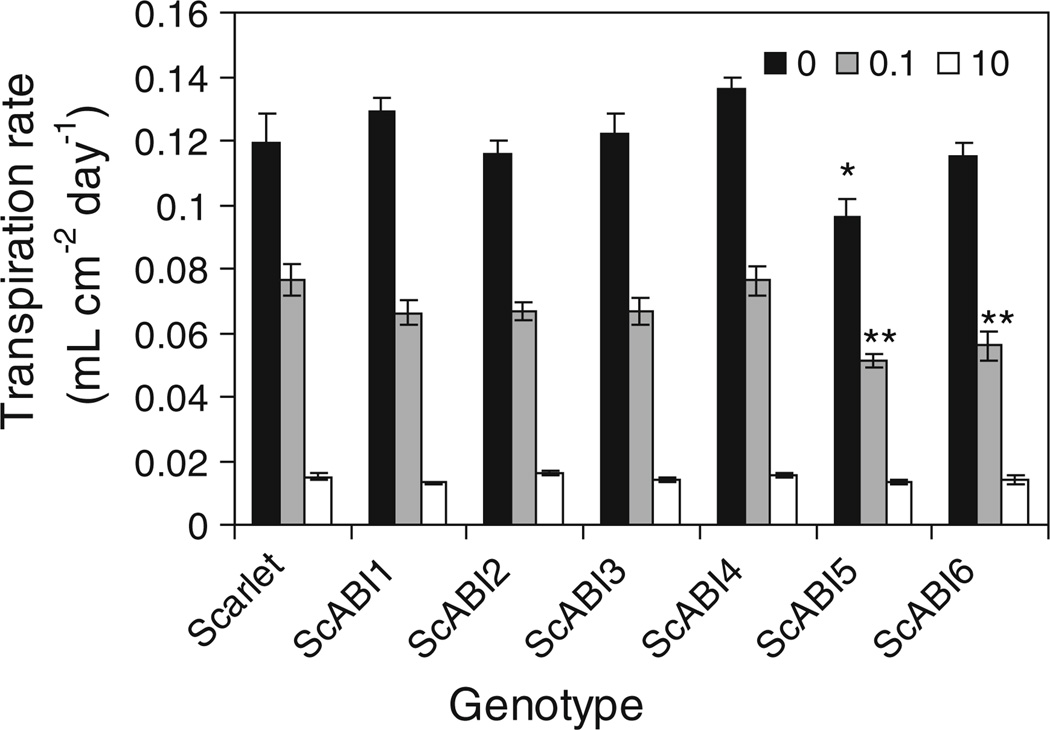
The ScABI mutants were examined to determine if they are associated with decreased vegetative transpiration in response to exogenous ABA. This was assayed using a detached leaf assay (Raskin and Ladyman 1988). ScABI1 and ScABI4 both showed a slight but non-significant increase in transpiration rate in the absence of ABA (Fig. 5). All of the mutants responded to ABA with decreased transpiration rates suggesting that none of these mutations result in a significant decrease in vegetative ABA sensitivity. ScABI5 and ScABI6 result in reduced transpiration at one or more ABA concentrations compared to Scarlet wild type.
Fig. 5.
Transpiration rate of detached leaves of ScABI mutants and Scarlet wild type. Transpiration rate of detached leaves from plants at stem elongation treated with three ABA concentrations: 0 µM (black), 0.1 µM (gray), or 10 µM (white). Significance determined by analysis of variance with Dunnett’s multiple comparison adjustment; *P < 0.05; **P < 0.01. Error bars represent standard error of the mean, with 4–5 plants per genotype per concentration
Discussion
Isolation of ScABI mutants with decreased ABA sensitivity and seed dormancy
When the goal is to isolate and characterize mutants with reduced ABA sensitivity and increased germination capacity is expected, a background with some dormancy is desirable to maximize the difference between mutants and wild type. Therefore, the moderately dormant hard red spring Scarlet was chosen for identification of ABA-insensitive mutants. Previous research showed that Scarlet grain fails to germinate efficiently when fully dormant, is sensitive to ABA when partly after-ripened, and ABA insensitive after prolonged after-ripening for 8–10 months (Schramm et al. 2010). Therefore, dormant mutagenized grain after-ripened for only 3 weeks was screened for the ability to germinate when treated with ABA, conditions under which wild type Scarlet would not germinate. Eleven mutants were initially isolated in the M2, and six mutants consistently showed higher germination efficiency on ABA than Scarlet over three generations (Table 1).
The mutants isolated can be classified based on ABA dose–response, dormancy, and secondary phenotypes. ScABI3 and ScABI4 are the most ABA insensitive showing increased germination in the absence of ABA and over all ABA concentrations tested in two dose–response experiments (Table 1; Fig. 1; Online Resource 1). The mutants ScABI1 and ScABI2 have an intermediate ABA insensitive germination phenotype, whereas ScABI5 and ScABI6 are weaker mutants showing increased germination in the absence of ABA and at low ABA concentrations (Fig. 1b), but not at higher ABA concentrations. It is possible that this weak phenotype results not from altered ABA signaling, but from altered seed morphology or development since ScABI5 and ScABI6 are associated with changes in seed shape and size. ScABI5 has a longer, narrower, darker colored, and somewhat shriveled seed compared to Scarlet, and ScABI6 has a shorter and narrower seed (Online Resource 2). ScABI5 resembles the fusca3 mutation of Arabidopsis that results in darker seed coat color associated with a reduction in seed dormancy (Keith et al. 1994). Alternatively, since these mutants affect primarily dormancy rather than ABA sensitivity, it is possible that there is some structural change affecting seed coat permeability, which may result in increased germination. Future work will need to examine whether these secondary phenotypes co-segregate with the germination phenotype.
Genetic analysis of ScABI3 and ScABI4
Based on the genetic pooling strategy of the mutant screen, the six mutants recovered from independent M1 plants likely represent independent mutations. The drastic reduction in dormancy and ABA sensitivity in ScABI3 and ScABI4 facilitated segregation analysis by choosing a point in after-ripening and germination where nearly 100 % of mutant seeds and close to 0 % of wild-type seeds germinated on ABA. Both ScABI3 and ScABI4 consistently appeared to be partly dominant in multiple F2 populations (Table 2; Fig. 2, Fig. 3; Online Resource 3). The isolation of dominant mutations is not surprising given that the allohexaploid nature of the wheat genome should make it difficult to recover single recessive mutations.
Most ScABI mutants appear to be seed specific
The ScABI mutants recovered in this study appear to be, for the most part, seed-specific in terms of ABA sensitivity. ABA response mutants in other species can be either seed-specific or may alter vegetative water relations due to the fact that ABA triggers stomatal closure in response to drought stress. Scarlet ABA insensitive mutants were examined for possible effects on water relations using a detached leaf transpiration assay (Fig. 5). None of the ScABI lines appear to be significantly insensitive to ABA in leaf transpiration. The expectation is that ABA insensitive mutants should show increased transpiration rate. Surprisingly, the two weak ABA-insensitive mutants ScABI5 and ScABI6 show a significant decrease in transpiration rate at one or more ABA concentrations. This may result from unlinked background mutations, or this may result from some developmental defect that somehow influences transpiration rate. For example, leaf structure may be affected, altering stomatal density as in the Arabidopsis sdd-1 mutant (Schlüter et al. 2003).
Some minor secondary phenotypes were detected. ScABI1, ScABI2, and ScABI4 were found to have average heights of 92, 82, and 79 %, respectively, of wild type height (Table 3), and ScABI1, ScABI2, and ScABI3 were associated with delays of 3–4 days in flowering (Table 4). The reduction in height in ScABI4, based on a ScABI4 backcross population, appears to be unlinked to the mutation causing increased germination. Whether the remaining secondary phenotypes are the result of background mutations from the mutagenesis will need to be examined.
The role of ABA response in wheat grain dormancy, after-ripening, and pre-harvest sprouting
This study and others indicate that altered ABA responsiveness results in altered grain dormancy in wheat (Kawakami et al. 1997; Rikiishi and Maekawa 2010; Schramm et al. 2010). The Warm (Wheat ABA responsive mutants) in Chinese Spring wheat result in ABA hypersensitive seed germination accompanied by higher grain dormancy and a need for longer after-ripening to break seed dormancy (Schramm et al. 2010). Conversely, the ScABI mutants result in ABA insensitive seed germination accompanied by reduced dormancy and rapid after-ripening (Table 1; Figs. 1, 4). The reduction in the time required for after-ripening directly correlates with the degree of ABA insensitivity (Figs. 1, 4). While this does not constitute proof, it is suggestive of a causal relationship. This result is consistent with previous studies that isolated EH47-1 and RSD32 (Reduced Seed Dormancy) wheat mutants based on reduced seed dormancy and subsequently found that these mutants were less sensitive to ABA (Kawakami et al. 1997; Kobayashi et al. 2006; Rikiishi and Maekawa 2010). Taken together, the fact that ABA hypersensitive wheat mutants are more dormant and that ABA insensitive mutants are less dormant suggests that variation for ABA sensitivity may be one mechanism controlling wheat grain dormancy and pre-harvest sprouting resistance.
Although the ScABI mutants after-ripen more rapidly than Scarlet wild type, they do not completely abolish seed dormancy. ScABI3 and ScABI4 seeds freshly harvested at physiological maturity fail to germinate and some seed dormancy is observed at 3 weeks of after-ripening. This may be due in part to genetic redundancy in hexaploid wheat. However, given that both ScABI3 and ScABI4 are partially dominant, we may speculate that ABA signaling is not the sole mechanism determining dormancy in the hard red spring Scarlet. Such mechanisms may include red testa color and other hormone signaling pathways such as jasmonic acid, gibberellin, ethylene, and brassinosteroids (Lalonde and Saini 1992; Ghassemian et al. 2000; Steber and McCourt 2001; Appleford et al. 2007; Barrero et al. 2009; Dave et al. 2011). Because the ScABI3 and ScABI4 mutations increase the rate of after-ripening without decreasing initial dormancy levels, these mutations may be useful in breeding red wheat cultivars that emerge better when planted within a few weeks of harvest without compromising pre-harvest sprouting resistance.
Given that wheat loses ABA sensitivity with after-ripening, it is difficult to be certain that the ABA insensitive mutants isolated are the result of mutations in ABA signaling genes rather than mutations that alter dormancy through another mechanism. It is interesting that Scarlet reaches the same germination efficiency as ScABI1 and ScABI2 with 13–14 weeks of after-ripening in the absence of ABA, but is more sensitive to ABA than the mutants at the same after-ripening time point (Fig. 4). This suggests that the increased germination on ABA is a result of reduced ABA signaling rather than differences in degree of dormancy. ScABI3 and ScABI4 are the most ABA insensitive during seed germination and so are good candidates for true ABA signaling mutants of wheat.
Possible mechanisms leading to reduced ABA sensitivity in ScABI wheat
ABA insensitive phenotypes can result either from increased ABA turnover or from reduced ABA signaling. For example, increased accumulation of the ABA catabolic enzyme ABA 8′-hydroxylase results in increased ABA turnover in Arabidopsis and increased turnover results in an ABA insensitive phenotype in Arabidopsis and in barley (Visser et al. 1996; Okamoto et al. 2006). Higher level expression of the gene encoding ABA 8′ -hydroxylase in barley is associated with loss of dormancy during after-ripening (Millar et al. 2006). In contrast, the reduced dormancy mutant TL43 on barley chromosome 6H shows an ABA insensitive germination phenotype without any change in endogenous ABA levels (Molina-Cano et al. 1999; Romagosa et al. 2001; Prada et al. 2005). Thus it is possible to alter seed dormancy and ABA sensitivity in cereals without any apparent change in ABA hormone accumulation.
Both ScABI3 and ScABI4 appear to be semi-dominant mutations resulting in ABA insensitive seed germination, but not ABA insensitive leaf transpiration. The Arabidopsis mutants abi3/vp1, abi4, and abi5 are seed-specific ABA insensitive mutants (reviewed by Finkelstein et al. 2008), but unlike ScABI3 and ScABI4, these mutants are recessive. The ABA-insensitive mutations in the Arabidopsis PP2C subfamily including ABI1, ABI2, and HAB1, are semi-dominant, but unlike ScABI lines, these mutants result in both ABA insensitive seed germination and stomatal response (Koornneef et al. 1984; Allen et al. 1999; Dupeux et al. 2011). Other members of this PP2C family, AHG1 and AHG3, appear to regulate only seed germination due to the fact that they are expressed only in seeds (Nishimura et al. 2004, 2007; Yoshida et al. 2005). It is possible that mutations in a PP2C of wheat with seed-specific expression could result in the phenotypes observed here. Alternatively, ScABI mutants could result from gain-of-function mutants in other negative regulators of ABA signaling.
Acknowledgments
The authors would like to thank K. Kidwell for providing seeds of Scarlet wheat, and S. Abrams for providing (+)-ABA. Thanks are due to R. Parveen and A. Burke for expert technical assistance, to K. Garland Campbell for advice and assistance, and to C. Walker for assistance with statistical analysis. This work was supported by NIH Protein Biotechnology training grant funding (ECS), a fellowship from ARCS (Seattle chapter) (ECS), and USDA CSREES grant number 2005-01099 (CMS).
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s10681-012-0669-1) contains supplementary material, which is available to authorized users.
Contributor Information
Elizabeth C. Schramm, Department of Crop and Soil Sciences and the Molecular Plant Sciences Program, Washington State University, Pullman, WA 99164-6420, USA
Sven K. Nelson, Department of Crop and Soil Sciences and the Molecular Plant Sciences Program, Washington State University, Pullman, WA 99164-6420, USA
Camille M. Steber, Department of Crop and Soil Sciences and the Molecular Plant Sciences Program, Washington State University, Pullman, WA 99164-6420, USA Wheat Genetics, Physiology, Biochemistry, and Quality Unit, USDA-ARS, Pullman, WA 99164-6420, USA, csteber@wsu.edu, URL: http://public.wsu.edu/~csteber/.
References
- Abramoff MD, Magalhaes PJ, Ram SJ. Image processing with ImageJ. Biophotonics Int. 2004;11:36–42. [Google Scholar]
- Allen GJ, Kuchitsu K, Chu SP, Murata Y, Schroeder JI. Arabidopsis abi1-1 and abi2-1 phosphatase mutations reduce abscisic acid-induced cytoplasmic calcium rises in guard cells. Plant Cell. 1999;11:1785–1798. doi: 10.1105/tpc.11.9.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleford NEJ, Wilkinson MD, Ma Q, Evans DJ, Stone MC, Pearce SP, Powers SJ, Thomas SG, Jones HD, Phillips AL, Hedden P, Lenton JR. Decreased shoot stature and grain α-amylase activity following ectopic expression of a gibberellin 2-oxidase gene in transgenic wheat. J Exp Bot. 2007;56:3213–3226. doi: 10.1093/jxb/erm166. [DOI] [PubMed] [Google Scholar]
- Barrero J, Talbot MJ, White RG, Jacobsen JV, Gubler F. Anatomical and transcriptomic studies of the coleorhiza reveal the importance of this tissue in regulating dormancy in barley. Plant Physiol. 2009;150:1006–1021. doi: 10.1104/pp.109.137901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bewley JD. Seed germination and dormancy. Plant Cell. 1997;9:1055–1066. doi: 10.1105/tpc.9.7.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bewley JD, Black M. Seeds: physiology of development and germination. New York: Plenum Press; 1994. [Google Scholar]
- Chono M, Honda I, Shinoda S, Kushiro T, Kamiya Y, Nambara E, Kawakami N, Kaneko S, Watanabe Y. Field studies on the regulation of abscisic acid content and germinability during grain development of barley: molecular and chemical analysis of pre-harvest sprouting. J Exp Bot. 2006;57:2421–2434. doi: 10.1093/jxb/erj215. [DOI] [PubMed] [Google Scholar]
- Dave A, Hernández ML, He Z, Andriotis ME, Vaistij FE, Larson TR, Graham IA. 12-oxo-phytodienoic acid accumulation during seed development represses seed germination in Arabidopsis . Plant Cell. 2011;23:583–599. doi: 10.1105/tpc.110.081489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubcovsky J, Dvorak J. Genome plasticity a key factor in the success of polyploid wheat under domestication. Science. 2007;316:1862–1866. doi: 10.1126/science.1143986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupeux F, Antoni R, Betz K, Santiago J, Gonzalez-Guzman M, Rodriguez L, Rubio S, Park S-Y, Cutler SR, Rodriguez PL, Márquez JA. Modulation of abscisic acid signaling in vivo by an engineered receptor-insensitive protein phosphatase type 2C allele. Plant Physiol. 2011;156:106–116. doi: 10.1104/pp.110.170894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R, Reeves W, Ariizumi T, Steber C. Molecular aspects of seed dormancy. Annu Rev Plant Biol. 2008;59:387–415. doi: 10.1146/annurev.arplant.59.032607.092740. [DOI] [PubMed] [Google Scholar]
- Flintham JE. Different genetic components control coat-imposed and embryo-imposed dormancy in wheat. Seed Sci Res. 2000;10:43–50. [Google Scholar]
- Galili S, Avivi Y, Millet E, Feldman M. RFLP-based analysis of three RbcS subfamilies in diploid and polyploid species of wheat. Mol Genet Genomics. 2000;263:674–680. doi: 10.1007/s004380051216. [DOI] [PubMed] [Google Scholar]
- Ghassemian M, Nambara E, Cutler S, Kawaide H, Kamiya Y, McCourt P. Regulation of abscisic acid signaling by the ethylene response pathway in Arabidopsis. Plant Cell. 2000;12:1117–1126. doi: 10.1105/tpc.12.7.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groot SPC, Karssen CM. Dormancy and germination of abscisic acid-deficient tomato seeds: studies with the sitiens mutant. Plant Physiol. 1992;99:952–958. doi: 10.1104/pp.99.3.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler F, Millar AA, Jacobsen JV. Dormancy release, ABA and pre-harvest sprouting. Curr Opin Plant Biol. 2005;8:183–187. doi: 10.1016/j.pbi.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Himi E, Mares DJ, Yanagisawa A, Noda K. Effect of grain colour gene (R) on grain dormancy and sensitivity of the embryo to abscisic acid (ABA) in wheat. J Exp Bot. 2002;53:1569–1574. doi: 10.1093/jxb/erf005. [DOI] [PubMed] [Google Scholar]
- Himi E, Maekawa M, Miura H, Noda K. Development of PCR markers for Tamyb10 related to R-1, red grain color gene in wheat. Theor Appl Genet. 2011;122:1561–1576. doi: 10.1007/s00122-011-1555-2. [DOI] [PubMed] [Google Scholar]
- Holdsworth MJ, Bentsink L, Soppe WJJ. Molecular networks regulating Arabidopsis seed maturation, after-ripening, dormancy and germination. New Phytol. 2008;179:33–54. doi: 10.1111/j.1469-8137.2008.02437.x. [DOI] [PubMed] [Google Scholar]
- Jiang J, Gill BS. New 18S·26S ribosomal RNA gene loci: chromosomal landmarks for the evolution of polyploid wheats. Chromosoma. 1994;103:179–185. doi: 10.1007/BF00368010. [DOI] [PubMed] [Google Scholar]
- Karssen CM, Laçka E. A revision of the hormone balance theory of seed dormancy: studies on gibberellin and/or abscisic acid-deficient mutants of Arabidopsis thaliana . In: Bopp M, editor. Plant growth substances 1985: proceedings of the 12th international conference on plant growth substances; New York: Springer; 1986. pp. 315–323. [Google Scholar]
- Kawakami N, Miyake Y, Noda K. ABA insensitivity and low ABA levels during seed development of non-dormant wheat mutants. J Exp Bot. 1997;48:1415–1421. [Google Scholar]
- Keith K, Kraml M, Dengler NG, McCourt P. fusca3: a heterochronic mutation affecting late embryo development in Arabidopsis. Plant Cell. 1994;6:589–600. doi: 10.1105/tpc.6.5.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidwell KK, Shelton GB, Morris CF, Line RF, Miller BC, Davis MA, Konzak CF. Registration of ‘Scarlet’ wheat. Crop Sci. 1999;39:1255. [Google Scholar]
- Klinger JP, Batelli G, Zhu J-K. ABA receptors: the START of a new paradigm in phytohormone signalling. J Exp Bot. 2010;61:3199–3210. doi: 10.1093/jxb/erq151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi F, Takumi S, Egawa C, Ishibashi M, Nakamura C. Expression patterns of low temperature responsive genes in a dominant ABA-less-sensitive mutant line of common wheat. Physiol Plantarum. 2006;127:612–623. [Google Scholar]
- Koornneef M, Jorna ML, Brinkhorst-van der Swan DLC, Karssen CM. The isolation of abscisic acid (ABA) deficient mutants by selection of induced revertants in non-germinating gibberellin sensitive lines of Arabidopsis thaliana (L.) heynh. Theor Appl Genet. 1982;61:385–393. doi: 10.1007/BF00272861. [DOI] [PubMed] [Google Scholar]
- Koornneef M, Reuling G, Karssen CM. The isolation and characterization of abscisic acid-insensitive mutants of Arabidopsis thaliana . Physiol Plantarum. 1984;61:377–383. [Google Scholar]
- Kushiro T, Okamoto M, Nakabayashi K, Yamagishi K, Kitamura S, Asami T, Hirai N, Koshiba T, Kamiya Y, Nambara E. The Arabidopsis cytochrome P450 CYP707A encodes ABA 8′-hydroxylases: key enzymes in ABA catabolism. EMBO J. 2004;23:1647–1656. doi: 10.1038/sj.emboj.7600121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalonde S, Saini HS. Comparative requirement for endogenous ethylene during seed germination. Ann Bot Lond. 1992;69:423–428. [Google Scholar]
- Leung J, Merlot S, Giraudat J. The Arabidopsis ABSCISIC ACID-INSENSITIVE2 (ABI2) and ABI1 genes encode homologous protein phosphatases 2C involved in abscisic acid signal transduction. Plant Cell. 1997;9:759–771. doi: 10.1105/tpc.9.5.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Szostkiewicz I, Korte A, Moes D, Yang Y, Christman A, Grill E. Regulators of PP2C phosphatase activity function as abscisic acid sensors. Science. 2009;324:1064–1068. doi: 10.1126/science.1172408. [DOI] [PubMed] [Google Scholar]
- McCallum JA, Walker JRL. Phenolic biosynthesis during grain development in wheat: changes in phenylalanine ammonia-lyase activity and soluble phenolic content. J Cereal Sci. 1990;11:35–49. [Google Scholar]
- McCarty DR, Hattori T, Carson CB, Vasil V, Lazar M, Vasil IK. The Viviparous-1 developmental gene of maize encodes a novel transcriptional activator. Cell. 1991;66:895–905. doi: 10.1016/0092-8674(91)90436-3. [DOI] [PubMed] [Google Scholar]
- McKibbin RS, Wilkinson MD, Bailey PC, Flintham JE, Andrew LM, Lazzeri PA, Gale MD, Lenton JR, Holdsworth MJ. Transcripts of Vp-1 homeologues are misspliced in modern wheat and ancestral species. Proc Natl Acad Sci USA. 2002;99:10203–10208. doi: 10.1073/pnas.152318599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar AA, Jacobsen JV, Ross JJ, Helliwell CA, Poole AT, Scofield G, Reid JB, Gubler F. Seed dormancy and ABA metabolism in Arabidopsis and barley: the role of ABA 8′-hydroxylase. Plant J. 2006;45:942–954. doi: 10.1111/j.1365-313X.2006.02659.x. [DOI] [PubMed] [Google Scholar]
- Miyamoto T, Everson EH. Biochemical and physiological studies of wheat seed pigmentation. Agron J. 1958;50:733–734. [Google Scholar]
- Molina-Cano JL, Sopena A, Swanston JS, Casas AM, Moralejo MA, Ubieto A, Lara I, Pérez-Vendrell AM, Romagosa I. A mutant induced in the malting barley cv Triumph with reduced dormancy and ABA response. Theor Appl Genet. 1999;98:347–355. [Google Scholar]
- Morris CF, Moffatt JM, Sears RG, Paulsen GM. Seed dormancy and responses of caryopses, embryos, and calli to abscisic acid in wheat. Plant Physiol. 1989;90:643–647. doi: 10.1104/pp.90.2.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura N, Yoshida T, Murayama M, Asami T, Shinozaki K, Hirayama T. Isolation and characterization of novel mutants affecting the abscisic acid sensitivity of Arabidopsis germination and seedling growth. Plant Cell Physiol. 2004;45:1485–1499. doi: 10.1093/pcp/pch171. [DOI] [PubMed] [Google Scholar]
- Nishimura N, Yoshida T, Kitahata N, Asami T, Shinozaki K, Hirayama T. ABA-Hypersensitive Germination1 encodes a protein phosphatase 2C, an essential component of abscisic acid signaling in Arabidopsis seed. Plant J. 2007;50:935–949. doi: 10.1111/j.1365-313X.2007.03107.x. [DOI] [PubMed] [Google Scholar]
- Nishimura N, Sarkeshik A, Nito K, Park S-Y, Wang A, Carvalho PC, Lee S, Caddell DF, Cutler SR, Chory J, Yates JR, Schroeder JI. PYR/PYL/RCAR family members are major in vivo ABI1 protein phosphatase 2C–interacting proteins in Arabidopsis. Plant J. 2010;61:290–299. doi: 10.1111/j.1365-313X.2009.04054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto M, Kuwahara A, Seo M, Kushiro T, Asami T, Hirai N, Kamiya Y, Koshiba T, Nambara E. CYP707A1 and CYP707A2, which encode abscisic acid 8′-hydroxylases, are indispensible for proper control of seed dormancy and germination in Arabidopsis. Plant Physiol. 2006;141:97–107. doi: 10.1104/pp.106.079475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okubara PA, Steber CM, DeMacon VL, Walter NL, Paulitz TC, Kidwell KK. Scarlet-Rz1, and EMS-generated hexaploid wheat with tolerance to the soilborne necrotrophic pathogens Rhizoctonia solani AG-8 and R. oryzae . Theor Appl Genet. 2009;119:293–303. doi: 10.1007/s00122-009-1038-x. [DOI] [PubMed] [Google Scholar]
- Park S-Y, Fung P, Nishimura N, Jensen DR, Fujii H, Zhao Y, Lumba S, Santiago J, Rodrigues A, Chow T-FF, Alfred SE, Bonetta D, Finkelstein R, Provart NJ, Desveaux D, Rodriguez PL, McCourt P, Zhu J-K, Schroeder JI, Volk-man BF, Cutler SR. Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science. 2009;324:1068–1071. doi: 10.1126/science.1173041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prada D, Romagosa I, Ullrich SE, Molina-Cano JL. A centromeric region on chromosome 6 (6H) affects dormancy in an induced mutant in barley. J Exp Bot. 2005;56:47–54. doi: 10.1093/jxb/eri005. [DOI] [PubMed] [Google Scholar]
- Raskin I, Ladyman JAR. Isolation and characterization of a barley mutant with abscisic acid-insensitive stomata. Planta. 1988;173:73–78. doi: 10.1007/BF00394490. [DOI] [PubMed] [Google Scholar]
- Rikiishi K, Maekawa M. Characterization of a novel wheat (Triticum aestivum L.) mutant with reduced seed dormancy. J Cereal Sci. 2010;51:292–298. [Google Scholar]
- Romagosa I, Prada D, Moralejo MA, Sopena A, Muñoz P, Casas AM, Swanston JS, Molina-Cano JL. Dormancy, ABA content and sensitivity of a barley mutant to ABA application during seed development and after ripening. J Exp Bot. 2001;52:1499–1506. doi: 10.1093/jexbot/52.360.1499. [DOI] [PubMed] [Google Scholar]
- Schlüter U, Muschak M, Berger D, Altmann T. Photo-synthetic performance of an Arabidopsis mutant with elevated stomatal density (sdd1-1) under different light regimes. J Exp Bot. 2003;54:867–874. doi: 10.1093/jxb/erg087. [DOI] [PubMed] [Google Scholar]
- Schramm EC, Abellera JC, Strader LC, Campbell KG, Steber CM. Isolation of ABA-responsive mutants in allo-hexaploid bread wheat (Triticum aestivum L.): drawing connections to grain dormancy, preharvest sprouting, and drought tolerance. Plant Sci. 2010;179:620–629. [Google Scholar]
- Schwartz SH, Tan BC, Gage DA, Zeevaart JAD, McCarty DR. Specific oxidative cleavage of carotenoids by Vp14 of maize. Science. 1997;276:1872–1874. doi: 10.1126/science.276.5320.1872. [DOI] [PubMed] [Google Scholar]
- Steber CM, McCourt P. A role for brassinosteroids in germination in Arabidopsis. Plant Physiol. 2001;125:763–769. doi: 10.1104/pp.125.2.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torada A, Amano Y. Effect of seed coat color on seed dormancy in different environments. Euphytica. 2002;126:99–105. [Google Scholar]
- Tuteja N. Abscisic acid and abiotic stress signaling. Plant Signal Behav. 2007;2:135–138. doi: 10.4161/psb.2.3.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser K, Vissers APA, Çağirgan MI, Kijne JW, Wang M. Rapid germination of a barley mutant is correlated with a rapid turnover of abscisic acid outside the embryo. Plant Physiol. 1996;111:1127–1133. doi: 10.1104/pp.111.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker-Simmons M. ABA levels and sensitivity in developing wheat embryos of sprouting resistant and susceptible cultivars. Plant Physiol. 1987;84:61–66. doi: 10.1104/pp.84.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner RL, Kudrna DA, Spaeth SC, Jones SS. Dormancy in white-grain mutants of Chinese Spring wheat (Triticum aestivum L.) Seed Sci Res. 2000;10:51–60. [Google Scholar]
- Yoshida T, Nishimura N, Kitahata N, Kuromori T, Ito T, Asami T, Shinozaki K, Hirayama T. ABA-Hypersensitive Germination3 encodes a protein phosphatase 2C (AtPP2CA) that strongly regulates abscisic acid signaling during germination among Arabidopsis protein phosphatase 2Cs. Plant Physiol. 2005;140:115–126. doi: 10.1104/pp.105.070128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Jia W, Yang J, Ismail AM. Role of ABA in integrating plant responses to drought and salt stresses. Field Crop Res. 2006;97:111–119. [Google Scholar]